Benign strictures are frequent complications following colorectal surgery, with an incidence of up to 20%. Endoscopic treatment is safe and effective but there is not enough evidence for establishing stricture management at that anatomic level.
AimTo determine the risk factors associated with the development of stricture in patients with colorectal cancer and describe endoscopic treatment in those patients.
Materials and methodsA retrospective study was conducted on patients with colorectal cancer that underwent surgery and anastomosis, evaluated through colonoscopy, within the time frame of 2014 to 2019.
ResultsOf the 213 patients included in the study, 18.3% presented with stricture that was associated with the type of surgery. Intersphincteric resection was a risk factor (OR = 18.81, 95% CI: 3.31-189.40, p < 0.001). A total of 69.2% patients with stricture had a stoma, identifying it as a risk factor for stricture (OR = 7.07, 95% CI: 3.10-16.57, p < 0.001). Mechanical anastomotic stapling was performed in 87.4% of the patients that did not present with stricture, identifying it as a protective factor (OR = 0.41, 95% CI: 0.16-1.1, p = 0.04).
Endoscopic treatment was required in 69.2% of the patients and provided favorable results in 83.3%. Only 2.6% of the patients had recurrence. No complications were reported.
ConclusionIntersphincteric resection and the presence of a stoma were independent risk factors for stricture, and mechanical anastomosis was a protective factor against stricture development. Endoscopic treatment was safe and effective.
Las estenosis benignas son complicaciones frecuentes posterior a la cirugía colorectal con una incidencia hasta del 20%. El tratamiento endoscópico es seguro y efectivo pero no hay evidencia suficiente para establecer el manejo de las estenosis a este nivel.
ObjetivoDeterminar los factores de riesgo asociados al desarrollo de estenosis en pacientes con cáncer y describir el tratamiento endoscópico en estos pacientes
Material y métodosEs un estudio retrospectivo en pacientes con cáncer colorectal sometidos a cirugìa y anastomosis valorados por colonoscopia entre el 2014 y 2019.
ResultadosSe incluyeron 213 pacientes, 18.3% con estenosis, la cual se asoció con el tipo de cirugía, siendo la RIE un factor de riesgo OR = 18.81 (IC95% 3.31- 189.40, p < 0.001). La presencia de estoma fue de 69.2% en los pacientes con estenosis identificándose como factor de riesgo para estenosis OR = 7.07 (3.10 - 16.57, p < 0.001). Las anastomosis se hicieron de forma mecánica en el 87.4% de los pacientes sin estenosis, siendo identificado como factor protector OR = 0.41 (IC 0.16 - 1.1, p = 0.04).
El 69.2% requirieron tratamiento endoscópico con resultados favorables en el 83.3% y recurrencia en el 2.6% únicamente. No se reportaron complicaciones.
ConclusiónLa RIE y la presencia de un estoma son factores de riesgo independiente para estenosis y la conformación mecánica de la anastomosis como un factor protector contra el desarrollo de estenosis. El tratamiento endoscópico es una opción segura y efectiva.
Benign strictures of the colon are a relatively frequent entity, with a reported incidence of 5.8 to 20% in patients with anastomosis after colorectal resection. Strictures have been observed to be more frequent, the closer they are to the anal verge.1
Strictures are not an insignificant complication, given that they can cause fecal urgency or incontinence, and in extreme cases, intestinal obstruction, importantly impacting patient quality of life.2
Obstructive symptoms appear in only 2-5% of cases and the majority of those patients present with abdominal pain, constipation and/or incontinence.3
Strictures have been reported to occur from 6 months to 11 years after surgery.4
The most accepted theory as to the pathogenesis of strictures is tissue ischemia. The risk factors that have been associated with increased risk for stricture are obesity, bleeding, anastomotic leak, abdominal collections, and adjuvant radiation.5
Different authors have reported male sex, body mass index > 25 kg/m2, a history of smoking, type of surgery (low or ultralow anterior resection), and the presence of a protective stoma as factors that predispose to stricture development.6
Surgical treatment consists of a resection of the stricture area and re-anastomosis. That procedure involves a high level of morbidity, with considerable risk for death. For several decades, endoscopic dilation has been proposed as a safe and effective alternative to surgical treatment.7
At present, the precise pathophysiology of strictures is unknown, resulting in a lack of standardized treatment for those patients. Therefore, knowledge of the risk factors in our patient population can aid in the prevention of stricture development and guide opportune treatment.8
The primary aim of our study was to identify the risk factors associated with the postoperative development of stricture in patients that underwent colorectal anastomosis as treatment for colorectal cancer and describe the endoscopic treatment of said stricture.
Materials and methodsA retrospective, longitudinal study was conducted, in which the case records were reviewed of patients diagnosed with colorectal cancer that underwent surgical resection and anastomosis, evaluated through colonoscopy, within the time frame of January 1, 2014 and January 1, 2019 at the Instituto Nacional de Cancerología in Mexico City.
Inclusion criteriaPatients above 18 years of age, diagnosed with colorectal cancer, and that underwent surgery with resection and anastomosis.
Exclusion criteriaPatients with colorectal resection not due to colorectal cancer, patients that underwent the Hartmann procedure, abdominal-perineal resection, or that had a permanent stoma.
All the patients were studied by means of flexible colonoscopy, determining the distance of the tumor from the anal verge in cm. Computed axial tomography or magnetic resonance imaging, and in some selected cases, endorectal endoscopy, were performed. Patients considered to have a locally advanced clinical status received neoadjuvant chemotherapy and/or radiotherapy at doses of 45 to 50.4 Gy in 28 fractions.
Patients that were surgical candidates underwent resection, according to tumor location, with an open or laparoscopic approach, and manual or mechanical anastomosis. A protective stoma was placed depending on the surgeon’s criterion and the conditions of the patient.
After the surgery, the patients were classified according to pathologic stage and those with lymph node invasion or residual disease received adjuvant chemotherapy or radiotherapy.
Stricture was defined in patients that underwent colonoscopy after the surgery if the diameter of the anastomosis was smaller than the exploratory finger at digital rectal examination or smaller than the diameter of the colonoscope (13.2 mm).
All patients with a reduced stricture diameter were treated according to the percentage of stricture and preference of the endoscopist through mechanical dilation, hydrostatic balloon dilation, radial incisions, or dual therapy (balloon dilation plus radial incisions).
Hydrostatic balloons were utilized for balloon dilation. The size of the balloon was selected, based on the initial diameter of the stricture and depending on the increase of caliber achieved, initial stricture size, presence of ulcerations or secondary tears, and patient discomfort. Insufflation was sustained for one minute for each diameter. After bowel preparation with split-dose polyethylene glycol (4 l), the procedures were performed, under direct vision or fluoroscopic control, on the sedated patient. Strictures were reevaluated endoscopically every 2 weeks, and treatment was established according to the findings.
Statistical analysisThe categorical variables were compared utilizing chi-square tests and the linear variables were analyzed employing the Student’s t test and the Mann-Whitney U test. All variables with a p < 0.05 were considered statistically significant. The statistical analysis was performed using the SPSS version 21.0 software.
Ethical considerationsInformed consent was not required for the publication of the present study, given that no personal data were published that could identify the patients. The bioethics committee of the hospital approved the study protocol, as well as the review and analysis of the case records.
The present work abided by the current regulations for bioethics research and authorization was obtained from the corresponding ethics committee of the healthcare institution where the study was conducted.
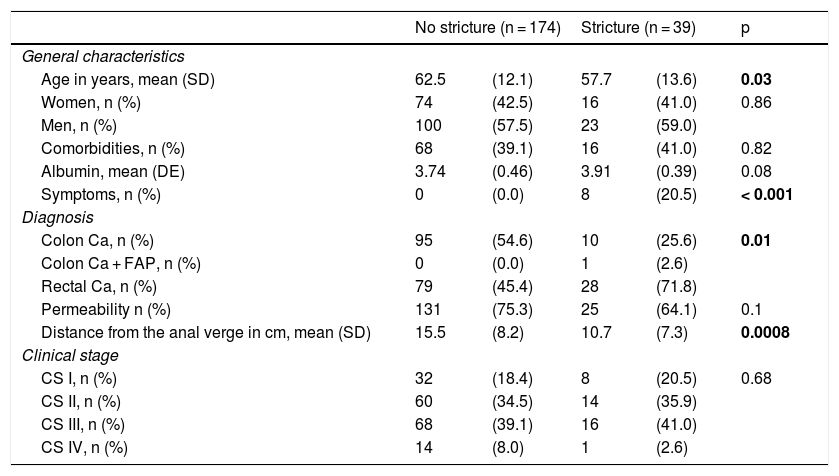
ResultsThe data of 213 patients were included and 18.3% of them presented with anastomotic stricture. Table 1 shows the clinical-pathologic variables analyzed in the two groups.
Correlation between anastomotic stricture and demographic variables.
| No stricture (n = 174) | Stricture (n = 39) | p | |||
|---|---|---|---|---|---|
| General characteristics | |||||
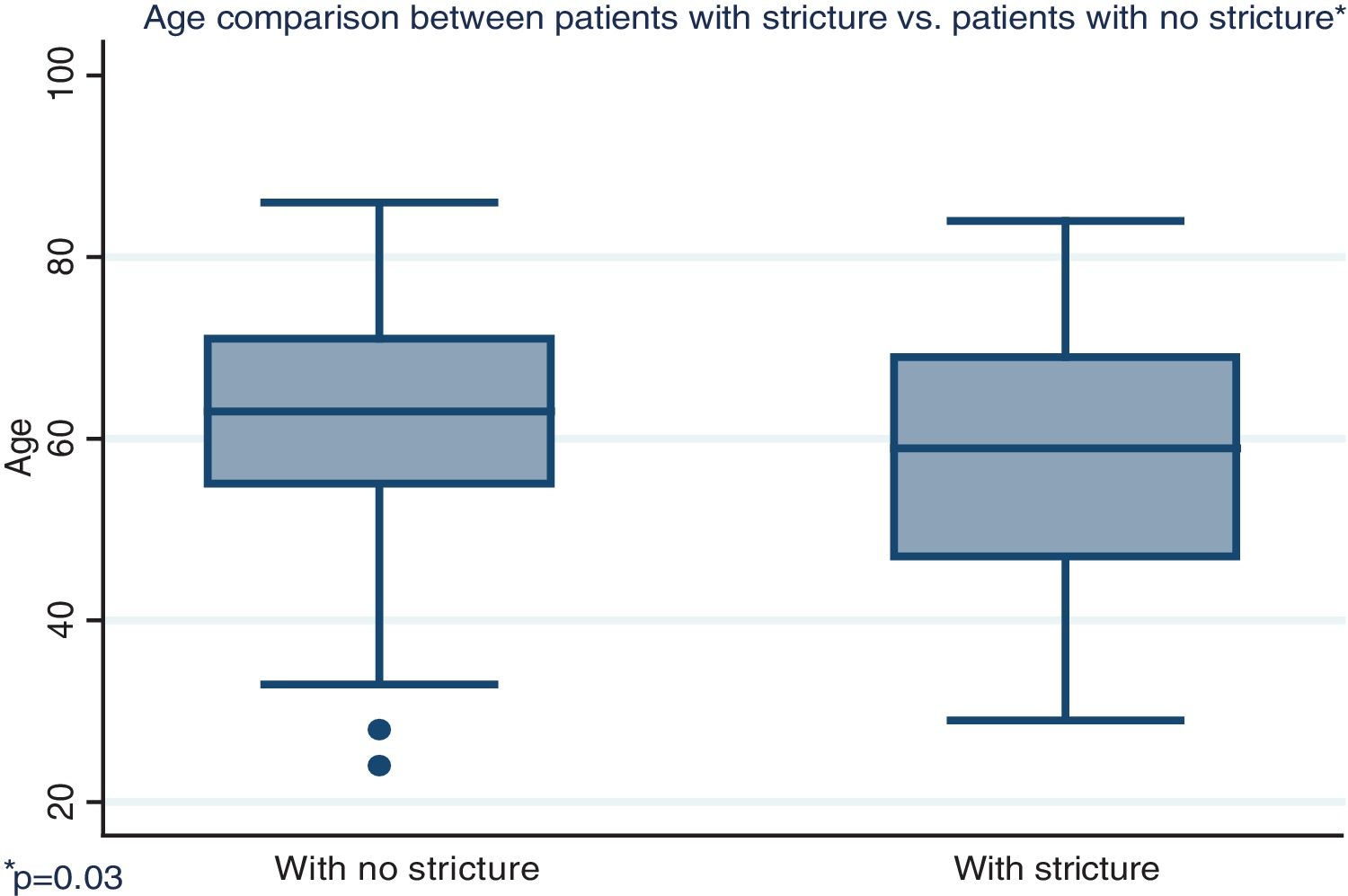
| Age in years, mean (SD) | 62.5 | (12.1) | 57.7 | (13.6) | 0.03 |
| Women, n (%) | 74 | (42.5) | 16 | (41.0) | 0.86 |
| Men, n (%) | 100 | (57.5) | 23 | (59.0) | |
| Comorbidities, n (%) | 68 | (39.1) | 16 | (41.0) | 0.82 |
| Albumin, mean (DE) | 3.74 | (0.46) | 3.91 | (0.39) | 0.08 |
| Symptoms, n (%) | 0 | (0.0) | 8 | (20.5) | < 0.001 |
| Diagnosis | |||||
| Colon Ca, n (%) | 95 | (54.6) | 10 | (25.6) | 0.01 |
| Colon Ca + FAP, n (%) | 0 | (0.0) | 1 | (2.6) | |
| Rectal Ca, n (%) | 79 | (45.4) | 28 | (71.8) | |
| Permeability n (%) | 131 | (75.3) | 25 | (64.1) | 0.1 |
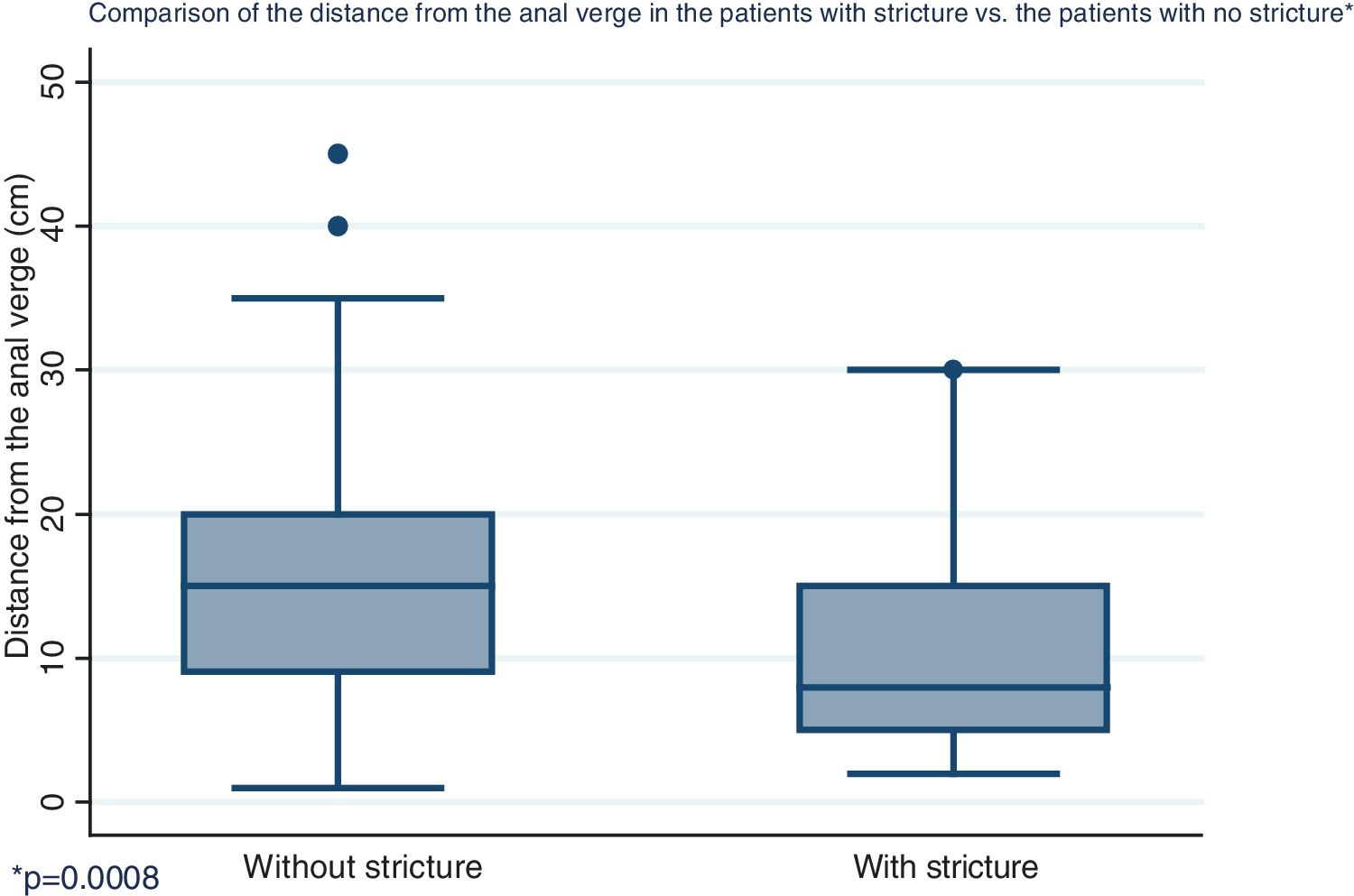
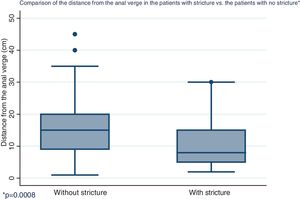
| Distance from the anal verge in cm, mean (SD) | 15.5 | (8.2) | 10.7 | (7.3) | 0.0008 |
| Clinical stage | |||||
| CS I, n (%) | 32 | (18.4) | 8 | (20.5) | 0.68 |
| CS II, n (%) | 60 | (34.5) | 14 | (35.9) | |
| CS III, n (%) | 68 | (39.1) | 16 | (41.0) | |
| CS IV, n (%) | 14 | (8.0) | 1 | (2.6) | |
Ca: cancer; CS: clinical stage; FAP: familial adenomatous polyposis; n: number; SD: standard deviation.
Statistically significant values are in bold text.
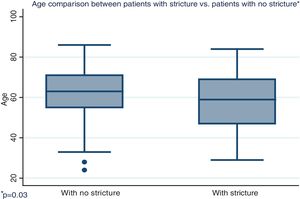
Distribution by sex of the patient total resulted in 57.7% men and 42.3% women, with a mean patient age of 61 ± 12.4 years (Fig. 1). Initial tumor location in cm, with the anal verge as the reference point, was measured through colonoscopy, with a mean 15.5 cm in the patients with no stricture and 10.7 cm in the group with stricture (Fig. 2).
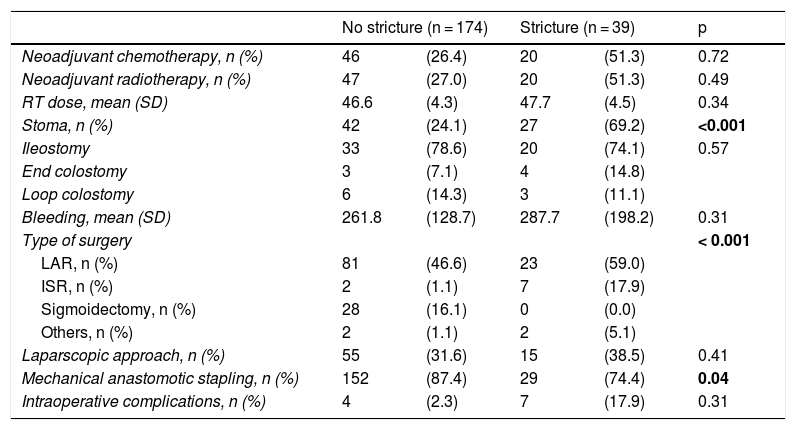
A total of 24.1% of the patients with no stricture had some type of stoma, as did 69.2% of the patients with stricture. Mechanical anastomosis was performed on 87.4% of the patients with no stricture and 74.4% of the patients with stricture (Table 2).
Correlation between anastomotic stricture and oncologic treatment.
| No stricture (n = 174) | Stricture (n = 39) | p | |||
|---|---|---|---|---|---|
| Neoadjuvant chemotherapy, n (%) | 46 | (26.4) | 20 | (51.3) | 0.72 |
| Neoadjuvant radiotherapy, n (%) | 47 | (27.0) | 20 | (51.3) | 0.49 |
| RT dose, mean (SD) | 46.6 | (4.3) | 47.7 | (4.5) | 0.34 |
| Stoma, n (%) | 42 | (24.1) | 27 | (69.2) | <0.001 |
| Ileostomy | 33 | (78.6) | 20 | (74.1) | 0.57 |
| End colostomy | 3 | (7.1) | 4 | (14.8) | |
| Loop colostomy | 6 | (14.3) | 3 | (11.1) | |
| Bleeding, mean (SD) | 261.8 | (128.7) | 287.7 | (198.2) | 0.31 |
| Type of surgery | < 0.001 | ||||
| LAR, n (%) | 81 | (46.6) | 23 | (59.0) | |
| ISR, n (%) | 2 | (1.1) | 7 | (17.9) | |
| Sigmoidectomy, n (%) | 28 | (16.1) | 0 | (0.0) | |
| Others, n (%) | 2 | (1.1) | 2 | (5.1) | |
| Laparscopic approach, n (%) | 55 | (31.6) | 15 | (38.5) | 0.41 |
| Mechanical anastomotic stapling, n (%) | 152 | (87.4) | 29 | (74.4) | 0.04 |
| Intraoperative complications, n (%) | 4 | (2.3) | 7 | (17.9) | 0.31 |
IST: intersphincteric resection; LAR: low anterior resection; n: number.
Statistically significant values are in bold text.
The presence of a stoma was identified as an independent risk factor for stricture, with an OR of 7.07 (95% CI 3.10-16.57, p < 0.001), and mechanical anastomosis was identified as a protective factor, with an OR of 0.41 (95% CI 0.16-1.1, p = 0.04).
Stricture development in the colorectal anastomoses was associated with the type of surgery, and intersphincteric resection (ISR) was the highest risk factor (OR = 18.81, 95% CI: 3.31-189.40, p < 0.001).
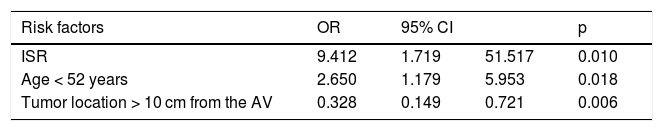
The significant variables were included in the construction of the multivariate logistic regression models for the presence of stricture. The independent risk factors were ISR (OR = 9.4, 95% CI: 1.17-51.5, p = 0.01); age under 52 years (OR = 2.6, 95% CI: 1.17-5.9, p = 0.018), and tumor location more than 10 cm from the anal verge (OR = 0.32, 95% CI: 0.14-0.72, p = 0.006) (Table 3).
Of the patients that developed stricture, 8 (20.5%) had symptoms, and of those, 3 (37.5%) had a stoma. The mean reduction of the lumen reported by colonoscopy was 79.7% ± 18.6%.
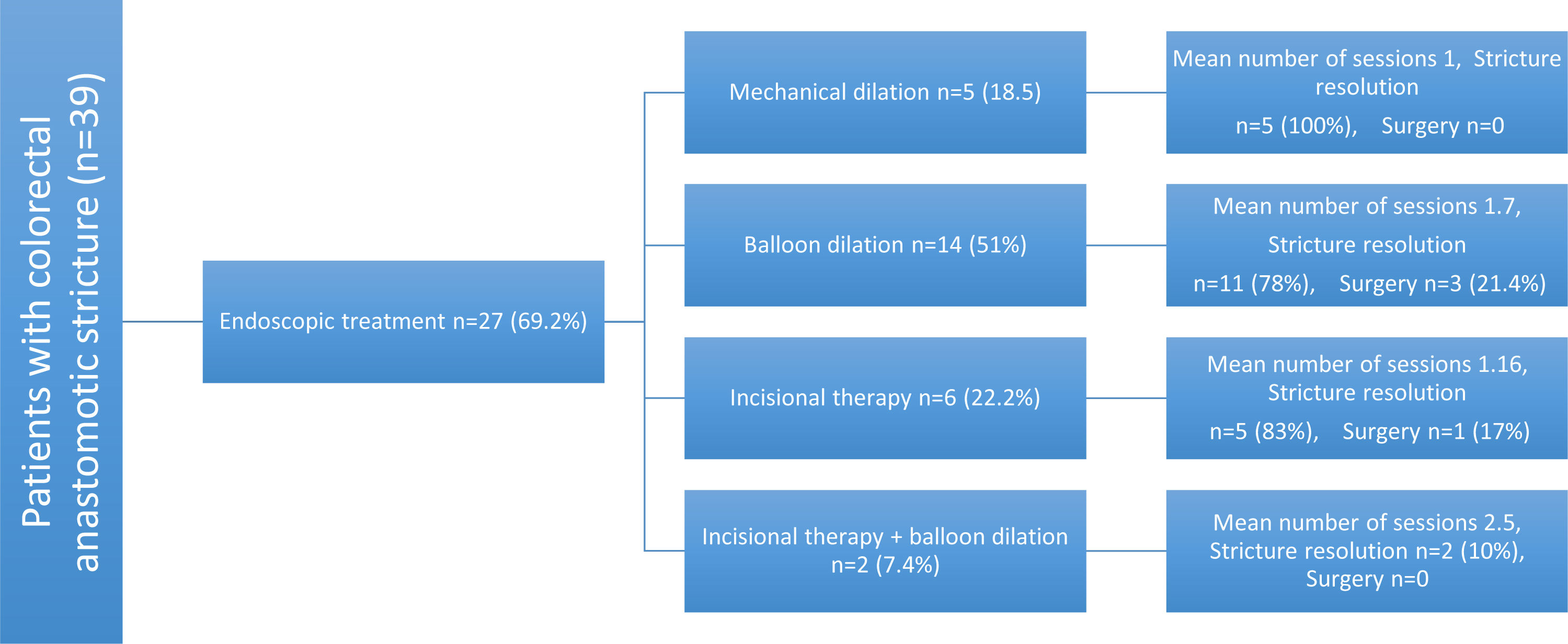
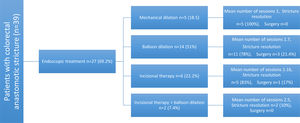
Endoscopic treatment was required for 69.2% patients. Hydrostatic balloon dilation was performed in 51% of them, 22.2% had radial incisions, 18.5% had mechanical dilation, and 7.4% received dual therapy with radial incisions and balloon dilation (Fig. 3). The mean number of sessions needed was 1.57 (1 minimum, 4 maximum). A total of 83.3% of the patients had favorable results and only 2.6% presented with recurrence. No complications associated with the procedure were reported.
The patients retained their stomas for a mean 13.5 months, with a minimum of 3 months and a maximum of 46 months. Bowel transit was restored in 23 (85.1%) of the patients with stricture and stoma.
DiscussionRisk factors for the development of stricture in colorectal anastomosis after oncologic resection were analyzed. Intersphincteric resection and the presence of a protective stoma were the independent risk factors for developing stricture.
Likewise, mechanical stapling of the anastomoses was identified as a protective factor against stricture development.9,10
Those results are consistent with that reported in the literature. Some risk factors described in other studies, such as radiotherapy before or after surgery, were not confirmed in our population.11
Radiotherapy has been proposed as a risk factor, given that it has been histologically confirmed to cause obliterating endarteritis, and therefore ischemia and necrosis, resulting in transmural fibrosis and stricture.12,13 Systemic chemotherapy before or after surgery was included as a variable to analyze but did not appear to influence the development of stricture.
The majority of the patients with stricture (69.2%) underwent endoscopic treatment, the most frequent of which was balloon dilation. The success rate was high, with recurrence in only one patient, no reported complications, and bowel transit was restored in 85.1% of the patients.
Benign stricture of colorectal anastomosis after resection is the most frequent complication in patients with cancer,14 and incidence at our hospital center was 18.3%, which is comparable to that reported in the literature.
Nevertheless, at present there is no consensus that defines what constitutes stricture in the upper gastrointestinal tract. Some studies define it as difficulty in passing the colonoscope or rectosigmoidoscope through the anastomosis, with variations in the diameters utilized. Due to the different criteria, incidence is difficult to compare between studies.15,16
Endoscopic treatment has currently been proposed as the first treatment option in those patients. Frequent complications, such as intestinal perforation or rupture of the anastomosis, are known to occur with the use of rigid dilators.17,18
The majority of studies describe balloon dilation as the tool that is most widely used, as well as being safe and effective.19 Radial incisions have been described as a safe and efficient technique for the treatment of upper gastrointestinal anastomotic stricture and said incisions continue to be used in case series to treat colorectal anastomotic strictures. Self-expanding metallic stents have also been described as a therapeutic option.20
The present study has limitations. First, the sample size of the patients with stricture (n = 39) was relatively low, which could be an obstacle in determining the results of each therapeutic option. Second, the therapeutic modality for resolving the strictures was the choice of the endoscopist, given that there is no standardized treatment.
A standardized and widely accepted definition to define and classify colorectal anastomotic strictures is needed for future studies on incidence, risk factors, and management.
ConclusionsIn conclusion, in the patients with colorectal cancer that underwent resection and anastomosis, intersphincteric resection and having a protective stoma were identified as independent risk factors for the development of anastomotic stricture. Mechanical stapling of the anastomosis was a protective factor against stricture development.
The endoscopic treatment of colorectal anastomotic stricture is a safe and effective option prior to intestinal transit restoration in those patients.
Further evidence is needed to determine which endoscopic therapy is the safest and most efficacious for the treatment of colorectal anastomotic stricture.
Financial disclosureNo financial support was received in relation to this study/article.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Picazo-Ferrera K, Jaurrieta-Rico C, Manzano-Robleda M, Alonso-Lárraga J, de la Mora-Levy J, Hernández-Guerrero A, et al. Factores de riesgo y tratamiento endoscópico para estenosis de anastomosis posterior a resección en pacientes con cáncer colorrectal. Revista de Gastroenterología de México. 2021;86:44–50.