The polyp detection rate is defined as the percentage of colonoscopies in which one or more polyps are detected, and has been shown to be highly correlated with the adenoma detection rate. The aim of the present study was to evaluate the polyp detection rate at the Endoscopy Unit of the Kasr Al-Ainy Hospital, Cairo University, Egypt, through the i-SCAN, Endocuff, and underwater colonoscopy techniques.
Materials and methodsThe study was conducted on 100 Egyptian subjects over 50 years of age. Their polyp detection rate was measured through 4 different colonoscopic techniques. An equal number of patients were divided into 4 groups: i-SCAN, Endocuff, underwater colonoscopy, and controls. The control group was examined using standard white light colonoscopy. The colonoscopy evaluation included the type of agent utilized for bowel preparation, preparation grade, and colonoscopy withdrawal time.
ResultsThe general polyp detection rate was 48%. The i-SCAN technique had the highest rate (56%), followed by the underwater (52%) and the Endocuff (48%) techniques.
ConclusionThe i-SCAN and underwater colonoscopy techniques produced higher polyp detection rates than the Endocuff-assisted and standard techniques, but with no statistical significance.
La tasa de detección de pólipos (PDR por sus siglas en inglés) se define como el porcentaje de colonoscopias con uno o más pólipos detectados. Se ha demostrado que está altamente correlacionado con la tasa de detección de adenoma (ADR por sus siglas en inglés). Este estudio tuvo como objetivo la evaluación de la tasa de detección de pólipos en la Unidad de Endoscopia "Kasr Al-Ainy", Facultad de Medicina, Universidad de El Cairo, Egipto a través de las técnicas i-SCAN, Endocuff y colonoscópica subacuática.
Materiales y métodosEl estudio se realizó en 100 candidatos egipcios mayores de 50 años para quienes se midió la tasa de detección de pólipos a través de 4 técnicas colonoscópicas diferentes. Los pacientes fueron distribuidos en números iguales en 4 grupos; i-SCAN, Endocuff, técnica subacuática y el grupo control, quienes fueron examinados por medio de colonoscopio de luz blanca estándar. La evaluación colonoscópica incluyó el tipo de agente utilizado para la preparación, el grado de preparación intestinal y también el tiempo de retirada.
ResultadosLa PDR general fue del 48%. La técnica i-SCAN presentó el nivel más alto (56%), seguido de la técnica subacuática (52%) y luego la Endocuff (48%).
ConclusiónLas técnicas de exploración i-SCAN y colonoscópica subacuática mostraron una PDR más alta; aunque estadísticamente insignificante, que con Endocuff o la técnica estándar.
Colonic polyps are protrusions occurring in the lumen of the colon and more commonly are sporadic or part of other syndromes. Polyps are classified as diminutive if they measure ≤ 5 mm in diameter, small if they measure 6-9 mm in diameter, and large if they are ≥ 1 cm in diameter.1 They are usually classified as neoplastic (adenomatous) and non-neoplastic (hyperplastic, inflammatory, hamartomatous).2
Hyperplastic polyps are the most common type of colorectal polyps. They lack potentiality for malignancy, yet multiple lines of evidence suggest that serrated variants are premalignant.3 Thus, colonoscopic surveillance is recommended every 3 years in at-risk patients, particularly those with a strong family history, to reduce the risk of conversion to colorectal carcinoma (CRC). When such is not the case, the surveillance intervals are longer and frequent colonoscopies are unnecessary.4
Adenomatous polyps are common, especially in Western countries, accounting for 20-40% of screening colonoscopies in persons above 50 years of age,5 with at least one polyp detected in 34.3% of asymptomatic patients undergoing a screening colonoscopy.6 All types of adenomas exhibit some degree of dysplasia that correlates with polyp size and villous macroscopy.7
The incidence of inflammatory polyps (pseudopolyps) is 10-20% in ulcerative colitis patients and they typically occur in the second and third decades of life in patients with inflammatory bowel disease.8 Endoscopically, they cannot be distinguished from adenomatous polyps, making biopsy mandatory.9
Hamartomas are commonly sporadic (juvenile polyps), but may occur as part of a hamartomatous polyposis syndrome, such as Peutz-Jeghers syndrome, juvenile polyposis, Cowden disease, or Cronkhite-Canada syndrome.10
The polyp detection rate (PDR) is defined as the percentage of colonoscopies in which one or more polyps are detected.11 It is a surrogate for the adenoma detection rate (ADR), which is the percentage of patients ≥ 50 years of age, undergoing a first-time screening colonoscopy, in whom one or more conventional adenomas are detected and removed. The PDR has been shown to correlate well with the ADR, but the measurement of the former is more feasible because it does not require histologic verification.12 However, a poor correlation for polyps in the distal colon has been described.13
Colonoscopy is the gold standard for detecting colonic polyps, but its sensitivity is not 100%. Its miss rate for polyps of any size was reported at 22% and its miss rate for adenomas, by size, was 2.1% for adenomas ≥ 10 mm, 13% for adenomas 5-10 mm, and 26% for adenomas 1-5 mm.14 Full colonoscopy is recommended as a screening strategy every 10 years, beginning at the age of 50.15
The PDR can be affected by various factors, including age, sex, bowel preparation grade, the endoscopist’s experience, withdrawal time, cecal intubation rate, and retroflexion.16,17 Novel colonoscopic techniques have been developed to increase the ADR because 17-24% of polyps are missed during colonoscopy.18
In the i-SCAN technique, spectral features are modified by narrowing the band width of spectral transmittance, using filters adjusted to the characteristics of hemoglobin absorption.19 It has a greater ability to detect non-protruding polyps, leading to an overall higher detection rate for all colorectal polyps, especially flat lesions, which are a major factor influencing missed polyp detection.20
In underwater colonoscopy, a water pump is utilized to deliver water inside the colon. Compared with air insufflation, water infusion is safe.21 Water exchange improves bowel preparation quality, and a water-filled colon provides a unique perspective during insertion that can facilitate polyp detection. Because the bowel is less distended with water than with air, polyps appear less flattened. Moreover, water has a magnifying effect that may improve polyp visibility.22 In addition, water reduces pain in patients that are lightly sedated or unsedated and it also facilitates cecal intubation and enhances vision.23,24
The Endocuff is a flexible plastic cuff with 2 rows of soft wings that help flatten the colonic mucosa during withdrawal.25 The benefit of Endocuff-assisted colonoscopy is that it is a safe measure that improves the PDR and ADR in a screening population, with no severe adverse events, even in patients with diverticulosis, by spreading out the colonic folds. Furthermore, cecal intubation time is not lengthened, and a significant number of small polyps can be detected at the right side of the colon.26
The aim of our study was to evaluate the colonoscopic i-SCAN, Endocuff, and underwater techniques, comparing them with conventional white light colonoscopy, in relation to the PDR.
Materials and methodsA prospective, randomized study was conducted at the Kasr El-Ainy Hospital’s Endoscopy Unit to assess the PDR through the i-SCAN, Endocuff, and underwater colonoscopic techniques versus conventional white light colonoscopy.
The present study, which included 100 Egyptian subjects, was approved by the ethics committee of the Endemic Medicine Department and the Faculty of Medicine of Cairo University.
Inclusion criteria: a) both sexes and b) age ≥ 50 years.
Exclusion criteria: a) patients with familial adenomatous polyposis, CRC, or inflammatory bowel disease and b) patients with poor bowel preparation.
An equal number of patients were divided into each of the 4 colonoscopic technique groups, according to block sequencing randomization. The PDR was then separately calculated for all patients and for each group.
All the patients included in the study underwent: 1) a complete clinical history, with special attention given to alarm features (e.g., asthenia, rectal bleeding, anorexia, significant weight loss, body mass perception, microcytic hypochromic anemia), 2) clinical examination (e.g., organomegaly, any palpable masses, lymphadenopathy, anemic manifestations, and cachexia), 3) informed consent (all patients signed written statements of informed consent), and 4) colonoscopy.
Each colonoscopy included a) bowel preparation: a split-dose polyethylene glycol solution was used for bowel preparation in the majority of patients, perhaps due to its availability in Egypt, and the endoscopists assessed bowel preparation quality, according to the Boston bowel preparation score,27 b) sedation: deep sedation was achieved, with midazolam as preinduction sedation followed by propofol; vital signs were monitored (heart rate and oxygen saturation), using a pulse oximeter throughout the procedure, c) position: the patient was in the left lateral position, and under certain conditions, changed to the supine position to facilitate further introduction of the colonoscope, d) examination: the first step was inspection of the perianal region and digital rectal examination; standard lubricant was sometimes used to facilitate insertion of the endoscope, e) the performance of one of the following colonoscopic techniques: i) i-SCAN (the Pentax EPK-i5000 ® i-SCAN was used during colonoscope withdrawal); ii) Endocuff (this device flattens the colonic mucosa during withdrawal by means of its flexible plastic cuff with 2 rows of soft wings); iii) underwater colonoscopy (Olympus® OFP) (a water pump delivers water inside the colon through a catheter inserted in a channel in the colonoscope, enabling the colon to be inflated with water, instead of air); iv) conventional white light colonoscopy, utilizing an Olympus CLV-U40® colonoscope. Instruments are passed through its working channels to perform biopsies, remove polyps, or cauterize bleeding. Air, water, and suction can be applied to provide a clearer visual field for inspection; f) full colonoscopic examination up to the cecum was performed by an expert colonoscopist in all cases, with a minimum withdrawal time of approximately 7 min. The PDR was assessed in each of the techniques, and g) histopathologic examination: snare biopsy was taken from the detected polyps, either through hot snaring or cold snaring.
Statistical methodsThe data were coded and entered, using the SPSS® version 25 program. The information was summarized, using mean and standard deviation for the quantitative variables and frequencies (number of cases) and relative frequencies (percentages) for the categorical variables. Comparisons between groups were made using the analysis of variance (ANOVA) post-hoc test with multiple comparisons.28 The chi-square test was performed to compare the categorical data but an exact test was used when the expected frequency was less than 5.29 Means were compared through the t test and a p < 0.05 was considered statistically significant.
Ethical ConsiderationsThe present study was approved by the ethics committee of the Tropical Medicine Department. All patients enrolled in the study signed written statements of informed consent that included possible colonoscopy complications. The forms were not uploaded to prevent disclosing any patient data.
ResultsThe study was conducted on 100 Egyptian subjects of both sexes that were ≥ 50 years of age. They were enrolled from the Colonoscopy room of the Gastrointestinal Endoscopy Unit, within the time frame of August 2017 to March 2018.
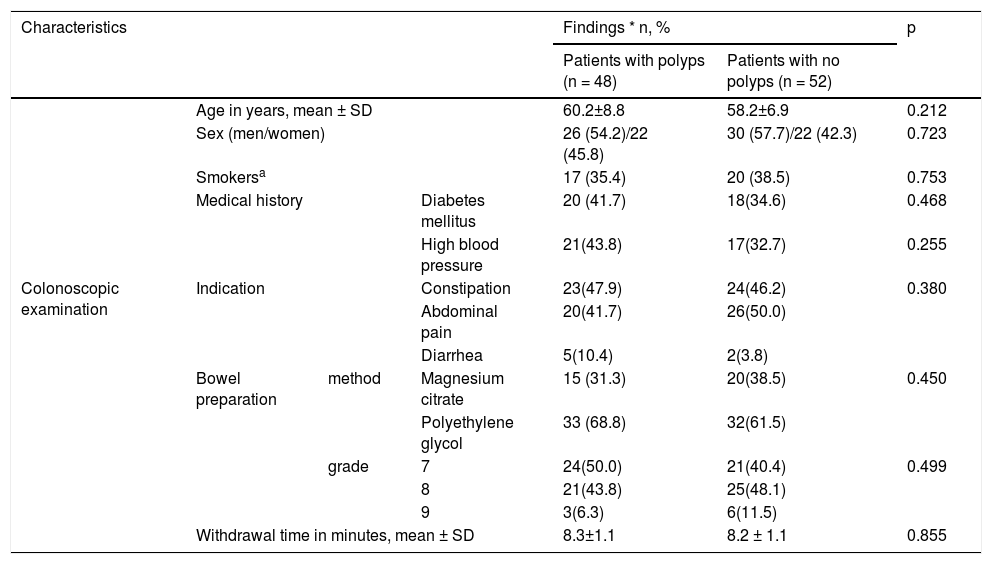
Table 1 shows the baseline parameters, general demographics, and colonoscopic examinations of all the study patients. Bowel preparation was carried out using polyethylene glycol in 65% of the patients and magnesium citrate in 35%.
Baseline parameters of all study subjects.
| Characteristics | Findings * n, % | p | ||||
|---|---|---|---|---|---|---|
| Patients with polyps (n = 48) | Patients with no polyps (n = 52) | |||||
| Age in years, mean ± SD | 60.2±8.8 | 58.2±6.9 | 0.212 | |||
| Sex (men/women) | 26 (54.2)/22 (45.8) | 30 (57.7)/22 (42.3) | 0.723 | |||
| Smokersa | 17 (35.4) | 20 (38.5) | 0.753 | |||
| Medical history | Diabetes mellitus | 20 (41.7) | 18(34.6) | 0.468 | ||
| High blood pressure | 21(43.8) | 17(32.7) | 0.255 | |||
| Colonoscopic examination | Indication | Constipation | 23(47.9) | 24(46.2) | 0.380 | |
| Abdominal pain | 20(41.7) | 26(50.0) | ||||
| Diarrhea | 5(10.4) | 2(3.8) | ||||
| Bowel preparation | method | Magnesium citrate | 15 (31.3) | 20(38.5) | 0.450 | |
| Polyethylene glycol | 33 (68.8) | 32(61.5) | ||||
| grade | 7 | 24(50.0) | 21(40.4) | 0.499 | ||
| 8 | 21(43.8) | 25(48.1) | ||||
| 9 | 3(6.3) | 6(11.5) | ||||
| Withdrawal time in minutes, mean ± SD | 8.3±1.1 | 8.2 ± 1.1 | 0.855 | |||
SD: standard deviation.
Quantitative parameters are expressed in mean ± standard deviation, and qualitative parameters are expressed in numbers (percentage).
Table 2 shows the characteristics of the polyps detected by the colonoscopic techniques evaluated in the study. The PDR was 48% and the ADR was 17%. None of the polyps were malignant.
Characteristics of the colonic polyps detected by the colonoscopic techniques evaluated.
| Polyp characteristics | PDR n, % | Colonoscopic technique n, %a | ||||
|---|---|---|---|---|---|---|
| i-SCAN | Endocuff | Underwater | White light | |||
| Polyps detected | 48 (48) | 14 (56/ 29.2) | 12 (48/ 25) | 12(48/ 25) | 10 (40/20.8) | |
| Site | Right/descending | 17 (35.4) | 6 (24/35.3) | 3 (12/ 17.6) | 5 (20/ 29.4) | 3 (12/ 17.6) |
| Left/ascending | 25 (52.1) | 6 (24/ 24) | 8 (32/32) | 5 (20/20) | 6 (24/24) | |
| Synchronous/right and left | 6 (12.5) | 2 (8/ 33.3) | 1 (4/ 16.7) | 2 (8/ 33.3) | 1 (4/ 16.7) | |
| Number | Single | 34 (70.8) | 9 (64.3/ 26.5) | 9 (75/26.5) | 10 (83.3/ 29.4) | 6(60/ 17.6) |
| Multiple | 14 (29.2) | 5 (35.7/ 35.7) | 3 (25/ 21.4) | 2 (16.7/ 14.3) | 4 (40/ 28.6) | |
| Histo-pathology | Hyperplastic | 27 (56.3) | 5 (35.7/ 18.5) | 10 (83.3/ 37) | 6 (50/ 22.2) | 6 (60/ 22.2) |
| Adenomatous | 17 (35.4) | 9 (64.3/ 53) | 2 (16.7/ 11.8) | 2 (16.7/ 11.8) | 4 (40/ 23.5) | |
| Inflammatory | 4 (8.3) | 0 | 0 | 4 (33.3/ 100) | 0 | |
PDR: Polyp detection rate.
The colonoscopic techniques assessed had no statistically significant higher values than the conventional procedure, but the highest rate was reported with the i-SCAN technique (56%, p = 0.26), followed by underwater colonoscopy (48%, p = 0.6) and Endocuff-assisted colonoscopy (48%, p = 0.4). None of the parameters were significantly related to any of the techniques.
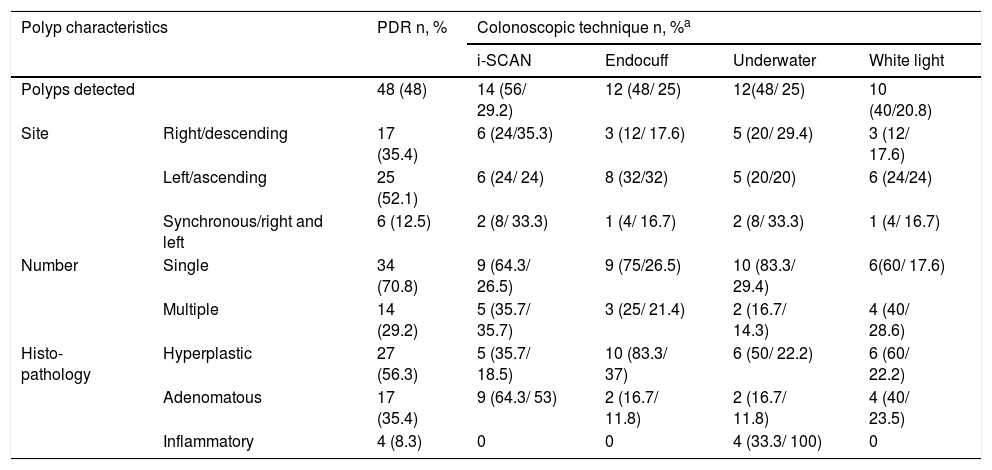
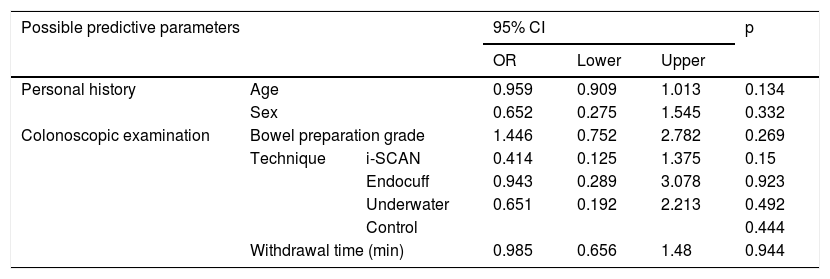
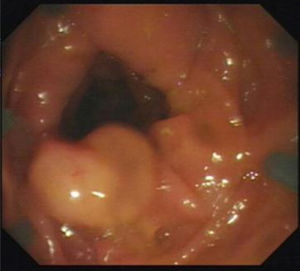
Figs. 1, 2 and 3 show samples of the polyps detected by the i-SCAN, underwater, and Endocuff-assisted colonoscopic techniques.
Table 3 shows the results of the univariate logistic regression analysis for polyp detection predictors.
Predictors of polyp detection by univariate logistic regression.
| Possible predictive parameters | 95% CI | p | ||||
|---|---|---|---|---|---|---|
| OR | Lower | Upper | ||||
| Personal history | Age | 0.959 | 0.909 | 1.013 | 0.134 | |
| Sex | 0.652 | 0.275 | 1.545 | 0.332 | ||
| Colonoscopic examination | Bowel preparation grade | 1.446 | 0.752 | 2.782 | 0.269 | |
| Technique | i-SCAN | 0.414 | 0.125 | 1.375 | 0.15 | |
| Endocuff | 0.943 | 0.289 | 3.078 | 0.923 | ||
| Underwater | 0.651 | 0.192 | 2.213 | 0.492 | ||
| Control | 0.444 | |||||
| Withdrawal time (min) | 0.985 | 0.656 | 1.48 | 0.944 | ||
95% CI: 95% confidence interval; OR: odds ratio.
Colonic polyps are protrusions in the lumen of the colon. They most commonly appear sporadically or as part of other syndromes and are classified as neoplastic and non-neoplastic.1
Our study included 100 Egyptian subjects seen at the Colonoscopy room of the Kasr El-Ainy Endoscopy Unit. They were equally divided into 4 groups to compare 3 different colonoscopic techniques (i-SCAN, underwater, and Endocuff) with conventional white light colonoscopy.
The i-SCAN technique appears to have greater sensitivity for detecting non-protruding polyps. Thus, the procedure is surmised to have an improved neoplastic polyp detection rate, resulting from its increased ability to detect non-protruding polyps, leading to an overall higher detection rate for all colorectal polyps, including neoplastic, hyperplastic, and inflammatory polyps.20
In underwater colonoscopic examination, the infused water remains in the colon, facilitating colonoscope insertion, and is removed mainly during colonoscope withdrawal. That method focuses primarily on reducing pain during unsedated or sedated sigmoidoscopy or colonoscopy. However, there are concerns that water contaminated with residual feces could lower the ability to detect subtle mucosal lesions because of impaired visibility.21 Water infusion has been found to enhance the ADR. Hsieh et al.22 reported an overall gain in the ADR with water exchange, compared with air insufflation (56.7 vs. 43.3%). The positive results with underwater colonoscopy could be due to improved bowel preparation quality and the fact that the bowel is less distended when filled with water, compared with gas, so polyps appear less flattened. In addition, water has a magnifying effect that might improve polyp visibility.
Endocuff-assisted colonoscopy may result in a higher overall ADR and PDR. It may also be more effective in detecting right-sided adenomas and enable better inspection of the proximal colonic folds. The detection of polyps that are more likely to be hidden from view, especially on the reverse side of the mucosal folds, could also make polypectomy easier to perform. Greater friction could prevent the sudden slippage of the colonoscope because the distal row of the Endocuff arms can be used to expose the polyp. Additionally, the Endocuff device does not impair the direct wide-angle view of the colonoscope and causes no major complications.30 A study conducted to evaluate the effect of Endocuff-assisted colonoscopy on detection rates revealed a marked improvement in the PDR (PDR: 55.4 vs. 38.4%).31
The basic parameters in our study patients that presented with polyps were similar to those of the patients that did not present with polyps, implying that, despite the lack of significant specific characteristics, polyp detection should be considered for all candidates above 50 years of age. That concurs with the US Preventive Services Task Force on CRC screening for individuals at average risk and the 2017 American Society of Gastrointestinal Endoscopy recommendations, which state that colonoscopy is one of the most efficient screening modalities. The National Polyp Study demonstrated a 76% reduction in CRC incidence and a 53% reduction in mortality in patients that underwent colonoscopic polypectomy.32
There was a comparable predominance of men in our patients with and without polyps (54.2 and 57.7%, respectively) that was similar to the findings of Lieberman et al.33 and Kashiwagi et al.34 Those authors reported a respective 52 and 69% predominance in men. Said preponderance could be explained by the fact that men seek that type of medical consultation more frequently than women, many of whom find colonoscopic examination painful and embarrassing, and bowel preparation difficult. In addition, numerous diseases of the colon are more common in men. In contrast, Ritvo et al.35 demonstrated 60% female predominance in their study.
The PDR, the percentage of colonoscopies in which one or more polyps is detected, is a surrogate for the ADR.36 The minimum standard PDR is estimated at 40%, which corresponds to an ADR of 25%.37 In the present study, the PDR was 48% and single polyp detection was more than twice as high as multiple polyp detection (70.8 vs. 29.2%, respectively) but with no statistically significant difference. Our PDR was similar to those reported in previous studies from Spain (45.8%)38 and the United States (49%).39 Slightly lower rates were reported in France (35.5%)40 and Iran (23.5%).41 The latter rate was attributed to the younger age structure in the Iranian patients.42 On the other hand, much lower rates were reported in Kuwait (20%), Oman (12.1%) and Malaysia (11.5%), which could be related to the relatively young mean age of those study populations.41
Polyp detection was higher in men (55%) in our study, in agreement with the results of Kim et al.,42 who reported a 1.5-fold increased rate of polyps in men, compared with age-matched women. That could be attributed to the higher prevalence of smoking, considered a risk factor for polyp development, in men than in women. None of the women in the present study were smokers.43
Histopathologically, the hyperplastic polyps had the highest prevalence (56.25%) in our study, followed by adenomatous polyps (35.4%) and inflammatory polyps (8.35%). Uraoka et al.44 and Kumar et al.45 reported similar results, finding a greater predominance of hyperplastic polyps than adenomatous polyps (41 vs. 34%, respectively). Contrastingly, Vişovan et al.46 and Filho et al.47 described a predominance of adenomatous polyps over hyperplastic polyps (66 vs. 32%, and 58.9 vs. 34.7%, respectively).
Most adenomatous polyps convert into colon carcinoma over a 10-year period. The National Polyp Study showed a 76% reduction in CRC incidence and a 53% reduction in mortality in patients that underwent colonoscopic polypectomy.32
In our study, the difference regarding polyps detected in advanced-age patients from those in whom polyps were not detected was insignificant, i.e., advanced age per se is a potentiality for harboring colonic polyps. Increasing age is also a risk factor for right-sided polyps.48
The PDR was higher than the ADR (48 vs. 17%, respectively) in the present study and conventional white light colonoscopy had the lowest detection rate (40%).
Colonoscopies with the i-SCAN technique had the highest PDR (56%) in our study. Heresbach et al.20 and Bisschops et al.49 stated that i-SCAN had a greater ability to detect non-protruding polyps, leading to an overall higher detection rate for all colorectal polyps, especially the easily missed flat lesions. However, in our results, the PDR with the i-SCAN was not significantly higher than the PDR with white light colonoscopy. That was concordant with the Cochrane review and meta-analysis conducted by Nagorni et al.50 and Dinesen et al.,51 who found no significant difference in the ADR or PDR, when comparing the i-SCAN technique with white light colonoscopy. To the contrary, Kim et al.42 reported a significantly higher PDR in colonoscopy with i-SCAN than with the standard white light procedure (52 vs. 38.1%, p = 0.004). One explanation is the fact that the i-SCAN device has a greater ability to detect non-protruding polyps, which is a major factor influencing the missed detection of polyps.20
We also reported a higher, albeit not statistically significant, PDR for underwater colonoscopy (52%), compared with the standard white light colonoscopy. That was similar to the findings of Cadoni et al.52 and Patel et al.,53 who described a higher PDR with water exchange.
In addition, a higher, but not statistically significant, PDR was observed in our Endocuff group (48%), compared with the standard white light colonoscopy group, concurring with the German studies by Floer et al.31 and Biecker et al.54 Those authors favored the Endocuff-assisted technique over conventional colonoscopy, for its higher PDR (55.4 vs. 38.4% and 56 vs. 42%, respectively). The higher rates could be explained by the greater unfolding of the colonic mucosa during withdrawal of the colonoscope. In contrast, a Dutch study conducted by Van Doorn et al.55 revealed no advantage to Endocuff use, regarding the number of patients with at least one adenoma (ADR: 52% for both study arms).
We conclude that i-SCAN colonoscopy is a very efficient technique, but we recognize the need for a study with a larger number of patients to be carried out.
Study limitationsOur study was conducted at a single, albeit large, referral center and our patient sample was small.
Author contributionsM. Abdelbary: lead author and study design; S. Hamdy and H. Shehab: colonoscopic examination of the enrolled patients; N. ElGarhy: clinical evaluation, patient enrollment, and manuscript drafting; M. Menesy: data analysis and interpretation; R. Marzaban: literature review and manuscript revision.
Financial disclosureNo financial support was received in relation to this study.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Abdelbary M, Hamdy S, Shehab H, ElGarhy N, Menesy M, Marzaban R. Técnicas colonoscópicas para la detección de pólipos: un estudio egipcio. Revista de Gastroenterología de México. 2021;86:36–43.