During the clinical course of inflammatory bowel disease, different causes can compromise kidney, liver, and bone marrow function and increase the risk for osteoporosis, infections, and neoplasias. The aim of the present study was to describe the follow-up of Mexican patients with inflammatory bowel disease in relation to their vaccination regimen, treatment-associated risks, and cancer screening.
Materials and methodsA retrospective cross-sectional study was conducted within the time frame of February and June 2017. One hundred patients that had a histopathologic diagnosis of inflammatory bowel disease were surveyed about their follow-up vaccination regimen, treatment-associated risks, and cancer screening. SPSS v24 software was employed for the statistical analysis.
ResultsOne hundred patients with inflammatory bowel disease were studied (90% with ulcerative colitis and 10% with Crohn's disease; 60% women, 40% men): 75% stated that they had no vaccination regimen. A total of 71.4% of the women had at least one Pap smear in their lives and 28.6% did not have them done annually. Twenty-four percent of the patients wore sun block daily. A total of 18.2% of the patients with more than a 10-year progression of ulcerative colitis had an annual colonoscopy. Yearly kidney function was registered in 57.1% of the patients, 92.9% had a yearly complete blood count, and 78.6% had yearly liver function tests. A total of 34.8% of patients had no bone densitometry in their case records.
ConclusionsThese results are a red flag suggesting the need to reinforce the role of the primary healthcare providers in relation to vaccination follow-up and the need to improve the education of the patient in relation to inflammatory bowel disease.
Durante el curso clínico de la enfermedad inflamatoria intestinal (EII) diversas causas pueden comprometer la función renal, hepática y medular e incrementar el riesgo de osteoporosis, infecciones y neoplasias. El objetivo de este estudio es describir el seguimiento que llevan los pacientes mexicanos con EII respecto a su esquema de vacunación, riesgos asociados a tratamiento y cáncer.
Material y métodosEstudio transversal, retrospectivo. Se encuestó a 100 pacientes con diagnóstico histopatológico de EII entre febrero y junio de 2017 acerca del seguimiento de esquema de vacunación, riesgos asociados al tratamiento y cáncer. Se realizó el análisis estadístico en SPSS v. 24.
ResultadosSe estudiaron 100 pacientes con EII (el 90% con colitis ulcerosa crónica idiopática, y el 10% con enfermedad de Crohn; el 60% eran mujeres y el 40%, hombres): el 75% negaron poseer un carnet de vacunación; el 71.4% de las mujeres se habían realizado al menos una citometría cervical en su vida, el 28.6% no se la realizan de forma anual; el 24% utilizan protector solar diariamente; el 18.2% con más de 10 años de evolución de una colitis ulcerosa crónica idiopática tiene una colonoscopia anual; anualmente se registra función renal en el 57.1%, biometría hemática en el 92.9%, función hepática en el 78.6% y el 34.8% no tienen densitometría ósea en el expediente.
ConclusionesEstos resultados son un foco rojo que indica la necesidad de reforzar el papel del primer nivel de atención respecto al seguimiento vacunal y la necesidad de mejorar la educación al paciente con relación a la EII.
Ulcerative colitis (UC) and Crohn's disease (CD) are the 2 main types of inflammatory bowel disease (IBD).1 Its etiology is still unknown, but it is considered a multifactorial disease, in which the reciprocal interactions between the genetics of the host, environmental factors, the microbiota, and immune responses that normally would mediate mucosal homeostasis, appear deregulated and induce or perpetuate chronic inflammation.2,3 Patients diagnosed with IBD require the use of drugs that have different risks, including the 5-aminosalicylates that are used as first-line treatment.4,5 Moreover, at some time, 80% of the patients will require treatment with corticosteroids, 40% with thiopurines, and around 20% with biologic drugs. Therefore, due to the medications they receive and the intrinsic factors of the disease, patients with IBD have an increased risk for acquiring infections,6,7 diseases that are preventable through vaccination,8 and the development or recurrence of cancer.9,10
The safety profile of the aminosalicylates, especially mesalazine, is similar to that of placebo, but they are not exempt from risks, such as nephrotoxicity, interstitial nephritis, or allergy,11 and therefore annual kidney function control is recommended.12 Thiopurines have some dose-independent adverse effects, such as the development of pancreatitis, and predisposition to it can only be determined through genetic studies and preventing their use in selected cases. Other adverse effects are dose-dependent, such as myelotoxicity and hepatic toxicity, which we should monitor through complete blood count and liver function tests before treatment and during treatment follow-up.13 Likewise, thiopurine use increases the risk for opportunistic infections and malignancy.14,15
One of the most important risks in IBD, not only due to steroid use, but also to the effect of bone inflammation, malabsorption of calcium and vitamin D, and low body mass index,16 is the alteration in bone mineral density, whose prevalence varies from 22 to 77% for osteopenia and from 17 to 41% for osteoporosis.17 The use of those drugs can also present, albeit less frequently, risks for glaucoma and cataracts.16 Finally, biologic treatment results in an increased risk for latent tuberculosis, among other things, making it necessary to perform a tuberculin test or an interferon-gamma release assay before treatment and every year during treatment.6
Likewise, the use of those types of drugs and the intrinsic factors involved in IBD increase the risk for skin cancer, and so patients should be advised to use broad-spectrum sunscreen.18 In relation to UC, there is also an increased risk for colon cancer, making it necessary to have annual control colonoscopy starting from the eighth year of disease progression.19 Women in particular are at greater risk for presenting with abnormal Pap smears associated with human papillomavirus20 and cervical cancer, underlining the importance of strict follow-up through annual cervical cytology.21 With respect to infectious risks, some are preventable through vaccines.16 Nevertheless, it appears that only 14% of gastroenterologists adequately inform their patients with IBD about vaccinations to prevent those diseases.22
There are no studies that guide the follow-up of those aspects in Mexican patients with IBD. Therefore, the aim of the present study was to describe the follow-up of Mexican patients with IBD in relation to their vaccination regimen, the risks associated with treatment, and the risk for cancer.
Materials and methodsA retrospective, cross-sectional study was conducted within the time frame of February and June 2017. One hundred patients with the definitive diagnosis of IBD from the IBD Clinic of the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán were interviewed. Other clinical, sociodemographic, and laboratory data were retrospectively collected from the case records for their later concentration and analysis using the SPSS v24 software.
Active patients at the IBD Clinic consultation were asked if they kept a vaccination record card as an important document at home; if they remembered having had the following vaccines and their application dates: chicken pox, herpes zoster, measles/mumps/rubella, diphtheria and pertussis, tetanus, influenza, human papillomavirus, hepatitis A, hepatitis B, meningococcal vaccine, pneumococcal vaccine; and in the case of women, if they had ever had a Pap smear, the last date of the test, if they knew the test result, the frequency with which they had a Pap smear (every year, every 2 years, every 3 years, every 5 years, or at intervals greater than 5 years); if the patients visited a dermatologist (once in their lives, once a year, once every 2 years, or never); if they wore sunscreen daily; and if they smoked or not, the number of years they had smoked, and the number of cigarettes smoked a day to calculate the smoking index (the number of cigarettes a day times the number of years smoking, divided by 20).
Other clinical and sociodemographic data were collected from the case records and they included: age, sex, histopathologic diagnosis of UC or CD, clinical pattern of CD (inflammatory, stricturing, or fistulizing), as well as the extension of UC (proctitis, proctosigmoiditis, left colitis, or pancolitis), the number of years of disease progression, a family history of colon cancer, cervical cancer, or skin cancer, a personal history of diabetes, dyslipidemia, cataracts, or glaucoma; the presence of extraintestinal manifestations, such as arthritis, arthralgias, sacroiliitis, ankylosing spondylitis, pyoderma gangrenosum, erythema nodosum, primary sclerosing cholangitis, or uveitis; the tuberculin test or purified protein derivative (PPD) skin test recorded in the case record, as well as the date and result (positive >10mm or negative <10mm), and finally, if they had ophthalmology or dermatology follow-up (at least one note in the case file, once a year, once every 2 years, or never), and the documented diagnosis of cataracts, glaucoma, or avascular necrosis of the head of the femur as a consequence of steroid use.
With respect to laboratory follow-up, the interval (once a year, once every 3 years, or every 5 years or more) between complete blood count, kidney function tests (urea, creatinine), and liver function tests (total bilirubin, direct bilirubin, indirect bilirubin, and transaminases) were registered. The results of hip and spinal densitometry (classifying the result as normal, osteopenia, osteoporosis, or not documented) were also registered, along with the date and report of the last colonoscopy and intervals between the registered studies (maximum every year, maximum every 3 years, or every 5 years or more, or not documented) and the history or presence of colon cancer registered in the case record.
Finally, the data were collected on the pharmacologic treatment of IBD, such as current and previous treatments stated in the clinical history (aminosalicylates, steroids, immunomodulators, or biologic agents), including the mean doses, years of treatment, and calculation of the accumulated dose of each drug (number of treatment days times the mean dose).
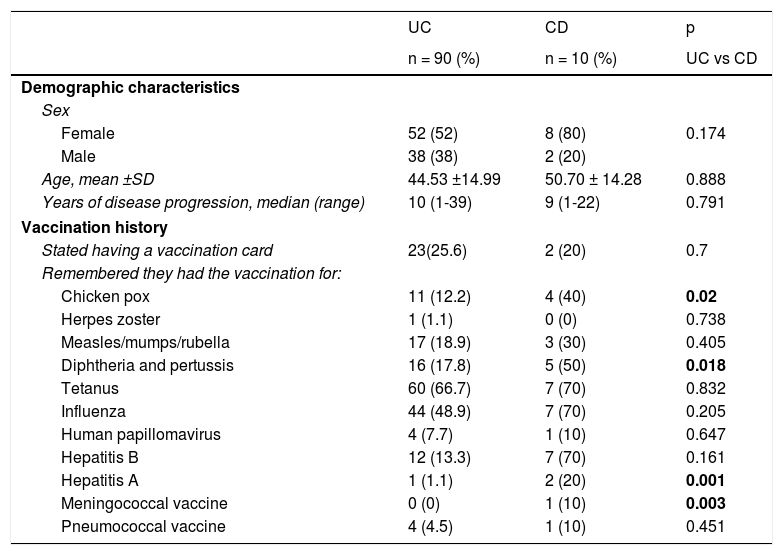
ResultsOne hundred patients with IBD were interviewed. Ninety percent of them had UC and 10% had CD, 60% were women, and 40% were men. In relation to vaccination regimen follow-up, it was striking that 75% of the patients stated they did not have an immunization record card. The demographic characteristics and vaccination history are detailed in Table 1.
Demographic characteristics of Mexican patients with inflammatory bowel disease and their vaccination regimen follow-up history.
| UC | CD | p | |
|---|---|---|---|
| n = 90 (%) | n = 10 (%) | UC vs CD | |
| Demographic characteristics | |||
| Sex | |||
| Female | 52 (52) | 8 (80) | 0.174 |
| Male | 38 (38) | 2 (20) | |
| Age, mean ±SD | 44.53 ±14.99 | 50.70 ± 14.28 | 0.888 |
| Years of disease progression, median (range) | 10 (1-39) | 9 (1-22) | 0.791 |
| Vaccination history | |||
| Stated having a vaccination card | 23(25.6) | 2 (20) | 0.7 |
| Remembered they had the vaccination for: | |||
| Chicken pox | 11 (12.2) | 4 (40) | 0.02 |
| Herpes zoster | 1 (1.1) | 0 (0) | 0.738 |
| Measles/mumps/rubella | 17 (18.9) | 3 (30) | 0.405 |
| Diphtheria and pertussis | 16 (17.8) | 5 (50) | 0.018 |
| Tetanus | 60 (66.7) | 7 (70) | 0.832 |
| Influenza | 44 (48.9) | 7 (70) | 0.205 |
| Human papillomavirus | 4 (7.7) | 1 (10) | 0.647 |
| Hepatitis B | 12 (13.3) | 7 (70) | 0.161 |
| Hepatitis A | 1 (1.1) | 2 (20) | 0.001 |
| Meningococcal vaccine | 0 (0) | 1 (10) | 0.003 |
| Pneumococcal vaccine | 4 (4.5) | 1 (10) | 0.451 |
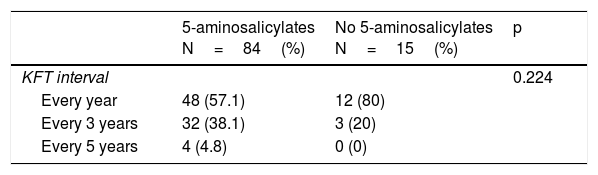
Table 2 describes the surveillance of risks associated with medication use. Eighty-four percent of the patients were treated with 5-aminosalicylates. Of those patients, 57.1% had annual kidney function tests, 38.1% had them every 3 years, and 4.8% had them every 5 years or more. Forty-six percent of the patients were treated with steroids. Of those patients, none had cataracts, glaucoma, or femoral head avascular necrosis, 4.3% of them had at least one note per year from the ophthalmology service, 34.8% had no register of bone densitometry in their case records, 15.2% had one every year, and 32.6% had one every 5 years or more. Twenty-eight percent of the patients were treated with thiopurines. Of those patients, 92.9% had at least one complete blood count yearly and 78.6% had annual liver function tests. Three percent of the patients were treated with biologic agents, all of whom had a negative PPD skin test.
Surveillance of risks associated with medication use in Mexican patients with inflammatory bowel disease.
| 5-aminosalicylates N=84(%) | No 5-aminosalicylates N=15(%) | p | |
|---|---|---|---|
| KFT interval | 0.224 | ||
| Every year | 48 (57.1) | 12 (80) | |
| Every 3 years | 32 (38.1) | 3 (20) | |
| Every 5 years | 4 (4.8) | 0 (0) | |
| Steroids N = 46 (%) | No steroids N = 54 (%) | p | |
|---|---|---|---|
| Ophthalmology follow-up | 0.082 | ||
| Never | 44 (95.7) | 46 (85.2) | |
| One ophthalmology note | 2 (4.3) | 8 (14.8) | |
| Densitometry | 0.592 | ||
| Normal | 13 (43.3) | 13 (50) | |
| Osteopenia | 12 (40) | 11 (42.3) | |
| Osteoporosis | 5 (16.7) | 2 (7.7) | |
| Densitometry interval | 0.223 | ||
| Never | 16 (34.8) | 28 (51.9) | |
| Every year | 7 (15.2) | 7 (13) | |
| Every 3 years | 8 (17.4) | 5 (9.3) | |
| Every 5 years | 15 (32.6) | 14 (25.9) | |
| Thiopurines N = 28 (%) | No thiopurines N = 72 (%) | p | |
|---|---|---|---|
| CBC interval | 0.130 | ||
| Every year | 26 (92.9) | 71 (98.6) | |
| Every 3 years | 2 (7.1) | 1 (1.4) | |
| LFT interval | 0.821 | ||
| Every year | 22 (78.6) | 56 (77.8) | |
| Every 3 years | 6 (21.4) | 15 (20.8) | |
| Biologic agent N = 3 (%) | No biologic agent N = 97 (%) | p | |
|---|---|---|---|
| PPD skin test | 3 (100) | 24 (24.7) | 0.735 |
CBC: Complete blood count; KFT: Kidney function tests; LFT: Liver function tests PPD: Purified protein derivative
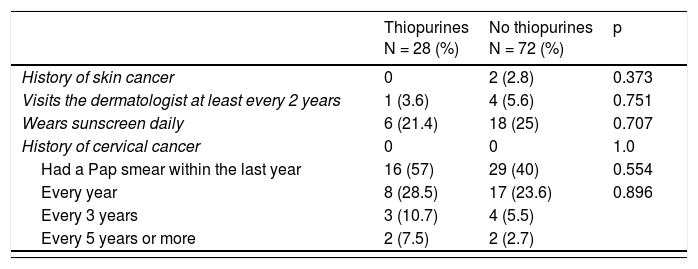
With respect to the surveillance and prevention of the development of cancer, detailed in Table 3, 71.4% of the women stated they had had at least one Pap smear in their lifetimes, 28.6% did not have it done annually, and 14.3% had a Pap smear every 3 years, but none of them reported positivity for human papillomavirus infection. Seventeen percent of the patients stated they visited the dermatologist at least once a year and 24% wore sunscreen daily. Eighteen percent of the patients were smokers. Upon analyzing those variables in relation to thiopurine use, 3.6% of the patients that took those drugs saw a dermatologist at least every 2 years and 21.4% wore sunscreen daily. Likewise, 28.5% had an annual Pap smear. A total of 18.2% of the patients with disease progression of more than 10 years had a yearly colonoscopy and none of them had a personal history of colon cancer.
Surveillance of cancer development in Mexican patients with inflammatory bowel disease.
| Thiopurines N = 28 (%) | No thiopurines N = 72 (%) | p | |
|---|---|---|---|
| History of skin cancer | 0 | 2 (2.8) | 0.373 |
| Visits the dermatologist at least every 2 years | 1 (3.6) | 4 (5.6) | 0.751 |
| Wears sunscreen daily | 6 (21.4) | 18 (25) | 0.707 |
| History of cervical cancer | 0 | 0 | 1.0 |
| Had a Pap smear within the last year | 16 (57) | 29 (40) | 0.554 |
| Every year | 8 (28.5) | 17 (23.6) | 0.896 |
| Every 3 years | 3 (10.7) | 4 (5.5) | |
| Every 5 years or more | 2 (7.5) | 2 (2.7) |
| 10 or more years of UC progression N = 48 (%) | Fewer than 10 years of UC progression N = 42 (%) | p | |
|---|---|---|---|
| History of colon cancer | 0 | 0 | 1.0 |
| Colonoscopy | |||
| Once a year | 8 (18.2) | 22 (53.7) | 0.006 |
| Once every 2 years | 16 (36.4) | 6 (14.6) | |
| Every 3 years | 11 (25) | 7 (17.1) | |
| Every 5 years | 9 (20.5) | 6 (14.6) | |
Seventy-five percent of the Mexican patients with IBD did not have an immunization record card, 28.6% of the female patients did not have an annual Pap smear, and 16.3% of the patients with disease progression of more than 10 years had one annual colonoscopy. Those results suggest the need for reinforcing the role of the primary healthcare provider and the gastroenterologist in educating the patient about his or her disease and the importance of vaccination follow-up, so that he or she has a greater commitment to IBD follow-up, thus facilitating the road to remission. That responsibility does not belong only to the subspecialist, given that surveillance and follow-up are also part of primary healthcare.16
In the present study, 75% of the patients with IBD did not have an immunization record card. The vaccines most frequently applied were those for tetanus and influenza (66.7 and 70%, respectively). It is known that the low vaccination rates reflect the lack of awareness about vaccination on the part of the patient with IBD, which places that population at a substantial risk for developing infections.23
There are numerous obstacles to increasing the vaccination rates, such as general apathy, fear and concern about the side effects of vaccination, and even logistic barriers of healthcare center location, as well as the long waiting period to see a physician.24 One of the limitations of the present study to objectively know the vaccination regimen of each patient, was the fact that the majority of the patients did not have an immunization record card. Therefore, the importance of that document needs to be stressed to the patients.25 One of the strategies to get patients interested in vaccination is to educate them in a simple and practical manner about the opportunistic infections they can acquire, such as chicken pox, herpes zoster, influenza, pneumococcal pneumonia, diphtheria and tetanus, hepatitis B, and meningococcal infections. Fatal cases of those infections have been reported in patients with IBD.25–29
Regarding the surveillance of adverse effects of the medications used, in the present study it appears that there is good laboratory follow-up in general, with annual complete blood count, liver function tests, and kidney function tests. However, patients with IBD also have an increased risk for developing skin cancer, uveitis and episcleritis, glaucoma, or cataracts.30–32 The results of the present study revealed that less than 10% of the patients were seen by an ophthalmologist or dermatologist, and therefore we emphasize the importance of follow-up by those specialists.
Thirty percent of the patients in our study had a bone densitometry at some point in the course of their disease, which we find alarming, given that IBD patients have an increased risk for developing osteoporosis or osteopenia, not only because of steroid use, but also because of their higher risk for presenting with vitamin D and calcium deficiencies, in addition to the intrinsic factors of the disease and their consequent chronic inflammation.33,34 Therefore, we suggest that gastroenterologists reinforce the performance of routine bone densitometry, meaning every year.
In the specific cases of patients in whom pharmacologic treatment with a biologic agent is indicated, before beginning treatment, a PPD skin test is suggested, to rule out latent tuberculosis,35,36 and to administer the adequate drug treatment before using the biologic agent.35,37 In our sample of patients with IBD that were given a biologic agent, a PPD skin test and chest x-ray were registered, demonstrating adequate surveillance in that respect.
Eighty-one percent of the women in the present study had an annual Pap smear, but there is an area of opportunity and improvement in relation to the 19% of women who do not.
Only 18.2% of our study patients with more than 10 years of UC progression had a yearly colonoscopy for dysplasia surveillance. The increased risk for colorectal cancer in IBD patients makes that result important. Cancer is the second most common cause of death in those patients, even though the rates of colorectal cancer have been decreasing. Patients with IBD are recommended to have surveillance colonoscopy 8-10 years after symptom onset. Surveillance colonoscopies should be performed in 1 to 3-year intervals, depending on whether the risk is low, intermediate, or high for developing colorectal cancer. Ideally, colonoscopy should be carried out when the patient is in clinical remission and surveillance should be annual in the patient with endoscopically persistent active disease, a history of dysplasia, a family history of colon cancer in a first-degree relative, or a history of primary sclerosing cholangitis.38–40 Thus, we suggest that the physicians treating patients with UC reinforce those measures.
Finally, it is important to emphasize the role each level of healthcare plays, in relation to the patient with IBD, as well as the responsibility each patient has to carry out the recommendations for the follow-up and surveillance of his or her disease and the surveillance of comorbidities that can present during the course of the disease.6 The present study underlines the importance of reinforcing follow-up and surveillance at all levels of healthcare, particularly in immunosuppressed patients with IBD. A complete clinical history should be obtained for each patient, with special care given to the family history of hereditary disease, especially in first-line cases of cancer. The vaccination regimen should be objectively evaluated using the immunization record card and adequate follow-up and surveillance of adverse effects from medications and risks for the disease should be carried out, as well as multidisciplinary management including the areas of dermatology and ophthalmology.6,16
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Financial disclosureNo specific grants were received from public sector agencies, the business sector, or non-profit organizations in relation to this study.
Conflict of interestDr. Jesús Kazuo Yamamoto Furusho is a speaker, opinion leader, and member of the advisory board committees for the following national and international laboratories: Abbvie, Ferring, Hospira, Janssen, Pfizer, and Takeda. He has been or is presently a speaker for the following laboratorios: Almirall, Danone, Farmasa, Grunenthal, and UCB. He has been or presently is the main researcher in international projects with the following laboratories: Abbvie, Allergan, Bristol, Ferring, Pfizer, Roche, Shire, and Takeda. He is currently the president of the Pan American Crohn's and Colitis Organisation (PANCCO).
The authors A. Sarmiento-Aguilar, N.N. Parra-Holguín, and K.E. Bozada-Gutiérrez declare that they have no conflict of interest.
Please cite this article as: Yamamoto-Furusho JK, Sarmiento-Aguilar A, Parra-Holguín NN, Bozada-Gutiérrez KE. Evaluación del esquema de vacunación y cuidados con relación al seguimiento y tratamiento de los pacientes con enfermedad inflamatoria intestinal. Revista de Gastroenterología de México. 2019;84:11–17.