The C-reactive protein/albumin ratio (CAR) is an inflammatory marker that is considered to have prognostic value in many diseases. Our aim in the present study was to investigate the diagnostic performance of the CAR in determining the clinical severity of acute severe ulcerative colitis (ASUC).
Materials and methodsA retrospective study on 200 UC patients that were admitted to the Gastroenterology unit of the Alexandria University, over an 8-year period from January 2012 to January 2020, was conducted. Patient demographic data, laboratory values, and clinical and endoscopic disease activity scores were evaluated. C-reactive protein (CRP) and albumin levels were recorded. The CAR was calculated to determine clinical severity.
ResultsOf the 200 patients, 135 (67.5%) were men and 65 (32.5%) were women. Mean age was 43.5 ± 9.8 years. Patients were divided into groups with mild, moderate, or severe disease activity and there were statistically significant differences in the CAR, the erythrocyte sedimentation rate (ESR), CRP levels, and albumin levels (p = 0.001; p < 0.05). With a cut-off value of 0.6, the CAR performed best in defining patients with severe disease, with an area under the curve (AUC) of 0.985, 98% sensitivity, 100% specificity, positive predictive value (PPV) of 100%, and negative predictive value (NPV) of 97%, p < 0.001. AUC values for the diagnosis of severe disease were 0.985, 0.88, 0.72, and 0.65 for the CAR, CRP, albumin, and the ESR, respectively.
ConclusionThere was a statistically significant association between the CAR and clinical disease activity in patients with UC. The CAR is a reliable and practical tool for detecting ASUC
El índice PCR / Albúmina (IPA) es un marcador inflamatorio que es considerado de valor para el pronóstico en muchas enfermedades. En este estudio, nuestro objetivo fue investigar el desempeño diagnóstico del IPA para determinar la gravedad clínica de la CUCI grave.
Materiales y métodosEstudio retrospectivo de 200 pacientes con CUCI ingresados en la unidad de Gastroenterología de la Universidad de Alejandría durante un período de 8 años, desde enero de 2012 hasta enero de 2020. En la evaluación se incluyeron los datos demográficos, los resultados de laboratorio y de las escalas endoscópicas y clínicas de actividad de la enfermedad. Se registraron los niveles de proteína C reactiva (PCR) y albúmina. Se calculó el IPA para determinar la gravedad clínica.
ResultadosDe los 200 pacientes, 135 (67.5%) pacientes fueron hombres y 65 (32.5%) pacientes fueron mujeres. La edad promedio fue de 43.5 ± 9.8 años. Los pacientes fueron divididos en grupos con enfermedad leve, moderada y severa. Hubo diferencias estadísticamente significativas entre el IPA, la velocidad de sedimentación globular (VSG) y los niveles de albúmina y de PCR (p = 0.001; p < 0.05). El IPA mostró un mejor desempeño, con un punto de corte de 0.6, para definir pacientes con enfermedad grave, con un área bajo la curva (AUC por sus siglas en inglés) de 0.985, 98% de sensibilidad, 100% de especificidad, valor predictivo positivo (VPP) 100% y valor predictivo negativo (VPN) 97%, p < 0.001. Los valores de AUC para el diagnóstico de enfermedad grave fueron 0.985, 0.88, 0.72, y 0.65 para IPA, PCR, albúmina y ESR, respectivamente.
ConclusiónSe encontró una relación estadísticamente significativa entre el IPA y la actividad clínica de la enfermedad en CUCI. El IPA es una herramienta confiable y práctica para detectar CUCI grave.
Ulcerative colitis (UC) is characterized by chronic, uncontrolled inflammation of the intestinal mucosa. UC is typically characterized by relapsing and remitting mucosal inflammation that starts in a continuous pattern from the rectum and extends to the proximal colon.1,2
The term “acute severe colitis” is preferred over “fulminant colitis” because “fulminant” is a term lacking a clear definition. Approximately 20% of UC patients with initial disease flares have severe UC.3
Acute severe ulcerative colitis (ASUC) is a medical emergency. According to the Truelove and Witts criteria, it is characterized by the presence of more than 6 bloody stools/day, together with any of the following: tachycardia >90 bpm, fever >37.8 °C, hemoglobin (Hb) <10.5 g/dL, and/or erythrocyte sedimentation rate (ESR) >30 mm/h.4
Elevated C-reactive protein (CRP) levels and low serum albumin levels are particularly associated with severe disease activity.5–9
The CRP/albumin ratio (CAR) is an inflammatory marker, whose predictive value has been utilized in relation to various cancers, sepsis, and acute pancreatitis.10,11 A high level of the CAR is associated with increased inflammatory burden, poor prognosis, and mortality.12
Determining disease activity by utilizing cost-effective, non-invasive, objective inflammatory indicators, could be a practical and unbiased method. Our aim in the present study was to investigate the diagnostic performance of the CAR in determining clinical disease severity in Egyptian UC patients.
Materials and methodsParticipantsRetrospective data was collected from a database of active UC patients that were admitted to the gastroenterology unit of the Alexandria Faculty of Medicine, over an 8-year period (January 2012 to January 2020). Of the 592 UC patients that were retrospectively analyzed, only 200 subjects met the criteria for inclusion in the present study. All patients had histologic confirmation of active UC. Patient demographic data, laboratory values, clinical disease activity, endoscopic activity scores, and treatment were evaluated. CRP and albumin levels were determined and recorded, and the CAR was calculated.
Exclusion criteriaMalignancy; recent surgical intervention of the small or large intestine within the last 6 months; infectious diarrhea, including bacterial, viral, and parasitic diarrhea; prolonged antibiotic or nonsteroidal anti-inflammatory drug use; corticosteroid use for the last 3 months; biologic therapy for the last 6 months; other autoimmune diseases; pregnancy; severe burn; sepsis; chronic kidney, liver, or heart diseases; diabetes mellitus; celiac disease; and missing albumin or CRP data at admission.
The present study was carried out according to the principles in the Declaration of Helsinki, and was approved by the Ethics and Research Committee of our hospital.
Disease severityUtilizing the Truelove-Witts criteria, ASUC was defined as more than 6 bloody stools/day, plus one or more of the following: temperature >37.8 °C; pulse >90 bpm; Hb <10.5 g/dl; ESR > 30 mm/h; or CRP > 30 mg/l; moderate activity was defined as 4 or more bloody stools/day, plus all of the following: temperature ≤ 37.8 °C; pulse ≤90 bpm; Hb ≥ 10.5 g/dl, and ESR ≤ 30 mm/h; and mild activity was defined as fewer than 4 bloody stools/day, plus all of the following: temperature <37.8 °C; pulse <90 bpm; Hb > 11.5 g/dl, ESR < 20 mm/h.9 Remission was defined as 3 bowel movements per day, with no bloody stools, and no abdominal pain or fecal urgency.
Colonoscopy was performed on all patients, to assess endoscopic disease activity and detect disease location. Endoscopic activity was assessed by experienced gastroenterologists, using standard-definition colonoscopes (Olympus CV-190, VP-4450HD system and EC-590WL Fujinon, Fujifilm, Tokyo, Japan). According to the Mayo endoscopic activity index (Mayo endoscopic subscore), normal endoscopic mucosal appearance was defined as Mayo 0; the presence of mucosal erythema, decreased vascular pattern, and mild friability was defined as Mayo 1; marked erythema, the absence of vascular pattern, and the presence of friability and erosions was defined as Mayo 2; and the presence of spontaneous bleeding and ulceration was defined as Mayo 3.13
Statistical analysisThe data were collected and entered into a personal computer. The statistical analysis was carried out, using Statistical Package for Social Sciences (SPSS/version 21) software. The continuous values were presented as the mean ± standard deviation, or in case of non-normally distributed data, as the median and 25th–75th percentiles. For the categorical variables, the chi-square test was utilized to compare the different groups. The F-test (ANOVA) for normally distributed quantitative variables was employed to compare more than two groups and a post hoc test (Tukey, LSD) was employed for pairwise comparisons. The Mann-Whitney test for abnormally distributed quantitative variables was utilized to compare two study groups, the Kruskal–Wallis test for abnormally distributed quantitative variables was utilized to compare more than two study groups, a post hoc test (Dunn’s multiple comparisons test) was used for pairwise comparisons, and the Student’s t test was employed to compare the mean of two study groups and to evaluate whether there was a statistically significant difference in the means of the two sets of data. Statistical significance was set at a p value ≤ 0.05. The cut-off levels, sensitivity, and specificity were calculated using the receiver operating characteristic (ROC) curve analysis. The cut-off level was calculated, in the event that the value of the area under the ROC curve (AUC) was above 0.89. As is typically advised, the null hypothesis (H0) was rejected if the corresponding p value was smaller than 0.05. A minimum sample size of 200 patients was required for the present study.
ResultsA total of 200 patients had clinically active UC, of whom 135 (67.5%) were men and 65 (32.5%) were women. The mean age of the study participants was 43.5 ± 9.8 years. According to the Truelove–Witts severity index, 40 (20%) patients had mild disease activity, 82 (41%) had moderate disease activity, and 78 (39%) had severe disease activity. None of the patients in the severe active group had fulminant colitis. Table 1 shows the demographic characteristics and clinical and endoscopic disease activity of the patients.
Demographic and basic clinical data of the study patients.
| Parameters | n = 200 | |
|---|---|---|
| n | % Percentage | |
| Age (years) | ||
| <30 | 13 | 6.5 |
| 30−40 | 65 | 32.5 |
| 40−50 | 57 | 23.5 |
| 50+ | 65 | 32.5 |
| Range | 28−60 | |
| Mean ± SD | 43.5 ± 9.8 | |
| Sex | ||
| Men | 135 | 67.5 |
| Women | 65 | 32.5 |
| Disease severity (Truelove severity index) | ||
| Mild (10−34) | 40 | 20 |
| Moderate (35−64) | 82 | 41 |
| Severe (65 and above) | 78 | 39 |
| Disease location | ||
| Proctitis | 16 | 8 |
| Left-sided colitis | 72 | 36 |
| Extensive colitis | 112 | 56 |
| Endoscopic severity index (Mayo subscore) | ||
| 0 | 2 | 1 |
| 1 | 27 | 13.5 |
| 2 | 61 | 30.5 |
| 3 | 110 | 55 |
| Treatment | ||
| Mesalazine | 159 | 79.5 |
| Sulfasalazine | 41 | 20.5 |
| Azathioprine | 63 | 31.5 |
Mean, arithmetic mean, SD, standard deviation.
There were statistically significant differences in the CAR, CRP and albumin levels, and the ESR, in the patients with different disease activity. Table 2 shows the comparison between the different clinical activity of UC and the biochemical markers of the CAR, CRP and albumin levels, and the ESR.
Comparison of the CAR, CRP and albumin levels, and ESR in the different clinical disease activity of ulcerative colitis.
| Clinical activity Markers | Mild Mean ± SD | Moderate Mean ± SD | Severe Mean ± SD | ANOVA test | p value |
|---|---|---|---|---|---|
| CRP (mg/dl) | |||||
| Range | 0.12−0.75 | 2.10−4.52 | 1.53−9.88 | ||
| Mean ± SD | 0.449 ± 0.182 | 3.42 ± 0.82 | 5.91 ± 2.82 | 88.58 | 0.0001* |
| CAR | |||||
| Range | 0.01−0.23 | 0.23−1.41 | 2.32−4.50 | 65.2 | 0.0001* |
| Mean ± SD | 0.13075 ± 0.068 | 0.82 ± 0.34 | 3.41 ± 0.62 | ||
| Albumin (g/dl) | |||||
| Range | 3.6−4.8 | 3.00−4.00 | 2.40−3.46 | 58.2 | 0.0002* |
| Mean ± SD | 4.2475 ± 0.386 | 3.56 ± 0.37 | 2.94 ± 0.32 | ||
| ESR (mm/h) | |||||
| Range | 11−27.8 | 15.90−48.30 | 34.70−80.00 | 61.2 | 0.0001* |
| Mean ± SD | 19.635 ± 4.894 | 36.30 ± 10.33 | 59.01 ± 13.89 |
CAR, CRP/albumin ratio; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; SD, standard deviation.
The differences in the CAR, CRP, and ESR values were higher, with statistical significance, even between patients with ASUC and those without severe UC, whereas albumin levels in the severe clinical activity group were significantly lower than in the other groups (p = 0.001; p < 0.05), as shown in Table 3.
The CAR, CRP and albumin levels, and ESR in non-severe ulcerative colitis and acute severe ulcerative colitis.
| Clinical activity Markers | Non-severe UC Mean ± SD | ASUC Mean ± SD | Student’s t test | p value |
|---|---|---|---|---|
| CRP (mg/dl) | ||||
| Range | 295.42−4.78 | 1.53−9.88 | 11.25 | 0.001* |
| Mean ± SD | 2.42 ± 1.55 | 5.91 ± 2.82 | ||
| CAR | ||||
| Range | 70.66−1.41 | 2.32−4.50 | 12.89 | 0.001* |
| Mean ± SD | 0.58 ± 0.42 | 3.41 ± 0.62 | ||
| Albumin (g/dl) | ||||
| Range | 459.50−4.80 | 2.40−3.46 | 10.6 | 0.001* |
| Mean ± SD | 3.77 ± 0.50 | 2.94 ± 0.32 | ||
| ESR (mm/h) | ||||
| Range | 3588.40−48.40 | 34.70−80.00 | 16.2 | 0.001* |
| Mean ± SD | 29.66 ± 11.57 | 59.01 ± 13.89 |
ASUC, acute severe ulcerative colitis; CAR, CRP/albumin ratio; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; SD, standard deviation; UC, ulcerative colitis.
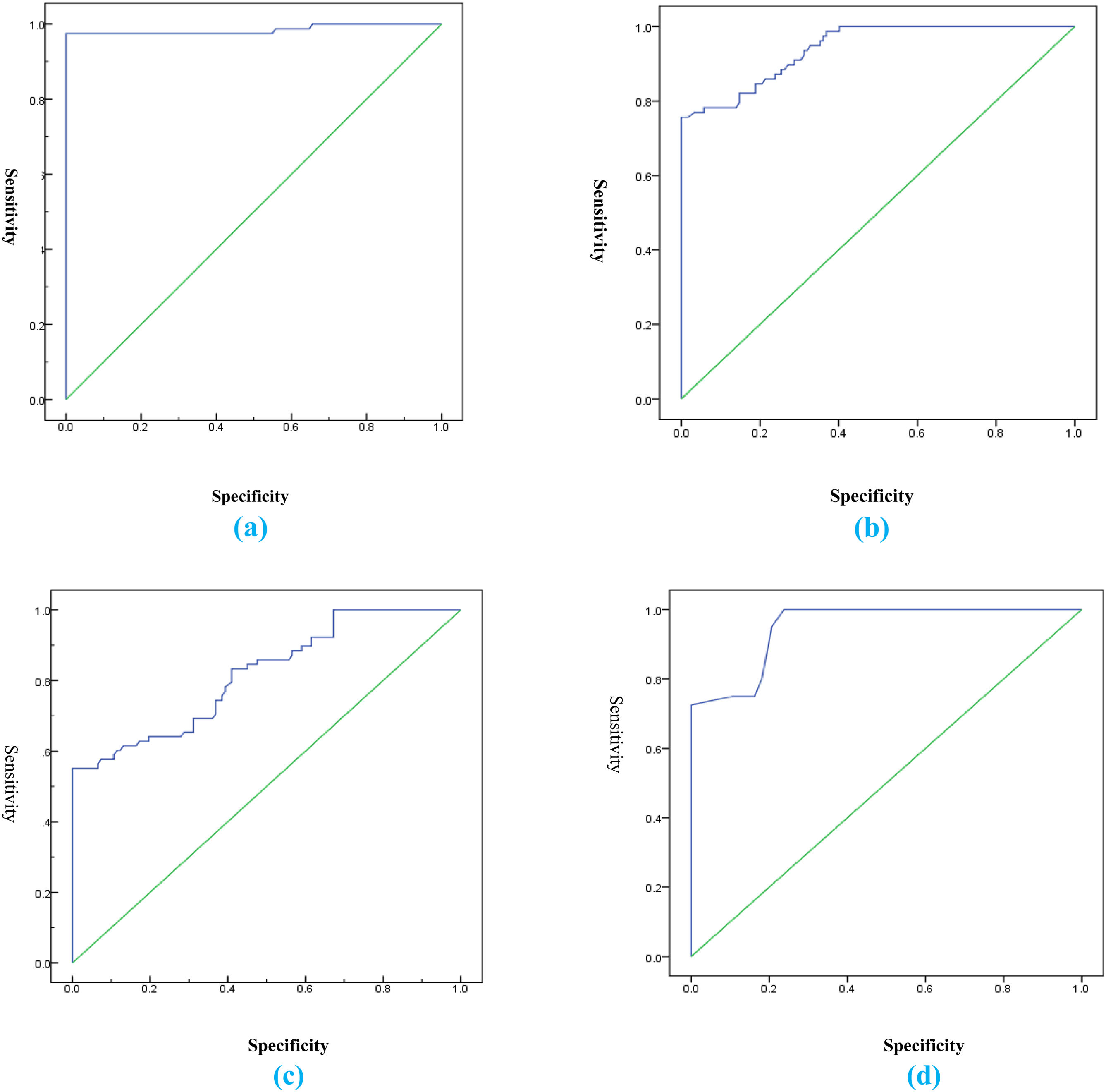
The cut-off value of 0.6 for the CAR showed the best performance in defining patients with severe disease, with an AUC of 0.985, 98% sensitivity, 100% specificity, positive predictive value (PPV) of 100%, and negative predictive value (NPV) of 97%, p < 0.001. The AUC values for the diagnosis of severe disease were 0.985, 0.88, 0.72, and 0.65 for the CAR, CRP, albumin, and the ESR, respectively (Table 4 and Fig. 1).
ROC analysis of the CAR, CRP, albumin, and the ESR, in the diagnosis of severe disease.
| Parameters | Cut-off | AUC | p | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|---|
| CAR | 0.6 | 0.985 | 0.001* | 98.0 | 100.0 | 100.0 | 97.0 |
| CRP | 2 | 0.88 | 0.001* | 90.0 | 95.0 | 94.0 | 91.0 |
| Albumin | 3.6 | 0.72 | 0.003* | 78.0 | 72.0 | 74.0 | 77.0 |
| ESR | 36 | 0.65 | 0.015* | 69.0 | 67.0 | 66.0 | 64.0 |
AUC, area under the receiver operating characteristic curve; CAR, CRP/albumin ratio; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; NPV, negative predictive value; PPV, positive predictive value.
(a) Receiver operating characteristic curve for the CAR showing 100% specificity; (b) Receiver operating characteristic curve for CRP showing 95% specificity; (c) Receiver operating characteristic curve for albumin showing 72% specificity; (d) Receiver operating characteristic curve for the ESR showing 67% specificity.
The cut-off values for the CAR, CRP, albumin, and the ESR were compared with the number of patients with different disease severity, according to the Truelove severity index (Table 5).
Comparison of the number of patients, classified according to the Truelove severity index, and utilizing cut-off points of the different parameters analyzed.
| Parameters | Cut-off | Disease severity (Truelove severity index) | |||||
|---|---|---|---|---|---|---|---|
| Mild (n = 40) | Moderate (n = 82) | Severe (n = 78) | |||||
| n | % | n | % | n | % | ||
| CAR | >0.6 | 8 | 20 | 82 | 99.1 | 78 | 100 |
| CRP | >2 | 0 | 0 | 82 | 97.9 | 78 | 99.8 |
| Albumin | <3.6 | 2 | 5 | 49 | 59.8 | 78 | 98.6 |
| ESR | >36 | 0 | 0 | 43 | 52.4 | 75 | 96.2 |
CAR, CRP/albumin ratio; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate.
Approximately 20–30% of UC patients have a lifetime risk of developing an acute flare during the course of their disease.14 In addition, colonoscopy is an invasive procedure that carries the risk of perforation in patients with ASUC.15 Thus, there is an urgent need for reliable, noninvasive, predictive markers in ASUC patients, given that, over time, they can develop absent or incomplete response to intravenous corticosteroids and require rescue therapy.16
Many laboratory markers are used to detect disease activity in UC patients, such as ESR, CRP, albumin level, fecal calprotectin, and others.
CRP is one of the most commonly used nonspecific inflammatory indicators and has a half-life of approximately 19 h. It is an acute-phase reactant synthesized by the liver that increases during disease activity due to a response to inflammatory cytokines, such as TNF-alpha, interleukin-6 (IL-6), and interleukin-1 (IL-1).17
Although CRP is an acute phase reactant, it may falsely correlate with the degree of activity of UC, with some endoscopically active patients having normal levels.18–20
The level of serum albumin is inversely proportional to the extent of the inflammation, due to a decrease in albumin synthesis by the liver during the inflammatory process and secondary to the synthesis of cytokines, such as TNF-alpha and IL-6.21 Serum albumin levels are also affected by nutritional status.22
Elevated CRP and low serum albumin are associated with the degree of inflammatory response.23 Karoui et al.24 found a positive correlation between clinical and endoscopic activity and CRP levels. Hindryckx et al.25 found that hypoalbuminemia was associated with UC activity, unresponsiveness to treatment, and increased risk of colectomy in patients with ASUC.
In our retrospective study, data were collected from a database that included 200 patients admitted to the Gastroenterology unit of the Alexandria Faculty of Medicine, spanning an 8-year period, from January 2012 to January 2020. All patients had histologic confirmation of UC and were admitted with active UC.
According to disease location in our study, 16 patients (8%) had proctitis, 72 patients (36%) had left-sided colitis, and 112 patients (56%) had extensive colitis. Disease severity of UC (Mayo subscore) showed that 171 patients had moderate and severe disease (85.5%, Mayo subscores 2 and 3). The results of our study showed statistically significant differences concerning the CAR, CRP, and serum albumin, at different phases of UC activity (mild, moderate, and severe activity), according to the Truelove-Witts criteria. CRP and the CAR showed a statistically significant increase, at different phases of UC.
The performance of the CAR, CRP, serum albumin, and the ESR in assessing disease severity, according to its clinical activity score, was evaluated. The CAR had higher sensitivity (98% vs 90%, 78%, 69%), specificity (100% vs 95%, 72%, 67%), PPV (100% vs 94%, 74%, 66%), NPV (97% vs 91%, 77%, 64%), and AUC value (0.941 vs 0.931, 0.883, 0.888), in determining severe activity, than the other parameters.
Similar to our study, Sayar et al.26 found a significant relationship between the CAR and disease activity, showing that the CAR had higher specificity and PPV than CRP, for predicting severe ulcerative colitis. Gibson et al.27 also found that, when measured on the third day of treatment, in patients with ASUC, the CAR was strongly predictive of non-responsiveness to steroid therapy and was superior to CRP and albumin. Chen et al.28 demonstrated in their study that the CAR was strongly related to disease activity. Compared with complete blood count parameters, the CAR had a higher discriminative capacity for active inflammatory bowel disease (IBD).
Our study has several limitations. First, the small sample size could likely have led to a loss of statistical significance at certain points and a high performance of the CAR in the results. Second, endoscopy was not performed on the patients at the different stages of disease activity and we depended solely on the Truelove-Witts severity criteria, and so could not match or correlate the endoscopic degree of activity with said criteria. Third, our study was designed as a retrospective single-center study, preventing firm conclusions from being drawn. In addition, the nutritional status of the patients was uncertain due to the retrospective nature of the study. Prospective studies are needed to provide more useful information on this subject. Nevertheless, our study serves as a base for further larger studies to show the CAR at different stages of disease activity in UC patients and to assess its level after treatment.
In conclusion, our results showed that the CAR was a helpful biomarker for differentiating UC disease activity, showing higher sensitivity, specificity, and PPV than CRP, for predicting severe ulcerative colitis. The CAR is an inexpensive, reliable, and easily calculated marker that can be used to detect activity in ASUC patients.
Ethical considerationsAll procedures in the study involving human participants were performed in accordance with the ethical standards of the institutional research committee (Medical Research Ethics Committee of the Alexandria Faculty of Medicine, Egypt) and the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Statement of Informed Consent: A written statement of informed consent was obtained from each patient in the study. In the case of underage patients, written informed consent was obtained from the guardians. The authors declare that this article contains no personal information that could identify the patients.
Financial disclosureNo specific grants were received from public sector agencies, the business sector, or non-profit organizations in relation to this study.
Author contribution statementDH and AE designed the study and performed the sample and data collections. DH and RA analyzed the data. RA, EB, and ME drafted the manuscript. All authors critically reviewed the manuscript, approved the final version to be published, and agreed to be accountable for all aspects of the work.
Conflict of interestThe authors declare that there is no conflict of interest.
The authors wish to thank Dr. Doaa Header, Dr. Ahmed EL Lakany and Dr. Reham Abo El Wafa for their support in the data analysis, study concept and design, and data acquisition.
Please cite this article as: Header DA, Aboelwafa RA, Elkeleny MR, Bedewy ES, Ellakany AI. El índice proteína C reactiva/albúmina como marcador para detectar colitis aguda ulcerosa grave en pacientes egipcios, Revista de Gastroenterología de México. 2022;87:447–454.