Chronic hepatitis C is one of the main causes of cirrhosis of the liver. Treatment with direct-acting antivirals (DAAs) improves survival. There is controversy as to whether AADs create an increased risk for the development of hepatocellular carcinoma (HCC). The aim of the present study was to determine the risk factors for developing HCC in patients with chronic hepatitis C treated with DAAs.
Materials and methodsA cohort study was conducted, within the time frame of June 2017 and June 2018, on patients >18 years of age, with chronic hepatitis C, genotypes 1 and 4, with one year of follow-up, to evaluate the presence of HCC.
ResultsWe analyzed 108 patients, 71 (65%) of whom were women. Mean patient age was 56.24 years (±10.6), 1b was the most frequent genotype (63%), and 49% of the patients received treatment with DAAs (ombitasvir/paritaprevir/ritonavir plus dasabuvir). Thirty-four (31%) patients were obese. Fifty-three percent (58) had cirrhosis and 82% (89) had Child-Pugh class A liver function. Sustained virologic response at 12 weeks was 100%. Eight (7%) patients developed HCC and 1b was the most frequently associated genotype (87%). The presence of regenerative nodules >10 mm (P < .05), esophageal varices (P < .05), cirrhosis of the liver (P < .05), Child-Pugh B-C (P < .05), and alpha-fetoprotein >20 IU/mL (P = 0.20) one year after treatment were associated with the development of HCC.
ConclusionsThe risk factors for developing HCC were the presence of cirrhosis of the liver, Child-Pugh class B liver function, esophageal and/or gastric varices, and genotype 1b.
La hepatitis C crónica es de las principales causas de cirrosis hepática. El tratamiento con antivirales de acción directa (AAD) mejora la supervivencia. Existe controversia si los AAD generan un incremento del riesgo para desarrollar carcinoma hepatocelular (CHC). El objetivo del trabajo es determinar los factores de riesgo para desarrollar CHC en pacientes con hepatitis C crónica tratados con AAD.
Material y métodosEstudio de cohorte realizado de junio de 2017 a junio de 2018, incluyó a pacientes >18 años con hepatitis C crónica, genotipo 1 y 4, tratados con AAD, con un año de seguimiento para evaluar la presencia de CHC.
ResultadosAnalizamos 108 pacientes, 71 mujeres (65%), edad media de 56.24 años (±10.6), el genotipo más frecuente 1b (63%), el 49% recibió tratamiento con (ombitasvir, paritaprevir, ritonavir, dasabuvir). Treinta y cuatro pacientes (31%) tenían obesidad. El 53% tenía cirrosis (58) y el 82% en Child-Pugh A (89). La respuesta viral sostenida a las 12 semanas fue del 100%. Ocho pacientes (7%) desarrollaron CHC y el genotipo más asociado fue 1b (87%). La presencia de nódulos de regeneración >10 mm (p < 0.05), várices esofágicas (p < 0.05), cirrosis hepática (p < 0.05), Child-Pugh B y C (p < 0.05) y alfafetoproteína > 20 UI/mL (p 0.20) un año postratamiento, se asociaron al desarrollo de CHC.
ConclusionesLos factores de riesgo para desarrollar CHC fue la presencia de cirrosis hepática, clase funcional Child-Pugh B, varices esofágicas y/o gástricas y genotipo 1b.
Hepatitis C is the main cause of liver transplantation in the United States (US) and worldwide. Prevalence in Mexico is 0.3–0.5% and it is one of the main causes of cirrhosis of the liver, together with alcohol consumption.1
Considering that the progression to cirrhosis is part of the natural history of the disease, in untreated hepatitis C, opportune treatment has achieved a positive impact on the prognosis of that group of patients.2
There has been an important evolution in treatment, with the advent of the direct-acting antiviral (DAA) agents, reporting a sustained virologic response (SVR) above 95%. Treatment goals are the prevention of the complications of cirrhosis, including hepatocellular carcinoma (HCC), which is considered the sixth most frequent cancer worldwide and the third cause of cancer-related death.3,4
Despite the high level of efficacy of the DAAs for eradicating hepatitis C virus infection, the impact on the development of HCC has been controversial. Some reviews identified an increase with the use of those medications, documenting an incidence of HCC of 7.4% in the first year, after achieving SVR,5,6 but there are conflicting results in the literature, as well.7
Treatment with DAAs is novel and the length of follow-up in patients treated with those drugs is short-term, thus whether there is an increase in the development of HCC needs to be defined.
The aim of the present study was to determine the risk factors for developing HCC in patients with chronic hepatitis C treated with DAAs.
Materials and methodsA cohort study was conducted at the Hospital de Especialidades Centro Médico Nacional Siglo XXI-IMSS, from June 2017 to June 2018. It included patients >18 years of age, diagnosed with hepatitis C, in the phase of chronic hepatitis or cirrhosis of the liver. Diagnoses were based on reliable clinical indicators (the presence of esophageal and/or gastric varices and FibroScan® >12.5 kPa). The patients had genotypes 1 or 4 and were treated with DAAs (ombitasvir/paritaprevir/ritonavir plus dasabuvir, or ledipasvir/sofosbuvir, with or without ribavirin), with one year of follow-up to evaluate the presence of HCC. All the patients, with or without cirrhosis, underwent abdominal ultrasound and alpha-fetoprotein determination at least 6 months prior to starting treatment. Those studies were repeated, according to the presence of cirrhosis of the liver (every 6 months). Patients with regenerative nodules in the baseline ultrasound study had triple-phase abdominal tomography to rule out HCC. Patients with suspected HCC nodules were eliminated from the analysis, given that they had closer follow-up, in accordance with clinical practice guidelines.
Patients whose medical records were incomplete, that did not sign statements of informed consent, patients that died, that were lost to follow-up at one year, and patients that had a previous diagnosis of HCC or nodules suspicious for HCC were excluded from the study.
Data were registered at the baseline, at the end of treatment, at reaching SVR12, and at one year after having finished treatment. All of the patients underwent ultrasound 6 months after finishing treatment.
Statistical analysisThe demographic characteristics were described as mean (±standard deviation [SD]), median (interquartile range [IQR]), and percentage. The two groups were compared, using the Student’s t test and the Mann-Whitney U test, according to the distribution of the variables. The dichotomous variables were analyzed using the chi-square test and the Fisher’s exact test. Logistic regression was carried out to define the risk factors for HCC. The analysis was performed using the SPSS® version 24.0 program.
Sample size was calculated with the formula for two proportions, with 95% sensitivity and 80% power, delta: 0.07, Z α: 1.96, 0.4% prevalence, 10% prevalence in the non-exposed, and 20% prevalence in the exposed.
Ethical considerationsThe present study was carried out according to the General Health Law regarding health research in Mexico, based on the current legal and ethical regulations, and was authorized by the institutional Local Ethics and Research Committee (R-2020-3601-012).
The requisites of the hospital administration and international norms of medical ethics in relation to research on humans were followed, including the Nuremberg code, which primarily focuses on patient rights, and the Declaration of Helsinki, which places special emphasis on researcher obligations.
Priority was given to the maintenance of privacy, by avoiding the management of personal data of the participants; confidentiality regarding what we could and could not do with their data; and anonymity, by not providing any information that could identity the participants or for which we did not have their consent. A number code was used and all forms and data, especially information with individual identifiers, were stored and are in the possession of the lead researcher, with the knowledge of the personnel of the Instituto Mexicano del Seguro Social, and they are electronically backed-up.
The written statement of informed consent included the participant’s signature and the following information: 1) the purpose of the research protocol; 2) the significance of each participation in the study; 3) maintenance of confidentiality; 4) the right to refuse to participate, without harming the relationship with the institution or the individuals affiliated with the study; 5) the right to interrupt their participation at any time of the study. Their authorization to collaborate in the study was requested, once the information was understood and all doubts were clarified. The present study did not include patients under 18 years of age.
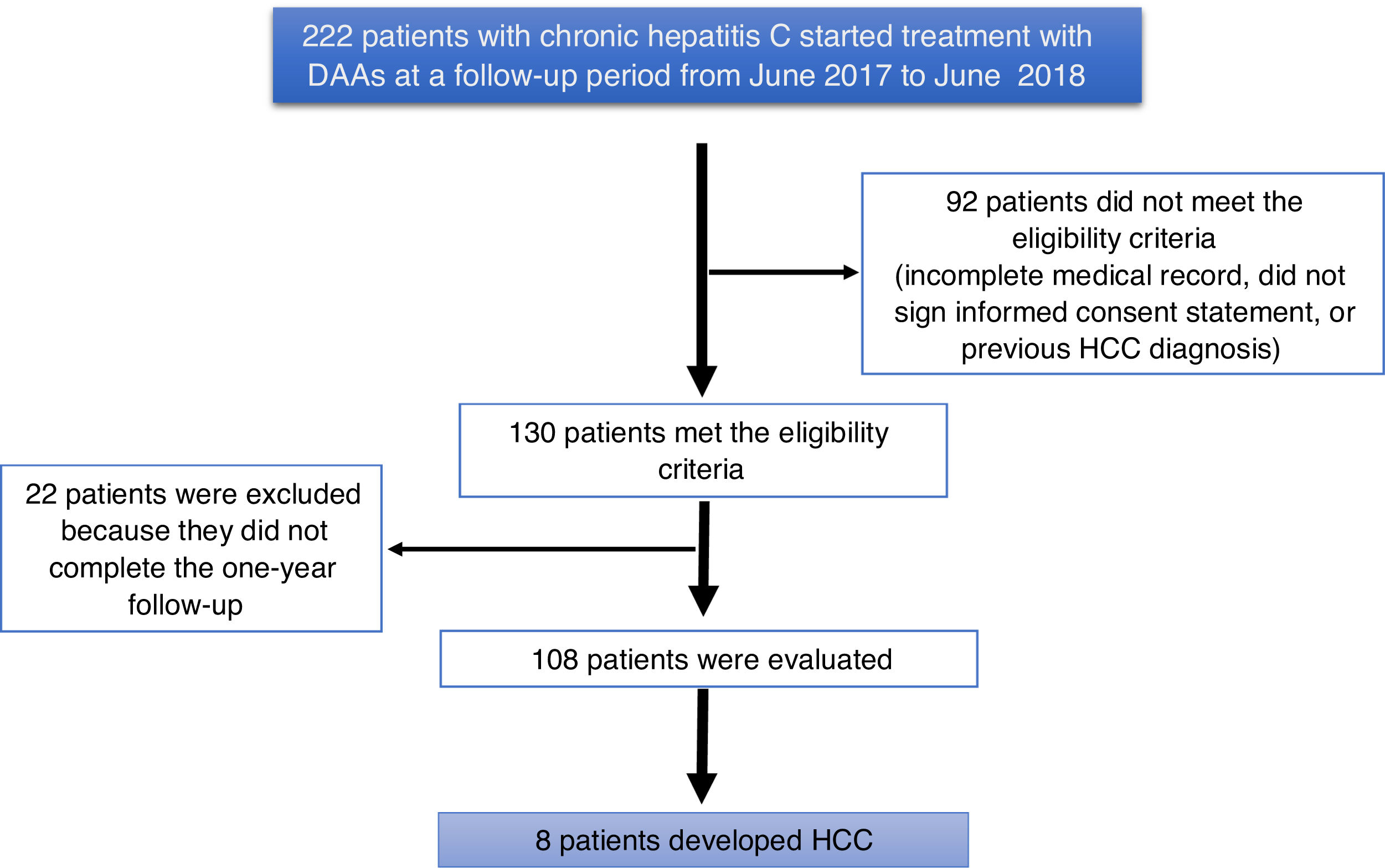
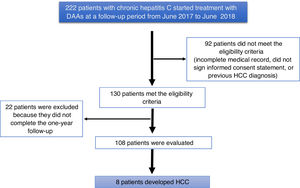
ResultsA total of 222 patients with chronic hepatitis C infection that started treatment with DAAs, within the time frame of June 2017 and June 2018, were studied. Ninety-two patients did not meet the eligibility criteria (they had incomplete medical records, did not sign a written statement of informed consent, or had a previous diagnosis of HCC). One hundred and thirty patients met the eligibility criteria, and 22 patients were excluded for not completing the one-year follow-up, resulting in a final total of 108 patients analyzed (Fig. 1).
The variables assessed were grouped into clinical, demographic, and biochemical variables and the therapeutic aspects were evaluated.
Table 1 shows the baseline characteristics of the patients with chronic hepatitis C virus that were treated with DAAs. Mean patient age was 56.24 (±10.6) years, and the majority of patients were women (65.7%). The most frequent genotype was 1b (69 patients, 63.9%), and the median viral load was 435428.50 IU/mL (IQR 136241.2–1095906.7 IU/mL). The most frequent comorbidity was obesity (34 patients, 31.5%), followed by diabetes mellitus (6 patients 5.6%). Fifty-eight (53.7%) patients were diagnosed with cirrhosis, 7.4% had regenerative nodules <10 mm (not documented before the study began), and 34.0% presented with small esophageal varices. Child-Pugh class A liver function was predominant (48 patients, 82.8%) and the median model for end-stage liver disease (MELD) score registered was 8 (IQR 7–11).
Characteristics of the patients with chronic HCV infection treated with direct-acting antivirals.
| Variables | n = 108 |
|---|---|
| Age (years) | 56.24 (±10.6)a |
| Sex (%) | |
| Male | 37 (34.3) |
| Female | 71 (65.7) |
| Genotipo (%) | |
| 1a | 39 (36.1) |
| 1b | 69 (63.9) |
| Alcohol consumption (%) | 2 (1.9) |
| Comorbidity (%) | |
| Diabetes mellitus (%) | 6 (5.6) |
| Obesity (%) | 34 (31.5) |
| Dyslipidemia(%) | 5 (4.6) |
| Coinfected (chronic hepatitis B) | 1 (0.9) |
| Cirrhosis (%) | 58 (53.7) |
| Regenerative nodules (%) | |
| Absent | 93 (86.1) |
| <10 mm | 8 (7.4) |
| >10 mm | 7 (6.5) |
| Esophageal varices (%) n = 97 | |
| Absent | 55 (56.7) |
| Small (<5 mm) | 33 (34.0) |
| Large (>5 mm) | 9 (9.3) |
| Child-Pugh class (%) n = 58 | |
| A | 48 (82.8) |
| B | 9 (15.5) |
| C | 1(21.7) |
| MELD | 8.00 (7.0–11.0)b |
| AST (U/l) | 49.50 (30.0–79.7)b |
| ALT (U/l) | 49.50 (31.2–71.0)b |
| Albumin (g/dl) | 4.25 (3.7–4.5)b |
| Platelets (cells/mm3) | 139000.00 (87000.0–203750.0)b |
| INR | 1.10 (1.0–1.2)b |
| Baseline alpha-fetoprotein (IU/mL) | 6.90 (3.9–13.9)b |
| Baseline viral load (IU/mL) | 435428.50 (136241.2–1095906.7)b |
AFP: alpha-fetoprotein; ALT: alanine aminotransferase; AST: aspartate aminotransferase; HCV: hepatitis C virus; INR: international normalized ratio; MELD: model for end-stage liver disease.
With respect to the biochemical characteristics, the median AST and ALT levels were 49.50 U/l for both enzymes (IQR 30.0–79.7 and 31.2–71.0, respectively) and the median albumin level was 4.25 g/dl (IQR 3.7–4.5). The median platelet level was 139,000 cells/mm3 (IQR 87000.0–203750.0) and the INR was 1.1 (IQR 1.0–1.2). Median alpha-fetoprotein was 6.90 IU/mL (IQR 3.9–13.9).
The DAA therapeutic regimens that the patients with chronic hepatitis C received were: ombitasvir/paritaprevir/ritonavir plus dasabuvir (53 patients, 49.1%), sofosbuvir/ledipasvir (34 patients, 31.5%), and ledipasvir/sofosbuvir plus ribavirin (21 patients, 9.4%). For the last group an OR of 4, 95% CI of 0.66–18.19, and a p value of 0.63 were reported.
The Fisher’s exact test was used for the bivariate analysis, documenting the following: 100% of the patients with HCC had a diagnosis of cirrhosis of the liver. Of the group of patients with cirrhosis of the liver, 7 presented with regenerative nodules > 10 mm (not documented before treatment was begun), with a p < 0.05. The demographic, clinical, and biochemical variables and their association with the presence of HCC were analyzed (Table 2). Patients with regenerative nodules >10 mm (OR 101, 95% CI 14.3–710.0; p < 0.05) had the highest risk for developing HCC, followed by patients with esophageal varices (OR 10, 95% CI 1.27–91.61; p < 0.05) and those with cirrhosis, with Child-Pugh class B and C liver function (OR 7.3, 95% CI 1.44–37.32; p < 0.05). The variable of viral load was categorized, taking the lower limit of normal of the third quartile as the cutoff point (OR 0.40, 95% CI 0.48–3.46; p 0.35).
Bivariate analysis of the patients that developed hepatocellular carcinoma.
| Variables | Hepatocellular carcinoma | No hepatocellular carcinoma | OR | 95% CI | p |
|---|---|---|---|---|---|
| n = 8 | |||||
| Age ≥ 65 years (n = 24) | 4 (16.7%) | 20 (83.3%) | 4 | 0.92–17.39 | 0.71 |
| Sex | |||||
| Male (n = 37) | 2(5.4%) | 35 (94.6% | 0.61 | 0.11–3.23 | 0.44 |
| Female (n = 71) | 6 (8.5%) | 65(91.5%) | |||
| Genotype | |||||
| 1a (n = 39) | 1 (2.6%) | 38 (97.4%) | 0.23 | 0.28–1.96 | 0.14 |
| 1b (n = 69) | 7 (10.1%) | 62 (89.9%) | |||
| Diabetes mellitus (n = 6) | 1 (16.7%) | 5 (83.3%) | 2.7 | 0.278–26.54 | 0.37 |
| Obesity (n = 34) | 1(2.9%) | 33 (97.1%) | 0.29 | 0.034–2.45 | 0.21 |
| Dyslipidemia (n = 5) | 0 | 5 (100%) | 0.67 | ||
| Cirrhosis (n = 58) | 8 (13.8%) | 50 (86.2%) | 0.86 | 0.778–0.956 | <0.05 |
| Regenerative nodules >10 mm (n = 7) | 7 (100%) | 0 | 101 | 14.3–710.0 | <0.05 |
| Esophageal varices (n = 42) | 7 (16.7%) | 35 (83.3%) | 10 | 1.27–91.61 | <0.05 |
| Child-Pugh | |||||
| B and C (n = 10) | 4 (40%) | 6 (60%) | 7.3 | 1.44–37.32 | <0.05 |
| MELD > 9 (n = 22) | 4 (18.2%) | 43 (18 81.8%) | 1.77 | 0.39–7.97 | 0.35 |
| AST > 120 U/l (n = 9) | 1(11.1%) | 8 (88.9%) | 1.64 | 0.179–15.07 | 0.51 |
| ALT > 120 U/l (n = 14) | 1 (7.1%) | 13 (92.9%) | 0.95 | 0.109–8.4 | 0.72 |
| Albumin < 3.5 g/dl (n = 34) | 2(15.4%) | 11(84.6) | 2.69 | 0.484–15.03 | 0.24 |
| Platelets < 100,000 cells/mm3 (n = 34) | 5(14.7%) | 29 (85.3%) | 4 | 0.91–18.19 | 0.63 |
| AFP > 20 IU/mL (n = 22) | 3 (13.6%) | 19 (86.4%) | 2.5 | 0.56–11.64 | 0.20 |
| Viral load <1095906.0 IU/mL (n = 81) | 7 (8.6%) | 74 (91.4%) | 0.40 | 0.48–3.46 | 0.358 |
| Type of treatment | |||||
| Ombitasvir/dasabuvir/paritaprevir plus ritonavir (n = 53) | 2 (38%) | 51 (96.2%) | 0.32 | 0.62–1.66 | 0.14 |
| Sofosbuvir/ledipasvir (n = 21) | 1 (4.8%) | 20 (95.2%) | 0.57 | 0.66–4.9 | 0.51 |
| Sofosbuvir/ledipasvir plus ribavirin (n = 34) | 5 (14.7%) | 29 (85.3%) | 4 | 0.66–18.19 | 0.63 |
ALT: alanine aminotransferase; AST: aspartate aminotransferase; MELD: model for end-stage liver disease.
The Fisher’s exact test was used.
Regenerative nodules were excluded from the multivariate analysis because they were an unstable variable for carrying out that statistical procedure. Nevertheless, the OR indicated risk and the p value was significant.
Of the 108 patients analyzed, 8 (7.4%) developed HCC within the first 3 months after having finished treatment.
The statistically significant variables were included in the multivariate analysis and none of the variables analyzed were independent risk factors for developing HCC. The model proposed had an R2 of 0.23, explaining 23% of the HCC cases (Table 3).
Logistic regression model for the variables associated with hepatocellular carcinoma.
| Variables | Multivariate analysis | |||
|---|---|---|---|---|
| OR | 95% CI | B coefficient | p | |
| Age ≥ 65 years | 1.44 | 0.840–21.61 | 4.2 | 0.08 |
| AFP > 20 IU/mL | 0.69 | 0.356–11.33 | 2 | 0.43 |
| Albumin < 3.5 g/dl | 0.19 | 0.18–8.22 | 1.21 | 0.84 |
| Esophageal and/or gastric varices | −2.2 | 0.11–1.00 | 0.10 | 0.51 |
AFP: Alpha-fetoprotein; OR: odds ratio; 95% CI: 95% confidence interval.
Chronic hepatitis C virus infection is currently a worldwide public health problem, with a great impact on morbidity and mortality. Therefore, implementing strategies directed at promoting health and preventing complications is imperative. The advent of treatment based on DAAs has modified the natural history of the disease and the prognosis in patients with cirrhosis of the liver, including overall survival,8 given that they are safe treatment regimens, with adequate tolerability and minimal adverse effects. There are few controlled trials that evaluate their long-term clinical impact at all stages of liver disease.
The relation of treatment with DAAs to HCC has been analyzed in different populations, and so far, only an association with patients that have had previous treatment has been found, not with patients with de novo treatment.9 In 2016, different publications released alarm signals about the early appearance of HCC in patients treated with DAAs that achieved SVR.10,11 One of the most accepted hypotheses on the relation between HCC and DAAs is the immune distortion associated with the rapid decrease in the viral load after starting treatment.12
Those reports started a revolution in the research on that theme, paving the way for uncertainty about the increased risk for HCC in cirrhotic patients treated with DAAs and whether the effects of the new antiviral drugs influenced the mortality rate, resulting in reviews with conflicting results. Thus, since then, the importance of conducting multicenter studies has been emphasized.7,13
At present, 2 recent multicenter studies, conducted by Kanwal et al. and Innes et al., have shown no association of HCC in patients treated with DAAs.14,15
The CirVir study explained the different confounding factors and identified certain characteristics, such as age or advanced stage of cirrhosis, as independent factors that increase the risk for developing HCC.16
Many of the limitations of the studies published up to now include selection bias, study populations in advanced stages of cirrhosis, suboptimal evaluation prior to starting treatment, and the absence of details regarding the application of HCC surveillance programs, and in turn, the omission of the diagnosis. In our study, we included patients with and without cirrhosis of the liver. In the cirrhotic patients, diagnosis was made through the presence of esophageal and/or gastric varices or through transition elastography, with a cutoff point >12.5 kPa, according to American Gastroenterological Association recommendations. Patients with cirrhosis made up 53.7% of our study population and their mean age was higher (56.24 ± 10.6 years) than that of previous cohorts. Unlike other studies, in which a large portion of the populations presented with comorbidities, the most frequent comorbidity in our cohort was obesity (31.5%).
The aim of the present project was to analyze the factors that influence the presence of HCC in patients that received treatment with DAAs, with a follow-up time of 12 months. All of the patients (100%) had abdominal ultrasound or abdominal tomography, if regenerative nodules were documented, as well as alpha-fetoprotein determination before starting treatment with DAAs, which are considered adequate strategies for ruling out the presence of HCC prior to receiving treatment.
Regenerative nodules were not documented in 86.1% of our study population during the 12 months of follow-up after finishing treatment. Of the 8 patients that developed HCC (7.4%), detection was early (within 3 months of having finished the treatment with DAAs) in 7 of them, a greater incidence than that published in international guidelines.12
The regenerative nodules in our patients were single, measuring from 10 to 35 mm (the largest) through abdominal ultrasound. The diagnosis of HCC was finally corroborated through triple-phase abdominal tomography.
The association of the time interval from starting the DAAs and the development of HCC, together with the association with the presence of non-characterized nodules in the baseline ultrasound, suggest that antiviral therapy causes a mechanism (most likely related to the immune system) that prepares the growth and clinical recognition of HCC in the first stages of follow-up.17
The most common genotype in the present study was 1b, concurring with that stipulated epidemiologically in Mexico.18 The SVR at 12 weeks was 100%, higher than that reported in the literature.4
Cirrhosis of the liver, in Child-Pugh class B or C liver function, continues to be the most important variant for the development of HCC, as has been reported by Calvaruso et al. and Kanwal et al.7,14 Another identified factor was the presence of esophageal and/or gastric varices.
Among the tumor markers, alpha-fetoprotein is an oncofetal marker that results in greater “cost-benefit”, which, according to the HCC guidelines of the European Association for the Study of the Liver (EASL),12 has >60% sensitivity and >80% specificity, depending on the cutoff point utilized. Even though its usefulness in HCC is debatable, in our study population, we found that it was within normal ranges prior to starting treatment, and at one year of follow-up, it was elevated in only 3 of the 8 patients that developed HCC. Therefore, according to the value proposed in the international guidelines, we took into account an alpha-fetoprotein cutoff point of >20 IU/mL at one year of follow-up, post-treatment, which was higher than the cutoff point ≥9.0 ng/mL proposed by Ogawa et al.19
Those data indicate that in a heterogeneous population of cirrhotic patients that achieve SVR after therapy with DAAs, the risk for developing HCC should be evaluated in relation to disease stage.20 However, treatment should be guaranteed to all patients with cirrhosis, at any functional stage, considering the inevitable and residual risk of HCC after viral eradication.
The main strength of the present cohort was the fact that this study is the first in Mexico to evaluate the association of HCC and the use of DAAs, as well as comprehensively assess the patients, utilizing abdominal ultrasound and laboratory tests prior to starting antiviral treatment, with a 12-month follow-up in all patients after the end of treatment.
A weakness of our study was the small population size, unlike that of other cohorts. In addition, there were no comparisons with a group of patients with cirrhosis due to other etiologies or patients that did not receive treatment.
Despite the fact that our results, statistically, were not independent factors for developing HCC, the risk for that cancer after virologic response is latent. We know that none of the biochemical techniques or standard imaging methods can offer 100% sensitivity in the detection of HCC,15 hence the importance of adhering to screening.14
Finally, we conclude that the risk factors for developing HCC in our group of patients were cirrhosis of the liver, Child-Pugh class B liver function, esophageal and/or gastric varices, and genotype 1b.
The diagnosis of HCC in patients treated with DAAs was early in our cohort, within the first 3 months after finishing treatment. Therefore, in patients with cirrhosis of the liver, we propose that surveillance be carried out every three months, through abdominal ultrasound and alpha-fetoprotein determination, in patients with the risk factors described above, during treatment and the first post-treatment year, to enable the identification of HCC at early stages. Clearly, more studies are needed to support our group’s proposal regarding screening for HCC every 3 months and include it in clinical practice guidelines.
Financial disclosureNo financial support was received in relation to this study.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Santana-Salgado I, Bautista-Santos A, Moreno-Alcántar R. Factores de riesgo para desarrollar carcinoma hepatocelular en pacientes tratados con antivirales de acción directa. Revista de Gastroenterología de México. 2022;87:455–461.