Helicobacter pylori (H. pylori) is associated with a higher risk of peptic ulcer and gastric cancer. The sole presence of the bacterium is not a determinant of clinical outcome, but rather the interaction of strain type and host factors determines the risk of disease. Our aim was to study the association between bacterial load, strain type, and gastric symptoms in H. pylori-positive subjects.
Materials and methodsIn a community survey, a diagnostic 13C-urea breath test for H. pylori was performed on 302 volunteers that were not taking antibiotics, antacids, or proton pump inhibitors one month prior to the test. The breath test produced 25 H. pylori-positive subjects, between 25 and 74 years of age, who then took a gastric symptoms survey and were tested for the presence of the cagA genotype in gastric juice, using the Entero-test®. Bacterial load was determined as a measure of urease activity, utilizing the delta over baseline (DOB) value, obtained in the 13C-urea breath test.
ResultsA total of 48% of the H. pylori-positive subjects were cagA+. A positive association was found between cagA status and high gastric urease activity (p<0.0001) and the latter was significantly associated with the presence of symptoms (p<0.0001).
ConclusionGastric urease activity was strongly associated with dyspeptic symptoms and cagA+H. pylori. Elevated 13C-DOB values could be used as indicators of a higher risk for gastric disease.
La Helicobacter pylori (H. pylori) está asociada con un mayor riesgo de úlcera péptica y cáncer gástrico. La presencia de la bacteria no es un factor determinante para el desenlace clínico, sino que la cepa y otros factores del huésped interactúan para determinar el riesgo a adquirir la enfermedad. El objetivo del presente estudio fue investigar la asociación entre la carga bacteriana, el tipo de cepa y los síntomas gástricos en personas con positividad a H. pylori.
Materiales y métodosEn una encuesta dirigida a la comunidad se contactaron 302 voluntarios que no estuvieran tomando antibióticos, antiácidos ni inhibidores de la bomba de protones un mes antes del diagnóstico de H. pylori utilizando la prueba de la ureasa en aliento. Se seleccionaron 25 sujetos con edades entre los 25 y los 74 años, positivos a H. pylori, para una encuesta de síntomas gástricos y determinar la presencia del genotipo cagA en el jugo gástrico obtenido con Entero-test®. La carga bacteriana se determinó como medida de la actividad de la ureasa utilizando el valor 13C-delta sobre el valor basal obtenido en la prueba de aliento.
ResultadosEl 48% de los sujetos positivos a H. pylori fueron cagA+. Se encontró una asociación positiva entre el estado de cagA y la alta actividad de la ureasa gástrica (p<0.0001), que además resultó significativamente asociada con la presencia de síntomas (p<0.0001).
ConclusiónLa actividad de la ureasa gástrica está fuertemente asociada con los síntomas de dispepsia y la presencia de H. pyloricagA+. Los valores elevados de 13C-delta sobre el valor basal pudieran ser usados como indicadores de mayor riesgo de enfermedad gástrica.
Helicobacter pylori (H. pylori) colonization is a well-recognized risk factor for the development of gastric disorders in the human stomach,1–3 even though most infected persons will remain asymptomatic throughout their lives. The seroprevalence of H. pylori infection in Mexico was reported at 66% and age was the strongest risk factor for infection.4 According to the fourth Mexican consensus on H. pylori,1 the association between functional dyspepsia and H. pylori infection is controversial. Several studies have suggested that the genetic variability of H. pylori and the host,5 as well as environmental factors, determine clinical outcome.6H. pylori is genetically diverse and type I strains, which are cytotoxin-associated gene A-positive (cagA+) and secrete the vacuolating cytotoxin A (VacA), are the most virulent.7,8 They are associated with duodenal ulceration,9,10 abdominal pain, bleeding, active gastritis,11 symptoms of dyspepsia,12,13 DNA damage in the gastric mucosa,14 and gastric carcinoma.15,16
Atherton et al. found histologic evidence of higher H. pylori density in the gastric mucosa colonized by cagA+ strains than in the epithelia colonized by cagA− strains.17 Other authors have also found a significant relationship between the density of H. pylori colonization and the presence of cagA+ and vacAs1 strains,18–20 suggesting the importance of bacterial load determination as a risk predictor of gastric disease. Perri et al. and Zagari et al. proposed the use of 13C-delta over baseline (DOB) as a predictor of the intragastric bacterial load and severity of H. pylori gastritis in patients referred for endoscopy.21,22 Matthews et al. also found that 13C-DOB values were significantly higher in symptomatic subjects with moderate and severe antral gastritis, compared with those that had mild gastritis or no inflammation.23 However, they found no correlation between H. pylori load, measured through bacterial culture and 13C-DOB values.23 In children with dyspeptic symptoms, Machado et al. also reported that 13C-DOB does not estimate the severity of histologic measurements of bacterial colonization.24 The lack of correlation may be due to the differing virulence of H. pylori strains, or to bacterial density estimates (through bacterial culture22 and histologic estimation24), which are not indicative of actual mucosal H. pylori load.
The non-invasive 13C-urea breath test (UBT) is the most sensitive and specific test for determining the presence of H. pylori.1 It uses the 13C-DOB value as the cut-off criterion, which is indicative of urease activity, and therefore, of bacterial load. Virulent H. pylori strain determination is made possible through complementary molecular or immunologic analyses. We hypothesized that a higher 13C-DOB value, in addition to indicating bacterial load, would also indicate the presence of the cagA+ genotype. Therefore, the primary aim of our study was to investigate the association between gastric urease activity (13C-DOB), symptoms of dyspepsia, and H. pyloricagA+, in an open population. We also explored the association of sociodemographic variables with the presence of H. pylori and determined the variables that best explained the 13C-DOB value.
Materials and methodsStudy populationsIn a community survey, 302 persons not taking antibiotics, antacids, or proton pump inhibitors for the past month, were recruited in Northern Mexico (Hermosillo, Sonora) to take a diagnostic urea breath test for H. pylori (within the time frame of June to November 2004). Twenty-five of those individuals were H. pylori-positive and their ages ranged from 25 to 74 years (mean age 34 years). They were randomly chosen to fill out a gastric symptoms survey and provide a gastric juice sample, using the Entero-test® (Enterotest HP, HDC Corporation, San Jose, CA, USA).
The subjects were clinically symptomatic, presenting with 2 or more symptoms of dyspeptic disease, such as epigastric pain, epigastric burning, postprandial fullness, early satiety, bloating, belching, nausea, and vomiting. The chronicity, variety, or intensity of symptoms were not considered in the symptom questionnaire.
Sociodemographic dataEducational level was evaluated, according to the following scale: 1, elementary school; 2, middle school; 3, high school; 4, semiprofessional; 5, professional; 6, postgraduate. Family income was expressed in monthly minimum wage units. Based on data in the literature, a score for the risk of presenting with H. pylori related to socioeconomic condition was calculated. Educational level, employment, family income, housing characteristics (construction material of the house, ceiling, and floor, presence and type of sewage disposal system, household drinking water), overcrowding (3 or more persons sharing one bedroom), the presence of domestic animals inside or outside the house, and animal breeding were all considered in the score. Characteristics associated with a high risk for presenting with H.pylori were coded numerically (no risk: 0–2; moderate risk: 3–5; high risk: >5).
Detection of Helicobacter pylori statusH. pylori status was determined through the 13C-UBT. Breath samples were collected before, 30min after, and 45min after the intake of 50mg of 13C-labeled urea, together with natural orange juice, to provide acidic gastric conditions. The 13C-UBT test has 98% sensitivity. The urease secreted by H. pylori in the stomach hydrolyses urea to release 13CO2 from the ingested labeled urea, which then enters into the body's bicarbonate pool and is excreted in breath. We measured the 13CO2/12CO2 ratio through isotope ratio mass spectrometry (BreathMAT Plus, 1998, Finnigan MAT GMBH, Bremen, Germany) and expressed the results as intensity ratios 13CO2/12CO2, 45/44). The 13C-DOB values considered positive for H. pylori were those ≥3.5‰.
Identification of cagA+Helicobacter pylori strainsGastric juice samples were taken from H. pylori-positive subjects after an overnight fast, using a string test (Entero-test® pediatric test, Enterotest HP, HDC Corporation, San Jose, CA, USA). The test consisted of a 90cm length of nylon fiber enclosed in a 2.5cm long weighted gelatin capsule that dissolves in the stomach. The string remained in the stomach for 1h, after which it was retrieved via the oral route and placed in 15ml of sterile saline for DNA isolation.
The string was vigorously shaken in the saline and centrifuged for DNA extraction from the resulting pellet, using the QIAamp DNA Mini Kit (Qiagen Inc., Valencia, CA, USA). DNA was subject to PCR amplification of the glmM and cagA genes, using the following available primers: cagA-F: 5′-ATAATGCTAAATTAGACAACTTGAGCGA-3′; cagA-R: 5′-AGAAACAAAAGCAATACGATCATTC-3′, product size: 120 bp25; glmM-F: 5′-GGATAAGCTTTTAGGGGTGTTAGGGG-3′; glmM-R: 5′-GCTTACTTTCTAACACTAACGCGC-3′, product size 300bp.26
PuReTaq Ready-to-go™ PCR Beads (Amersham Biosciences, GE Healthcare Life Sciences, Pittsburg, PA, USA) were used for PCR amplification. Each PCR bead reconstituted to 25μl contained 10pmol of the cagA-F and cagA-R or glmM-F and glmM-R primers (Sigma-Genosys, USA), 200μM of deoxy nucleoside triphosphates, 50mM KCl, 1.5mM MgCl2, 10mM Tris–HCl, and approximately 100ng of genomic DNA. Thirty-five cycles of 1min at 95°C, 1min at 52°C, and 1min at 72°C were performed. PCR products were visualized through standard procedure electrophoresis on 2% agarose gels.27
Statistical analysisData were analyzed using the 2007 Windows Number Cruncher Statistical System (Number Cruncher Statistical System for Windows, Kaysville, UT, USA). Descriptive statistics were used to characterize the population studied. A correlation analysis was performed to find associations between the sociodemographic variables and H. pylori infection markers, adjusting for sex (female, 0; male, 1) and age. To determine the variables that best explained the bacterial load (13C-DOB), a multivariate model was developed and included the following variables in the selection process: family income, overcrowding, housing characteristics, cagA+ H. pylori strain (absent, 0; present, 1), and presence of symptoms (absent, 0; present, 1), adjusted for age and sex.
ResultsTable 1 shows the sociodemographic characteristics of the participants. Only one of the 25 subjects identified as positive through the breath test was negative for the PCR amplification of glmM, the genetic marker of Helicobacter.
Sociodemographic characteristics of the subjects.
| Age (years) | 34.5 |
| Range | 25–74 |
| Sex, n | |
| Female | 7 |
| Male | 18 |
| Educationallevela | 6 |
| Monthly family income (minimum wage) | 5–10 |
| Percentage of participants at risk of presenting with H. pylori related to socioeconomicconditionb | |
| No risk | 44 |
| Moderate risk | 44 |
| High risk | 12 |
Educational level was evaluated using the following scale: 1, elementary school; 2, middle school; 3, high school; 4, semiprofessional; 5, professional; 6, postgraduate.
Calculated as described in the Materials and methods section, considering educational level, employment, family income, housing characteristics, overcrowding, the presence of domestic animals inside or outside the home, and breeding animals. Characteristics associated with a high risk of presenting with H.pylori were coded numerically (final scores were: no risk, 0–2; moderate risk, 3–5; and high risk, >5).
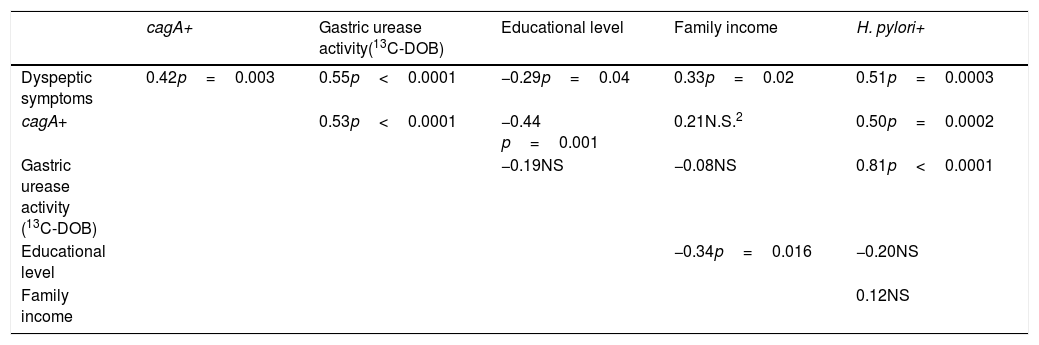
CagA+ H. pylori strains were found in 48% of the subjects. When divided by the presence of symptoms, 66% of the symptomatic subjects had cagA+ H. pylori strains, versus 33% of the asymptomatic subjects. The presence of cagA+ H. pylori strains was significantly associated with symptoms of dyspepsia (r=0.42; p=0.003) and negatively associated with educational level (r=−0.44; p=0.001) (Table 2).
Correlation coefficients and p values from the correlation analysis (adjusted by age and sex) for associations between the sociodemographic variables and H. pylori infection markers.
| cagA+ | Gastric urease activity(13C-DOB) | Educational level | Family income | H. pylori+ | |
|---|---|---|---|---|---|
| Dyspeptic symptoms | 0.42p=0.003 | 0.55p<0.0001 | −0.29p=0.04 | 0.33p=0.02 | 0.51p=0.0003 |
| cagA+ | 0.53p<0.0001 | −0.44 p=0.001 | 0.21N.S.2 | 0.50p=0.0002 | |
| Gastric urease activity (13C-DOB) | −0.19NS | −0.08NS | 0.81p<0.0001 | ||
| Educational level | −0.34p=0.016 | −0.20NS | |||
| Family income | 0.12NS |
DOB: delta over baseline; NS: not significant.
High gastric urease activity was associated with symptoms, given that 87.5% of the H. pylori positive participants with high gastric urease activity (13C-DOB values>20‰) had symptoms of dyspepsia, versus 35% of the H. pylori positive subjects with low gastric urease (13C-DOB values<20‰) and 12% of the H. pylori negative subjects.
Gastric urease activity, which is a marker of H. pylori load, showed a significant association with the presence of dyspepsia (r=0.55; p<0.0001) and cagA+ H. pylori strains (r=0.53; p<0.0001) (Table 2).
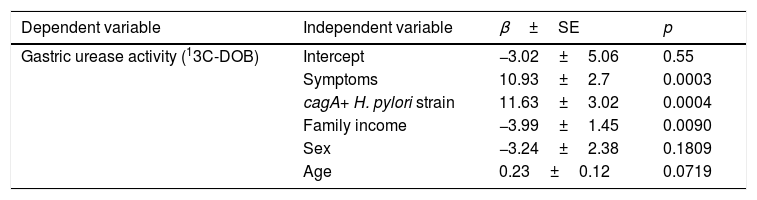
The variables that best explained the 13C-DOB value were the presence of symptoms (p=0.0003), cagA+ H. pylori strain (p=0.0004), and family income, adjusted for age and sex (p<0.01) (Table 3).
Multivariate regression analysis to assess gastric urease activity.
| Dependent variable | Independent variable | β±SE | p |
|---|---|---|---|
| Gastric urease activity (13C-DOB) | Intercept | −3.02±5.06 | 0.55 |
| Symptoms | 10.93±2.7 | 0.0003 | |
| cagA+ H. pylori strain | 11.63±3.02 | 0.0004 | |
| Family income | −3.99±1.45 | 0.0090 | |
| Sex | −3.24±2.38 | 0.1809 | |
| Age | 0.23±0.12 | 0.0719 |
DOB: delta over baseline; β±SE regression coefficient±standard error.
R2=0.5396 (p<0.0001).
The presence of cagA+ H. pylori strains was associated with cagA positivity and educational level (r=−0.44; p=0.001). In other words, cagA+ H. pylori carriers had a lower educational level than cagA−H. pylori carriers.
The cagA gene is a marker of the cag-pathogenicity island, and its presence is associated with more severe pathologies.11,28,29 The injection of CagA protein into gastric epithelial cells affects their spreading, migration, and adhesion, as well as other signal-transduction pathways related to proinflammatory responses, and induces interlukine-8 (IL-8) via the NF-κB signaling pathway.29 We found an association between cagA+ H. pylori colonization and the presence of symptoms. Similar results were obtained by Loffeld et al., who found that cagA+ H. pylori patients had more dyspeptic symptoms than patients with cagA−H. pylori strains.30
Utilizing gastric urease activity as an indicator of colonization intensity or bacterial load,22 we found that higher gastric activity correlated with greater symptoms, concurring with results reported by others.17–22
The main limitation of the present study was its small sample size. Nevertheless, to the best of our knowledge, it is the first analysis to investigate and show a positive association between 13C-DOB and cagA+ H. pylori strains, suggesting that an elevated 13C-DOB value could be indicative of pathogenicity. Another limitation of our study was the fact that the chronicity, variety, or intensity of dyspeptic symptoms were not included, preventing a more in-depth examination of the association between urease activity, H. pylori strain, and symptoms.
In conclusion, our data support the association between high gastric urease activity (elevated 13C-DOB values), cagA positive colonization, and the presence of gastric symptoms. High 13C-DOB values in patients could be a criterion for performing H. pylori genotype identification and deciding to initiate clinical treatment. Further studies aimed at establishing a possible cut-off value for pathologic risk are warranted.
Ethical considerationsProtection of human and animal subjectsOur study was evaluated and approved by the Ethics Committee of the Centro de Investigación en Alimentación y Desarrollo, A. C., based on the international standards stated in the Declaration of Helsinki and the General Health Law Regulation, related to the field of Health Research, of Sonora, Mexico.
Data confidentialityThe authors declare that they have treated all patient data with confidentiality and anonymity, following the protocols of their work center.
Right to privacy and informed consentAll participants signed written statements of informed consent, prior to enrollment. Strict data confidentiality was guaranteed, and the authors declare that the information contained in the study does not allow the identification of the participants.
Financial disclosureThis work was supported by the Acuerdo Regional de Cooperación para la Promoción de la Ciencia y Tecnología Nucleares en América Latina-Organismo Internacional de la Energía Atómica (ARCAL-OIEA), grant # ARCAL-RLA/6/042. MFMO received funding from the CONACYT Register No. 42473 (Mexico).
AuthorshipM.F. Moreno-Ochoa participated in the data acquisition, analysis, and interpretation and approval of the final version of the manuscript. M.E. Valencia participated in the study conception and design, data analysis and interpretation, drafting of the article, and approval of the final version of the manuscript. G.G. Morales-Figueroa participated in the data analysis and interpretation and approval of the final version of the manuscript. S.Y. Moya-Camarena participated in the study conception and design, data analysis and interpretation, drafting of the article, and approval of the final version of the manuscript.
Conflict of interestThe authors declare that there is no conflict of interest.
The authors wish to thank the study participants, Bertha Isabel Pacheco Moreno, Ana Cristina Gallegos Aguilar, and María Guadalupe Galaz Sánchez for their excellent technical assistance, and Dr. María Gloria Domínguez-Bello for her key contributions to the preparation of the study.
Please cite this article as: Moreno-Ochoa MF, Valencia ME, Morales-Figueroa GG, Moya-Camarena SY. Asociación de cepas de Helicobater pyloricagA+ con alta actividad de ureasa y dispepsia en adultos mexicanos. Revista de Gastroenterología de México. 2020;85:404–409.