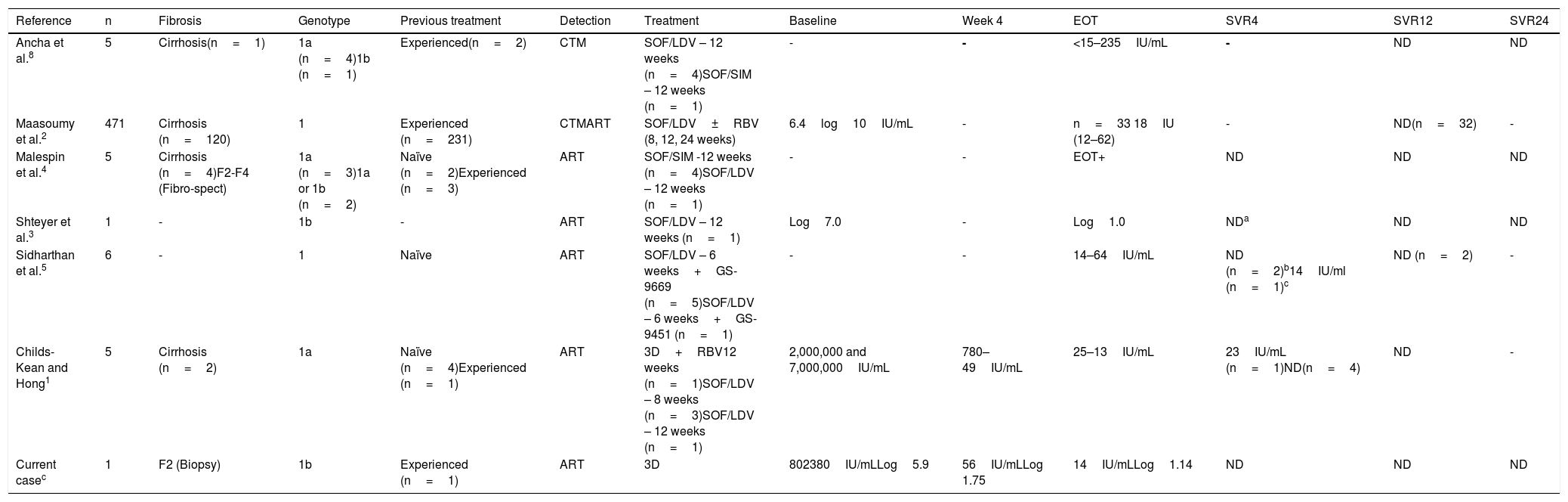
The treatment of hepatitis C virus (HCV) infection with regimens based on second generation direct-acting antivirals (DAAs) has been associated with high rates of sustained virologic response (SVR) and few secondary effects (1%). However, there is little information about the impact of detectable viral load on the SVR at the end of treatment with DAAs.1 Thus, we refer to the case of a 49-year-old Mexican man that had a history of failed treatment in 2006 with pegylated interferon and ribavirin for 48 weeks. The liver biopsy taken at that time reported grade 2 fibrosis (METAVIR F2). In 2016, the patient received 12 weeks of paritaprevir/ritonavir/ombitasvir/dasabuvir (3 D), with complete adherence, and no significant adverse events. Viral load at the end of treatment was detectable (Abbott Real Time PCR assay [ART]), with SVR 3 months later (Table 1).
Characteristics of patients with detectable viral load at the end of treatment and later sustained virologic response.
| Reference | n | Fibrosis | Genotype | Previous treatment | Detection | Treatment | Baseline | Week 4 | EOT | SVR4 | SVR12 | SVR24 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ancha et al.8 | 5 | Cirrhosis(n=1) | 1a (n=4)1b (n=1) | Experienced(n=2) | CTM | SOF/LDV – 12 weeks (n=4)SOF/SIM – 12 weeks (n=1) | - | - | <15–235IU/mL | - | ND | ND |
| Maasoumy et al.2 | 471 | Cirrhosis (n=120) | 1 | Experienced (n=231) | CTMART | SOF/LDV±RBV (8, 12, 24 weeks) | 6.4log10IU/mL | - | n=33 18IU (12–62) | - | ND(n=32) | - |
| Malespin et al.4 | 5 | Cirrhosis (n=4)F2-F4 (Fibro-spect) | 1a (n=3)1a or 1b (n=2) | Naïve (n=2)Experienced (n=3) | ART | SOF/SIM -12 weeks (n=4)SOF/LDV – 12 weeks (n=1) | - | - | EOT+ | ND | ND | ND |
| Shteyer et al.3 | 1 | - | 1b | - | ART | SOF/LDV – 12 weeks (n=1) | Log7.0 | - | Log1.0 | NDa | ND | ND |
| Sidharthan et al.5 | 6 | - | 1 | Naïve | ART | SOF/LDV – 6 weeks+GS-9669 (n=5)SOF/LDV – 6 weeks+GS-9451 (n=1) | - | - | 14–64IU/mL | ND (n=2)b14IU/ml (n=1)c | ND (n=2) | - |
| Childs-Kean and Hong1 | 5 | Cirrhosis (n=2) | 1a | Naïve (n=4)Experienced (n=1) | ART | 3D+RBV12 weeks (n=1)SOF/LDV – 8 weeks (n=3)SOF/LDV – 12 weeks (n=1) | 2,000,000 and 7,000,000IU/mL | 780–49IU/mL | 25–13IU/mL | 23IU/mL (n=1)ND(n=4) | ND | - |
| Current casec | 1 | F2 (Biopsy) | 1b | Experienced (n=1) | ART | 3D | 802380IU/mLLog5.9 | 56IU/mLLog 1.75 | 14IU/mLLog1.14 | ND | ND | ND |
3D: paritaprevir/ritonavir/ombitasvir/dasabuvir; ART: Abbott RealTime PCR assay; CTM: Cobas TaqMan HCV Test; EOT: end of treatment; ND: not detected; RBV: ribavirin; SOF/LDV: sofosbuvir/ledipasvir; SOF/SIM: sofosbuvir/simeprevir.
Previous analyses have reported a 5–7% detectable viral load at the end of treatment with SVR after different DAA regimens.1–4 We found 6 reports in relation to that interesting phenomenon, which are summarized in Table 1. To explain the viremia at the end of treatment, some authors suggest a mechanism involving viral kinetics, in which noninfectious viral particles or defective virions can be detected transitorily at the end of treatment.5 In addition, HCV infection is known to affect cell immunity, and a decrease in viral load after an effective treatment could subsequently restore the immune mechanisms that enable the clearance of residual viruses at the end of antiviral therapy.1,4 Strikingly, the majority of cases with positive viremia that later achieve SVR were described through the use of highly sensitive assays, such as real-time polymerase chain reaction.1,2 HCV virion clearance occurs at a rate of 10–12 virions per day, but apoptosis of the infected cells has been observed to extend for more than 70 days.6 We believe that our patient is not a case of a false positive, given that the viral loads were determined using the same method and they were not detectable 24 weeks after having finished treatment.
At present, predictors associated with detectable viral load at the end of treatment have not been reported. In the largest case series, conducted by Maasoumy et al.,2 neither the baseline viral load nor the regimen utilized, were associated with said phenomenon. The available information suggests that having a detectable viral load at the end of treatment is not clinically relevant, given that almost all the patients in the case series cited above, reached SVR (Table 1). In the recommendations of the 2018 EASL guidelines,7 the determination of viral load at the end of treatment is omitted, evaluating response 12 weeks later. That is based on the fact that efficacy of the DAA regimens is close to 100%.
Ethical disclosuresInformed consent was requested from the patient to receive the treatment. The present scientific letter meets the current bioethical research norms and was authorized by the ethics committee of the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán. The patient cannot be recognized or identified through the images or data contained in the article.
Financial disclosureNo financial support was received in relation to this article.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Toapanta-Yanchapaxi L, Páez-Zayas VM, Cuevas-Castillejos JE, Lizárraga-Gómez E, García-Juárez I. ¿Qué sabemos acerca de la carga viral detectable al final del tratamiento de virus de hepatitis C con respuesta viral subsecuente? Rev Gastroenterol Méx. 2019;84:526–528.