Hepatitis C virus (HCV) infection is a worldwide public health problem associated with significant morbidity and mortality. In the context of liver transplantation, the demand for organs continues to exceed the supply, prompting the consideration of using organs from HCV-positive donors in HCV-negative recipients. The introduction of direct-acting antivirals (DAAs), which have demonstrated great efficacy in eradicating the virus, has made transplantation of organs from donors with HCV infection possible. The present article provides a brief review of the current evidence on the use of organs from HCV-infected patients.
La infección por el virus de hepatitis C (VHC) es un problema de salud pública a nivel mundial asociado a una gran morbilidad y mortalidad. En el caso del trasplante hepático, la demanda de órganos continúa siendo mayor que la oferta. Este problema ha llevado a tomar en consideración el uso de órganos de donadores con VHC positivos en receptores negativos.
Gracias al advenimiento de los antivirales de acción directa (AAD), los cuales han mostrado ser altamente efectivos para la curación del virus, ha sido posible procurar y utilizar órganos de donadores con infección por VHC para ser trasplantados. En este artículo se hace una breve revisión de la evidencia actual del uso de órganos provenientes de pacientes infectados por VHC.
Hepatitis C virus (HCV) infection is a global public health problem associated with significant morbidity and mortality. According to estimates made in 2022 by the World Health Organization (WHO), around 58 million persons have chronic HCV infection worldwide, with an approximate incidence of 1.5 million new infections each year.1
In Mexico, according to the National Statistics and Geography Registry (INEGI, for the Spanish acronym),2 cirrhosis of the liver was the fifth cause of death in men and the seventh in women in 2022, and the only available cure at present is orthotopic liver transplant (OLT). There are different challenges in Mexico for organ transplantation, which include a low donation rate, donors with marginal organs, and the prevalence of fatty liver among donors. In addition, there is a significant discrepancy between organ demand and supply, for which the use of organs from HCV-infected donors could be of help.3 The use of organs with HCV infection requires universal access to direct-acting antivirals (DAAs). Said strategy could be implemented in Mexico, given that DDAs are required for the transplantation of organs from HCV-positive donors, in HCV-negative recipients.3 The results with this approach are similar to those of transplants performed with organs from donors that are negative for HCV.
On the other hand, the opioid epidemic is of great importance because it leads to a high mortality rate. Within the time frame of 1996 to 2019, approximately 500,000 deaths secondary to opioid overdose were reported.4 This epidemic has mainly affected North America, especially the United States, and more recently, Canada.4
In Mexico, the estimated prevalence of opioid use in the general population is under 1%. The most affected individuals are men under 45 years of age, particularly in the Mexican states bordering the United States. Given that intravenous drug use and abuse leads to the risk for infection, transmission, and development of HCV, it should be contemplated that these patients, in the long term, could be possible organ donors (due to the deaths associated with abuse). It must also be remembered that during the progression of opioid use, more than one out of every 3 drug users negative for HCV before their drug use, will develop HCV infection in the following year.5
Our aim was to carry out a review of the current evidence on the impact of the transplantation of organs from HCV-positive donors in HCV-negative recipients.
Type of organ donationWith respect to solid organ transplantation, demand continues to exceed supply, resulting in a considerable mortality rate in the patients on the waiting list, given that 25% of these patients are removed from the list or die, annually.6 This situation has made it necessary to look for alternatives other than the exclusive use of the brain-dead donor, resulting in the options of donors in controlled asystole (Maastricht III and IV), living donors, split organs, and organs from donors with chronic viral infections, especially hepatitis C. This last group accounted for 9.7% of the livers donated in 2019 and has recently increased.7
Impact of the direct-acting antiviralsThe arrival of the DAAs produced a revolution in the management prospects of hepatitis C,8 because they have been shown to be highly effective, achieving a sustained virologic response (SVR) above 95%, regardless of fibrosis grade and viral genotype. This had led to the worldwide development of HCV eradication programs.
It is well known that HCV recurrence after OLT is practically inevitable in infected patients, and if not treated, can lead to graft loss. However, DAAs have had a significant impact at all stages of liver disease. The achievement of SVR in patients with advanced liver disease stabilizes the disease and can delay transplantation. A considerable reduction in the number of patients with cirrhosis due to HCV on the waiting list for liver transplantation has also been observed.9
The high level of efficacy and tolerability of the currently available antivirals makes it possible to consider the use of organs from HCV-positive donors that previously would not have been considered viable. As a result, there is a higher percentage of this type of donor, increasing from 7 to 17% since 2015,10 posing new challenges and ethical considerations.11
The effect of drug use and hepatitis C virusThe opioid epidemic is extremely concerning, with a notable concentration in the United States and Canada in recent years. There has been a third wave of this problem since 2013 due to synthetic opioid use. In 2009, death due to drug use surpassed the automobile accident mortality rate, and between 1996 and 2019, more than 500,000 opioid-related deaths were registered. Importantly, since 2012, the number of opioid users has increased to approximately 225 million, with mortality at 11,000 persons per year, regardless of age, sex, race, or ethnicity.4
A study conducted during 2017 in the United States revealed that approximately 11.4 million persons stated that they had used drugs in the past year. Strikingly, 4.2% of that study population was only 12 years of age and the related mortality was 47,000 persons.12
Historically, Mexico has been considered a country with a low prevalence of drug use. However, in recent years, data have shown an increase in opioid use, associated with production. According to the National Survey on Drug, Alcohol, and Tobacco Use (ENCODAT, the Spanish acronym) conducted in 2016-2017, the prevalence of opioid use was below 1%, with a greater risk in men than in women; the most affected age group was 18 to 34 years.13
The opioid epidemic has been associated with an increase in parenterally transmitted infections, including hepatitis C. More than 30% of initially uninfected persons are known to end up positive for HCV after one year of being intravenous drug users (IDUs)4 and the development of the infection leads to a greater risk for death. Likewise, in 2017, the number of infected donors was observed to increase from 1.1 to 13.7%.14 These donors tend to be young and previously healthy, enabling the procurement of organs in excellent conditions.15
The phenomenon of opioid abuse is occurring in Mexico, which could lead to problems similar to those described in the United States and Canada.12 A study conducted on IDUs at Mexico’s border zones with the United States (Tijuana and Ciudad Juárez) found a general prevalence of antibodies against HCV of 94.6% and against human immunodeficiency virus (HIV) of 2.8%.16
Hepatitis C virus-positive donorsTo understand the reach of the use of organs from HCV-positive donors, the fact that there are 2 groups of infected patients must be underlined. The first group is made up of persons with positive antibodies and positive RNA for HCV (Ab+/RNA+) or with positive antibodies and a positive nucleic acid test (NAT) (Ab+/NAT+). The second group is made up of persons with positive antibodies and negative RNA (Ab+/RNA–).17 The relevance of this is in the fact that the group of donors with Ab+/RNA– has negative viremia and the risk for disease transmission is null.11 However, the majority of studies conducted before 2013 report “positive donors” as those with only a positive antibody test, and so the number of patients with viremia is unknown.
The use of organs from Ab+/RNA+ donors has been considered for many years in solid organ transplantation. However, given all the adverse effects caused by interferon (IFN), as well as a low SVR (under 50%), the implementation of this therapy has had suboptimal results. With the current increase in the need for OLT and the high cure rate with DAAs, the possibility of utilizing organs from IDUs and HCV-positive persons offers a great opportunity for increasing the number of donors.
The high prevalence of Ab+ individuals in Mexican border cities, such as Tijuana and Ciudad Juárez, should be considered an option for increasing the number of solid organ transplants in the different programs of our country.17 Given that the IDUs that die from a drug overdose are often young and with few comorbidities or none at all, they could be considered ideal candidates for organ donation.17
The criteria utilized in different studies that evaluate organs from HCV-positive donors vary. Some depend solely on the experience of the surgeon, given that an organ can be considered viable simply through its macroscopic appearance. However, there are also studies that employ stricter criteria, performing a baseline liver biopsy in organs from potential donors; the organs are considered adequate for donation if they have a fibrosis grade under 2 (18–20
Cost advantages in using organs from hepatitis C virus-positive donorsThe advantages in using organs from HCV-positive donors are not only reflected in OLT programs. Kidney transplantation provides the clearest example, taking into account that the time from starting dialysis to transplantation can be on average 4 years in developed countries, such as Canada and the United States, whereas in Mexico the interval can be even longer. Utilizing organs from donors that have HCV and are Ab+/RNA+can increase access to transplantation, as well as significantly reduce the time on the waiting list (years), with excellent results in graft function, converting this therapy into a cost-effective strategy in the medium and long terms. In Mexico, studies analyzing costs have been conducted on populations with chronic kidney disease, comparing renal replacement therapy with kidney transplantation. The result at follow-up year 3 was a significant cost reduction of more than 60%, in favor of transplantation.21 Another study analyzed the cost-benefit at follow-up year 5, evaluating the strategy of accepting an Ab+/RNA+ graft in HCV-negative recipients, who after the procedure, received a DAA regimen. Said intervention was effective and less costly ($138,000.00 USD), with 4.8 years of life, compared with the strategy of continuing on dialysis and staying on the waiting list until receiving a no-risk organ, with a cost that was over 200% higher ($329,000.00 USD) and 4.7 years of life.22
In patients with cirrhosis of the liver, the grade of decompensation is known to be proportional to the costs of medical care. In a study that analyzed mean yearly costs in USD per type of care at the Instituto Mexicano de Seguridad Social (IMSS), the costs were $4,269.00, $16,949.63, and $30,249.25 for patients classified as Child-Pugh A, Child-Pugh B, and Child-Pugh C, respectively. The estimated lifetime cost for a patient with cirrhosis was $65,520.19.23 The increase in the number of donors, and consequently, the number of OLTs, not only impacts survival and time on the waiting list, but also can significantly reduce the reported high costs of medical care for advanced liver disease in the Mexican public health systems.
Advantages in accepting hepatitis C virus-positive donors in hepatitis C virus-negative recipients in liver transplantationAs described above, DAAs confer a high rate of HCV eradication in the general population, as well as in the transplanted population. Because side effects and medication interactions are minimal, treatment with DAAs after transplantation is considered safe.24 As a result, broad experience in OLT with Ab+/RNA+donors has been accumulated in recent years. For example, in the study conducted by Cotter et al. that analyzed a cohort of patients that underwent transplantation within the time frame of January 2008 and January 2018, overall survival and graft survival at one and 2 years in recipients with Ab+/RNA+ donors were similar to results with non-viremic donors.25
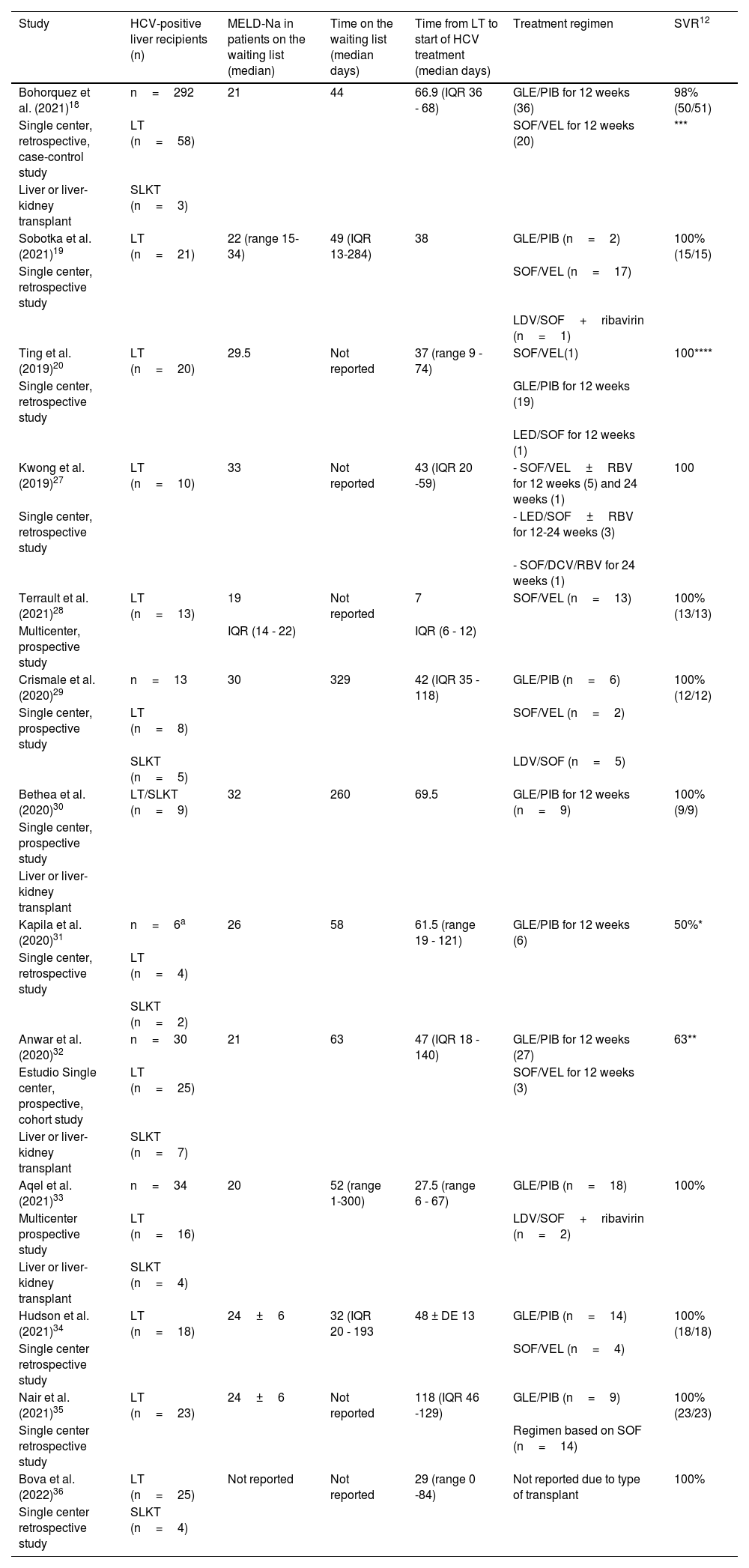
In addition, in a systematic review that included 15 studies conducted in the United States and Europe, with a sample size of more than 1,900 patients, there was no difference in overall survival and graft survival. Despite the fact that infected grafts can have a lower SVR and higher costs due to additional treatments, survival is known to improve in critically ill patients on the waiting list, especially in those that have a MELD score above 20 or even > 28 points.26Table 1 summarizes the impact of DAAs on OLTs from positive donors (D+)/negative recipients (R-) for HCV.
Liver transplantation from HCV-positive donors in HCV-negative recipients. Published trials.
| Study | HCV-positive liver recipients (n) | MELD-Na in patients on the waiting list (median) | Time on the waiting list (median days) | Time from LT to start of HCV treatment (median days) | Treatment regimen | SVR12 |
|---|---|---|---|---|---|---|
| Bohorquez et al. (2021)18 | n=292 | 21 | 44 | 66.9 (IQR 36 - 68) | GLE/PIB for 12 weeks (36) | 98% (50/51) |
| Single center, retrospective, case-control study | LT (n=58) | SOF/VEL for 12 weeks (20) | *** | |||
| Liver or liver-kidney transplant | SLKT (n=3) | |||||
| Sobotka et al. (2021)19 | LT (n=21) | 22 (range 15-34) | 49 (IQR 13-284) | 38 | GLE/PIB (n=2) | 100% (15/15) |
| Single center, retrospective study | SOF/VEL (n=17) | |||||
| LDV/SOF+ribavirin (n=1) | ||||||
| Ting et al. (2019)20 | LT (n=20) | 29.5 | Not reported | 37 (range 9 - 74) | SOF/VEL(1) | 100**** |
| Single center, retrospective study | GLE/PIB for 12 weeks (19) | |||||
| LED/SOF for 12 weeks (1) | ||||||
| Kwong et al. (2019)27 | LT (n=10) | 33 | Not reported | 43 (IQR 20 -59) | - SOF/VEL±RBV for 12 weeks (5) and 24 weeks (1) | 100 |
| Single center, retrospective study | - LED/SOF±RBV for 12-24 weeks (3) | |||||
| - SOF/DCV/RBV for 24 weeks (1) | ||||||
| Terrault et al. (2021)28 | LT (n=13) | 19 | Not reported | 7 | SOF/VEL (n=13) | 100% (13/13) |
| Multicenter, prospective study | IQR (14 - 22) | IQR (6 - 12) | ||||
| Crismale et al. (2020)29 | n=13 | 30 | 329 | 42 (IQR 35 - 118) | GLE/PIB (n=6) | 100% (12/12) |
| Single center, prospective study | LT (n=8) | SOF/VEL (n=2) | ||||
| SLKT (n=5) | LDV/SOF (n=5) | |||||
| Bethea et al. (2020)30 | LT/SLKT (n=9) | 32 | 260 | 69.5 | GLE/PIB for 12 weeks (n=9) | 100% (9/9) |
| Single center, prospective study | ||||||
| Liver or liver-kidney transplant | ||||||
| Kapila et al. (2020)31 | n=6a | 26 | 58 | 61.5 (range 19 - 121) | GLE/PIB for 12 weeks (6) | 50%* |
| Single center, retrospective study | LT (n=4) | |||||
| SLKT (n=2) | ||||||
| Anwar et al. (2020)32 | n=30 | 21 | 63 | 47 (IQR 18 - 140) | GLE/PIB for 12 weeks (27) | 63** |
| Estudio Single center, prospective, cohort study | LT (n=25) | SOF/VEL for 12 weeks (3) | ||||
| Liver or liver-kidney transplant | SLKT (n=7) | |||||
| Aqel et al. (2021)33 | n=34 | 20 | 52 (range 1-300) | 27.5 (range 6 - 67) | GLE/PIB (n=18) | 100% |
| Multicenter prospective study | LT (n=16) | LDV/SOF+ribavirin (n=2) | ||||
| Liver or liver-kidney transplant | SLKT (n=4) | |||||
| Hudson et al. (2021)34 | LT (n=18) | 24±6 | 32 (IQR 20 - 193 | 48 ± DE 13 | GLE/PIB (n=14) | 100% (18/18) |
| Single center retrospective study | SOF/VEL (n=4) | |||||
| Nair et al. (2021)35 | LT (n=23) | 24±6 | Not reported | 118 (IQR 46 -129) | GLE/PIB (n=9) | 100% (23/23) |
| Single center retrospective study | Regimen based on SOF (n=14) | |||||
| Bova et al. (2022)36 | LT (n=25) | Not reported | Not reported | 29 (range 0 -84) | Not reported due to type of transplant | 100% |
| Single center retrospective study | SLKT (n=4) |
DCV: daclatasvir; GLE: Glecaprevir; LT: liver transplant; PIB: pibrentasvir; RBV: ribavirin; SLKT: simultaneous liver-kidney transplant; SOF: sofosbuvir; SVR12: sustained virologic response week 12; VEL: velpatasvir.
Significantly, due to the wave of treatments with DAAs, the infected population that received treatment and achieved SVR is going to be Ab+for HCV. However, viremia or NAT will always be negative. This distinction is important, given that transmission of the virus is unusual in donors with a negative NAT test. Even though cases of transmission are rare, if they do occur, it is most likely due to an acute infection in patients that were in the “window period”.37
Currently, overall survival and graft survival in recipients of HCV-infected organs have been shown to improve with DAA use, even resulting in survival comparable to that of patients transplanted with organs from HCV-negative donors. Nevertheless, it is essential to always inform the potential recipient about the risks and benefits of this strategy.17,38 This should be done through informed consent, with the information provided by trained personnel in a clear and simple manner, with no coercion, and letting the recipient exercise his/her autonomy, given that all patients may not wish to accept this type of organ donation. A study conducted in the United States showed that, despite the abovementioned advantages, only 46% of patients accepted an organ from an HCV-positive donor, and of those patients only 60% were aware that HCV was curable.39
Direct-acting antiviral accessSince 2010, the World Health Assembly has recognized the hepatitis viruses as a public health problem, which is why the World Health Organization (WHO) established a global hepatitis C program, for the purpose of its eradication through different strategies.
In the same context, Mexico created the Specific Action for the Prevention, Diagnosis, and Treatment of Hepatitis C Program, 2016-2018, to achieve those goals in the medium term. Thanks to this program, Mexico has had universal access to effective treatments against HCV, making it possible to consider the strategy of organ donation from infected patients.40 However, there is currently no solid public policy in the country for accepting the use of infected organs, representing a challenge, not only for the National Transplant Center (CENATRA, the Spanish acronym), but also for every authorized transplant center and committee.
Impact of hepatitis C virus-positive donors on hepatitis C virus-negative recipients in non-liver transplantationNot only are there advantages with respect to OLT, but the efficacy of using HCV-positive donors in HCV-negative recipients in the transplantation of organs, such as the kidney, heart, and lung, among others, has also been shown.
Kidney transplantIn 2017, the first study utilizing viremic kidney grafts in 10 nonviremic recipients was described. One of the advantages found was a short time of only 58 days on the waiting list. The 10 patients received a DAA regimen based on elbasvir-grazoprevir, and all of them achieved SVR. In addition, graft function was excellent at the follow-up at six months.41
In another study on 10 patients, they received an organ from HCV-positive donors. The median time from being on the waiting list to undergoing transplant was only 30 days; therapy with DAAs was started before the transplant and continued for 12 weeks. In that study, 100% of the patients (n=10) achieved SVR, with no severe adverse effects related to treatment or immunosuppression.42
Heart transplantThere is also evidence regarding heart transplant that shows safety in utilizing HCV-infected organs. In a study by Schlendorf et al., they included 13 heart transplant patients that received an organ from HCV-positive donors. Nine of those patients developed viremia after transplantation and treatment with DAAs was begun once the patients remained clinically stable and were discharged. Eight of the 9 patients achieved SVR at 12 weeks after finishing the first regimen and one patient died due to pulmonary embolism. At the follow-up at 6 months, none of the patients presented with adverse events related to HCV or eradication treatment.43
In a study conducted by Kilic et al., the aim was to compare the results of heart transplant from HCV-positive donors versus HCV-negative donors. The study included 7,889 patients that received a heart transplant, within the time frame of 2016 to 2018 at 128 centers in the United States. HCV-positive organs were utilized in 343/7,889 (4.4%) of the transplants. There was no difference in overall survival at one year (90.2 vs. 91.1%; p = 0.86) or in the rejection rate at one year (22.1 vs. 21.1%; p = 0.84) between the two groups, demonstrating the safety in using that type of organ.44
Lung transplantSimilar to analyses of other organs, a study was conducted that included 8 patients with heart transplantation and 36 patients with lung transplantation from HCV-positive donors. The patients received treatment with DAAs a few hours after the procedure, for a duration of four weeks, and achieved SVR and survival of 100% at the follow-up at 6 months.45
In another study that included 22 lung transplant cases with HCV-positive organs, SVR was 100% and survival was 95% at the follow-up at 6 months.46
Other organsThere are reports of pancreas transplant or simultaneous pancreas and kidney transplant, especially in patients with type 1 diabetes, as well as multivisceral transplants, with the number of procedures increasing from 2.5 to 15% in recent years. Results have been very similar to those in other organs, with an impact on reducing the length of time on the waiting list, as well as an overall survival rate of more than 90% at one year of follow-up.47–49
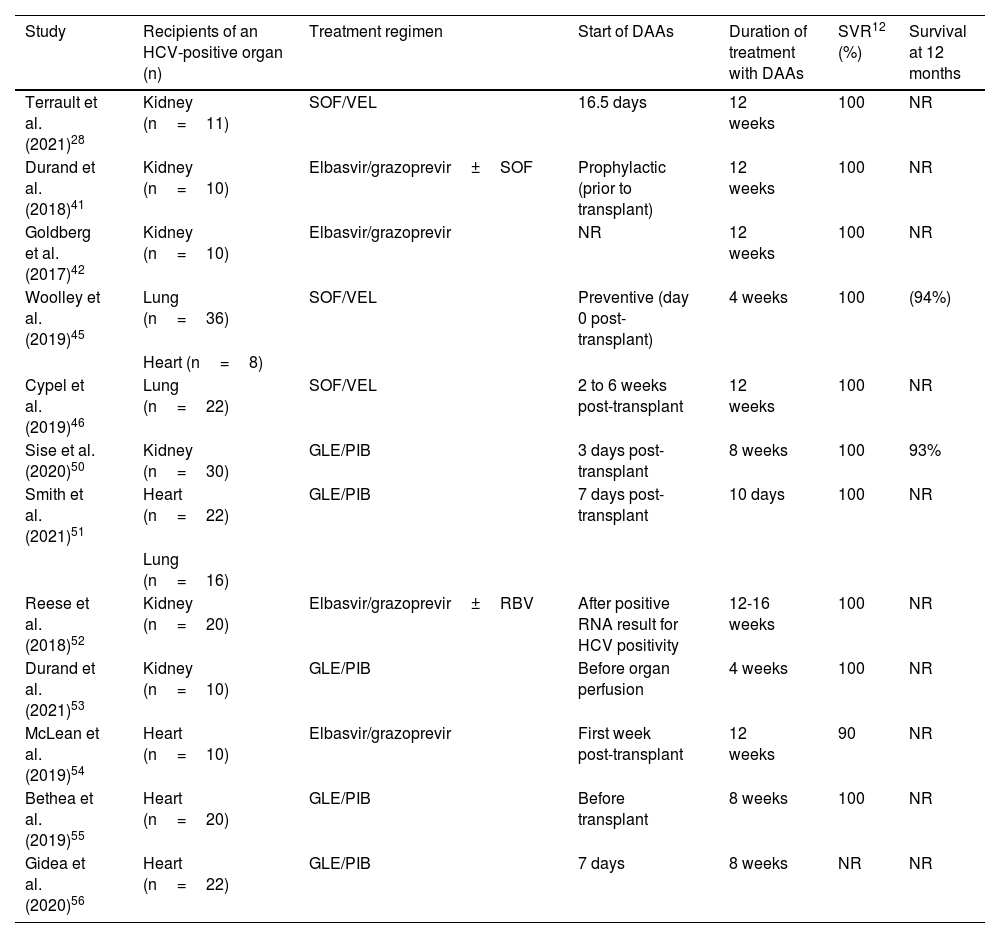
Table 2 summarizes the DAA regimens in HCV D+/R- non-liver transplants.
Organ transplants from HCV-positive donors in HCV-negative recipients. Published trials.
| Study | Recipients of an HCV-positive organ (n) | Treatment regimen | Start of DAAs | Duration of treatment with DAAs | SVR12 (%) | Survival at 12 months |
|---|---|---|---|---|---|---|
| Terrault et al. (2021)28 | Kidney (n=11) | SOF/VEL | 16.5 days | 12 weeks | 100 | NR |
| Durand et al. (2018)41 | Kidney (n=10) | Elbasvir/grazoprevir±SOF | Prophylactic (prior to transplant) | 12 weeks | 100 | NR |
| Goldberg et al. (2017)42 | Kidney (n=10) | Elbasvir/grazoprevir | NR | 12 weeks | 100 | NR |
| Woolley et al. (2019)45 | Lung (n=36) | SOF/VEL | Preventive (day 0 post-transplant) | 4 weeks | 100 | (94%) |
| Heart (n=8) | ||||||
| Cypel et al. (2019)46 | Lung (n=22) | SOF/VEL | 2 to 6 weeks post-transplant | 12 weeks | 100 | NR |
| Sise et al. (2020)50 | Kidney (n=30) | GLE/PIB | 3 days post-transplant | 8 weeks | 100 | 93% |
| Smith et al. (2021)51 | Heart (n=22) | GLE/PIB | 7 days post- transplant | 10 days | 100 | NR |
| Lung (n=16) | ||||||
| Reese et al. (2018)52 | Kidney (n=20) | Elbasvir/grazoprevir±RBV | After positive RNA result for HCV positivity | 12-16 weeks | 100 | NR |
| Durand et al. (2021)53 | Kidney (n=10) | GLE/PIB | Before organ perfusion | 4 weeks | 100 | NR |
| McLean et al. (2019)54 | Heart (n=10) | Elbasvir/grazoprevir | First week post-transplant | 12 weeks | 90 | NR |
| Bethea et al. (2019)55 | Heart (n=20) | GLE/PIB | Before transplant | 8 weeks | 100 | NR |
| Gidea et al. (2020)56 | Heart (n=22) | GLE/PIB | 7 days | 8 weeks | NR | NR |
DCV: Daclatasvir; GLE: Glecaprevir; NR: Not reported; PIB: Pibrentasvir; RBV: Ribavirin; SOF: Sofosbuvir; SVR12: sustained virologic response week12; VEL: Velpatasvir.
The different DAA treatment regimens for HCV have numerous advantages, including a short period of duration (12 weeks), few adverse effects, and a high eradication rate. With the great disparity between patients that need an organ transplant and organ availability, many transplantation programs worldwide have opted for using organs from HCV-positive donors, given the high response rate to DAAs.38
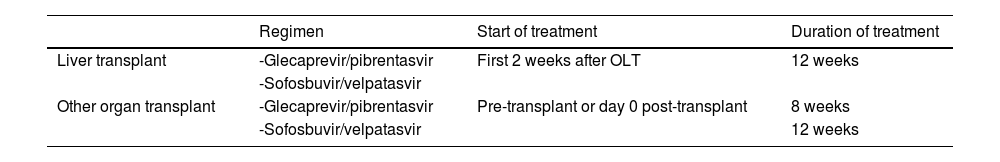
Liver transplantMultiple studies have shown the efficacy and safety of DAAs in the posttransplant period in recipients of HCV-positive and RNA-positive organs. The international guidelines recommend starting treatment early, preferably at the first or second posttransplant week, when the patient is clinically stable. The pangenotypic regimens currently recommended include a daily dose of the combination of glecaprevir (300 mg) and pibrentasvir (120 mg) or sofosbuvir (400 mg) and velpatasvir (100 mg), for 12 weeks. Both regimens are available in Mexico (Table 3).57
DAA treatment regimens in organ recipients from HCV-positive donors.
| Regimen | Start of treatment | Duration of treatment | |
|---|---|---|---|
| Liver transplant | -Glecaprevir/pibrentasvir | First 2 weeks after OLT | 12 weeks |
| -Sofosbuvir/velpatasvir | |||
| Other organ transplant | -Glecaprevir/pibrentasvir | Pre-transplant or day 0 post-transplant | 8 weeks |
| -Sofosbuvir/velpatasvir | 12 weeks |
DAA: direct-acting antiviral; HCV: hepatitis C virus; OLT: orthotopic liver transplant.
Different studies have examined outcomes in OLT from donors with positive HCV viremia. In their 2019 study, Kwong et al. reported the results from 10 HCV-negative patients that received an organ from donors with HCV and positive RNA. Of those recipients, 100% achieved SVR with the different DAA regimens, with a treatment duration of 12 to 24 weeks. The median time interval from transplant to the start of treatment was 43 days (interquartile range [IQR] of 20-59 days). Strikingly, there was a higher rate of acute posttransplant rejection events, possibly related to the interactions with immunosuppressants or changes in the immunologic profile after HCV eradication. However, there were no graft losses or deaths reported at the follow-up at 380 days (IQR 263-433).27
In another single center study, 61 patients transplanted with viremic organs were compared with 231 recipients of non-viremic organs. Only 56/61 patients received antiviral therapy and treatment was begun at a median of 66 days after transplant. Four patients (6.5%) died during the first year of follow-up; their deaths were unrelated to HCV or treatment. Of the 51 patients with complete treatment information, 64% were treated with glecaprevir-pibrentasvir and 36% received sofosbuvir-velpatasvir. SVR was achieved in 100% of the patients at 12 weeks, and only one patient (< 2%) required rescue therapy based on sofosbuvir-velpatasvir-voxilaprevir, after recurrence of the virus. There were no significant differences in the clinical outcomes, such as acute cell rejection, kidney function deterioration, or overall survival and graft survival, between the recipients of organs from HCV-viremic donors and from non-viremic donors.18
Another prospective, multicenter, clinical trial included 13 HCV-negative patients that received an organ from RNA-positive donors. The recipients were treated with sofosbuvir-velpatasvir for 12 weeks. The median time interval from transplantation to the start of antiviral treatment was 7 days. SVR was achieved in 100% of the patients. Four patients (31%) were reported to have severe adverse events, such as antibody-mediated rejection, idiopathic cardiomyopathy, intrahepatic biliary sclerosis, and graft-versus-host disease (which resulted in the death of the patient), but it was not clear whether the deaths were virus or treatment-related.28
In contrast to the transplantation of other organs, the use of short antiviral regimens of fewer than 12 weeks, is not recommended in OLT, due to the large reservoir of HCV in the transplanted organ. The most important goal is to eradicate the virus. Case reports have described extrahepatic lesions, such as acute kidney injury, within the first month, due to HCV-associated proliferative focal glomerulonephritis,58 exemplifying the risk for developing manifestations characteristic of HCV in the posttransplant period. Other less frequent complications are immunologic alterations, mainly rejection, albeit more studies are needed to evaluate the real risk, underlining the importance of strict surveillance during the follow-up of such patients.
Other organ transplantIn cases of non-liver transplant, two manners of starting different treatments with DAAs are recommended. The first is as prophylaxis, prior to knowing the results of viral RNA, generally before the transplant or on posttransplant day 0. Alternatively, preventive treatment can be offered, which is started from day 0 up to the first posttransplant week. The 2 pangenotypic regimens currently used are the combination of glecaprevir (300mg) and pibrentasvir (120mg) for 8 weeks, or sofosbuvir (400 mg) and velpatasvir (100mg) for 12 weeks (Table 3).57
The THINKER study included 10 HCV-negative recipients of kidneys from donors with positive viremia and genotype 1. All patients were treated with elbasvir-grazoprevir for 12 weeks, achieving 100% SVR and with no severe adverse effects.59
The prospective, multicenter MYTHIC trial included 30 HCV-negative recipients that underwent kidney transplant with viremic donors. Initial early treatment (within the first 3 posttransplant days) was with glecaprevir-pibrentasvir for 8 weeks and SVR was reached in 100% of the cases. In addition, there were no significant treatment-related adverse events.50 At one year, survival was 93% (with no deaths related to treatment or virus) and organ function was excellent (mean creatinine of 1.17; IQR 1.02-1.38mg/dl).60
In heart transplantation, a study with 22 recipients that received an organ from HCV-viremic donors evaluated the DAA regimen of glecaprevir-pibrentasvir for 8 weeks, starting between posttransplant days 6 and 11, once the viremia had developed. Two patients had a temporary 3-day interruption of treatment due to hyperbilirubinemia. Upon comparing the recipients of HCV-viremic organs with those that received non-viremic organs, there were no differences in overall survival or the development of complications, such as rejection.61
Regrading thoracic organ transplantation, in a study that included 38 recipients, 22 for heart transplant and 16 for lung transplant, treatment was started with glecaprevir-pibrentasvir, upon detecting positive viremia after transplantation (a mean 7 days for heart recipients and 3 days for lung recipients). SVR was achieved in 100% of the patients, despite the treatment interruption in the two cases due to the development of hyperbilirubinemia. One of those patients restarted treatment a few days after suspension, whereas the other patient only received a treatment regimen for 10 days.51
The goal of starting treatment with DAAs in HCV-negative non-liver organ recipients whose donors are viremic is to begin as soon as possible to minimize the duration of the viremia in the recipient, thus preventing the development of acute hepatitis and other non-liver complications.
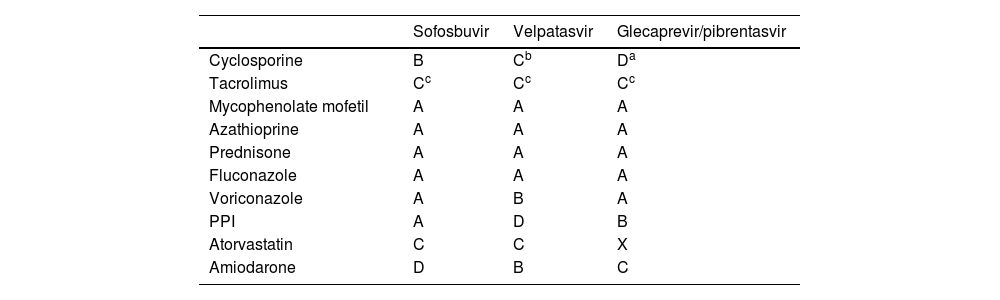
Direct-acting antiviral regimen interactions in transplantationIn the posttransplant patient population, it is important to be aware of possible drug interactions when choosing the treatment regimen. The majority of DAAs are metabolized by cytochrome P450 3A (CYP3A), which can give rise to interactions with immunosuppressants.
Caution must be taken, with respect to the glecaprevir-pibrentasvir regimen, due to possible interactions with the calcineurin inhibitors, especially cyclosporine. At high doses of 400 mg or more, cyclosporine can increase the serum level of glecaprevir by up to 5 times. Therefore, that combination is not recommended in patients that require stable doses of cyclosporine above 100 mg per day. With respect to tacrolimus, serum levels can be increased by a factor of 1.45, but adjusting the dose is not recommended. When given concomitantly, the patient should be monitored frequently, adjusting the dose, when necessary (Table 4).57,62
DAA regimen interactions in the transplanted population.
| Sofosbuvir | Velpatasvir | Glecaprevir/pibrentasvir | |
|---|---|---|---|
| Cyclosporine | B | Cb | Da |
| Tacrolimus | Cc | Cc | Cc |
| Mycophenolate mofetil | A | A | A |
| Azathioprine | A | A | A |
| Prednisone | A | A | A |
| Fluconazole | A | A | A |
| Voriconazole | A | B | A |
| PPI | A | D | B |
| Atorvastatin | C | C | X |
| Amiodarone | D | B | C |
Interaction characterization. A: not known; B: minor, can have minimal clinical effects and not require modification; C: moderate, can exacerbate the clinical condition or require treatment modifications; D: major, can cause damage or require management; X: contraindicated.
Interactions are not limited to immunosuppressants and they should also be verified with other drugs used in the transplanted population, and the corresponding adjustments or changes should be made.
We provide here a link where all interactions of DAAs with other drugs can be consulted: (https://hep-druginteractions.org/checker).
Overall evaluation for utilizing organs from hepatitis C virus-positive donors in MexicoIn Mexico, there are currently no centers that perform or have performed transplants from HCV-positive donors. With the accumulated experience available at present, transplants utilizing HCV-positive organs in HCV-negative recipients, followed by DAA therapy, provides excellent overall survival and graft survival, taking into account that evidence is limited in some areas.5 In very well selected cases, it appears to be an efficacious and well tolerated strategy. However, the safety of posttransplant DAA therapy is essential and patients must be made aware of the potential risks, including treatment failure, albeit that risk is lower than the risk of dying while on the waiting list.
On January 6, 2023, the CENATRA registry documented 160,406 patients on the waiting list for a kidney transplant, 241 for a liver transplant, and 40 for a heart transplant.63 We believe that the use of organs from HCV Ab+/RNA+donors in Mexico could be a feasible option in one of the Latin American countries with the lowest donation and transplant rates in general,3 keeping in mind that more studies need to be conducted.
Challenges in the futureWe understand that there are many obstacles to utilizing organs from HCV-positive that are not limited only to ethical concepts and certain controversial points. Even though access to DAAs is universal in Mexico and they offer the advantage of having few side effects and interactions with immunosuppressants, the best moment for starting treatment after transplant is not yet clear. With the currently available evidence, we conclude that the advantages outweigh the risks, and therefore, the use of these organs should not be limited in a country, such as Mexico, where the number of organ donations and transplants performed annually do not meet the needs of the population.
ConclusionsThe use of organs from HCV-positive donors provides a great opportunity for increasing the number of solid organ transplants. With the present accumulated experience, the high eradication rate with DAAs, and the excellent 6 and 12-month overall survival and graft survival, the use of these organs is a real option in Mexico and other countries, where, in general, the mortality rate due to cirrhosis exceeds the organ donation rate.
Financial disclosureNo financial support was received in relation to this article.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Torres-Díaz JA, Jasso-Baltazar EA, Toapanta-Yanchapaxi L, Aguirre-Valadez J, Martínez-Matínez L, Sánchez-Cedillo A, et al. Donantes de virus de la hepatitis C positivo en receptores negativo para trasplante hepático. ¿Es posible en México? Rev Gastroenterol Mex. 2023;88:392–403.