Fecal incontinence is the involuntary passage or the incapacity to control the release of fecal matter through the anus. It is a condition that significantly impairs quality of life in those that suffer from it, given that it affects body image, self-esteem, and interferes with everyday activities, in turn, favoring social isolation. There are no guidelines or consensus in Mexico on the topic, and so the Asociación Mexicana de Gastroenterología brought together a multidisciplinary group (gastroenterologists, neurogastroenterologists, and surgeons) to carry out the «Mexican consensus on fecal incontinence» and establish useful recommendations for the medical community.
The present document presents the formulated recommendations in 35 statements. Fecal incontinence is known to be a frequent entity whose incidence increases as individuals age, but one that is under-recognized. The pathophysiology of incontinence is complex and multifactorial, and in most cases, there is more than one associated risk factor. Even though there is no diagnostic gold standard, the combination of tests that evaluate structure (endoanal ultrasound) and function (anorectal manometry) should be recommended in all cases. Treatment should also be multidisciplinary and general measures and drugs (lidamidine, loperamide) are recommended, as well as non-pharmacologic interventions, such as biofeedback therapy, in selected cases. Likewise, surgical treatment should be offered to selected patients and performed by experts.
La incontinencia fecal es el paso involuntario o la incapacidad de controlar la descarga de materia fecal a través del ano, siendo una condición que deteriora significativamente la calidad de vida de los sujetos que la padecen, ya que afecta la imagen corporal, la autoestima e interfiere con las actividades cotidianas favoreciendo el aislamiento social. En nuestro país no existe una guía o consenso al respecto, por lo que la Asociación Mexicana de Gastroenterología reunió a un grupo multidisciplinario (gastroenterólogos, neurogastroenterológos y cirujanos), para que realizaran el Consenso mexicano sobre incontinencia fecal y se establecieran recomendaciones de utilidad para la comunidad médica.
Las recomendaciones emitidas fueron a través de 35 enunciados que se presentan en este documento. Se reconoce que la incontinencia fecal es una entidad frecuente, y cuya incidencia se incrementa conforme aumenta la edad, sin embargo, es poco reconocida. La fisiopatología de la incontinencia es compleja y multifactorial y en la mayoría de los casos existe más de un factor de riesgo asociado. Respecto al diagnóstico, se considera que, si bien no existe un estándar de oro, la combinación de pruebas que evalúen la estructura (p. ej., ultrasonido endoanal) y la función (manometría anorrectal) se debe de recomendar en todos los casos. El tratamiento debe ser también multidisciplinario, y se recomiendan medidas generales, fármacos (lidamidina, loperamida), y en casos seleccionados intervenciones no farmacológicas como la terapia de biorretroalimentación. De igual manera, el tratamiento quirúrgico debe ofrecerse a los pacientes seleccionados y debe ser brindado por los expertos.
Fecal incontinence (FI) is the involuntary passage or inability to control the release of fecal matter through the anus. It is a condition that significantly impairs the quality of life of the subjects that present with it, given that it affects body image and self-esteem, and interferes with daily activities, thus favoring social isolation. In Mexico, there are no guidelines or consensus on the topic. Therefore, the Asociación Mexicana de Gastroenterología brought together a multidisciplinary group (gastroenterologists, neurogastroenterologists, and colorectal surgeons) to formulate the “Mexican consensus on fecal incontinence” and establish useful recommendations for the medical community.
Specifically, the aim of this consensus was to prepare an up-to-date document on the epidemiology, diagnosis, and treatment of FI, with a practical application in Mexico. The recommendations are based on an extensive review of the literature and on the consensus opinion of the participating specialists.
MethodsThe Delphi process was utilized for developing the consensus, exactly as previously described.1 Two general coordinators and 2 associate coordinators (JMRT, ECA, KRGZ, and OGM) were designated and 13 experts were invited to participate. The coordinators carried out a thorough search on the following databases: CENTRAL (The Cochrane Central Register of Controlled Trials), MEDLINE (PubMed), EMBASE (Ovid), LILACS, CINAHL, BioMed Central, and the World Health Organization International Clinical Trials Registry Platform (ICTRP), considering data that appeared from January 1, 2010 to December 31, 2021. The search criteria included the term: “fecal incontinence” combined with the following terms: “epidemiology”, “incidence”, “prevalence”, “Mexico”, “pathophysiology”, “diarrhea”, “surgery”, “diagnosis”, “differential diagnosis”, “treatment”, “antibiotics”, “therapy”, “treatment”, “neurostimulation”, “biofeedback”, “management”, “review”, “guidelines”, and “meta-analysis”, and their Spanish equivalents. The entire bibliography was made available to the members of the consensus.
The coordinators then formulated 33 statements, which underwent a first anonymous electronic voting round (June 1 to July 15, 2022), whose purpose was to evaluate the drafting and content of the statements. The consensus participants voted, utilizing the following responses: a) in complete agreement, b) in partial agreement, c) uncertain, d) in partial disagreement, and e) in complete disagreement.
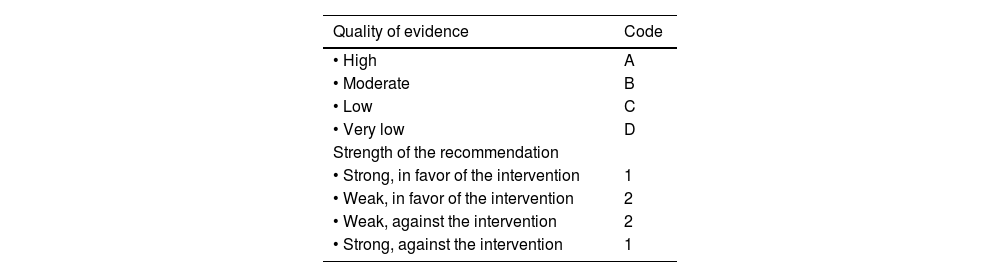
When the voting round was completed, the coordinators carried out the corresponding modifications. The statements that reached > 75% complete agreement were maintained, and the statements with > 75% complete disagreement were eliminated. The statements with ≤ 75% complete agreement and ≤ 75% complete disagreement were reviewed and restructured. The revised statements underwent a second anonymous electronic voting round (August 1 to 15, 2022). Based on the comments from the second voting round, the revised statements underwent a third voting round (September 22, 2022, as a hybrid [online/face-to-face] event), at which each of the resulting statements was drafted and the quality of evidence was evaluated for determining recommendation strength, employing the “Grading of Recommendations Assessment, Development and Evaluation” (GRADE) system.2 In the GRADE system, quality of evidence is not based solely on the study’s design or methodology, but also on a clearly expressed question related to an equally clearly formulated outcome measure,3 and so the quality can be high, moderate, low, or very low. In addition, the GRADE system establishes recommendation strength as strong or weak, in favor of or against the intervention or statement. Importantly, recommendation strength is only established when diagnostic tests and therapeutic interventions have been carried out. Table 1 shows the GRADE system codes, which utilize upper case letters for the quality of evidence followed by a number that indicates the strength of the recommendation, in favor of or against the intervention or statement.
GRADE system codes.
| Quality of evidence | Code |
|---|---|
| • High | A |
| • Moderate | B |
| • Low | C |
| • Very low | D |
| Strength of the recommendation | |
| • Strong, in favor of the intervention | 1 |
| • Weak, in favor of the intervention | 2 |
| • Weak, against the intervention | 2 |
| • Strong, against the intervention | 1 |
Source: adapted from Oñate-Ocaña and Ochoa-Carrillo.3
At this third meeting, the statements that obtained > 75% agreement were ratified. The statements that did not reach 75% agreement in the previous voting rounds were discussed to either reach a consensus or eliminate them, and another voting round was carried out. Once the final consensus statements were established, the coordinators formulated the present manuscript, which was reviewed and approved by all the consensus members.
ResultsThe coordinators initially proposed 33 statements. At the first voting round, 12 statements (36%) were revised, for not having reached consensus, but none were eliminated.
The second voting round was carried out on the 33 statements, and according to its results, only 2 statements (6%) did not reach consensus, and the addition of a statement on the pathophysiology of FI was proposed. The third voting round included 34 statements. At the end of this final round, one sentence was divided into two, resulting in a total of 35 consensus statements.
The final statements and the voting results are presented below.
General aspects, epidemiology, and risk factorsFI is defined as the involuntary passage of solid or liquid fecal matter, and even though the presence of frequent episodes (at least two within the last 3 months) considerably affects quality of life, there are authors that consider a single episode, in the absence of diarrhea, to be enough to be relevant.4,5 According to its clinical characteristics, FI can be classified into 3 types: 1) passive incontinence (inability to retain solid fecal matter); 2) urge incontinence (inability to contain the bowel movement), and 3) stool seepage (characterized by staining due to the leakage of small quantities of stool after a normal bowel movement). These 3 subtypes frequently overlap. Clinically distinguishing the 3 types is important, given that it will guide treatment.6 Each of the subtypes has specific characteristics. In passive incontinence, there is a loss of rectal sensation, with or without sphincter dysfunction; urge incontinence can be due to an inability of the rectum to retain stool or to lesions in the external anal sphincter (EAS); and stool seepage is associated with impaired rectal sensation.4
The overall prevalence of FI varies from 7-15% (range 2-35%) on average in Western countries.7 According to the National Health and Nutrition Examination Survey (NHANES), prevalence in non-institutionalized adults in the United States is 8.3%, and varies if the criterion is incontinence regarding liquid stools (6.2% prevalence), solid stools (1.6%), and/or stool seepage (3.1%).8 An overall prevalence of 16.1% was found in an online survey applied to 5,931 subjects in the United States, Canada, and the United Kingdom.7 When utilizing the Rome IV criteria, prevalence was 3.3%, but rose, when using less strict temporality criteria: 70.2% when there were fewer than 2 episodes per month and 29.8% with symptoms for fewer than 6 months.9
Epidemiologic studies estimate that FI affects 2% of the population under 65 years of age, 10% of the population above 65 years of age, and up to 50% of all patients that live in nursing homes.10 In Mexico, according to the SIGAME study, prevalence is 4.7% in the general population, with a mean age of 49.5 + 13 years and a predominance of women (67%).11 In the worldwide epidemiologic study conducted by the Rome Foundation on more than 73,000 subjects on the 5 continents, prevalence is 1.6% and can reach up to 2.3% in persons above 65 years of age.12
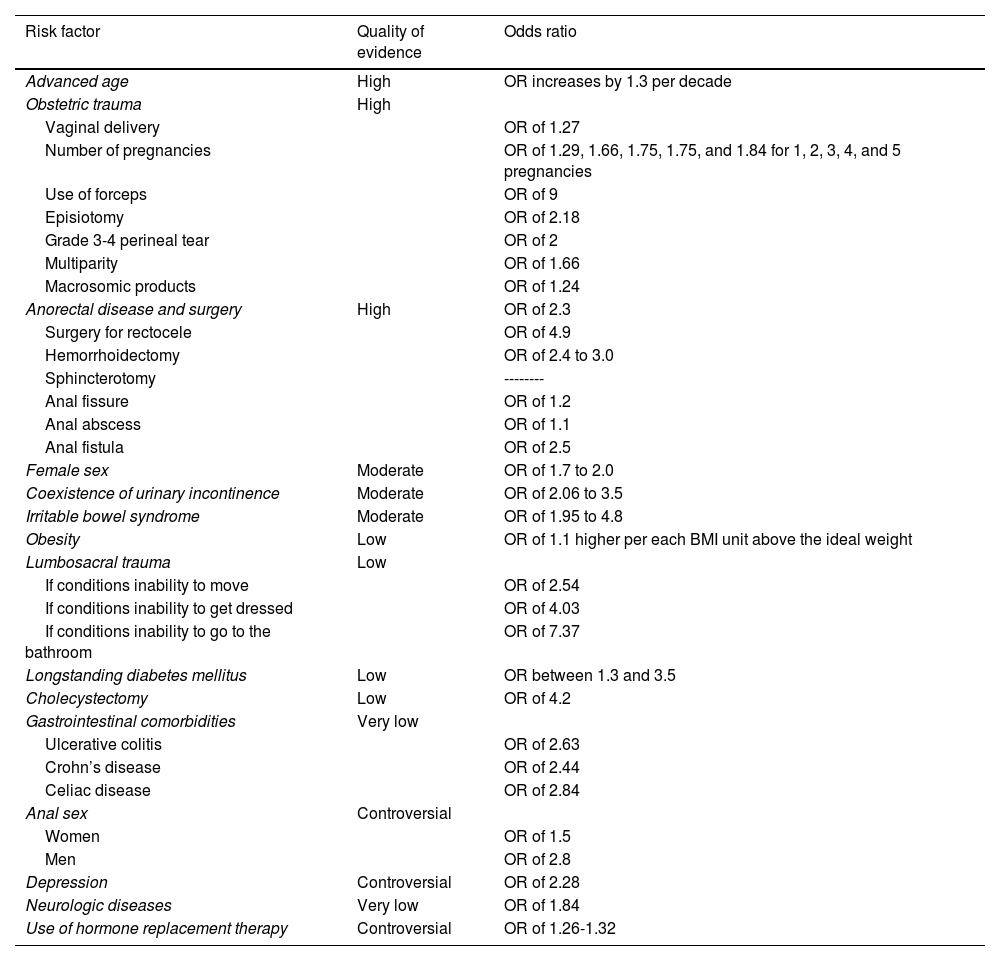
Numerous risk factors are associated with FI4,13 and they are described in the statements below and summarized in Table 2.
Risk factors for fecal incontinence.
| Risk factor | Quality of evidence | Odds ratio |
|---|---|---|
| Advanced age | High | OR increases by 1.3 per decade |
| Obstetric trauma | High | |
| Vaginal delivery | OR of 1.27 | |
| Number of pregnancies | OR of 1.29, 1.66, 1.75, 1.75, and 1.84 for 1, 2, 3, 4, and 5 pregnancies | |
| Use of forceps | OR of 9 | |
| Episiotomy | OR of 2.18 | |
| Grade 3-4 perineal tear | OR of 2 | |
| Multiparity | OR of 1.66 | |
| Macrosomic products | OR of 1.24 | |
| Anorectal disease and surgery | High | OR of 2.3 |
| Surgery for rectocele | OR of 4.9 | |
| Hemorrhoidectomy | OR of 2.4 to 3.0 | |
| Sphincterotomy | -------- | |
| Anal fissure | OR of 1.2 | |
| Anal abscess | OR of 1.1 | |
| Anal fistula | OR of 2.5 | |
| Female sex | Moderate | OR of 1.7 to 2.0 |
| Coexistence of urinary incontinence | Moderate | OR of 2.06 to 3.5 |
| Irritable bowel syndrome | Moderate | OR of 1.95 to 4.8 |
| Obesity | Low | OR of 1.1 higher per each BMI unit above the ideal weight |
| Lumbosacral trauma | Low | |
| If conditions inability to move | OR of 2.54 | |
| If conditions inability to get dressed | OR of 4.03 | |
| If conditions inability to go to the bathroom | OR of 7.37 | |
| Longstanding diabetes mellitus | Low | OR between 1.3 and 3.5 |
| Cholecystectomy | Low | OR of 4.2 |
| Gastrointestinal comorbidities | Very low | |
| Ulcerative colitis | OR of 2.63 | |
| Crohn’s disease | OR of 2.44 | |
| Celiac disease | OR of 2.84 | |
| Anal sex | Controversial | |
| Women | OR of 1.5 | |
| Men | OR of 2.8 | |
| Depression | Controversial | OR of 2.28 |
| Neurologic diseases | Very low | OR of 1.84 |
| Use of hormone replacement therapy | Controversial | OR of 1.26-1.32 |
BMI: body mass index; OR: odds ratio.
Strength of the recommendation: ------
Quality of evidence: B
Agreement reached: 76.5% complete agreement, 5.9% partial agreement, 11.8% uncertain, and 5.9% partial disagreement.
Female sex is an independent predictor of FI, mainly due to unique factors, such as the number of pregnancies and deliveries, or gynecologic maneuvers during labor. However, prevalence in men is underestimated because males tend to seek medical support less often, but some large-scale epidemiologic studies, including the NHANES, have reported similar rates: 8.9% in women vs. 7.7% in men.8,9,14 Several independent risk factors for FI have been described in women, and include advanced age, chronic diarrhea, liquid stools, multiple comorbidities, and urinary incontinence.9
The risk for presenting with FI increases with ageStrength of the recommendation: --------
Quality of evidence: A
Agreement reached: 94.1% complete agreement and 5.9% partial agreement.
Advanced age is the most well-established risk factor for FI. A linear progression associated with age in both men and women has been reported, signifying that the risk for FI becomes greater as age increases.15 This association persists after adjusting for other factors, such as health status, chronic-degenerative diseases, and activity level. A population study reported that the odds ratio (OR) increased 1.3-times for each decade of life,16 and the NHANES survey found a 2.6% increase in prevalence in persons under 29 years of age and a 15.3% increase in persons above 70 years of age.9 The Rochester epidemiologic project was a case-control study that compared 176 women with FI, with a similar control group, finding that 88% of the cases of FI first appeared in women over 40 years of age.17 A study conducted on 64,559 women, ranging in age from 62 to 87 years, reported a prevalence of FI involving liquid and/or solid stools of 9% in the group between 62-64 years of age; it increased to 17% in the group between 85 and 87 years of age. Urinary incontinence (UI) correlated strongly with FI, with a prevalence of 63% in the women with FI.18 The prevalence of FI in older populations varies according to place of residence, at 15-20% in ambulatory older adults, 18-33% in hospitalized adults, and up to 50-70% in nursing home residents.19,20 Different risk factors can aggravate the condition in nursing home residents, including less physical activity, loss of mobility and capacity for self-care, and less bathroom access, as well as functional constipation, dietary changes, and lower intake of liquids and fiber.11,12 There is less evidence on the incidence of FI, but it has been described in at least 2 studies. The first reported an incidence rate at 4 years of 17%, with a 6% monthly development, and the second reported a 7% rate at 10 years from the initial evaluation.21,22 The associated risk factors were UI, the development of urgency, diarrhea, sensation of incomplete bowel movement, and a history of pelvic radiation. Other factors associated with advanced age and FI are menopause, postmenopausal hormone therapy, age-related changes in the pelvic floor, and pudendal nerve neuropathy.20,23
Obesity/overweight can be a risk factor for fecal incontinenceStrength of the recommendation: --------
Quality of evidence: C
Agreement reached: 82.4% complete agreement and 17.6% partial agreement.
A 67% prevalence of FI has been described in morbidly obese women and a 16-63% prevalence in women referred for bariatric surgery.24 In the Rochester study, the multivariate analysis showed an OR of 1.1 (1.004-1.1), per unit of body mass index (BMI) above the ideal weight. In another study on older women, the BMI modestly increased the risk for FI, with an OR of 1.4 when the BMI was greater than 35, compared with a BMI between 21 and 23.24 In that study, reduced physical activity correlated with obesity, increasing the risk 1.58 times. Adults that underwent bariatric surgery, especially gastrojejunal bypass, have also been reported to have an increased risk for FI involving both liquid stools (48% women, 42% men) and solid stools (21% women, 30% men), and a structured weight reduction program was shown to reduce the frequency of FI episodes from 47.4 to 28.1%.25 Nevertheless, not all studies have confirmed that association.26,27 An analysis that compared 201 patients presenting with FI (67 obese patients versus 137 non-obese controls) found no significant differences between the two groups, with respect to symptom severity or quality of life, and even suggested that the obesity group had better anorectal function and that symptoms were more related to stool consistency.26 Another prospective study on 51,708 women found no greater risk for FI in obese women, but the women with moderate physical activity had 25% less risk for FI.27
Obstetric trauma (perineal tear, forceps, episiotomy) is a determining risk factor for fecal incontinenceStrength of the recommendation: --------
Quality of evidence: A
Agreement reached: 94.1% complete agreement and 5.9% partial agreement.
One of the most important anatomic factors conditioning FI is obstetric injury of the anal sphincter. A higher prevalence of FI has been described in women with a history of vaginal births, particularly those in which an instrument or maneuver to facilitate vaginal expulsion was used, compared with women with no history of giving birth.9,15,18,20 One study found a prevalence of 15.1% in women with > 4 deliveries vs. 5.9% in women with no previous deliveries. In that study, the number of deliveries was not significantly associated with FI after adjusting for age (p=0.09) or other risk factors (p =0.57). The number of pregnancies also increases the risk for FI, with an OR of 1.29, 1.66, 1.75, 1.75, and 1.84 for 1, 2, 3, 4, and 5 pregnancies, respectively.24 The maneuvers to extract the product can also increase the risk for FI, particularly the use of forceps, which according to the study, results in a 1.3 to 9-times greater risk (OR 9.0, 95% CI 5.6-14.4).6,15,28 Episiotomy is the second most common maneuver during labor that increases the risk for FI. Up to 56.2% of women with FI report a history of episiotomy, which is associated with a 2.18-times higher risk; 15% of women have reported a severe obstetric tear prior to symptom onset, with symptom duration longer than one year after the procedure in 86% of the cases.28 Although the mean age reported for FI onset, with a history of obstetric trauma, is 55 years, the majority of patients experience temporary postpartum FI: 18% of women that suffered grade 3 injury (involving the EAS) and 29% with grade 4 injury (involving the EAS and the internal anal sphincter [IAS]) developed FI at a mean 24 years after their first delivery, with an OR of at least 2.4,14 Initial obstetric injury is considered the first of a series of accumulated factors associated with structural perianal injury that involves the pudendal nerve, excessive perineal descent, and anal sphincter weakness that can finally lead to FI.4,14,15,18,20,28,29
Anorectal surgery (sphincterotomy, fistula, hemorrhoidectomy) increases the risk for fecal incontinence due to structural pelvic floor injuryStrength of the recommendation: --------
Quality of evidence: A
Agreement reached: 82.4% complete agreement and 17.6% partial agreement.
Similar to that occurring in cases of obstetric trauma in women, surgical procedures involving lesions near the anal sphincter increase the risk for FI in both men and women. A greater risk has been described in patients undergoing different anorectal surgical procedures, ranging from hemorrhoidectomy, anal sphincterotomy, and anal dilation to surgeries involving ileoanal anastomoses, with a 40% prevalence rate in this last group.6,30,31 In the Rochester study, the multivariate analysis found a higher risk for FI in patients that underwent rectocele correction, with an OR of 4.9 (1.3-19).16 In a study that compared variables associated with FI in men and women, the surgeries associated with a higher risk for FI were: transanal surgery (37%); prostate cancer therapy (14.7%), and lumbosacral surgery due to spinal cord injury (14.7%). Upon comparing sphincter defects, there was a lower prevalence of FI in men (35 vs. 70%, p=0.004).22 A Mexican study by Charúa et al.31 showed that 6.5% of patients that underwent partial lateral sphincterotomy developed minimum grade FI, 3 months after surgery.
Urinary incontinence can coexist in patients with fecal incontinenceStrength of the recommendation: --------
Quality of evidence: B
Agreement reached: 88.2% complete agreement and 11.8% partial agreement.
FI is frequently and significantly associated with urinary incontinence (UI), in both women and men. Up to 41% of women with FI report an association with UI, with an OR of 2.06-3.5.26 More than 30% of women experience UI during the second and third trimesters of pregnancy or during the first 3 postnatal months and 25% of them present with FI during the third trimester; up to 25% continue with symptoms during the following months and even one year after giving birth.32 Some epidemiologic studies have reported an association between FI and UI: 1.9% (1.5-2.4%) of patients with UI report FI: 1.1% in men and 2.7% in women.9 Inversely, UI is strongly correlated with FI: 63% of women with FI have UI at least once a month, compared with 45% of controls with no UI.18
Lumbar and/or sacral trauma is associated with fecal incontinenceStrength of the recommendation: --------
Quality of evidence: C
Agreement reached: 94.1% complete agreement and 5.9% partial agreement.
An association between FI and a history of trauma in the lumbosacral region has been reported. Among the mechanisms described are some that have a direct association, such as peripheral neuropathy, lumbosacral radiculopathy at the lumbosacral or cauda equina levels, spinal cord compression, and loss of the anocutaneous reflex, and others that have an indirect association and are particularly associated with poor mobility.15,29,30 A study on men with FI reported that 37.8% had a history of lumbosacral trauma or surgery, or a spinal cord malformation, albeit the study did not evaluate or specify a cause/effect relation. The inability to move has been associated with a 2.54-times greater risk for FI, the inability to dress oneself with a 4.03-times greater risk, and the inability to use the bathroom oneself up to a 7.37-times greater risk.33
Longstanding diabetes mellitus (especially in patients with inadequate glycemic control) is a risk factor for fecal incontinenceStrength of the recommendation: --------
Quality of evidence: C
Agreement reached: 94.1% complete agreement and 5.9% partial agreement.
Chronic diabetes mellitus (DM) can be associated with complications, such as visceral neuropathy and rectal hyposensitivity, and in turn, can increase the risk for FI. A higher risk for FI has been described in patients with longstanding diabetes, in both men and women. The NHANES survey reported a greater prevalence of FI in diabetic patients than in the general population (18.1 vs. 8.4%).9 In 7 studies, the risk factors associated with FI in DM were: advanced age (OR 1.3), depression (OR 2.0), UI (OR 3.5), and poor general status (OR 1.9).34 Another study on older women with FI also found a higher risk in patients with DM, as well as in those with DM associated with high blood pressure and smoking, with an OR between 1.2 and 1.7.28 Several pathophysiologic mechanisms associated with the development of FI in DM have been described, particularly visceral neuropathy, which when associated with rectal hyposensitivity increases the risk of FI by 3.3 times in diabetic patients.18,35 In addition, neurologic involvement in diabetics is recognized to not only be limited to the peripheral nervous system and its afferent pathways, but also to alterations in the efferent pathways, as well as in central nervous system processing. This comprehensively explains the hyperexcitability (afferent conduction), changes in sensory perception (hypersensitivity and hyposensitivity), changes in motility (efferent conduction) with both an increase (diarrhea) and decrease (constipation), in addition to changes in neuromuscular control, especially in the anorectal region, and impaired processing (diabetic encephalopathy) in patients with longstanding DM.4
Post-cholecystectomy diarrhea can be considered an associated risk factor for fecal incontinenceStrength of the recommendation: --------
Quality of evidence: C
Agreement reached: 76.5% complete agreement and 23.5% partial agreement.
Between 10-20% of patients undergoing cholecystectomy develop bile acid diarrhea (BAD).36 In general, patients with chronic diarrhea have been described to have a 3-times greater prevalence of FI than subjects without diarrhea, including conditions such as inflammatory bowel disease, celiac disease, irritable bowel syndrome (IBS), and BAD.17–37 In the majority of studies that evaluate BAD associated with other conditions, the evidence is indirect due to the low availability of diagnostic tests and the rapid response to bile acid sequestrants.36 One of the few studies evaluating the direct association between cholecystectomy and FI is the Rochester epidemiologic study, in which the multivariate analysis found that previous cholecystectomy was associated with FI, with an OR of 4.2 (1.2-15).17 One problem, with respect to BAD, is the implementation of tests for its diagnosis. There are different tests, such as SeHCAT imaging, which includes radiation; the measurement of serum C4, a product of bile acid synthesis that is elevated in conditions of diarrhea due to bile acid malabsorption (BAM); and the measurement of fibroblast growth factor 19 (FGF-19), which plays a role in inhibiting bile salt production, and thus is reduced in patients with BAM and is inversely correlated with C4.38 Unfortunately, this test has limited availability.
Fecal incontinence can coexist in patients with irritable bowel syndrome, especially in those with the diarrhea subtype (IBS-D)Strength of the recommendation: --------
Quality of evidence: C
Agreement reached: 76.5% complete agreement and 23.5% partial agreement.
Between 60-62% of patients that have IBS present with at least one episode of FI throughout their lives.39 A population survey in Australia applied to 396 patients revealed that 33% had a functional bowel disorder and/or FI; 11-12% of those patients presented with IBS.37 In the multivariate analysis of the Rochester epidemiologic project, there was a greater risk for presenting with FI in patients with IBS (OR 4.8, 95% CI 1.6-14) and chronic diarrhea (OR 53, 95% CI 6.1-471).17 Another study jointly conducted between the United States and Sweden on 168 patients with IBS based on the Rome III criteria, reported a prevalence of FI (> once a month) in IBS of 13.7% (Sweden) and 19.7% (the United States).40 The percentages rose to 29.8% and 43.4% upon removing the criterion of monthly frequency. Another study on 500 patients with IBS (Rome III) evaluated the prevalence of FI in the different IBS subtypes and found a greater prevalence in the mixed subtype (IBS-M), with the diarrhea subtype (IBS-D) predominating. However, one out of every 3 patients with predominant constipation (IBS-C) also presented with FI: 65.2% (IBS-D), 63.7% (IBS-M), and 37.9 (IBS-C).41 An observational study on 1,454 patients (71% women, Rome III) reported a prevalence of stool seepage of 8.5%, and in the logistical regression analysis, both IBS (OR 1.95) and functional diarrhea (OR 1.90) increased the risk for presenting with FI (6,19–22).42 Finally, the multivariate analysis of a study that attempted to clarify the pathophysiologic mechanisms associated with IBS in FI found a strong association with parity (p=0.007), vaginal deliveries (p=0.049), obstetric tears (p=0.007), fecal urgency (p=0.005), diarrhea (p=0.008), and hysterectomy (p=0.004), but not with episiotomy, prolapse, or UI.43
Other risk factors to considerEvidence is limited or controversial regarding certain risk factors that could be associated with FI, which, even though they were not voted on in the present consensus, should be mentioned. For example, there are inconsistent results as to whether anal sex can be a risk factor; some studies show that anoreceptive intercourse is associated with reduced resting anal canal pressures, but total pressures are normal and do not condition FI.44 Other studies report that FI is more frequent in women that have anal penetrative intercourse.45 However, more recent studies state that the risk for FI depends on the frequency with which anal sex is practiced (more than once a week), the coexistence with acquired immunodeficiency syndrome (AIDS), and practices such as “fisting”.46 In the National Institutes of Health survey applied to more than 4,000 subjects, the OR for developing FI in subjects that practice anal sex was established at 1.5 in women and 2.8 in men.47
Dementia and other neurologic disorders that condition neurogenic dysfunction, such as multiple sclerosis or transverse myelitis, can be associated with FI, but evidence is scarce.18 Some studies have reported that coexisting gastrointestinal diseases, such as ulcerative colitis, Crohn’s disease, and celiac disease, have been associated with FI.17,18
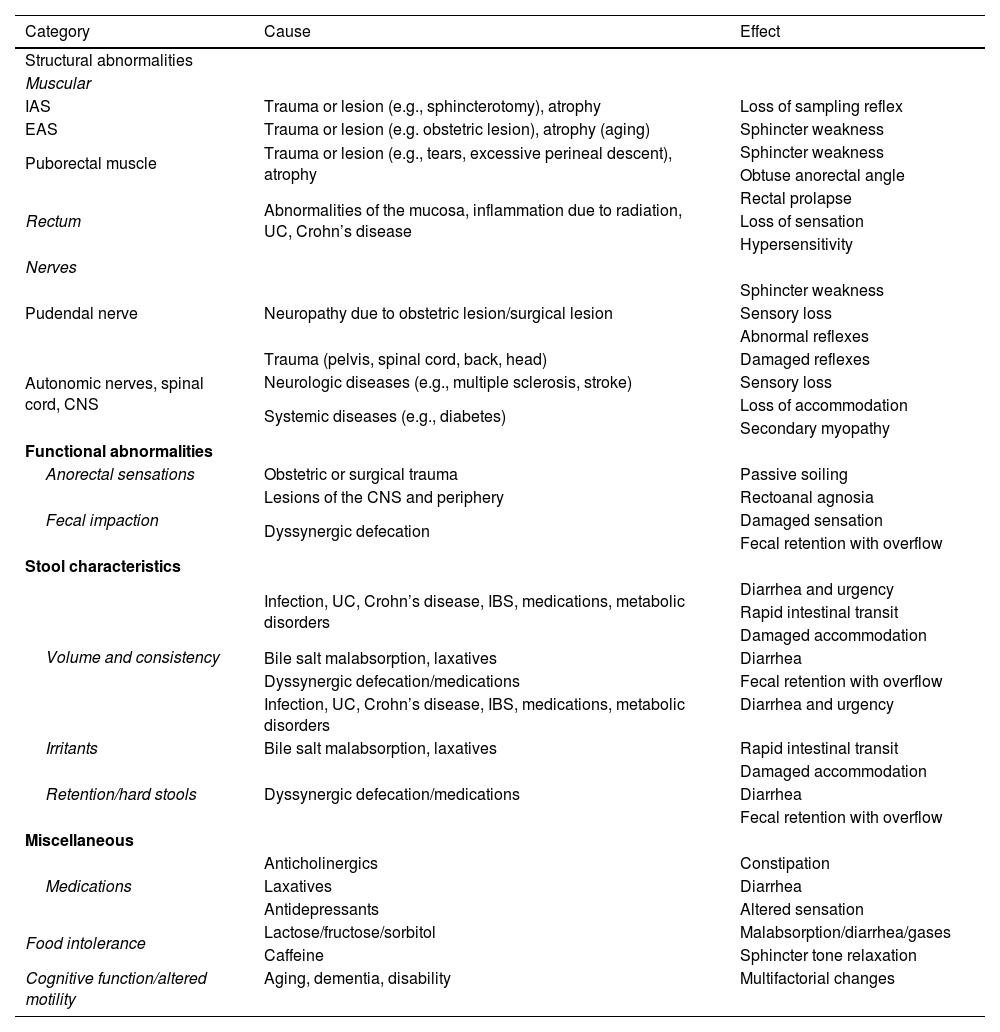
The pathophysiology of fecal incontinence is complex and multifactorial. Over 80% of patients have more than one alteration; thus, every effort should be made to determine the pathophysiology so that personalized treatment can be providedStrength of the recommendation: --------
Quality of evidence: B
Agreement reached: 94.1% complete agreement and 5.9% partial agreement.
The pathophysiology of FI is heterogeneous and there is more than one related mechanism in over 80% of cases (Table 3).13 Each individual can present with several of the pathophysiologic mechanisms, and expectedly, frequency and severity of episodes of FI would be greater, the higher the number of said mechanisms. In general terms, the pathophysiologic mechanisms that lead to FI can be grouped as follows:29
- a)
Anatomic/structural factors; they involve anal sphincter muscle lesions, rectal disorders (inflammation, laxity), puborectal muscle alterations (direct lesion), and neurologic alterations (damage to both the peripheral nerves [neuropathy] and the central nervous system [spinal cord injury]).
- b)
Functional factors; they include changes in rectal sensation due to different causes and problems in defecation dynamics (dyssynergic defecation), the latter impeding adequate stool expulsion.
- c)
Reduced stool consistency associated or not with rectal urgency and accelerated intestinal transit due to different etiologies (infectious causes, bacterial overgrowth, excess bile salts) can lead to more frequent FI episodes.
- d)
Miscellaneous causes, such as impaired cognitive function (dementia), psychiatric disorders (psychosis), and drug use (altered rectal sensation and changes in intestinal transit), as well as excessive consumption of fermentable carbohydrates that can lead to diarrhea and episodes of FI.
Pathophysiologic mechanisms of fecal incontinence.
| Category | Cause | Effect |
|---|---|---|
| Structural abnormalities | ||
| Muscular | ||
| IAS | Trauma or lesion (e.g., sphincterotomy), atrophy | Loss of sampling reflex |
| EAS | Trauma or lesion (e.g. obstetric lesion), atrophy (aging) | Sphincter weakness |
| Puborectal muscle | Trauma or lesion (e.g., tears, excessive perineal descent), atrophy | Sphincter weakness |
| Obtuse anorectal angle | ||
| Rectum | Abnormalities of the mucosa, inflammation due to radiation, UC, Crohn’s disease | Rectal prolapse |
| Loss of sensation | ||
| Hypersensitivity | ||
| Nerves | ||
| Pudendal nerve | Neuropathy due to obstetric lesion/surgical lesion | Sphincter weakness |
| Sensory loss | ||
| Abnormal reflexes | ||
| Autonomic nerves, spinal cord, CNS | Trauma (pelvis, spinal cord, back, head) | Damaged reflexes |
| Neurologic diseases (e.g., multiple sclerosis, stroke) | Sensory loss | |
| Systemic diseases (e.g., diabetes) | Loss of accommodation | |
| Secondary myopathy | ||
| Functional abnormalities | ||
| Anorectal sensations | Obstetric or surgical trauma | Passive soiling |
| Fecal impaction | Lesions of the CNS and periphery | Rectoanal agnosia |
| Dyssynergic defecation | Damaged sensation | |
| Fecal retention with overflow | ||
| Stool characteristics | ||
| Volume and consistency | Infection, UC, Crohn’s disease, IBS, medications, metabolic disorders | Diarrhea and urgency |
| Rapid intestinal transit | ||
| Damaged accommodation | ||
| Bile salt malabsorption, laxatives | Diarrhea | |
| Dyssynergic defecation/medications | Fecal retention with overflow | |
| Infection, UC, Crohn’s disease, IBS, medications, metabolic disorders | Diarrhea and urgency | |
| Irritants | Bile salt malabsorption, laxatives | Rapid intestinal transit |
| Retention/hard stools | Dyssynergic defecation/medications | Damaged accommodation |
| Diarrhea | ||
| Fecal retention with overflow | ||
| Miscellaneous | ||
| Medications | Anticholinergics | Constipation |
| Laxatives | Diarrhea | |
| Antidepressants | Altered sensation | |
| Food intolerance | Lactose/fructose/sorbitol | Malabsorption/diarrhea/gases |
| Caffeine | Sphincter tone relaxation | |
| Cognitive function/altered motility | Aging, dementia, disability | Multifactorial changes |
CNS: central nervous system; EAS: external anal sphincter; IAS: internal anal sphincter; IBS: irritable bowel syndrome; UC: ulcerative colitis.
The individualized evaluation of all pathophysiologic aspects will lead to a better understanding of the problem and a better choice of effective treatment modalities, improving patient symptoms.
DiagnosisClinical evaluationThe first step in evaluating FI is to establish a good doctor/patient relationship. A detailed clinical history should be obtained, given that patients are generally reluctant to admit their symptoms, and to directly question about the presence of FI is suggested.4,6 An evaluation of time and duration, nature (i.e., flatus incontinence, liquid or solid stool incontinence) and the impact on quality of life is important. The use of sanitary napkins or other devices and the ability to distinguish between solid stools, liquid stools, and gases should be documented. It is important to ask about the abovementioned risk factors, hygienic-dietary habits, and the coexistence of comorbidities. The use of clinical and quality of life questionnaires and scales can provide additional information on the frequency of bowel movements, the quantity (i.e., small, medium, or large amount), the type of leakage, and the presence of urgency, to provide an index of symptom severity.
Clinical questionnaires (the Wexner scale, FISS, etc.) are useful aids in making the diagnosis, providing follow-up, and establishing the severity of fecal incontinenceStrength of the recommendation: strong, in favor of
Quality of evidence: B1
Agreement reached: 88.2% complete agreement and 11.8% partial agreement.
Clinical questionnaires have demonstrated clinical utility in the initial evaluation of patients with FI, as well as in evaluating treatment response during follow-up, and they have a direct correlation with quality of life.48 The use of these questionnaires has been evaluated in different settings and therapeutic interventions, and despite the fact that all of them specifically measure similar parameters, there can be differences among them. A study that prospectively evaluated the St. Mark’s scale, the Wexner scale, the Pescatori scale, and the American Medical System scale found good correlation between them (r=0.79, p<0.001). However, the American Medical System scale was the exception, with respect to treatment response evaluation (p=0.09), whereas the other three scales correlated well for measuring treatment response (r=0.94, p<0.001), reaffirming their utility.49
Based on the use of these scales, their utility has even been suggested for grading response after an intervention. A study conducted at the Mayo Clinic evaluated the use of the Fecal Incontinence Severity Score (FISS), before and after treatment with clonidine or placebo, and reported that the grade of patient satisfaction was higher when the questionnaire revealed a reduction ≥ 50% in the number of FI episodes (clinical response) at the end of the intervention.50 From such results, the proposal is that these questionnaires should be applied and the changes associated with clinical improvement in patients that have undergone different treatments for FI should be defined.51
The use of quality-of-life scales (FIQLI, etc.) is recommended in the evaluation of all patients with fecal incontinenceStrength of the recommendation: strong, in favor of
Quality of evidence: B1
Agreement reached: 100% complete agreement.
Quality-of-life scales are important in evaluating the initial status of the patient with FI, as well as assessing the response to treatments for this problem, given the great negative impact the disease has on its sufferers. Three types of quality-of-life scales have been employed for this purpose: 1) general quality-of-life scales that evaluate all domains, 2) specialized scales focused on aspects related to depression or anxiety, and 3) specific scales on situations directly related to FI and its impact on quality of life.52 Each have their weaknesses and strengths, depending on what information is being sought, regarding the patient with FI. FI per se affects the domains related to social life, behavior in response to the problem, and the feelings related to having FI (embarrassment, shame). The most widely used instrument for evaluating aspects of quality of life specific to FI is the FIQLI. It can be used on the adult population and has been validated in different parts of the world. It evaluates 5 aspects: physiologic, cognitive, behavioral, affective, and social.53 The instruments that evaluate quality of life do not, on their own, evaluate the severity of the problem because they gather information more related to how the subject, in this case the person with FI, copes, adapts, or abstains from carrying out daily life activities.54
Without a doubt, the quality-of-life questionnaires aid in understanding the behaviors adopted by patients with FI. As a result, the problem is addressed and treated in a way that improves the most affected spheres, signifying that the solution is not necessarily directly related to the treatment of FI, but rather to the support given those patients, enabling them to better face their problem.
Digital rectal exam is an indispensable and useful maneuver in the evaluation of patients with fecal incontinenceStrength of the recommendation: strong, in favor of
Quality of evidence: B1
Agreement reached: 94.1% complete agreement and 5.9% partial agreement.
Physical examination is crucial in the evaluation of FI for defining the anatomy and baseline function. Before performing the digital rectal exam, the perineum is carefully inspected, identifying the presence of scars, skin ruptures, thinning of the perineal body, uncleanliness, fistulas, hemorrhoids and/or prolapse of the mucosa. The digital rectal exam is essential for evaluating gastrointestinal and anorectal problems, and even more so, in assessing the patient with FI. The rectal exam is necessary and should be routinely performed in all patients with FI. The examination should include the evaluation of the length of the anal canal, the tone of the resting sphincter and sphincter in contraction, and rectoanal coordination. Perianal sensitivity should also be evaluated. The anocutaneous reflex examines the integrity of the connection between the sensory nerves and the skin, the intermediate neurons in spinal cord segments S2, S3, and S4, and the motor innervation of the EAS. The absence of that reflex suggests either afferent or efferent neuronal damage.
Even though digital rectal exam is a crucial maneuver, a survey of medical students was conducted in the United States to emphasize its relevance and its low level of practice. The results showed that only 17% answered they had never performed the maneuver during their training and 48% were not confident in their interpretation of the findings.55 The most important findings in a digital rectal exam, such as the evaluation of the resting pressure and the increase in force during contraction, help clarify the pathophysiologic mechanisms leading to FI. There are barriers to performing this examination, but the majority come from the physician and not the patient. The diagnostic approach to patients is decisively improved through carrying out the exam.56 When compared with anorectal manometry findings, digital rectal examination results showed moderate agreement for anal squeeze pressure (κ=0.418, p=0.006) but poor agreement for anal resting tone (κ=0.079, p=0.368) between the two modalities, in patients with FI.57 With respect to dyssynergic defecation, which can coexist with FI and even be a mechanism leading to its development, digital rectal examination has been shown to have 75% sensitivity and 87% specificity for its identification.58
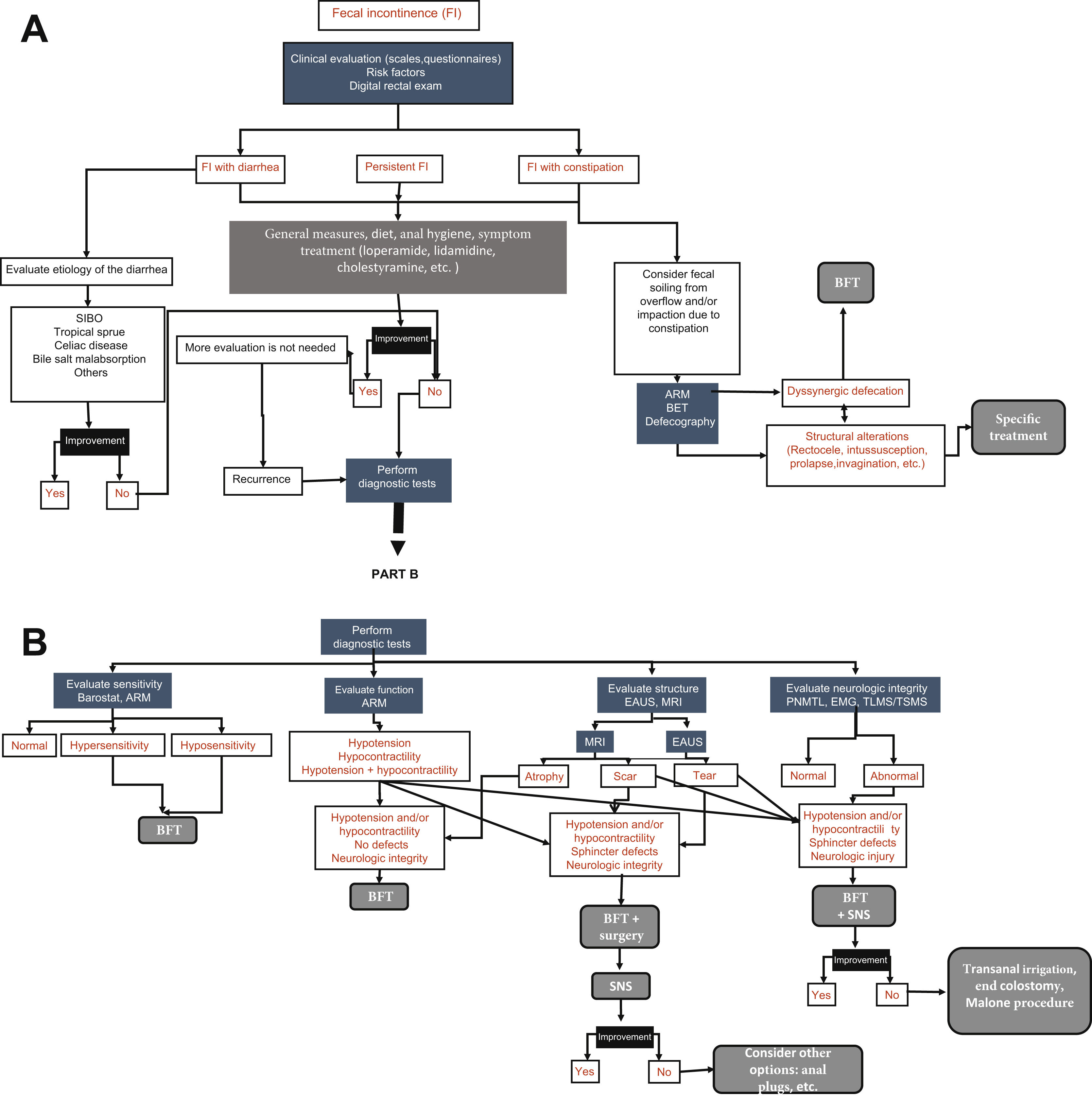
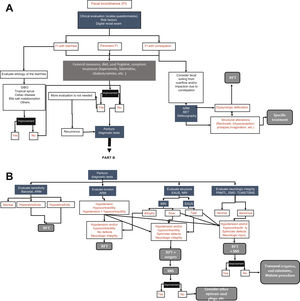
Diagnostic testsSeveral tests can be employed in the evaluation of the patient with FI. The selection of said tests depends on etiologic factors, symptom severity, impact on quality of life, and the age of the patient. Fig. 1 A and B shows the diagnostic/therapeutic algorithm recommended by the present consensus group.
Diagnostic algorithm for fecal incontinence.
A) Initial approach and management proposed for all patients with fecal incontinence (FI). ARM: anorectal manometry; BET: balloon expulsion test; BFT: biofeedback therapy; SIBO: small intestinal bacterial overgrowth. B) Diagnostic tests. There is no one single test, but rather the tests are complementary. EAUS: endoanal ultrasound; EMG: electromyography; MRI: magnetic resonance imaging; PNMTL: pudendal nerve motor terminal latency; SNS: sacral neurostimulation; TLMS/TSMS: translumbar and transsacral magnetic stimulation.
Strength of the recommendation: strong, in favor of
Quality of evidence: B1
Agreement reached: 88.2% complete agreement and 11.8% partial agreement.
Since its implementation at the beginning of the 1990s, endoanal ultrasound (EAUS) has been considered the gold standard for morphologic diagnosis, especially for defects of the anal canal, IAS, and EAS in patients with FI because it can evaluate lesions (scars or defects) of the anal sphincter, a decrease in its thickness, and its complete or partial atrophy. The test is also widely available and well tolerated.59 EAUS has improved over time, and currently can provide three-dimensional images (3D-EAUS), enabling the detection of small lesions of the sphincter that would otherwise go undetected or misinterpreted, given that the natural separation of the puborectal muscle tends to be confused with rupture of the sphincter. In addition, four-dimensional (4D) EAUS enables the length, thickness, area, and volume of the sphincter to be measured through the production of a digital volume that can be visualized at any plane, appearing as multiplane images or through tomographic sections, providing a more accurate view of defects. Transperineal ultrasound has recently emerged, which can provide additional information, especially in women, but it is limited by the diagonal viewing angle. Several studies published in the last decade have shown a significant correlation between 3D-EAUS and transperineal ultrasound findings when used for detecting anal sphincter lesions.60–62 The fact that EAUS is the best method for visualizing the IAS should be emphasized, but its sensitivity and accuracy in identifying lesions of the sphincter are subjects of debate and are operator-dependent. A case series of 51 patients reported good interobserver agreement for the diagnosis of IAS lesions and interobserver discrepancy of up to 5mm in the evaluation of EAS thickness. Therefore, if lesions that are difficult to define through EAUS or atrophy of the EAS are suspected, endoanal magnetic resonance imaging should be performed.63 Although it is a test that is often easily accessible, recognizing that it is operator-dependent is important.
When available, magnetic resonance imaging studies are useful in evaluating patients with fecal incontinenceStrength of the recommendation: weak, in favor of
Quality of evidence: B2
Agreement reached: 76.5% complete agreement and 23.5% partial agreement.
Endoanal magnetic resonance imaging (MRI) is a relatively noninvasive diagnostic modality with no exposure to radiation that can evaluate atrophy and defects of the anal sphincter complex, as well as overall pelvic floor movement, in real time.64 MRI is more sensitive for identifying EAS lesions because muscles, scars, and adipose tissue, thanks to the contrast material, can be well distinguished due to the different signal intensities in T2-weighted images. This facilitates the identification of local atrophy, something that cannot be done with EAUS, and provides a more accurate description of its extension and the affected structures in complex lesions, with 89% sensitivity and 94% specificity. In addition, it differentiates a tear in the EAS from a scar.65 MRI can obtain 360º images by inserting the coil into the anal canal (endorectal coil) or through an external phase matrix image.66 A retrospective study on 22 women that underwent sphincter repair found endoanal MRI to be superior to EAUS for diagnosing EAS lesions. Neither technique was advantageous for diagnosing said lesions.67
Additionally, magnetic resonance defecography is utilized for evaluating anorectal movement and the pelvic floor compartments in real time during defecation and contraction. The main indications for magnetic resonance defecography are to identify structural or “functional” problems in patients with FI and refractory symptoms.68
Despite their benefits, magnetic resonance studies are not widely available, they are costly, and they are contraindicated in patients with devices or hardware that are incompatible with MRI.
Anorectal manometry is an indispensable test and should be carried out in all patients with fecal incontinenceStrength of the recommendation: strong, in favor of
Quality of evidence: B1
Agreement reached: 100% complete agreement.
Anorectal manometry (ARM) is one of the better-established diagnostic tools for evaluating motor function and anorectal sensitivity in patients with FI, and for many authors, is the gold standard for evaluating anorectal function. Despite the factors of rectal reservoir function, stool form, adequate propulsive force, and cognitive or physical capacity, especially in men and women with no evidence of anal sphincter injury, anal sphincter dysfunction continues to be one of the most important pathophysiologic mechanisms in FI. ARM can evaluate motor function and determine whether the resting tone (which reflects IAS function) and contraction pressure (which reflects EAS function) are within or outside of the normal range. The introduction of high-resolution ARM (HR-ARM) and/or high-definition ARM (HD-RM) has increased the spatial resolution for obtaining data and providing continuous visualization of activity, with respect to anorectal pressures. To facilitate the comparison of diagnostic findings between centers, the International Anorectal Physiology Working Group (IAPWG) published a consensus in 2018 that proposed the London Classification, a practical and standardized protocol for the performance of ARM, its interpretation, and the terminology to be employed.69
Different manometric findings can be related to FI, such as reduced anal resting pressure (hypotonia) and reduced voluntary contraction pressure (hypocontractility). These alterations are classified as major disorders of anal tone and contractility. However, until recently, the prevalence of anal sphincter motor dysfunction, diagnosed through ARM in patients with FI, was uncertain. In their systematic review and meta-analysis, Rasijeff et al.70 reported that anal sphincter dysfunction was the most prevalent pathophysiologic finding. According to the combined results, 44% of women and 27% of men presented with anal hypotonia and 69% of women and 36% of men presented with anal hypocontractility. Those results support the concept that barrier dysfunction (whether of structural or neurologic/functional origin) is the main cause of FI in women. On the other hand, only a minority of men presented with anal sphincter motor dysfunction, and so other mechanisms (suprasphincteric) should be considered in that particular group. Nevertheless, resting tone and contraction pressures are measurements that could lack sensitivity for transmitting all the grades of anal sphincter dysfunction, and so these factors should be taken into account when making the interpretation. In Mexico, ARM is becoming increasingly accessible and there are numerous referral centers to which patients with FI that require ARM can be referred.
The evaluation of rectal sensitivity (through manometry and/or barostat) is recommended in all patients with fecal incontinenceBarostatStrength of the recommendation: strong, in favor of
Quality of evidence: B1
Agreement reached: 100% complete agreement.
Even though anal sphincter dysfunction is the most common cause of FI, the role of sensory dysfunction is key to the approach in these patients. Tests for evaluating rectal sensation, such as the rectal barostat or simple balloon distension, are essential, given that sensory alterations and/or altered biomechanical function (generally evaluated through measuring rectal compliance) are frequently found in cases of FI, justifying sensory function evaluation. The barostat is a computerized system that enables the distension of an adaptable balloon, increased through pressure, and provides a much faster method for eliciting rectal sensory perception. It is evaluated by registering each of the three sensory thresholds reported by the patient: volume of the first detectable sensation, sensation of fecal urgency, and sensation of discomfort or pain (maximum tolerable volume), as well as an optional fourth threshold (sustained urgency volume).59
Rectal hyposensitivity (elevation of the sensory thresholds above the normal range) has been related to frequency in patients with FI.71 It has also been reported to be a predictor of worse treatment outcomes, whether with biofeedback or surgery. Nevertheless, the presence of hyposensitivity is rarely taken into account when making medical or surgical management decisions. On the other hand, hypersensitivity can be the result of altered rectal distensibility (“compliance”), sensibilization of the extrinsic peripheral pathways and/or dysfunction of the central afferent mechanisms, or abnormalities in the perceptual and behavioral processes that cause hypervigilance. This often leads to urgency incontinence, associated with the inability to postpone defecation.72
ARMStrength of the recommendation: weak, in favor of
Quality of evidence: C2
Agreement reached: 100% complete agreement.
Changes in rectal sensation can affect continence. Routine determination of rectal sensitivity is recommended. It can be carried out during ARM by manually distending the balloon, which is secured to the ARM equipment, and registering the volumes needed to produce the series of rectal sensations. Hypersensitivity (greater sensory perception) and hyposensitivity (reduced sensory perception) are classified as major sensitivity disorders, according to the London Classification, and both can significantly affect continence. Altered rectal sensitivity, especially in the presence of anal hypotonia or hypocontractility, can lead to incontinence due to the reflex inhibition of the IAS before the patient perceives the presence of stool in the rectum.68,69 This can be one of the most important mechanisms for some patients that mainly present with passive incontinence.73 A recent meta-analysis reported a higher prevalence of rectal hyposensitivity in men, compared with women, whereas rectal hypersensitivity was more prevalent in women than in men.70
Electromyography is a complementary test that provides useful information in patients with severe fecal incontinence, especially when nerve injury is suspectedStrength of the recommendation: weak, in favor of
Quality of evidence: C2
Agreement reached: 88.2% complete agreement and 11.8% partial agreement.
Anal sphincter electromyography (EMG) is a neurophysiologic test that is utilized to evaluate anorectal function, particularly the function of the EAS and puborectal striated muscle, as well as to evaluate denervation-reinnervation potentials (indicative of neural injuries).68 Even though it is currently used less frequently in clinical practice, due to the introduction of less invasive methods, it continues to be an important tool for evaluating neurophysiologic anorectal function, given that pudendal nerve branches are responsible for providing sensory and motor innervation to the pelvic floor; they are vulnerable to injury due to stretching (during the third trimester, the second stage of delivery, and forceps-assisted vaginal birth), increasing the risk for EAS denervation and FI. In general, EMG is always carried out together with other diagnostic tests (e.g., anorectal manometry), for an overall evaluation of the pathophysiologic mechanisms involved and to aid in treatment planning. The technique can also be useful in performing retraining therapy.
In patients with severe fecal incontinence and nerve injury, the use of tests that evaluate neural integrity (pudendal nerve latency, translumbar and/or transsacral magnetic stimulation), when available, is suggestedPudendal nerve latencyStrength of the recommendation: weak, in favor of
Quality of evidence: C2
Agreement reached: 76.5% complete agreement, 17.6% partial agreement, and 5.9% uncertain.
The pudendal nerve terminal motor latency test measures the neuromuscular integrity between the terminal end of the pudendal nerve and the EAS. It is relatively easy to perform but its main limitation is the fact that it is not available at all centers. In addition, it is important to keep in mind that its correlation with clinical symptoms is controversial and a normal result does not rule out neuropathy. Likewise, its values are operator-dependent and are not standardized. Therefore, it should not be used as the only test in the investigation of nerve damage.74
Magnetic stimulationStrength of the recommendation: weak, in favor of
Quality of evidence: D2
Agreement reached: 76.5% complete agreement, 17.6% partial agreement, and 5.9% uncertain.
The arrival of minimally invasive magnetic stimulation of the lumbar and sacral plexus nerves has enabled the evaluation of the spinal-anorectal pathways that control anorectal neuronal function in patients with FI and spinal cord injury. A study75 revealed that 65% of patients with FI and spinal cord injury presented with conduction delay and had a two-times higher prevalence of anal neuropathy. In addition, the translumbar and transsacral motor evoked potentials of the rectum and anus provided better delimitation of the peripheral neuromuscular lesions in individuals with FI and spinal cord injuries, compared with pudendal nerve latency. Magnetic stimulation is recommended in patients with FI and spinal cord injury because it is a safe study and provides valuable information on the neuropathophysiologic mechanisms that could explain anorectal dysfunction.
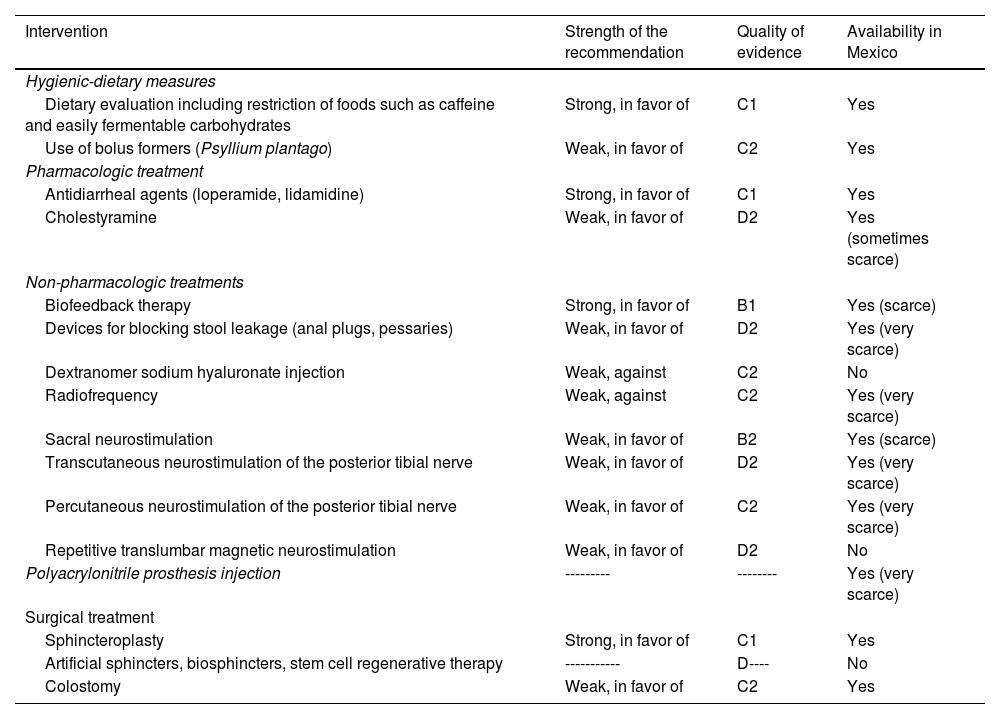
TreatmentTable 4 summarizes the recommendations issued by this consensus, with respect to treatment.
Recommendations regarding the treatment of fecal incontinence.
| Intervention | Strength of the recommendation | Quality of evidence | Availability in Mexico |
|---|---|---|---|
| Hygienic-dietary measures | |||
| Dietary evaluation including restriction of foods such as caffeine and easily fermentable carbohydrates | Strong, in favor of | C1 | Yes |
| Use of bolus formers (Psyllium plantago) | Weak, in favor of | C2 | Yes |
| Pharmacologic treatment | |||
| Antidiarrheal agents (loperamide, lidamidine) | Strong, in favor of | C1 | Yes |
| Cholestyramine | Weak, in favor of | D2 | Yes (sometimes scarce) |
| Non-pharmacologic treatments | |||
| Biofeedback therapy | Strong, in favor of | B1 | Yes (scarce) |
| Devices for blocking stool leakage (anal plugs, pessaries) | Weak, in favor of | D2 | Yes (very scarce) |
| Dextranomer sodium hyaluronate injection | Weak, against | C2 | No |
| Radiofrequency | Weak, against | C2 | Yes (very scarce) |
| Sacral neurostimulation | Weak, in favor of | B2 | Yes (scarce) |
| Transcutaneous neurostimulation of the posterior tibial nerve | Weak, in favor of | D2 | Yes (very scarce) |
| Percutaneous neurostimulation of the posterior tibial nerve | Weak, in favor of | C2 | Yes (very scarce) |
| Repetitive translumbar magnetic neurostimulation | Weak, in favor of | D2 | No |
| Polyacrylonitrile prosthesis injection | --------- | -------- | Yes (very scarce) |
| Surgical treatment | |||
| Sphincteroplasty | Strong, in favor of | C1 | Yes |
| Artificial sphincters, biosphincters, stem cell regenerative therapy | ----------- | D---- | No |
| Colostomy | Weak, in favor of | C2 | Yes |
Quality of evidence and strength of recommendation codes according to Table 1. A: high; B: moderate; C: low; D: very low; 1: strong, in favor of or against; 2 weak, in favor of or against; ------: not sufficient evidence.
Strength of the recommendation: strong, in favor of
Quality of evidence: B1
Agreement reached: 100% complete agreement.
The treatment goal in patients with incontinence is to restore continence and improve quality of life. As described in statement 11, more than one pathophysiologic mechanism is involved in the majority of cases and their identification is important for developing individualized treatment for each patient. Thus, the therapeutic proposal involves general, dietary, pharmacologic, non-pharmacologic and/or surgical measures. They should be carried out by a multidisciplinary group that includes gastroenterologists, neurogastroenterologists, nutritionists, physical therapists, and surgeons, depending on the case. Before considering pharmacologic therapy or surgical treatments, all patients should be offered support measures that include dietary modifications (see further ahead), adequate skin hygiene (the use of moisturizers, skin protectants, or a combination of the two; they have beneficial effects for preventing and treating the dermatitis associated with incontinence, compared with the use of soap and water), techniques for improving bowel movements, programmed bathroom use, pelvic floor exercises to strengthen the musculature, and management of concomitant diseases.13
Hygienic-dietary measuresA dietary evaluation should be carried out to identify foods (e.g., lactose, coffee, nonabsorbable sugars, etc.) associated with episodes and/or crises of fecal incontinenceStrength of the recommendation: strong, in favor of
Quality of evidence: C1
Agreement reached: 100% complete agreement.
The consumption of caffeine or foods containing easily fermentable sugars (lactose, disaccharides, raffinose, sorbitol, fructose) has been related to FI symptoms, especially liquid stools and fecal urgency. Thus, a diet with a reduced intake of those foods is recommended. Even though the evidence is limited, its quality is sufficient for issuing a recommendation in its favor. For example, in a study that included 65 patients following a low fermentable oligosaccharide, disaccharide, monosaccharide and polyol
(FODMAP) diet, 65% of them reported a significant decrease in symptoms on the low-FODMAP diet.76 Similar results were reported by patients with diarrhea-predominant IBS (IBS-D).77 With respect to this dietary intervention, patients should consult with a gastroenterologist and a nutritionist or registered dietician to receive instructions and monitor symptoms. Regular follow-up with health professionals enables patients to successfully incorporate adequate changes into their lifestyle.78
Fibers that increase the fecal bolus (e.g., Psyllium plantago) are recommended in patients with fecal incontinence and liquid or semi-liquid stoolsStrength of the recommendation: weak, in favor of
Quality of evidence: C2
Agreement reached: 94.1% complete agreement and 5.9% partial agreement.
Supplements of fiber, such as psyllium, are frequently recommended in an attempt to increase stool volume and reduce liquid stools.13 In a systematic review that examined the effects of fiber on FI, supplementation with 25g of psyllium or gum arabic was shown to improve stool consistency and produce fewer liquid stools, compared with placebo.79 Another study found that psyllium, but not guar gum or methyl cellulose, reduced symptoms associated with FI.80 A controlled clinical trial that included 43 patients recently described the effects of supplementation with psyllium, compared with a low-FODMAP diet.81 There was no statistically significant difference in the number of treatment responders (> 50% reduction in episodes of FI, compared with the beginning of treatment) during the 1-4 weeks of treatment (38.9% on the low-FODMAP diet, 50% in the psyllium group, p=0.33). However, the subjects in the psyllium group reported a greater reduction in general FI episodes, whereas the low-FODMAP group reported improvement, regarding FI severity and quality of life.
Importantly, fiber intake is useful in patients with fecal staining, which is mostly secondary to overflow soiling due to constipation.82
Pharmacologic treatmentThe use of antidiarrheic agents (loperamide, lidamidine) is recommended in patients with fecal incontinence and diarrheaStrength of the recommendation: strong, in favor of
Quality of evidence: C1
Agreement reached: 94.1% complete agreement and 5.9% partial agreement.
There are several medications for controlling diarrhea in patients with FI (e.g., loperamide, diphenoxylate/atropine, anticholinergics, and clonidine). They slow bowel transit, reduce the secretion of intestinal fluid, increase absorption, and improve anal sphincter pressure and tone.13 According to a Cochrane review, loperamide, diphenoxylate/atropine, and codeine significantly improve symptoms, help restore fecal continence, reduce fecal urgency, and reduce pad use.83 Loperamide 4mg thrice daily or diphenoxylate/atropine 5mg four times a day can temporarily be of help in patients with diarrhea and FI. Loperamide has even been described to improve rectal sensitivity.84 Other agents that have been shown to be less efficacious are topical phenylephrine, lidamidine, amitriptyline, and sodium valproate.
The use of cholestyramine is suggested as a coadjuvant in patients with severe fecal incontinence, especially in those with diarrhea due to bile salt malabsorptionStrength of the recommendation: weak, in favor of
Quality of evidence: D2
Agreement reached: 82.4% complete agreement and 17.6% partial agreement.
Patients with diarrhea and FI secondary to bile salt malabsorption can benefit from the use of ion-exchanging resins, such as cholestyramine or colestipol. In a study in which 21 patients received cholestyramine, together with biofeedback therapy (BFT), the administration of the drug produced a decrease in the frequency of bowel movements (p<0.01) and improved both stool consistency (p=0.001) and the number of incontinence episodes (p<0.04), compared with the group of patients that only received BFT.85 The mean dose of cholestyramine used was 3.6g; 13 subjects (62%) needed dose adjustment and 7 (33%) subjects reported minor side effects.
Non-pharmacologic treatmentPatients with fecal incontinence that are nonresponders to conservative treatment should receive biofeedback therapy, given that it is not harmful and provides numerous benefitsStrength of the recommendation: strong, in favor of
Quality of evidence: B1
Agreement reached: 88.2% complete agreement and 11.8% partial agreement.
BFT is a safe and effective treatment that improves FI symptoms, restores quality of life, and objectively improves anorectal physiology.86 Thus, in patients with FI secondary to weakness of the sphincter muscle apparatus, with or without sensory abnormalities of the rectum, it should be considered a first-line therapeutic measure. According to a group of experts from the American and European neurogastroenterology associations, the evidence for recommending BFT is Grade II B.86 The principle of BFT is based on the acquisition of new behaviors through the process of trial and error. If the new behavior is repeatedly reinforced, the possibility of success increases considerably. The aims of BFT are to: a) improve anal sphincter strength, b) improve the coordination between the pelvic floor muscles and the anal sphincter, and c) improve anorectal perception. Because each aim requires a specific training method, treatment must be individualized for each patient, according to the predominant pathophysiologic mechanism involved. At least 6 sessions are recommended, each one carried out every 15 days. After each session, the patient should be instructed on how to perform the pelvic floor exercises (Kegel exercises) at home. Importantly, pelvic floor exercises, alone, should not be considered BFT and their isolated performance has shown no benefit in FI management.87
The effectiveness of BFT on FI symptoms varies from 64 to 89%.13 Long-term studies show that BFT reduces the frequency of bowel movements and the number of FI episodes, improves quality of life, and increases anal sphincter pressure and rectal capacity.88 The study by the Australian group of Mazor et al.89 has shown longer-term efficacy of BFT in a cohort of 89 patients, in which over half the patients reported FI symptom control for more than 7 years. Fecal urgency has recently been described as one of the symptoms that is significantly improved with BFT.90 Curiously, randomized trials have compared biofeedback with different treatment focuses, such as pelvic floor exercises, counseling, and education, but there are no randomized controlled trials that compare biofeedback with the simulated therapy. A limitation of BFT is its restricted availability and the fact that it must be performed in a hospital setting. Nevertheless, devices and protocols utilizing ambulatory equipment have recently been described, showing that ambulatory BFT is as effective as conventional BFT.91
Importantly, the presence of severe FI, severe pudendal nerve neuropathy, extensive anal sphincter injury, and the coexistence of systemic neurologic diseases and significant cognitive alterations (e.g., dementia) are factors associated with a poor response to BFT. In Mexico, BFT is performed at some referral centers, but it is vital to train more personnel so that this therapy can be carried out in more areas of the country.
In selected patients that are nonresponders to conservative measures and biofeedback, the use of devices to block the passage of stool (anal plugs, pessaries, etc.) could be useful because they improve quality of life, but their availability in Mexico is limitedStrength of the recommendation: weak, in favor of
Quality of evidence: D2
Agreement reached: 88.2% complete agreement, 5.9% partial agreement, and 5.9% uncertain.
Anal plugs are mechanical blocking devices, with numerous presentations. In Mexico, clinical evidence is scarce, and their availability is nonexistent. According to the most recent Cochrane review, data suggest that anal plugs can be difficult to tolerate.92 Nevertheless, if they are tolerated, they can be useful in preventing incontinence. Thus, anal plugs could be useful in a selected group of persons, whether as a substitute for other forms of treatment or as an adjuvant treatment option. The plugs most widely described in the literature are Renew®, Peristeen®, Protec®, and Eclipse®. Renew® is a disposable anal plug. In a study that included 30 patients with FI, 20% did not like the device, 23% had no symptom changes, and 12% reported worsening of FI symptoms. However, 57% of the patients wanted to continue using the device.93 In a second study, the Renew® device was used in 15 patients that had an ileoanal pouch. Renew® was acceptable in 53%, and 40% considered it effective.94 Peristeen® is a device that is introduced into the anal canal and prevents accidental leakage by acting as an absorbent plug. It is similar to a suppository and is covered by a transparent membrane that dissolves on contact with rectal mucosa humidity, expanding to form a plug and preventing fluid or stool leakage. Its efficacy is close to 50% but up to 70% of patients report discomfort with its use.95,96 ProTect® is a relatively simple medical device, designed for selected patients presenting with severe FI. It consists of a flexible silicone catheter with an inflatable balloon that seals the rectum at the anorectal junction, acting like a plug.97 The proximal part of the catheter has two contacts that monitor the rectum, in search of stool. The patient is alerted about an imminent bowel movement, and a possible fecal accident, through a beeping sound. Like the other devices, even though it improves quality of life and FI frequency in some patients, not all patients tolerate it. It is more useful in controlling bowel movements with semi-formed stool.98 The vaginal intestinal control device, Eclipse®, is a balloon that is inserted into the vagina and acts like a blocking mechanism (pessary), compressing the anterior wall of the rectum. The correct size of the balloon must be selected for each patient, and manual dexterity for deflating, inflating, inserting, and removing the device is required. Two case series have reported improvement from 50 to 86%, and even though relevant adverse effects have not been reported, up to 21% of patients report vaginal discomfort.99,100
Importantly, patients with passive incontinence for small quantities of stool can benefit from cotton perianal plugs (pads) for absorbing humidity and reducing the uncontrollable passage of gas. However, there are no formal studies on this intervention.
Evidence on the injection of substances (dextranomer sodium hyaluronate) or prostheses in patients with fecal incontinence is controversial, and so we do not recommend their useStrength of the recommendation: weak, against
Quality of evidence: C2
Agreement reached: 82.4% complete agreement, 11.8% partial agreement, and 5.9% uncertain.
For the purpose of increasing the resting pressure of the anal canal, injection into the submucosa or the intersphincteric space of several substances or prostheses (GateKeeper®, SphinKeeper®) has been tested for FI management. Efficacy varies, depending on the product tested, but few studies have compared the substances with other simulated injections. At present, the injection of dextranomer microspheres with hyaluronic acid (NASHA Dx®) is the only product approved by the Food and Drug Administration (FDA) for FI; it has also shown a significant difference versus placebo.13,101 In fact, a decrease in more than 50% of the numbers of FI episodes has been reported in 52% of patients that received NASHA Dx®, compared with 31% of patients that received placebo.101 Nevertheless, a later study found no significant difference in the number of FI episodes or in quality of life improvement in the group of patients that received the injectable agent vs. a simulated procedure.102 Although a large number of adverse effects have been reported, the majority are not serious (proctalgia, fever, and rectal bleeding). This intervention is not available in Mexico.
The use of the injection of prostheses made out of Hyexpan (polyacrylonitrile, SphinKeeper™), a self-expanding material with “shape memory”, which after 48h of implantation expand; the prostheses absorb physiologic fluids and increase their volume up to 730% of their original size.102 Thanks to their “shape memory” effect, the prostheses return to their initial shape, following the movement of the anal sphincters. SphinKeeper® has shown efficacy and safety in trials with limited samples. This technique is available in Mexico, but more better-quality trials are needed before issuing a clinical recommendation.
Radiofrequency (the Secca® procedure) is not recommended in patients with fecal incontinenceStrength of the recommendation: weak, against
Quality of evidence: C2
Agreement reached: 88.2% complete agreement and 11.8% partial agreement.
The Secca® procedure consists of the application of temperature-controlled radiofrequency energy (465kHz, 2-5W), especially on the anal canal quadrants.4,30 The released heat is suggested to induce tissue contraction and anal canal remodeling through the formation of retractile fibrosis, with the subsequent collagen deposition, thus inducing contraction of the IAS.103,104 Initial studies reported improvement of FI and quality of life, but more recent studies show contradictory results and its long-term efficacy appears to rapidly decrease over time.105,106
Sacral neurostimulation is recommended in patients with moderate-to-severe fecal incontinence that have had failed biofeedback therapyStrength of the recommendation: weak, in favor of
Quality of evidence: B2
Agreement reached: 94.1% complete agreement and 5.9% partial agreement.
Continuous sacral nerve stimulation (SNS) has become a treatment for patients that present with at least one episode of FI a week (moderate-to-severe) and that do not respond to conservative therapies or BFT.13,95 Indications for SNS include idiopathic FI with no sphincter injury; patients with FI and UI, post-obstetric perineal injuries (anal sphincter tears or pudendal neuropathy); and neurologic FI, whether central or peripheral.107 Sacral stimulation appears to improve somatosympathetic spinal cord reflexes, but also has a certain effect at the central level, improving neuroplasticity.108 Given that the results of surgical repair of FI show deterioration during the 5-year follow-up, SNS has been suggested as a valid alternative or a complement to surgical repair in the treatment of FI in patients with anal sphincter injury.109
This treatment permanently stimulates the sacral nerves through an electrode implanted in a sacral foramen on a spinal nerve site. The device is configurated in 2 stages. The first stage, called peripheral nerve evaluation, is a testing period. At 2 to 3 weeks, the electrode is implanted, near the S3 root and is linked to an external stimulator. At this stage, the stimulation parameters are adjusted (frequency and intensity) until obtaining the desired effects. The second stage involves the definitive implantation of an impulse generator under the skin, which is done only if the patient has a 50% reduction of FI episodes during the first stage. In general, according to a recent literature review, SNS appears to be efficacious in approximately 60-70% of patients in whom conservative treatment has failed.110 In the most recent network meta-analysis, SNS was found to improve the incontinence score, the capacity to defer bowel movements, improve the majority of the SF-36 and FIQL domains, and improve mean anal pressures.111 The therapeutic effect lasts over time, despite a 10% decrease in efficacy during the first 5 years. The most common adverse events are pain and infection at the insertion site, which occurs in 10% of patients.
This neurostimulation therapy is available in Mexico and its elevated cost is a limitation, but it has been shown to be cost-beneficial in other countries.112 In terms of cost-benefit, sacral neurostimulation can vary, depending on several factors, such as the cost of the procedure, the effectiveness of the treatment, and the individual results of each patient. Some studies have shown that sacral neurostimulation can be effective in improving symptoms in certain cases, which could result in a significant improvement in patient quality of life. However, it should be kept in mind that sacral neurostimulation is not first-line treatment. In general, it is recommended after other more conservative treatments, such as dietary changes, physical therapy, or medications, have not been effective. The cost of the procedure, which includes the surgery for implanting the device and its long-term maintenance, can be considerable.
In general, the cost-benefit of sacral neurostimulation should be evaluated on an individual basis. Factors, such as symptom severity, expected results, costs involved, and alternative treatment options, are important to consider in making an informed decision about treatment selection.
Posterior tibial neurostimulation (transcutaneous, percutaneous) are options that could be useful in some patients with fecal incontinencePercutaneous stimulationStrength of the recommendation: weak, in favor of
Quality of evidence: C2
Agreement reached: 100% complete agreement.
Transcutaneous stimulationStrength of the recommendation: weak, in favor of
Quality of evidence: D2
Agreement reached: 100% complete agreement.
It is a simple, noninvasive, inexpensive technique. There are two stimulation methods: percutaneous, using needle electrodes, and transcutaneous, using adhesive surface electrodes. Two electrodes are placed on the posterior tibial nerve pathway and are connected to a neurostimulator that can be controlled by the patient. The mechanism involved in the treatment is still poorly understood but it can certainly be inferred that the stimulation improves or affects somatosympathetic reflexes. Despite the fact that there are only a few published studies with results, the percutaneous technique is reported to reduce FI episodes in 63 to 82% of the patients treated, with follow-up ranging from one to 30 months.113,114 The evidence on transcutaneous stimulation shows no difference, when compared with placebo.115 According to the most recent meta-analyses,95 percutaneous posterior tibial nerve stimulation is efficacious for reducing the mean number of episodes of FI per week. However, FI severity and quality of life are not significantly different, when compared with placebo. In addition, even though studies show a significant reduction in FI episodes per week, the study with the largest sample failed to show that this therapy reduces FI episodes per week by more than 50%, which should be taken into account when opting for this therapy. At present, there is still no consensus on treatment duration, frequency/rhythm stimulation, or the need to repeat treatment. Nevertheless, this technique can be recommended for patients that are nonresponders to other noninvasive techniques or that present with FI.
Translumbar and transsacral magnetic stimulation could be useful in certain patients with fecal incontinenceStrength of the recommendation: weak, in favor of
Quality of evidence: D2
Agreement reached: 100% complete agreement.
The appearance of the translumbosacral anorectal magnetic stimulation (TAMS) test that utilizes minimally invasive magnetic stimulation of the lumbar and sacral plexus nerves to register the rectal and anal motor-evoked potentials (MEPs) has enabled better detection of neuropathy in patients with FI.75 Utilizing this same device and starting from the hypothesis that repetitive translumbar magnetic neurostimulation therapy (TMNT) at one or more frequencies could significantly improve FI symptoms through the production of neuroplasticity, the group of Dr. Rao has explored the efficacy of this intervention.116 In the pilot study, the authors included 31 patients that were randomized to receive 6 weekly sessions of TMNT. Treatment consisted of 600 repetitive magnetic stimulations at each of the 2 lumbar sites and 2 sacral sites, at a frequency of 1, 5, or 15Hz. Stool diaries, FI severity scales, neurophysiology, anorectal sensorimotor function, and quality of life (QOL) scores were compared. The results were positive, demonstrating that this therapy significantly improves FI symptoms in the short term. In general, frequency at 1Hz was better than at 5 and 15Hz. Both anorectal neuropathy and physiology improved significantly, showing that this is a promising novel noninvasive therapy that is a safe and efficacious treatment for FI. The proposed mechanism of action is the capacity to improve both peripheral and central neural excitability.117
Surgical treatmentPatients with severe FI that have had failed medical treatment or have large sphincter defects are the ones that benefit from surgical treatment. Nevertheless, a careful and detailed preoperative evaluation to determine FI etiology is the cornerstone of patient selection. The statements regarding surgical treatment follow below.
Sphincteroplasty is the best surgical treatment for repairing recent structural defects (lesions > 90 degrees, but < 150 degrees) in patients with fecal incontinenceStrength of the recommendation: strong, in favor of
Quality of evidence: C1
Agreement reached: 88.2% complete agreement and 11.8% partial agreement.
Sphincteroplasty (surgical repair in which the separated ends of the sphincter are joined or overlapped and sutured together to heal) shows good-to-excellent results in the majority of patients that present with an adequate residual muscle mass.118 If defects are larger than 150 degrees, the technique is usually difficult to perform. Success with this procedure varies from 35 to 70%.119 Despite the fact that 70-80% of patients report initial improvement, it decreases by 50% in the long term. Postoperative complication rates are generally low and wound infection is more commonly reported, varying from 6 to 35%.118 Among the factors to be taken into account to ensure a successful result are: a) the presence of neuromuscular integrity (including pudendal nerve integrity) and clinically detectable voluntary contraction, b) if the first repair has failed, at least three months should pass before attempting a second sphincteroplasty, c) scar tissue should not be removed from the damaged muscles, d) the internal and external sphincters should not be separated, and e) a protective colostomy is not needed.
Even though artificial sphincters, biosphincters, and stem cell injection are promising options, they are therapies that are still under evaluation, thus, no recommendation can be issuedStrength of the recommendation: --------
Quality of evidence: D
Agreement reached: 82.4% complete agreement, 11.8% partial agreement, and 5.9% uncertain.
The use of a magnetic anal sphincter composed of a system of magnetic beads, joined by titanium wire, placed in a tunnel around the anal sphincter, creating negative pressure in the anal lumen, has recently been described.120 During bowel movements, the beads separate, allowing the stool to pass. In 2012, Wong et al.120 reported the results of a nonrandomized study, showing the magnetic anal sphincter to be as efficacious as sacral nerve stimulation, with respect to improvement of symptoms and quality of life. Morbidity was similar with the two techniques. Long-term efficacy of this intervention is reported at close to 50%. Even though the results are promising, the efficacy of the magnetic anal sphincter should be confirmed in larger studies and with better methodologies.121
A recently described innovative therapy (regenerative therapy) is the use of stem cells derived from muscle or adipose tissue that is injected directly into the sphincter.122 From 2017 to the present date, 3 studies have evaluated this therapy and shown that the mean number of episodes of FI per week is lower than with placebo in the first 4 weeks, but the effect is gradually lost over the following weeks.123–125 Even though the findings are promising, more and better-quality studies on the subject are needed.
A technique that has been in development for decades is the perianal implantation of a sphincter designed through bioengineering.126 The process of developing a bioengineered anal sphincter generally involves the culturing of specialized cells in a laboratory, to then be incorporated into a structural matrix that provides a physical support for the cells and enables their growth and differentiation into a functional tissue similar to the anal sphincter. The fact that bioengineered anal sphincters are still in the developmental stages and are not widely available as a treatment option must be taken into account. Research in this field continues and clinical trials are being conducted to evaluate the safety and efficacy of these devices.
A terminal stoma is recommended in patients with severe fecal incontinence that are nonresponders to other treatments because it is a good option that prevents complications and improves quality of lifeStrength of the recommendation: weak, in favor of
Quality of evidence: C2
Agreement reached: 94.1% complete agreement and 5.9% partial agreement.
Fecal diversion through the creation of a colostomy or ileostomy offers definitive treatment for FI in patients with failed conservative treatment or those not suitable for it, particularly patients with spinal cord injury. Despite the esthetic implications and those of popular belief, many patients experience significantly improved quality of life after this procedure. In an exemplary study, 83% of the 69 subjects felt that a stoma restricted their lives “a little” or “not at all”, and 84% “probably” or “definitely” would choose to have a stoma (compared with their previous treatment); additionally, overall satisfaction with the stoma was graded as 9/10.127 However, this option should be individualized. For example, it can be a good option in patients with severe neurologic injuries. This decision should be made together with the patient, considering the morbidity and mortality associated with the procedure.
Other therapiesOther interventions not considered in the statements because of their limited efficacy, controversial evidence, and use in special situations are mentioned below.
Transanal irrigation (TAI) is recommended in patients that present with severe chronic neurologic diseases (such as a severed spinal cord) as second-line adjuvant therapy, in addition to dietary measures and medical treatment.128 The goal is to empty the colon through regular irrigation, achieving this through the use of a catheter that has an inflatable rectal balloon for forming a hermetic system. This method improves FI between 40 and 75% of patients that have chronic neurologic diseases.128 It also helps improve patient quality of life, increasing their independence and apparently reducing the risk of urinary tract infections. Nevertheless, this treatment can work, only if both the patient and his/her relatives are committed to it. Based on the same principle as TAI, the aim of anterograde irrigation (the Malone procedure) is to restore continence by maintaining an empty colon. The procedure consists of performing a cecostomy and placing a catheter that enables patients to carry out the irrigations themselves. With this technique, 80 to 90% of patients report having “pseudo” continence, but some of the complications make it necessary to explant the device.129 This method has also been shown to be efficacious when combined with an artificial urinary sphincter in patients that present with double incontinence. The performance of endoscopic cecostomies has presently been described to have similar results, albeit further studies on this novel technique are needed.129
If the anal sphincter is severely injured, a neosphincter can be built through reinforcement of the existing sphincter with autologous skeletal muscle, frequently the gracilis muscle (dynamic graciloplasty) and/or the buttocks, accompanied by neurostimulation. The success of these procedure varies from 42 to 85%, and the most common events are infection (28%), stimulator malfunction (15%), and leg pain (13%). This procedure is not currently recommended.130
ConclusionsIn the present consensus, recommendations have been issued for the appropriate diagnosis and treatment of FI in Mexico. FI is known to be a frequent entity whose incidence increases in relation to patient age; however, it is poorly recognized. The pathophysiology of incontinence is complex and multifactorial, and in the majority of cases, there is more than one associated risk factor. With respect to diagnosis, even though there is no gold standard, the combination of tests that evaluate the structure (e.g., endoanal ultrasound) and function (anorectal manometry) should be recommended in all cases. In addition, treatment should be multidisciplinary and general measures, drugs (lidamidine, loperamide, cholestyramine), and in selected cases, non-pharmacologic interventions, such as BFT, are recommended. Likewise, surgical treatment should be offered to selected patients and performed by experts.
Ethical considerationsBecause this is a consensus document based on the best published scientific evidence available and is not a research study on patients, informed consent from the patients for receiving treatment to participate in the study was not required. No experiments on humans or animals were carried out.
Given the descriptive nature of the document and the fact that it is a position document of the Association, authorization by an ethics committee was not required.
The authors declare that this article contains no personal information that could identify patients.
Financial disclosureThis consensus was carried out with the support of the Asociación Mexicana de Gastroenterología which enabled the participation, transportation, and housing, with respect to the face-to-face voting. No honoraria were received.
Conflict of interestDr. José María Remes-Troche and Dr. Enrique Coss-Adame have been speakers for Medtronic. The remaining authors declare they have no conflict of interest related to the formulation of the present work.
Please cite this article as: Remes-Troche JM, Coss-Adame E, García-Zermeño KR, Gómez-Escudero O, Amieva-Balmori M, Gómez-Castaños PC, et al. Consenso mexicano sobre incontinencia fecal. Rev Gastroenterol Mex. 2023;88:404–428.