Since the publication of the 2008 guidelines on the diagnosis and treatment of diverticular disease of the colon by the Asociación Mexicana de Gastroenterología, significant advances have been made in the knowledge of that disease. A systematic review of articles published in the medical literature from January 2008 to July 2018 was carried out to revise and update the 2008 guidelines and provide new evidence-based recommendations. All high-quality articles in Spanish and English published within that time frame were included. The final versions of the 43 statements accepted in the three rounds of voting, utilizing the Delphi method, were written, and the quality of evidence and strength of the recommendations were established for each statement, utilizing the GRADE system. The present consensus contains new data on the definition, classification, epidemiology, pathophysiology, and risk factors of diverticular disease of the colon. Special emphasis is given to the usefulness of computed tomography and colonoscopy, as well as to the endoscopic methods for controlling bleeding. Outpatient treatment of uncomplicated diverticulitis is discussed, as well as the role of rifaximin and mesalazine in the management of complicated acute diverticulitis. Both its minimally invasive alternatives and surgical options are described, stressing their indications, limitations, and contraindications. The new statements provide guidelines based on updated scientific evidence. Each statement is discussed, and its quality of evidence and the strength of the recommendation are presented.
Desde la publicación en 2008 de las guías de diagnóstico y tratamiento de la enfermedad diverticular del colon de la Asociación Mexicana de Gastroenterología ha habido avances significativos en el conocimiento de esta enfermedad. Se realizó una revisión sistemática de la literatura en PubMed de enero de 2008 a julio de 2018 con el fin de revisar y actualizar las guías 2008 y proporcionar nuevas recomendaciones basadas en la evidencia. Se incluyeron todas las publicaciones en español e inglés, de alta calidad. Se redactaron los enunciados, que fueron votados utilizando el método Delphi. Se estableció la calidad de la evidencia y la fuerza de las recomendaciones según el sistema GRADE para cada enunciado. Cuarenta y tres enunciados fueron finalmente votados y calificados. Se informan nuevos datos sobre definición, clasificación, epidemiología, fisiopatología y factores de riesgo. Se revisó con especial énfasis la utilidad de la tomografía computarizada y de la colonoscopia, así como los métodos endoscópicos para el control de la hemorragia. Se discutió sobre el tratamiento ambulatorio de la diverticulitis no complicada, el papel de la rifaximina y la mesalazina, en el manejo de la diverticulitis aguda complicada tanto en sus alternativas mínimamente invasivas hasta las opciones quirúrgicas con énfasis en sus indicaciones, limitaciones y contraindicaciones. Los nuevos enunciados proporcionan directrices basadas en la evidencia actualizada. Se presentan la discusión, el grado y la fuerza de la recomendación de cada uno de ellos.
The detection of diverticula in the colon is one of the most common incidental findings during the performance of a colonoscopy.1 The incidence of diverticulosis and diverticular disease of the colon has increased worldwide in recent years and is affecting persons at a younger age. Due to the associated morbidity and mortality, those pathologies are becoming a significant burden for national health systems.2 Diverticular disease of the colon is a common condition whose clinical presentation varies. Its clinical spectrum ranges from the sole presence of symptoms to the development of different complications.3
In 2008, the Asociación Mexicana de Gastroenterología brought together a multidisciplinary group of specialists that formulated the guidelines for the diagnosis and treatment of diverticular disease of the colon.4–6 Since then, new concepts about the disorder have emerged that include its better classification, epidemiology, pathophysiology, diagnosis, and the development of effective alternative therapies for each condition on the clinical spectrum. All those advances warranted the creation of a document that complements the 2008 diagnosis and treatment guidelines.
In April of 2018, the Asociación Mexicana de Gastroenterología summoned three coordinators (RRG, NSN, RCS) and 18 participants to form the consensus group and carry out a review of the advances in different aspects of the disease, evaluate the evidence, formulate statements on the current status of the pathology, and discuss them until reaching the level of agreement necessary for their approval. All the works were supervised by a general coordinator (JMRT).
The aim of the present document is to present a consensus review of the current status of diverticular disease of the colon to update the 2008 diagnosis and treatment guidelines, integrating new internationally published scientific evidence.
MethodsThe present consensus was formulated utilizing the Delphi method.7 The consensus coordinators carried out a review of the medical literature using the words “diverticular disease”, “diverticular colon disease”, “diverticular disease colon”, “diverticular”, “diverticulosis”, “diverticulitis”, “acute diverticulitis”, “symptomatic uncomplicated diverticular disease” and “complicated diverticulitis” as the search criteria, combined with the following terms: “epidemiology”, “incidence”, “prevalence”, “pathophysiology”, “risk”, “bleeding”, “diagnosis”, “differential diagnosis”, “treatment”, “therapy”, “diet”, “prevention”, “management”, “review”, “guidelines” and “meta-analysis”, as well as the equivalent terms in Spanish. The search was carried out in PubMed and included all articles in English and Spanish published from January 2007 to July 2018. Preference was given to consensuses, guidelines, systematic reviews, and meta-analyses, but selection was not limited to those types of articles. Complementary electronic and manual searches were carried out on all publications that the coordinators considered relevant, up to July 2018. The entire bibliography collected was placed at the disposal of the consensus group so they could consult it at any time throughout the process.
After the review was completed, 49 statements were formulated. They were put to a first round of anonymous, electronic voting from April 23 to 29, 2018. The votes were cast utilizing the following responses: a) in complete agreement, b) in partial agreement, c) uncertain, d) in partial disagreement, and e) in complete disagreement. When agreement was equal to or above 75%, the statement remained unchanged for the next round of voting. Statements in which disagreement was 75% or higher were eliminated from the document. The statements that did not reach agreement or disagreement of 75% were restated by the coordinator of each theme, taking into account the comments of the participants. The second round of electronic distant voting (from May 14 to 21, 2018) included 48 statements, following the same system. The face-to-face round of voting took place in the city of San Luis Potosí, in the State of the same name, Mexico, on June 28, 2018, in which the statements were voted upon and graded by the consensus group, resulting in the 43 final statements.
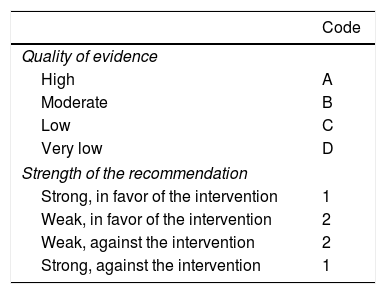
Once the final consensus statements were determined, the coordinators were responsible for establishing the quality of evidence sustaining each statement and they gave a corresponding grade of recommendation to all the statements that involved a diagnostic or therapeutic intervention, employing the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system8 (Table 1). That system came about in an effort to overcome the limitations of previous systems, optimizing the quality of evidence and the grade of strength of the recommendations, and has been used in the most recent consensuses of the Asociación Mexicana de Gastroenterología. In the GRADE system, the quality of evidence is not established solely by the methodology of the study analyzed, but it is also classified based on the design employed to respond to a specific question that has previously been posed.8,9 Consequently, the quality of evidence is “high” when the publication of further research studies will not change our confidence in the estimate of effect, “moderate” when the publication of further research studies may modify our confidence in the estimate of effect, “low” when the publication of further research studies is very likely to have an important impact on our confidence in the estimate of effect, and “very low” when any estimate of effect is uncertain. In addition, the GRADE system establishes the strength of recommendation as strong or weak and in favor of or against an intervention or statement. The system utilizes classification codes in which an upper case letter describes the quality of evidence, followed by a number indicating the strength of a recommendation in favor of or against the intervention or statement.8,9 In the statements referring to definition, epidemiology, and pathophysiology, only the quality of evidence was graded.
Classification of the quality of evidence and the strength of the recommendations according to the GRADE system.
| Code | |
|---|---|
| Quality of evidence | |
| High | A |
| Moderate | B |
| Low | C |
| Very low | D |
| Strength of the recommendation | |
| Strong, in favor of the intervention | 1 |
| Weak, in favor of the intervention | 2 |
| Weak, against the intervention | 2 |
| Strong, against the intervention | 1 |
Modified from Oñate-Ocaña and Ochoa-Carrillo.8
For the face-to-face meeting, a total of 48 statements were presented. After several of them were revised, eliminated, or combined, the resulting total was 43 statements. The final statements and the corresponding voting results are presented below.
Definitions, epidemiology, and pathophysiology- 1.
The small sacs formed by herniations of the mucosa and submucosa that protrude through the muscle layers of the colonic wall are known as diverticula of the colon. Strictly speaking, they are pseudodiverticula
Quality of evidence: A
Level of agreement: in complete agreement 95%, in partial disagreement 5%
Diverticula are herniations of the mucosa and submucosa through zones where blood vessels penetrate, and they occur due to increased intracolonic pressure. Because they do not contain all the layers of the colonic wall, they are called pseudodiverticula. The diverticula of approximately 90% of individuals develop in the left colon (sigmoid colon). In contrast, right-side diverticula are true diverticula.10
- 2.
Diverticulosis is the presence of diverticula in the colon. When it presents with symptoms, it is known as diverticular disease
Quality of evidence: A
Level of agreement: in complete agreement 90%, in partial agreement 5%, in partial disagreement 5%
Traditionally, the presence of diverticula in the colon has been called “diverticulosis”, and by definition, the diverticula are asymptomatic in all patients. Although they have commonly been associated with constipation, there are no studies demonstrating that diverticulosis causes a specific symptom. On the other hand, approximately 20% of individuals with diverticulosis develop symptoms, characterizing it as a disease: diverticular disease of the colon.11
- 3.
Diverticular disease is classified as symptomatic uncomplicated diverticular disease and complicated diverticular disease
Quality of evidence: A
Level of agreement: in complete agreement 95%, in partial agreement 5%
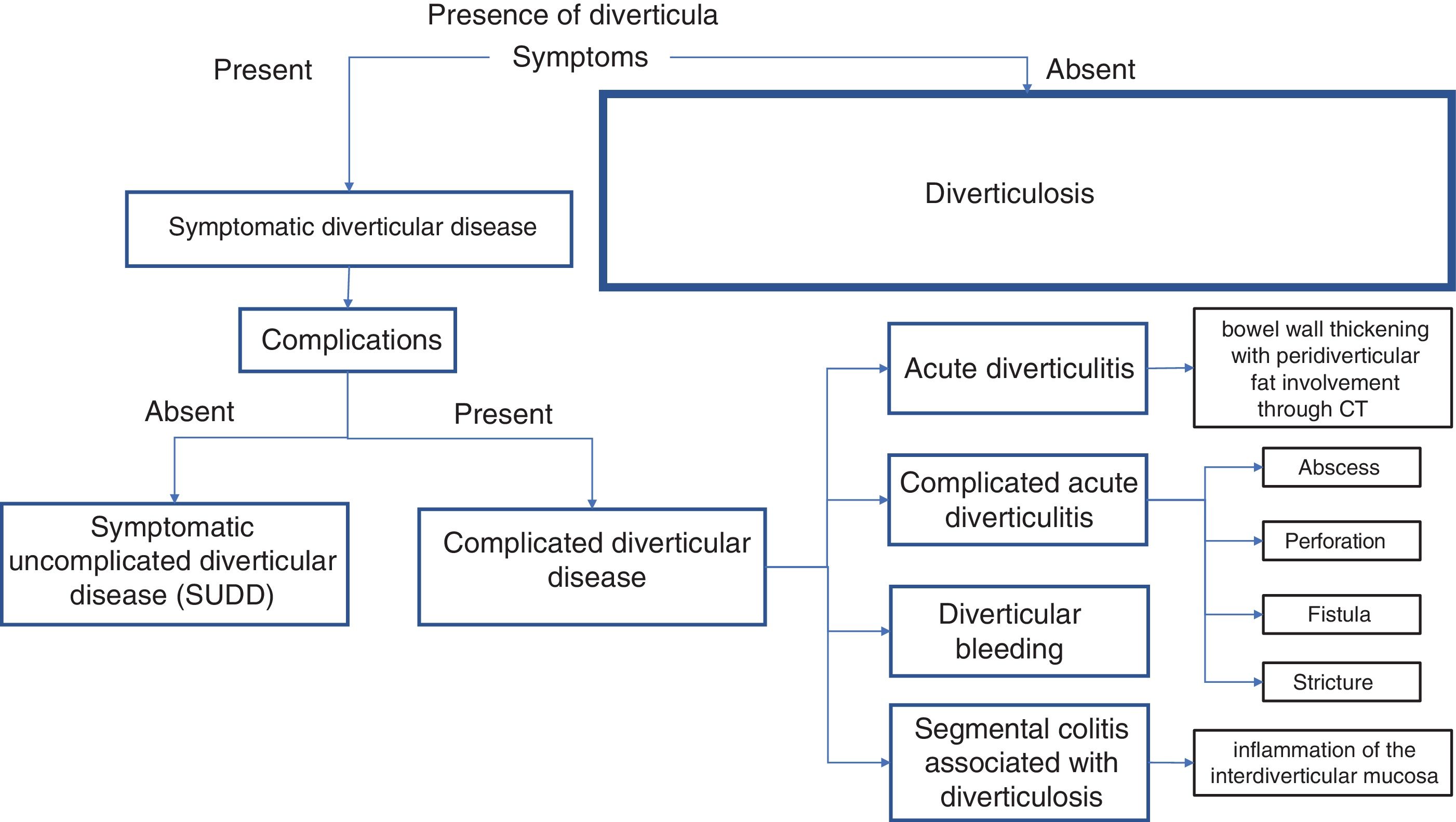
Diverticular disease (DD) is classified as uncomplicated and complicated. The presence of symptoms defines DD and is synonymous with symptomatic uncomplicated diverticular disease (SUDD), as long as there are no complications.11 When DD has macroscopic, radiologic, or serologic signs of inflammation, it is known as “complicated diverticular disease”, which includes uncomplicated acute diverticulitis, complicated acute diverticulitis, diverticular bleeding, and segmental colitis associated with diverticulosis.2,3,11,12
- 4.
Symptomatic uncomplicated diverticular disease refers to the appearance of symptoms similar to irritable bowel syndrome (abdominal pain and bloating associated with changes in bowel habit), in the presence of diverticula with no macroscopic inflammatory alterations
Quality of evidence: A
Level of agreement: in complete agreement 90%, in partial agreement 5%, in complete disagreement 5%
Symptomatic uncomplicated diverticular disease (SUDD) is defined as the presence of abdominal pain and bloating associated with changes in bowel habit and the presence of diverticula, with no other apparent cause of the symptoms.13 The manifestations are indistinguishable from irritable bowel syndrome (IBS) and the overlapping of the two disorders is a practical problem, given that 10 to 66% of patients with diverticula meet the Rome III diagnostic criteria for IBS.13 However, there are clinical differences between the two conditions. Both diseases tend to present at different stages of life, with peak presentation of IBS observed between 20 and 40 years of age, whereas SUDD symptoms usually appear starting at 60 years of age. IBS is predominant in women, whereas there is a greater frequency of SUDD in men. The abdominal pain associated with IBS is visceral and diffuse, whereas the pain related to SUDD is somatic and more localized. Relapses are more frequent in IBS and remission is more prolonged in SUDD. Despite those differences, there is overlap between SUDD and IBS, predominantly in middle-aged patients. In those cases, it is difficult to discern whether the symptoms are caused by the presence of diverticula or due to IBS overlap. Biomarkers, such as fecal calprotectin, can be useful because that test is negative in IBS and can be positive in SUDD, but its practical usefulness has not been clearly established.13–15
- 5.
Complicated diverticular disease includes acute diverticulitis, complicated acute diverticulitis (abscess, perforation, fistula, and obstruction, in the case of stricture), diverticular bleeding, and segmental colitis associated with diverticulosis
Quality of evidence: A
Level of agreement: in complete agreement 100%
Diverticular disease (DD) is divided into uncomplicated (SUDD) and complicated disease. Complicated DD includes acute diverticulitis, which is mainly characterized by pain and colonic wall thickening, as well as by involvement of the peridiverticular fat detected through abdominal tomography.2 Complicated acute diverticulitis includes the presence of peridiverticular abscesses, free perforation with fecal peritonitis, or the development of fistulas whose inflammatory phenomena can penetrate neighboring organs, enabling the passage of bowel content into the bladder, vagina, etc.2,16 Diverticular bleeding is more prevalent in the ninth decade of life and approximately 10% of patients can have bleeding recurrence the following year.2,17
- 6.
Segmental colitis associated with diverticulosis is characterized by inflammation of the interdiverticular mucosa, with no involvement of the diverticular orifices (not affecting the segments with no diverticula), and can present with abdominal pain, chronic diarrhea, and intermittent hematochezia
Quality of evidence: A
Level of agreement: in complete agreement 90%, in partial agreement 5%, uncertain 5%
Inflammation in the zones of diverticulosis, between the diverticula, without affecting the diverticular orifices, is known as “segmental colitis associated with diverticulosis” (SCAD) or “diverticular colitis”. It tends to develop almost exclusively in older adults, predominantly in men, and is usually located in the sigmoid colon and the descending colon.18,19 The rectum and the right colon are free from the disease, both macroscopically and histologically. Although the precise physiopathogeny of SCAD is not known, most likely it is heterogeneous and includes mechanisms that also play a role in inflammatory bowel disease (IBD). The histologic characteristics are very similar to those of ulcerative colitis, but the mucin depletion and increase in plasma cells, lymphocytes, and histiocytes present in ulcerative colitis are absent in SCAD and differentiate it from inflammatory bowel disease.18–20 Despite that, it is still not clear whether SCAD is a separate entity or belongs on a spectrum with IBD. 18–20
- 7.
The prevalence of diverticulosis is variable. It depends on the group under study and increases with patient age. In accordance with age group, it is prevalent in women (50 years of age or older) or in men (50 years of age or younger). The incidence and prevalence of diverticulosis have increased in recent years, but their rates in Mexico are not known
Quality of evidence: C
Level of agreement: in complete agreement 80%, in partial agreement 10%, uncertain 5%, in partial disagreement 5%
Several studies have shown that diverticula of the colon present with greater frequency in Western populations and that their prevalence increases with patient age (35% in patients older than 60 years of age, 65% in patients 80 years of age or older). The number of patients with more than 10 diverticula also increases with age (8% in patients above 50 years of age, 15% in patients between 51 and 60 years of age, and 30% in those above 60 years of age). Much has been said about the different prevalence of diverticulosis between women and men according to age (women older than 50 years of age and men younger than 50 years of age), but there does not appear to be a significant difference in the overall prevalence of diverticulosis in relation to sex.21 The anatomic distribution of the diverticula seems to vary according to race. In white people, 75% of diverticula are found in the sigmoid colon, 11% in the descending colon, 6% in the transverse colon, and only 8% in the ascending colon, whereas in black people, 64% are observed in the sigmoid colon, 8% in the descending colon, 7% in the transverse colon, and 20% in the ascending colon (p = 0.0008).22 Nevertheless, the true prevalence of diverticulosis is difficult to establish because the majority of individuals are asymptomatic and only 10 to 20% present with clinical symptoms, whether as diverticulitis or diverticular bleeding.
- 8.
In the past decade, an increase in hospitalizations and healthcare service costs due to acute diverticulitis has been reported worldwide, but there are no figures in that regard in Mexico
Quality of evidence: C
Level of agreement: in complete agreement 90%, in partial agreement 10%
The estimated cost of diverticular disease (DD) and its complications is considerable worldwide. The data obtained in 2010 from the United States National Hospital Ambulatory Medical Care Survey and the National Ambulatory Medical Care Survey place DD as the eighth most frequent gastrointestinal diagnosis in outpatients, with more than 2.7 million consultations per year. According to the 2012 Nationwide Inpatient Sample Report, diverticulitis with no bleeding was the cause of more than 216,000 hospital admissions, with an increase of 21%, compared with data from 2003, generating a cost of 2.2 billion USD. Admissions due to diverticular bleeding were above 500,000 and DD was the sixteenth cause of death reported.23 Ito et al.24 divided the costs due to diverticular bleeding into two groups: below 500,000 JPY (the equivalent of 83,815 MXN) and more than 500,000 JPY. The risk factors associated with greater expense were age, low hemoglobin level, and the need for blood transfusion.
- 9.
The presence of comorbidities in elderly patients that present with complicated diverticular disease is responsible for the increase in mortality
Quality of evidence: B
Level of agreement: in complete agreement 80%, in partial agreement 15%, in complete disagreement 5%
Diverticulosis of the colon presents in at least 60% of patients 80 years of age or older and it progresses to acute diverticulitis in 20%, requiring emergency surgery for colectomy, with or without colostomy. Morbidity and mortality in those cases are 60 and 20%, respectively. Age is an independent risk factor for mortality, given that those patients have more probability of postoperative complications, such as septic shock, prolonged mechanical ventilation, and acute kidney failure.25 In a retrospective study, Bostock et al.26 reviewed medical records utilizing the American College of Surgeons National Surgical Quality Improvement Project (ACS-NSQIP) from 2005 to 2013 to identify patients above 80 years of age diagnosed with acute diverticulitis and evaluate the risk factors for death. A total of 2,986 patients diagnosed with diverticulitis were included in the study, 464 of whom died, with a postoperative mortality rate at 30 days of 19.6%. In the subgroup analysis, the authors found that age above 80 years was an independent risk factor for death, a finding reported by other authors. Other risk factors were the presence of ascites, previous heart surgery, a partially dependent or totally dependent functional status, albumin level under 3g/dl, and an American Society of Anesthesiologists (ASA) class above 3. The univariate analysis showed that septic shock, the development of pneumonia, the need for reintubation, respiratory failure, the need for dialysis, cardiac arrest, and the use of postoperative transfusion were independent factors associated with a higher mortality rate.26 Several studies conducted in England and Scotland have demonstrated that younger patients with no comorbidity have a lower standardized mortality rate, at an average of 4.95% for those 55 years of age or younger that underwent emergency surgery, compared with only 2.8% in patients of the same age that underwent elective surgery.23 Finally, in a multicenter study conducted by Broersen et al.,27 whose aim was to evaluate mortality due to steroids in immunosuppressed patients (nonusers, users, and new users) with perforated acute diverticulitis, the risk for death in nonusers was 4.4% after 7 days and 15.6% after one year. The mortality rate in users was 14.2% after 7 days and 47.6% after one year. In new users, the risk increased from 15.7% after 7 days to 52.5% after one year from diagnosis, showing a real increase in mortality simply due to corticosteroid use.
- 10.
Alterations in the enteric nervous system and smooth muscle, as well as low-grade inflammation, may be part of the pathophysiology of diverticular disease
Quality of evidence: C
Level of agreement: in complete agreement 85%, in partial agreement 5%, uncertain 10%
The enteric nervous system (ENS) maintains gastrointestinal function control independent from the brain and spinal cord. Several elements are required to carry out that function. The myenteric plexus located between the circular and longitudinal muscle fibers is composed of primary afferent neurons. The interstitial cells of Cajal (ICCs) serve as pacemakers of the gastrointestinal tract, mediating the information between the enteric nerve and the smooth muscle. The ICCs are located in the submucosa (ICC-SM), on the surface of the circular muscle (ICC-MY), and intramuscularly (ICC-IM). The ICC-SM and ICC-MY produce slow waves, whereas the ICC-IM carry information to the ENS.28 Furthermore, the intestinal glial cells regulate the enteric neurons. Alterations in the function of those cells and their interaction with smooth muscle are responsible for the morphologic and motility changes in diverticular disease (DD).29
In addition, immunity, the microbiota, and the metabolome may play an essential role in DD. Barbara et al.30 studied patients with diverticulosis (asymptomatic cases), patients with SUDD, and healthy controls through colonoscopy with biopsies to immunohistochemically quantify the immunocytes. The patients with diverticula had an increase in macrophages of the colon above 70% (regardless of symptoms). In contrast, Peery et al.31 recently compared 255 patients with colonic diverticula and 364 controls through biopsies of the colonic mucosa, measuring interleukin 6 and 10 levels, as well as tumor necrosis factor, and quantified the immune cells through immunohistochemistry. The researchers found no association between diverticulosis of the colon and inflammation of the mucosa or gastrointestinal symptoms, and they found no inflammation of the mucosa in the patients with SUDD.
- 11.
Evidence regarding the role of a low-fiber diet in the development of diverticulosis is inconsistent, but a high-fiber diet is likely to be beneficial in reducing the complications of diverticular disease
Quality of evidence: C
Level of agreement: in complete agreement 95%, uncertain 5%
Burkitt and Painter were the first to propose that deficient fiber consumption was the cause of diverticular disease (DD), based on the observation that diverticulitis was practically nonexistent in rural Africa.32 The hypothesis was that reduced dietary fiber resulted in smaller intestinal content and a decrease in the intestinal lumen, causing the muscle contraction pressure to be transmitted onto the colon wall instead of the luminal content. The increased pressure on the weakest points of the wall, the sites of blood vessel (vasa recta) penetration, resulted in the formation of diverticula. After that postulation, high-fiber diets were considered beneficial in the prevention of DD, as well as its complications, and the recommendation of their consumption was widely accepted.
Over time, the analysis of the evidence sustaining that recommendation revealed inconsistencies and deficient quality.33 More recent studies have shown that the incidence of diverticulosis in persons that consume high-fiber diets is the same as that in persons whose diets are low in fiber.34 However, the role of fiber in the development of SUDD and in the prevention of complications is more controversial. A cohort study conducted on more than 47,000 inhabitants in England demonstrated that a vegetarian diet and high dietary fiber consumption were associated with a lower risk for hospitalizations and deaths due to DD.35 Crowe et al.36 studied more than 690,000 women and found a significant reduction in the risk for DD with the increase in dietary fiber, as well as a possibly preventive role for the development of SUDD with certain substrates, particularly fruits and cereals. In contrast, Strate et al.,37 in a systematic review from the American Gastroenterological Association, found an uncertain effect of a high-fiber diet versus a low-fiber diet, in relation to the recurrence of DD, complications, need for surgery, and the presence of chronic pain. In a systematic review that included 19 studies, the benefit of dietary or supplementary fiber in patients with SUDD could not be established or ruled out, due to substantial methodological limitations, heterogeneity of the therapeutic regimens employed, and a lack of studies with an ad hoc design.38 Therefore, the benefit of dietary or supplementary fiber in reducing the symptoms or complications of diverticular disease has not yet been clearly established.
- 12.
The ingestion of red meat, nuts, and seeds, as well as the presence of constipation, are not risk factors for diverticulitis
Quality of evidence: B
Level of agreement: in complete agreement 85%, uncertain 10%, in partial disagreement 5%
Several possible mechanisms through which red meat could have an influence on the risk for diverticulitis have been postulated. Red meat promotes low-grade inflammation, contains products such as heme, N-nitroso compounds, and heterocyclic amines that alter the homeostasis of the colonic epithelium and thus can contribute to obesity if consumed in large quantities. Since the publication of the study by Crowe et al.,35 red meat has been considered a risk factor for diverticular disease (DD), but their study never showed that meat consumption increased the risk for DD. Rather, it demonstrated that a vegetarian diet conferred a lower risk for hospitalizations and deaths due to DD. Cao et al.39 showed that excessive and constant consumption of unprocessed red meat increased the risk for diverticulitis in men (RR: 1.51). However, the simple substitution of a ration of unprocessed meat with a ration of poultry or fish reduced the risk, indicating that it is not the red meat itself, but the fact that it is unprocessed, and the quantity and frequency with which it is consumed, that confers the risk.
Nuts include hazelnuts, chestnuts, acorns, almonds, cashews, pecans, and pistachios (among others), whereas seeds include corn kernels, popcorn, sunflower seeds, or pumpkin seeds. Historically, persons with DD have been advised to avoid eating nuts and seeds, based on the argument that they can become lodged inside a diverticulum, obstruct the neck, and erode the mucosa, precipitating inflammation or bleeding. A prospective study conducted on more than 47,000 men with no known diverticular disease showed that the consumption of nuts, corn, and popcorn did not increase the risk for diverticulitis or diverticular bleeding during 18 years of follow-up.40 Those findings make it necessary for us to reconsider the recommendation of avoiding those foods to prevent diverticular complications.41
Diverticulosis has traditionally been considered a direct consequence of constipation. Peery et al.34 conducted a case-control study in which participants underwent colonoscopy and an evaluation of diet, physical activity, and bowel habits, and showed that constipation (self-defined by the participants) was not associated with a higher risk for diverticulosis. A more recent study with a similar design that compared more than 500 cases of diverticulosis with over 1,000 controls, matched by age and sex, demonstrated that neither constipation nor hard stools were associated with a higher risk for diverticulosis, regardless of the location of the diverticula.42 Those findings have been replicated in different populations,43,44 making it necessary to rethink that supposed association.
- 13.
Some studies suggest that the microbiota and its metabolic products may play an important role in both the symptoms and complications of diverticular disease.
Quality of evidence: B
Level of agreement: in complete agreement 76%, in partial agreement 10%, in partial disagreement 14%
The importance of the gut microbiota in the etiology and pathophysiology of a broad spectrum of diseases, especially of the digestive tract, has been recognized in recent years. Despite that fact, only a few studies with respect to microbial composition in diverticular disease (DD) have been conducted. The abovementioned study by Barbara et al.30 demonstrated depletion of the microbiota members that have anti-inflammatory activity, in subjects with DD. There was a decrease in Clostridium cluster IV in the composition of the fecal microbiota and symptomatic patients showed reduced Clostridium cluster IX, Fusobacterium, and Lactobacillaceae. The profiles of the metabolome were related to the inflammatory pathways and neuromotor dysfunction of the intestine and were capable of discriminating the diverticular subgroups of the controls with more than 95% accuracy.
Another observational study whose aim was to compare the gut bacterial diversity in the colonic mucosa between cases and controls, showed that the cases with DD had a larger quantity of Enterobacteriaceae.45 Tursi et al.46 compared the microbiota of 44 women (15 with SUDD, 13 with diverticulosis, and 16 healthy subjects) and found no bacterial overgrowth or differences in the majority of the bacterial groups studied, except in the quantity of Akkermansia muciniphila, which was significantly higher in the groups with diverticula, compared with the healthy controls. A pilot study conducted on 28 patients with SUDD showed that the symptoms were significantly correlated with the characteristics of the fecal microbiota. For example, bloating was associated with a relative abundance of Ruminococcus and inversely related to a relative abundance of Roseburia.47 Those results suggest the possibility of a future finding of a microbiologic or metabolomic profile that characterizes DD. They also strengthen the possible usefulness of targeted therapies for modifying the gut microbiota.
- 14.
Even though the gene responsible for diverticulosis has not been demonstrated, genes involved in the unfavorable progression of complicated acute diverticulitis have been recognized
Quality of evidence: B
Level of agreement: in complete agreement 90%, in partial agreement 5%, in partial disagreement 5%
Several arguments have been written about the role of heredity in the pathogenesis of diverticular disease (DD). Turkish immigrants in Holland have less DD than the native Dutch population. Similarly, Japanese immigrants in Hawaii continue to present with diverticula located predominantly on the right side, in spite of adopting a Western diet. Although much has been said about the familial tendency of DD, there are no large studies that can corroborate it.
Two population studies appear to demonstrate the role of genetics in DD. The first was conducted on twins in Sweden and showed that if one twin had DD, the risk for the other presenting with it was higher in monozygotic twins versus dizygotic twins (OR 7.15 vs. 3.2, respectively).48 The other study was conducted in Denmark, in which incident cases of DD were found in a broad population register, and the authors found that the relative risk for developing DD in siblings of index cases was 2.92 (95% CI: 2.50-3.39), compared with the general population. The relative risk for having DD in a twin whose other twin had it was 14.5 (95% CI: 8.9-23) for monozygotic twins, compared with 5.5 (95% CI: 3.3-8.6) for dizygotic twins.49 Finally, Reichert et al.50 reported that a variant of the COL3A1 (rs3134646) gene that encodes for the formation of collagen in connective tissue was associated with an increased risk for the development of diverticulosis in white men.
- 15.
The pathophysiology of segmental colitis associated with diverticulosis is unknown, but it shares characteristics with inflammatory bowel disease
Quality of evidence: C
Level of agreement: in complete agreement 95%, in partial disagreement 5%
The pathophysiology of segmental colitis associated with diverticulosis (SCAD) is unknown. Nevertheless, there are similarities and differences with inflammatory bowel disease (IBD). SCAD presents predominantly in advanced-age patients, whereas IBD presents in younger subjects. The rectum and proximal colon are spared in SCAD, whereas ulcerative colitis affects the rectum and Crohn's disease affects any segment of the digestive system. The progression of SCAD is benign, with a low rate of surgeries, whereas IBD has a higher complication rate. Differentiating the two pathologies is usually difficult, given that neither histology nor tumor necrosis factor-alpha expression can distinguish them.20
Clinical presentation and diagnosis- 16.
Uncomplicated diverticular disease and complicated diverticular disease are different spectra, not phases, of the same disease. In general, disease progression is favorable, given that the complication rate is low
Quality of evidence: B
Level of agreement: in complete agreement 85%, in partial agreement 10%, uncertain 5%
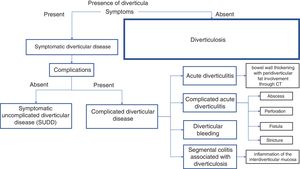
The current status and most accepted classification of diverticular disease (DD) is shown in Figure 1. Most patients with diverticulosis (up to 89%) are asymptomatic. The presence of abdominal pain and bloating, associated with changes in bowel habit with a predominance of diarrhea, is called DD that could be uncomplicated (SUDD) or complicated. Fifteen to 20% of patients with diverticula will present with SUDD and approximately 50% will meet the Rome criteria for IBS, but by definition, they will not have complications. Of all the patients with diverticulosis, 4% will develop complicated DD, which does not require the pre-existence of SUDD to manifest itself, and it is divided into uncomplicated acute diverticulitis, complicated acute diverticulitis (abscesses, perforation, fistula, or stricture), diverticular bleeding, and segmental colitis associated with diverticulosis (SCAD).51 In recent years, that classification of DD has caused confusion because many authors consider that DD is synonymous with SUDD, leading to the belief that DD can be complicated and progress linearly to complicated or uncomplicated acute diverticulitis, bleeding, and SCAD. Other authors have simplified their definitions by not using the classification of complicated DD, simply calling it acute diverticulitis, but that does not clear up the confusion between DD and SUDD.
- 17.
Complicated diverticular disease refers to the presence of variable signs and symptoms, such as abdominal pain, bleeding, or fever, leukocytosis, and radiologic or endoscopic signs, and includes acute diverticulitis, segmental colitis associated with diverticulosis, and diverticular bleeding
Quality of evidence: A
Level of agreement: in complete agreement 100%
Uncomplicated acute diverticulitis results from microperforation of the diverticulum and is clinically characterized by abdominal pain in the left iliac fossa, bloating, fever, and leukocytosis. Computed tomography reveals thickening of the wall of the colon and infiltration of pericolonic fat, without the presence of free air or abscesses.
The etiology of complicated diverticular disease (DD) has not yet been clarified. Complicated acute diverticulitis is diagnosed when free pericolonic air, abscesses (pericolonic or adjacent to the colon), pneumoperitoneum, or fecal peritonitis are detected. Fistula is diagnosed when there are clinical data of acute diverticulitis in the presence of gas in the neighboring organs (generally the bladder or vagina), as well as pneumaturia, fecal material in the urine, or fecal discharge from the vagina.52,53
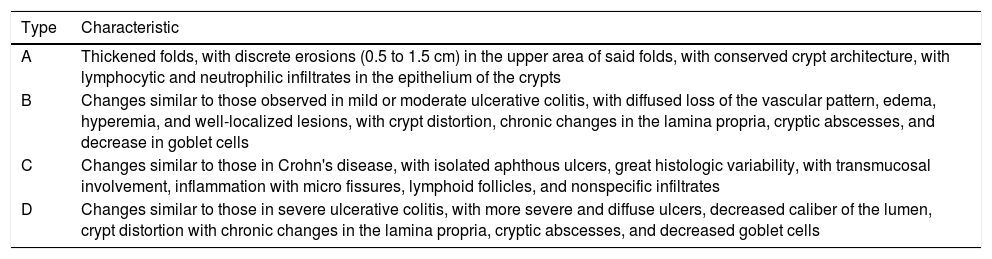
Diverticular bleeding is produced by traumatic rupture of the vasa recta in the lumen of the diverticulum. It is characterized by hematochezia, hemodynamic status alterations (according to the magnitude of the bleeding), and alterations in hemoglobin and hematocrit levels. Emergency colonoscopy can identify the bleeding diverticulum or diverticula, for their treatment.54 Finally, segmental colitis associated with diverticulosis (SCAD) is classified into four subtypes, according to endoscopic and morphologic findings, and are described in Table 2.55
- 18.
Acute diverticulitis usually presents as intense and prolonged abdominal pain that is generally in the lower left quadrant, a change in bowel habit, increased temperature, leukocytosis, and signs of systemic inflammatory response. It can be self-limited or complicated with perforation, abscess, or fistula
Endoscopic classification of segmental colitis associated with diverticulosis (SCAD).
| Type | Characteristic |
|---|---|
| A | Thickened folds, with discrete erosions (0.5 to 1.5 cm) in the upper area of said folds, with conserved crypt architecture, with lymphocytic and neutrophilic infiltrates in the epithelium of the crypts |
| B | Changes similar to those observed in mild or moderate ulcerative colitis, with diffused loss of the vascular pattern, edema, hyperemia, and well-localized lesions, with crypt distortion, chronic changes in the lamina propria, cryptic abscesses, and decrease in goblet cells |
| C | Changes similar to those in Crohn's disease, with isolated aphthous ulcers, great histologic variability, with transmucosal involvement, inflammation with micro fissures, lymphoid follicles, and nonspecific infiltrates |
| D | Changes similar to those in severe ulcerative colitis, with more severe and diffuse ulcers, decreased caliber of the lumen, crypt distortion with chronic changes in the lamina propria, cryptic abscesses, and decreased goblet cells |
Modified from Søreide et al.55
Quality of evidence: A
Level of agreement: in complete agreement 95%, in partial agreement 5%
Acute diverticulitis involves an inflammatory phenomenon of the diverticular sacs. The changes that are observed in the adjacent mesenteric fat are a reflection of the natural attempt to control the process.55 The susceptibility of the diverticula to inflammation is explained by local ischemia, the translocation of pathogens due to the retention of feces, and trauma from fecaliths and microperforations.56 The signs and symptoms of acute diverticulitis are not specific for the disease, but the location of the process, together with the rest of the clinical data, is vital for establishing diagnostic suspicion. Well-contained microperforations are common in the course of the disease and the majority of cases can be treated conservatively.2,57 However, the more severe inflammation of the intestinal wall can cause necrosis, loss of bowel integrity, and perforation. An abscess typically manifests as a collection of loculated fluid that contains air and can be detected in up to 30% of the cases of acute diverticulitis. In some cases, it is distant from the site of the primary inflammation. Fistula occurs when a diverticular abscess ruptures the integrity of the wall of an adjacent anatomic structure, such as the bladder, ureter, other bowel segments, the gallbladder, uterus, Fallopian tubes, the vagina, skin, or perianal region. In descending order of frequency, fistulas due to diverticulitis are usually colovesical, coloenteric, or colouterine.57
- 19.
Diverticular bleeding is usually manifested by nonpainful hematochezia that may or may not produce hemodynamic involvement in the patient
Quality of evidence: A
Level of agreement: in complete agreement 80%, in partial agreement 10%, uncertain 5%, in partial disagreement 5%
Less than 5% of the patients that have diverticulosis present with diverticular bleeding, which is due to an asymmetric rupture of the distended vasa recta in the diverticular dome and is not related to inflammation.56,58 That explains the absence of pain and signs of local inflammation or systemic inflammatory response. Diverticular bleeding stops spontaneously in 70-90% of the cases. Nevertheless, patients that present with severe bleeding and hemodynamic instability, that have persistent bleeding after 24h, that have a decrease in their hemoglobin level of more than 2g/dl, or that need transfusion, should undergo immediate study, as soon as they are stabilized.59
- 20.
Diverticula can be detected through different imaging studies. Multi-slice computed tomography is the method of choice when acute diverticulitis is suspected, because it enables diagnosis, the acquisition of extraintestinal information, and the detection of complications
Quality of evidence: A
Strength of the recommendation: 1, strong, in favor of the intervention
Level of agreement: in complete agreement 100%
The presence of diverticula in the colon can be detected through different imaging methods, such as double-contrast barium enema, abdominal ultrasound, virtual colonography, or multi-slice computed tomography. However, strictly speaking, the diagnosis of diverticular disease (DD) requires a sectioned imaging study that has few associated undesirable effects and is cost-effective for diagnostic purposes. Computed tomography has the advantage of being an operator-independent, reproducible, and widely available method that offers a high degree of diagnostic accuracy, provides extraintestinal images, and makes it possible to classify each case, with the consequent treatment and outcome implications. Therefore, it is considered the standard study in the diagnosis of complicated DD.57,60 Ultrasound imaging is less sensitive for detecting complications, it does not enable the classification of each case, and it is operator-dependent. To be of value in the context of complicated diverticular disease, the operator must be highly qualified in relation to the disorder, which limits its availability.61 Significant differences have been shown in the comparison of the two methods for the diagnosis of the disease: the sensitivity of ultrasound for acute diverticulitis was 61%, compared with the 81% sensitivity of tomography (p < 0.01).62
- 21.
The Hinchey classification and its modifications have been shown to aid in determining the best therapeutic focus and predicting complications in patients that require surgery
Quality of evidence: A
Strength of the recommendation: 1, strong, in favor of the intervention
Level of agreement: in complete agreement 95%, uncertain 5%
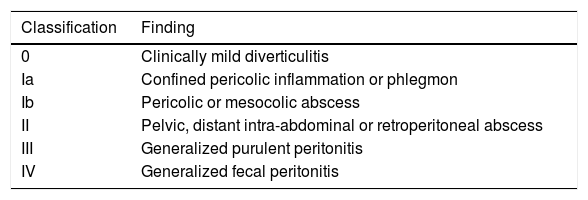
Since the first classification for complicated acute diverticulitis with perforation proposed by Hinchey in 1978, different modifications and new classification systems have been suggested for the purpose of updating the approach to the disease. The wide use of tomography resulted in the beginning of modifications to the Hinchey classification, as well as the appearance of new classifications. Each classification emphasized its strengths and limitations, attempting to guide the physician toward the best form of treatment for each particular case.63 Together, the new descriptions have redefined the use of new treatment strategies, such as tomography-guided percutaneous drainage or laparoscopic drainage.64,65 Currently, the most widely used classification in acute diverticulitis is the Hinchey classification modified by Wasvary, which is slightly more complex than the original description (Table 3). That modified classification enables patients with acute diverticulitis (AD) to be categorized and several studies have clearly demonstrated the impact of that classification for determining the best therapeutic focus and predicting perioperative complications in the patients that require surgery. The physician should be familiar with the Wasvary modification of the Hinchey classification so that patient care and management can be improved, given that each category has a specific therapeutic focus.65,66
- 22.
Colonoscopy is useful for the diagnosis and treatment of diverticular bleeding and for the diagnosis of segmental colitis associated with diverticulosis. The routine use of colonoscopy is not recommended in the evaluation of acute diverticulitis
Hinchey classification modified by Wasvary.
| Classification | Finding |
|---|---|
| 0 | Clinically mild diverticulitis |
| Ia | Confined pericolic inflammation or phlegmon |
| Ib | Pericolic or mesocolic abscess |
| II | Pelvic, distant intra-abdominal or retroperitoneal abscess |
| III | Generalized purulent peritonitis |
| IV | Generalized fecal peritonitis |
Modified from Klarenbeek et al.65
Quality of evidence: A
Strength of the recommendation: 1, strong, in favor of the intervention
Level of agreement: in complete agreement 95%, in partial agreement 5%
Colonoscopy can be used for both the diagnosis and treatment of diverticular bleeding of the colon.67 The identification of recent stigmata enables the bleeding site to be located and a method of endoscopic hemostasis to be applied.68 Colonoscopy is also useful for the differential diagnosis of other forms of chronic colitis (particularly for discerning between Crohn's disease and SCAD) because it enables the direct evaluation of all bowel segments and the performance of biopsies. Its usefulness for confirming the diagnosis of uncomplicated diverticulitis is debatable and its systematic use for that purpose is unjustified, given that the risk for advanced colonic neoplasia in patients with uncomplicated acute diverticulitis is similar to that of the average-risk population.69–71 In contrast, colonoscopy is recommended after the resolution of an episode of complicated diverticulitis or in patients that complain of persistent symptoms. In subjects with a recent episode of complicated acute diverticulitis, cases of colorectal carcinoma and advanced adenomas can be identified through colonoscopy performed 4 to 8 weeks after the episode, albeit that indication is under constant revision.37,71,72
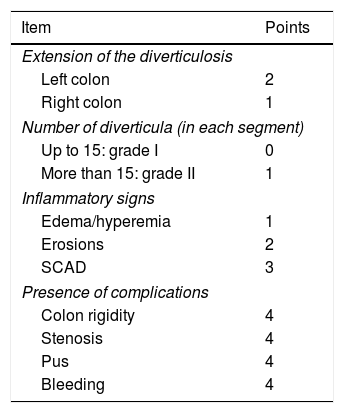
Finally, colonoscopy can evaluate diverticular disease (DD) through the so-called Diverticular Inflammation Complication Assessment (DICA), which has been shown to predict disease progression and select therapeutic strategies.73 The DICA grade is the sum of the scores for diverticulosis extension, the number of diverticula per region, the presence and type of inflammation, and the presence and type of complications (Table 4). Retrospective studies have demonstrated that DICA is a simple, reproducible, easy-to-use method that correlates well with other inflammation indicators. It aids in the selection of patients with DD that truly require treatment and in the selection of the best treatments in terms of cost/effectiveness for the prevention of acute diverticulitis, its recurrence, and the need for future surgery.74–76 However, the DICA endoscopic classification has not been validated in the Mexican population.
- 23.
The C-reactive protein and fecal calprotectin biomarkers can be useful in the diagnosis of complicated acute diverticulitis and segmental colitis associated with diverticulosis, but more studies are needed to demonstrate their sensitivity and specificity
Endoscopic DICA classification.
| Item | Points |
|---|---|
| Extension of the diverticulosis | |
| Left colon | 2 |
| Right colon | 1 |
| Number of diverticula (in each segment) | |
| Up to 15: grade I | 0 |
| More than 15: grade II | 1 |
| Inflammatory signs | |
| Edema/hyperemia | 1 |
| Erosions | 2 |
| SCAD | 3 |
| Presence of complications | |
| Colon rigidity | 4 |
| Stenosis | 4 |
| Pus | 4 |
| Bleeding | 4 |
DICA 1 (from 1 to 3points), DICA 2 (from 4 to 7points), DICA 4 (>7points).
Modified from Tursi et al.75
Quality of evidence: B
Strength of the recommendation: 2, weak, in favor of the intervention
Level of agreement: in complete agreement 95%, uncertain 5%
There has been a growing interest in recent years about the possible role of biologic markers in DD as noninvasive, reliable, and low-cost tools for accurate and early diagnosis of acute diverticulitis. The majority of studies that have evaluated C-reactive protein have shown that it strongly supports the diagnosis of acute diverticulitis at values > 50mg/l and correlates with histologic severity, the risk for perforation, and treatment response.77 Case-control studies have demonstrated that fecal calprotectin determination distinguishes healthy controls and patients with irritable bowel syndrome from those with SUDD, uncomplicated AD, and SCAD.78–80 Despite those promising results, the usefulness of biomarkers for predicting the recurrence of diverticulitis or treatment response has not yet been clearly established.
Treatment- 24.
There is no way to prevent the development of diverticula and asymptomatic patients do not require treatment
Quality of evidence: A
Strength of the recommendation: 1, strong, in favor of the intervention
Level of agreement: in complete agreement 95%, in partial disagreement 5%
The underlying pathologic mechanisms that cause the formation of colonic diverticula have not yet been clarified, hindering the establishment of effective measures to prevent their appearance.2 For a long time, a high-fiber diet was thought to prevent or delay the presence of diverticulosis. However, even though numerous studies have documented greater fiber intake in Western populations, the incidence of diverticular disease has not decreased, and complications have increased.81 Studies carried out in the past decade have shown that a high-fiber diet does not prevent diverticulosis. In fact, it has been associated with a higher prevalence of the pathology, changing the paradigms in relation to that entity.81–83
- 325.
The use of aspirin and nonsteroidal anti-inflammatory agents should be avoided whenever possible in patients with a history of diverticulitis
Quality of evidence: B
Strength of the recommendation: 2, weak, in favor of the intervention
Level of agreement: in complete agreement 90%, in partial agreement 10%
The gastrointestinal toxicity of the nonsteroidal anti-inflammatory drugs (NSAIDs) is well-known, but their harmful effect on the colon has been studied less. Research conducted in recent decades has clarified the relation between NSAID use and complicated diverticular disease, as well as the use of aspirin at low doses.84 At least three systematic reviews and meta-analyses that included more than 70 articles have shown that NSAIDs and aspirin increase the risk for presenting with diverticular bleeding of the colon. One of the reviews found an increase in the risk for presenting with abscesses and diverticular perforation.85–87 Moreover, suspending those drugs has been shown to significantly reduce the risk for recurrence of diverticular bleeding, without increasing the incidence of cerebrovascular accidents.88 Unfortunately, NSAID and aspirin use is very frequent, and sometimes indispensable, in persons in the age groups with the highest prevalence of diverticulosis. The consensus group recommends avoiding those drugs whenever possible.
- 26.
Mesalazine can improve both symptoms and relapses of symptomatic uncomplicated diverticular disease. Its efficacy in the prevention of the first episode of diverticulitis and recurrent diverticulitis has not been clarified
Quality of evidence: B
Strength of the recommendation: 2, weak, in favor of the intervention
Level of agreement: in complete agreement 80%, in partial agreement 10%, uncertain 5%, in complete disagreement 5%
The justification for the use of mesalazine in SUDD and in the prevention of acute and recurrent diverticulitis is based on the role of low-grade inflammation and the inflammation that manifests in those clinical settings. A systematic review that included 6 controlled clinical trials and more than 1,000 patients concluded that mesalazine was effective for alleviating symptoms and preventing the first event of diverticulitis in patients with SUDD.89 Data from uncontrolled studies suggest that mesalazine is beneficial in patients with SUDD, whereas data from randomly controlled trials showed some evidence of symptom improvement, albeit with contrasting results.90 The conclusion reached in another systematic review that included 6 controlled clinical trials and more than 1,900 patients was that mesalazine did not prevent the recurrence of diverticulitis in patients with SUDD.91 In the most recent Cochrane meta-analysis that was made up of 7 controlled clinical trials and more than 1,800 patients, there was no evidence of a favorable effect of mesalazine vs. placebo in the prevention of recurrent diverticulitis (31.3 vs. 29.8%; RR: 0.69, 95% CI: 0.43-1.09).92 The role of mesalazine in the prevention of acute and recurrent diverticulitis cannot be defined with certainty due to the heterogeneity and methodological bias of the studies published at present.
- 27.
Probiotic use in diverticular disease is controversial. Their usefulness for preventing new episodes of diverticulitis has not been demonstrated at present
Quality of evidence: C
Strength of the recommendation: 2, weak, in favor of the intervention
Level of agreement: in complete agreement 90%, in partial agreement 5%, uncertain 5%
Dysbiosis and low-grade inflammation have been identified as pathophysiologic factors in diverticular disease (DD) and have become potential therapeutic targets. Probiotics can improve symptoms by modifying the gut microbiota and are considered promising treatments in the pathology. In a systematic review that included 11 studies and more than 750 patients with DD, no conclusions regarding their efficacy were reached due to the heterogeneity of the designs and the variables of primary interest.93 As in many other digestive disorders, there are ongoing studies being conducted that have good methodological designs, using specific probiotics in different phases of DD, that have shown promising results.94 More research is needed to establish the role of probiotics in diverticular disease.
- 28.
Outpatient treatment of uncomplicated acute diverticulitis is safe and cost-effective in well-selected patients
Quality of evidence: A
Strength of the recommendation: 1, strong, in favor of the intervention
Level of agreement: in complete agreement 100%
Acute diverticulitis has become a frequent motive for emergency room consultations, even though the majority of cases are mild and do not present with complications. Uncomplicated acute diverticulitis was traditionally treated in the hospital, with bowel rest, liquids, and intravenous antibiotics. In recent years, an increasing number of articles have attempted to determine whether care could be carried out in the community, with earlier enteral feeding and oral antibiotics. Several studies have currently shown that outpatient treatment with antibiotics was safe and effective. At least three systematic reviews published in recent years have examined the results of outpatient treatment in uncomplicated acute diverticulitis.95–97 The first, which included 9 studies and more than 400 patients, showed that 97% were successfully treated as outpatients.95 The second systematic review consisted of 11 studies, and the success rate of outpatient treatment varied from 91.5 to 100%. Less than 8% of the patients were readmitted to the hospital and with no more complications, compared with the patients treated in the hospital.96 In the most recent systematic review, which included 10 studies, there were no differences in the medical treatment failure rates (6.5 vs. 4.6%, p = 0.32) or recurrence rates (13.0 vs. 12.1%, p = 0.81) between outpatients and inpatients.97 Important economic savings resulting from outpatient care were reported in two of the three reviews.96,97 Oral diet intolerance and lack of family or social support were commonly used exclusion criteria in those studies, but severe comorbidities were not considered definitive exclusion criteria in all of them.
A more liberal strategy has been evaluated in the last few years in which patients with tomographically demonstrated uncomplicated acute diverticulitis were hospitalized exclusively on clinical grounds and given only supportive measures and no antibiotics (DIABOLO study).98–100 At present, the reported results show that the omission of antibiotics in the treatment of uncomplicated acute diverticulitis was not associated with more complicated diverticulitis, recurrent diverticulitis, or a higher number of resections of the sigmoid colon in the long-term follow-up. Nevertheless, those results must still be confirmed, and that conduct cannot yet be generally accepted. Therefore, the selective use of antibiotics is recommended.100,101
Outpatient treatment of uncomplicated acute diverticulitis is safe, efficacious, and economically efficient when applied to adequately selected patients.
- 29.
The patient with complicated acute diverticulitis should be hospitalized, receive broad-spectrum antibiotics, and be under close surveillance by the surgical team
Quality of evidence: A
Strength of the recommendation: 1, strong, in favor of the intervention
Level of agreement: in complete agreement 95%, in partial agreement 5%
Contrary to what happens in uncomplicated acute diverticulitis, there is general agreement as to beginning a more aggressive treatment in cases of complicated acute diverticulitis and closely watching patients with complications, especially if they present signs of severity, comorbidities, or immunosuppression.102–107 Even though that conduct is currently being carried out, it is clear that management of complicated acute diverticulitis is constantly evolving and more evidence is increasingly accumulating on the potential usefulness of conservative treatment in the initial phases of inflammation. Some experts have proposed ambulatory and conservative treatment for patients with “mildly complicated diverticulitis” (with abscesses < 4cm or pneumoperitoneum < 2cm) but always accompanied by antibiotic administration.108 The consensus group recognizes those advances and trends toward conservative treatment in well-selected cases, but until there is more and better evidence, it recommends a more proactive and vigilant conduct in the cases of complicated diverticulitis.
- 30.
The percutaneous drainage of abscesses in appropriately equipped centers and with trained personnel in interventional radiology can be considered a reasonable treatment option in selected cases
Quality of evidence: A
Strength of the recommendation: 1, strong, in favor of the intervention
Level of agreement: In complete agreement 100%
Initial treatment of complicated diverticulitis with abscesses has gone from being a surgical emergency to being treated conservatively with antibiotics and percutaneous drainage, followed by deferred resection in selected cases. In a systematic review and meta-analysis that included 22 studies and more than 1,000 patients with complicated diverticulitis, percutaneous drainage was performed with a 49% success rate in abscesses smaller than 3cm, albeit with a high probability of recurrence during follow-up.109 Another more recent systematic review that included 42 observational studies and more than 8,700 patients found that only 2.5% of the patients that underwent percutaneous drainage experienced complications related to the drainage and 15.5% needed drain adjustment or replacement.110 Even though 25% of the patients treated non-surgically had recurrence in the long-term follow-up, drainage was associated with a lower frequency of recurrence (15.9 vs. 22.2%).110 Good drainage results primarily depend on adequate patient selection, as well as the experience of the center.
- 31.
Certain long-term treatment regimens that include rifaximin-alpha and mesalazine reduce the possibility of new symptoms of acute diverticulitis, compared with mesalazine, alone
Quality of evidence: B
Strength of the recommendation: 2, weak, in favor of the intervention
Level of agreement: in complete agreement 80%, in partial agreement 10%, uncertain 10%
Treatment to prevent new symptomatic episodes of acute diverticulitis has not been clearly defined.111 In a comparative study, rifaximin plus mesalazine was shown to be more efficacious than rifaximin, alone, in the treatment of recurrent and complicated diverticulitis of the colon. Tursi et al.112 studied a series of 218 consecutive cases with diverticulitis and divided them into two groups. Group A was made up of 109 patients treated with 400mg of rifaximin bid plus 800mg of mesalazine tid for 7 days, followed by 400mg of rifaximin bid and 800mg of mesalazine bid for 7 days each month. Group B was composed of 109 patients treated only with 400mg of rifaximin bid for 7 days, followed by 400mg of rifaximin bid for 7 days each month. Colonoscopy was performed at 3, 6, and 12 months of treatment. Symptoms and bowel habit improved significantly in group A, compared with group B, from the third month of treatment. Recurrence of symptomatic diverticulitis presented in 3 group A patients, compared with 13 group B patients, during follow-up (p < 0.005). Their study clearly showed that rifaximin plus mesalazine was more effective than rifaximin, alone, for resolving the symptoms of diverticulitis and preventing its recurrence.
But rifaximin without mesalazine has also been shown to be effective in treating the symptoms of SUDD. Several open and retrospective studies have shown the effectiveness of the antibiotic, alone, or supplemented with fiber, for symptom control in SUDD. In a meta-analysis that included 4 studies and 1,660 patients, the administration of rifaximin at a dose of 400mg twice daily in cycles of 7 days per month for one year, together with fiber, was more effective than fiber, alone, for symptom control with a number needed to treat of 3.113 More recently, Moniuszko et al.114 demonstrated that the administration of rifaximin, in cycles of 7 days per month was highly effective in SUDD symptom control from the first month, with a maximum effect at 3 months.
- 32.
The majority of cases of diverticular bleeding are self-limited and only require supportive management. Early colonoscopy should be performed on patients with persistent bleeding that are hemodynamically stable
Quality of evidence: A
Strength of the recommendation: 1, strong, in favor of the intervention
Level of agreement: in complete agreement 80%, in partial agreement 15%, uncertain 5%
Diverticular bleeding of the colon is the most common cause of distal gastrointestinal bleeding and, as mentioned before, colonoscopy can be used for both its diagnosis and treatment.115 Between 3 and 15% of the patients with diverticulosis will present with diverticular bleeding at some point in their lives.116 In a systematic review that included 11 uncontrolled studies, 80% of the cases of bleeding stopped spontaneously and the definitive diagnosis of diverticular bleeding was always made through urgent colonoscopy.117 However, recurrence was observed in 13-48% of the cases.116 Colonoscopy is recommended within the first 24h, once the patient is stable and has had bowel cleansing, but it should be repeated in patients with evidence of recurrent bleeding.118
- 33.
Some of the most widely used methods for the endoscopic hemostasis of diverticular bleeding are adrenaline injection, band application, and endoclip placement.
Quality of evidence: A
Strength of the recommendation: 1, strong, in favor of the intervention
Level of agreement: in complete agreement 90%, in partial agreement 5%, uncertain 5%
Different endoscopic therapy modalities have been used to control diverticular bleeding and there are numerous reports on the potential benefit of adrenaline injection, electrocoagulation, band application, and endoclip placement, among others. Nevertheless, given the small number of comparative studies, the optimum technique for acute or long-term control of bleeding cannot be determined by the presently available evidence. Most of the reports are based on the preferences of the endoscopist or on their availability.119 A meta-analysis of 15 studies and 1,156 patients that compared the efficacy of the application of clips versus injection with or without thermocoagulation, showed that the successful application of hemoclips was superior to injection, alone, but comparable to thermocoagulation in achieving definitive hemostasis. No differences were found with respect to mortality.120 A more recent systematic review and meta-analysis that included 16 studies and more than 380 patients with diverticular bleeding showed that coagulation and the application of clips or ligature were similar in terms of bleeding control and the prevention of early recurrence. However, the need for arterial embolization or surgery was significantly lower with ligature, compared with the other methods.121 One of the few prospective studies that have evaluated the long-term efficacy of two endoscopic methods of hemostasis showed that the probability of bleeding recurrence at one year was 11.5% with ligature, compared with 37% of the patients treated with hemoclip application (p = 0.018). There was no difference in the complication rates or need for surgery.122 New devices have been tested in that clinical setting, but there is still a lack of comparative studies and long-term follow-up.123,124 Until there is more and better evidence coming from comparative studies, endoscopic hemostatic method selection will continue to be based on the preference and experience of the endoscopist, as well as on availability.
- 34.
The use of barium enemas to control acute diverticular bleeding has been reported anecdotally and with insufficient evidence and therefore cannot be recommended
Quality of evidence: D
Strength of the recommendation: 2, weak, in favor of the intervention
Level of agreement: in complete agreement 95%, uncertain 5%
The application of barium enemas has been used in patients in whom it was not possible to identify the precise site of diverticular bleeding through endoscopy. The mechanism of action has not been clarified, but impaction of the diverticular sac has been proposed to produce a hemostatic effect directly on the ulcerations or erosions of the dome by plugging up the bleeding vessel. The measure has been reported with success in case series and anecdotally.125–127 Two comparative studies have evaluated the use of barium enemas in the prevention of recurrent bleeding with apparent benefit, although the quality of the studies is questionable.128,129 The accumulated evidence at present is insufficient for recommending barium enema use. Perforation, viewing difficulty in a new endoscopy, or an event conditioning potential surgery are possible undesirable effects of barium enema application that should be taken into account.127
- 35.
Massive diverticular bleeding is that which causes severe hemodynamic compromise and cannot be treated endoscopically. Those cases can be treated through angiography or emergency surgery, if the former is not available or has not been effective
Quality of evidence: C
Strength of the recommendation: 1, strong, in favor of the intervention
Level of agreement: in complete agreement 95%, in partial agreement 5%
There is no question that therapeutic colonoscopy is the standard treatment for the management of diverticular bleeding in the colon. However, in elderly patients with comorbidities and polypharmacy, bleeding can be severe and often recurrent, and therefore endoscopic methods may not be sufficient for controlling it. Information on the risk factors associated with massive bleeding and its optimum treatment has come out of retrospective studies and has not been completely clarified. The use of nonsteroidal anti-inflammatory drugs, anticoagulants, old age, cerebrovascular disease, chronic nephropathies, bilateral diverticulosis, and right-sided diverticulosis are some of the associated factors.130–134 In those cases, angiography has become an important tool for locating the bleeding site and treating it. It can detect bleeding with a flow of 0.3ml/min and has a sensitivity of 50 to 86% and a specificity of 92 to 95%. Some studies have even shown a sensitivity of 100% and a specificity of 96%.135 Very few centers in Mexico are equipped with an angiography room and the trained personnel necessary for performing it, limiting its widespread use as a viable alternative for controlling those emergencies. Therefore, emergency resection surgery should be considered the only alternative to colonoscopy for treating massive bleeding.
- 36.
The indication for surgical treatment in patients with a history of recurrent uncomplicated diverticulitis should include the consideration of previous symptomatic episodes, absence from work due to future episodes, the possibility of access to specialized healthcare services, the patient's physical status and comorbidities, immunosuppression, and even patient preference, among others
Quality of evidence: B
Strength of the recommendation: 1, strong, in favor of the intervention
Level of agreement: in complete agreement 100%
Based on the results of large retrospective case series, after an event of uncomplicated diverticulitis, only one-third of patients are estimated to have a relapse, and of those patients, only one-third will have a new recurrence. Today we know that the real recurrence rate is from 13 to 23% and that the possibility of a subsequent severe episode or the need for emergency surgery is low (6%).135 Thus, the indication for elective surgery after two uncomplicated symptomatic episodes has been eliminated. Elective surgical treatment is currently reserved for patients with symptom persistence whose quality of life or work absenteeism is importantly impacted, and patient opinion is taken into consideration when making the treatment decision. Nevertheless, special care should be taken with immunosuppressed patients, transplanted patients, or those undergoing transplantation protocol that require life-long treatment, and in whom a new episode of diverticulitis could be very severe. At present, surgical treatment is considered a priority after the first event of diverticulitis in that special group of patients.136,137
- 37.
In patients with complicated acute diverticulitis, morbidity and mortality are greater in the patients that undergo emergency surgery, compared with those that can be operated on once the symptomatic episode has been resolved
Quality of evidence: A
Strength of the recommendation: 1, strong, in favor of the intervention
Level of agreement: in complete agreement 100%
The scenario of the patient that requires emergency surgery is similar to that of a patient with complicated disease. For many years, the procedure of choice in those cases was the Hartmann procedure (sigmoidectomy with end colostomy). However, today we know that sigmoidectomy with primary anastomosis and protection ileostomy can be carried out in selected patients. The severe reconnection complications in the Hartmann procedure are more frequent than those related to ileostomy closure (20 vs. 0%).136,138 The factors associated with the performance of a terminal stoma in emergency surgery are: body mass index (BMI) > 30; peritoneal Mannheim score > 10, immunosuppressed patients, and Hinchey III and IV classifications.139,140 Protection stomas are rarely used in a patient undergoing an elective procedure, and if required, the patient can receive nutritional support and the necessary measures for improving his/her conditions prior to surgery and preventing complications. The goal is to resolve the acute symptoms with a minimum of treatment, without putting the patient at risk, and to offer an elective procedure with fewer complications.
- 38.
The laparoscopic approach is a viable option for diverticular disease, as long as it is performed by a surgeon with experience in the procedure
Quality of evidence: B
Strength of the recommendation: 1, strong, in favor of the intervention
Level of agreement: in complete agreement 95%, uncertain 5%
The laparoscopic approach in diverticular disease (DD), the same as resection of the colon due to other causes, offers better short-term results than open surgery. Among its advantages are: less bleeding, less pain, shorter hospital stay, a quicker return of bowel transit, a lower complication rate, and better patient quality of life. Nevertheless, laparoscopic surgery for DD is a complicated and difficult procedure that has even been compared to oncologic resection, due to the inflammatory process in the colon. The recommendation is for those procedures to be performed at high-volume centers, given that the learning curve is calculated at 40 to 60 cases.141,142
- 39.
Age is a factor that does not need to be considered in the decision to perform surgical treatment.
Quality of evidence: A
Strength of the recommendation: 1, strong, in favor of the intervention
Level of agreement: in complete agreement 90%, in partial agreement 5%, uncertain 5%
It was historically thought that if a young patient presented with symptoms of diverticulitis, even uncomplicated disease, it would be aggressive, with a higher number of recurrences and a greater need for emergency surgery. Thus, for a long time, the recommendation in patients under 50 years of age with diverticulitis was for them to undergo sigmoidectomy. However, that conduct has been shown to be guided by poor quality evidence.143,144 It is now known that young patients with diverticulitis have a recurrence percentage similar to that of elderly patients (27%) and the possibility of requiring an emergency procedure is also similar (7.5%). Therefore, the term “diverticulitis in young patients” is no longer used as a synonym for more aggressive disease, nor is age considered a factor for offering surgical treatment.135
- 40.
Emergency surgical treatment is indicated in patients with purulent or fecal peritonitis and includes resection with the Hartmann procedure or resection with colon-to-rectum anastomosis, with or without a protection stoma
Quality of evidence: A
Strength of the recommendation: 1, strong, in favor of the intervention
Level of agreement: in complete agreement 95%, in partial agreement 5%
It was customary for patients with symptoms of complicated diverticulitis to undergo three surgeries: first, a derivative stoma, second, resection of the affected segment, and third, bowel transit reconnection. That strategy has now been abandoned and the most frequent treatment is a sigmoidectomy with the Hartmann procedure. An evidence-based proposal as an alternative to the Hartmann procedure is sigmoidectomy with a colon-to-rectum anastomosis and protection ileostomy. However, given that there are cases in which said procedure is difficult, the following predictive factors for an end colostomy have been established: hemodynamic instability, BMI > 30, Mannheim peritonitis index > 10, immunosuppressed patients, or a Hinchey III or IV classification.145,146 When a procedure with a stoma is performed, the morbidity associated with the reconnection of bowel transit should always be considered. In the comparison of major complications in a Hartmann reconnection versus ileostomy closure, the difference was 20 vs. 0%, respectively. In addition, patients that undergo a Hartmann procedure more frequently prefer not to be reconnected (57 vs. 90% of patients with reconnection).140
- 41.
Laparoscopic lavage for Hinchey III complicated diverticulitis is not recommended, except as part of a research study authorized by an ethics committee
Quality of evidence: A
Strength of the recommendation: 1, strong, in favor of the intervention
Level of agreement: in complete agreement 90%, in partial agreement 5%, uncertain 5%
The DILALA study is the first to evaluate laparoscopic lavage for diverticular disease that has an adequate methodology. It compared 36 patients that underwent laparoscopic lavage with 39 that underwent the Hartmann procedure, and laparoscopic lavage was shown to be feasible and safe in the short term.147 The SCANDIV study followed, and demonstrated that laparoscopic lavage did not reduce severe postoperative complications and reported worse results in all the secondary aims of the study.148 More recently, the LOLA arm of the LADIES study was halted by the ethics committee due to the high incidence of complications in the laparoscopic lavage group.149 Therefore, we consider that laparoscopic lavage for Hinchey III complicated diverticulitis should not be performed as widely and frequently as has occurred in Mexico. It is the opinion of the present consensus group that it should only be carried out within research protocol, with the approval of an ethics committee. We believe it should be pointed out that even though the nature of the present consensus does not have the obligatory characteristics of clinical guidelines, there is sufficient evidence to call for the prudent and protocolized use of the procedure.
- 42.
Laparoscopic lavage for Hinchey II complicated diverticulitis is an option for abscess drainage in patients that do not respond to medical treatment or interventional treatment, or when the latter is not available
Quality of evidence: B
Strength of the recommendation: 2, weak, in favor of the intervention
Level of agreement: in complete agreement 95%, uncertain 5%
Different case series have shown laparoscopic lavage as treatment for diverticular disease (DD) to be a safe and reproducible procedure. Nevertheless, the majority of the time it was not utilized in cases of generalized purulent peritonitis, but rather in cases of Hinchey II diverticulitis with localized abscesses, in which it is less probable to find a free undetected perforation, which is the reported cause of increased mortality. Thus, laparoscopic lavage can be considered an alternative for the treatment of abscesses due to DD when the resources necessary for performing a tomography-guided puncture or an ultrasound-guided puncture are nonexistent.150,151
- 43.
Standard resection of the sigmoid colon involves a colon-to-rectum anastomosis, ideally at the height of the promontorium or up to the healthy tissue. The proximal edge does not include the resection of all the diverticula, but it does include the resection of the sigmoid colon in its entirety
Quality of evidence: A
Strength of the recommendation: 1, strong, in favor of the intervention
Level of agreement: in complete agreement 95%, in partial agreement 5%
The extension of resection should include the complete resection of the sigmoid colon and the anastomosis should be performed in healthy tissue, which is why a colon-to-rectum anastomosis is recommended. The upper rectum sometimes presents with inflammation and a lower anastomosis is required. The proximal margin should include all the tissue in which there is inflammation, although not necessarily all the diverticula. Care must always be taken that no diverticulum is left in the anastomosis. The possibility of recurrence of diverticulitis in the proximal segment is low.152
ConclusionDiverticular disease of the colon is a common and increasingly frequent condition whose clinical presentation ranges from the presence of symptoms to the development of different complications. We consider it very important to be familiar with the changes and advances made in recent years, with respect to the knowledge of this disease. We present herein a consensus review that reaffirms, renews, and complements the information published in the 2008 guidelines of the Asociación Mexicana de Gastroenterología on diverticular disease.
Financial disclosureNo financial support was received in relation to the present manuscript.
Conflict of interestDr. Jaime de Jesús Aguilera Carrera: is a Speaker for Takeda.
Dr. Carlos Agustín Arnaud Carreño: is a Speaker for Menarini.
Dr. Ramón I. Carmona Sánchez: is an Advisory Board Member of Asofarma and a Speaker for Mayoly-Spindler, Asofarma, and Chinoin.
Dr. Enrique Coss Adame: is an Advisory Board Member of Takeda Pharmaceuticals, Asofarma, Grünenthal, and Ferrer and is a Speaker for Alfasigma, Takeda, Asofarma, Grünenthal, and Ferrer.
Dr. Francisco Esquivel Ayanegui: is a Speaker for Menarini.
Dr. Aurelio López Colombo: is a Speaker for Menarini and Takeda.
Dr. Alejandra Noble Lugo: is a Speaker for Takeda, Menarini, Alfa Wassermann, Asofarma, and Astra-Zeneca.
Dr. José María Remes-Troche: is an Advisory Board Member of Takeda Pharmaceuticals, Menarini, and Asofarma. He received research funds from Sanfer and is a Speaker for Takeda, Asofarma, Alfa-Wassermann, Menarini, Carnot, Sanofi, and Astra-Zeneca.
Dr. Ricardo Humbero Raña Garibay: is a Speaker for Menarini, Alfa Sigma, and Astra-Zeneca.
Dr. Noel Salgado Nesme: is a Speaker for Johnson & Johnson.
Dr. Manuel Vallejo Soto: is a Speaker for Menarini.
Dr. Luis Alonso Sánchez, Dr. Luis Charúa Gunidic, Dr. David R. Espinosa Medina, Dr. Janett Sofía Jacobo Karam, Dr. Fernando Rojas Mendoza, Dr. Luis Ignacio Muñoz Torres, Dr. Federico Roesch Dietlen, Dr. Miguel Stoopen Rometti, Dr. Jorge Suazo Barahona, Dr. Elba Torres Flores, and Dr. Omar Vergara-Fernández declare that they have no conflict of interest.
Please cite this article as: Raña-Garibay R, Salgado-Nesme N, Carmona-Sánchez R, Remes-Troche JM, Aguilera-Carrera J, Alonso-Sánchez L, et al. Consenso mexicano sobre el diagnóstico y tratamiento de la enfermedad diverticular del colon. Revista de Gastroenterología de México. 2019;84:220–240.