Cholangiocarcinoma accounts for 3% of gastrointestinal tumors and is the second most frequent hepatic neoplasia after hepatocellular carcinoma. The primary aim was to evaluate the median disease-free period and survival in patients with cholangiocarcinoma diagnosis through the comparison of R0 and R1 resection margins.
Materials and methodsA retrospective analysis was conducted on 36 patients that underwent some type of surgical resection due to cholangiocarcinoma diagnosis, within the time frame of 2000-2017, at a center specializing in hepatopancreatobiliary surgery. Population, preoperative, and oncologic variables were included. The IBM Statistical Package for the Social Sciences for Mac, version 16.0, software (IBM SPSS Inc., Chicago, IL, USA) was employed.
ResultsThirty-one patients underwent hepatectomy, the Whipple procedure, or bypass surgery, depending on tumor location. The statistical significance of survival between patients with positive margins and those with negative margins was evaluated through the Mann-Whitney U test, with a P<.05 as the reference value. No statistically significant difference was found. The overall morbidity rate was 58.06% (n=18) and the mortality rate was 12.9% (n=4).
ConclusionsNo statistically significant difference in relation to the incidence of disease recurrence or general survival resulted from the comparison of microscopically positive surgical margins (R1) and negative surgical margins (R0). There was also no correlation between preoperative CA 19-9 levels and disease prognosis.
El colangiocarcinoma representa el 3% de los tumores gastrointestinales y es la segunda neoplasia hepática más frecuente después del carcinoma hepatocelular. El principal objetivo es evaluar la mediana del periodo libre de enfermedad y sobrevida en los pacientes con este diagnóstico al comparar aquellos que tuvieron una resección R0 frente a los que tuvieron márgenes R1.
Material y métodosAnálisis retrospectivo de 36 pacientes sometidos a algún tipo de resección quirúrgica por diagnóstico de colangiocarcinoma en un centro especializado en cirugía hepatopancreatobiliar entre los años 2000-2017. Se incluyeron variables poblacionales, variables prequirúrgicas y variables oncológicas. El software empleado fue IBM Statistical Package for the Social Sciences versión 16.0 para Apple (IBM SPSS Inc., Chicago, IL, EE. UU.).
ResultadosA 31 pacientes se les realizó una hepatectomía, procedimiento de Whipple o derivación biliodigestiva, dependiendo de la localización del tumor. El test de la U de Mann-Whitney fue utilizado para evaluar la significación estadística entre la sobrevida de los pacientes con márgenes positivos y negativos, considerándose un valor de referencia de p<0.05; sin embargo, no se encontró diferencia. La morbilidad global fue de 58.06% (18), mientras que la mortalidad fue del 12.9% (4).
ConclusionesNo existe una diferencia estadísticamente significativa dejando márgenes quirúrgicos microscópicamente positivos (R1) en comparación con márgenes negativos (R0) en la incidencia de recurrencia de la enfermedad ni en la sobrevida general. Tampoco se encontró correlación entre los niveles prequirúrgicos de CA 19.9 y el pronóstico de la enfermedad.
Cholangiocarcinoma is a heterogeneous neoplasia that originates in the epithelium of the intrahepatic or extrahepatic bile ducts. It accounts for 3% of gastrointestinal tumors and is the second most common primary malignant tumor of the liver after hepatocellular carcinoma. It tends to present in the seventh decade of life and shows a preference for the male sex.1
Cholangiocarcinoma has 2 distinct forms of presentation: intrahepatic and extrahepatic. The latter, in turn, is subdivided into distal or proximal (also called perihilar) and their clinical relevance lies in the different characteristics that determine their stage and resectability.2 Fifty percent of all the cases of cholangiocarcinoma are perihilar, 40% are distal, and the remaining 10% are intrahepatic. The majority of cholangiocarcinomas appear as a de novo mutation but parasitic infections, primary sclerosing cholangitis, hepatolithiasis, liver cirrhosis, hepatitis C virus and hepatitis B virus infections, obesity, tobacco, and alcohol have been described as predisposing factors.3
Intrahepatic cholangiocarcinoma is defined as that which is located proximal to the secondary bile ducts. The proximal and distal portions are determined by the flow of bile. Perihilar lesions are found between the secondary ducts and the insertion of the cystic duct into the common bile duct, whereas the distal lesions are between the common bile duct and the ampulla of Vater.4
Intrahepatic cholangiocarcinoma, the least frequent subtype, has a 5-year survival rate of 35%.5 Symptoms usually present late, thus diagnosis is made at advanced disease stages.
Hilar cholangiocarcinomas are known as Klatskin tumors, in honor of the pathologist who first described them in 1965.6 Their management is quite complex, due to their location in the biliary tree, as well as to their advanced stage at the time of disease presentation.
Different factors have been associated with hilar cholangiocarcinoma. Those that stand out are primary sclerosing cholangitis, hepatolithiasis, and parasitic liver disease. More than 80% of proximal bile duct obstructions are secondary to Klatskin tumors.7
Surgical resection of cholangiocarcinoma is the only measure with intention-to-cure. However, only 10 to 40% of the patients are amenable to surgery at the time of diagnosis.1 The morbidity of cholangiocarcinoma has increased in recent years, its prognosis is poor, and survival tends to be under one year.8 Despite the fact that each subtype of cholangiocarcinoma has a different biology and behavior, our goal was to determine the impact of surgical treatment on the overall survival of patients with cholangiocarcinoma, as was done by Primrose et al. (the BILCAP study), in which they included all the resected cholangiocarcinomas, regardless of the initial location of the tumor. Those patients were randomly assigned to 2 groups: one received capecitabine as adjuvant therapy and the other was only under surveillance. Follow-up for the two groups was 36 months and the study conclusion favored the use of the chemotherapeutic agent.9
The aim of the present study was to ascertain the impact of a positive surgical margin on the general survival and recurrence rates of the disease.
Materials and methodsThe medical records of the patients diagnosed with cholangiocarcinoma, within the time frame of 1987 to 2017, were requested. A total of 452 patients were found that coincided with the search criteria, but only 36 patients were operated on. The remaining patients presented with clinical stage IV disease.
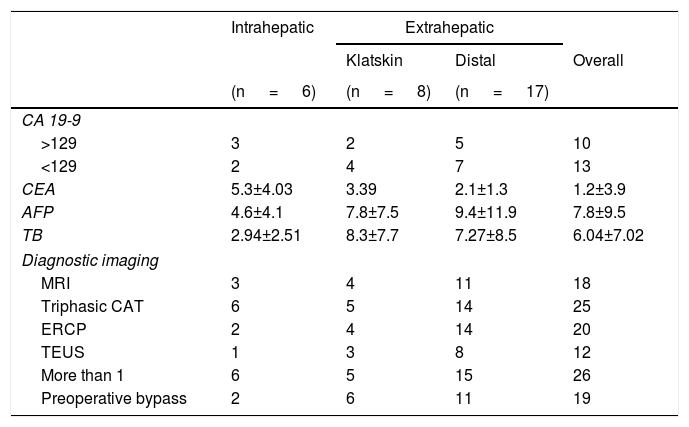
All the patients had a preoperative histopathologic report consistent with cholangiocarcinoma and all had at least one imaging study indicating the diagnosis. The different imaging studies utilized were triphasic computed tomography of the liver, cholangioresonance, transendoscopic ultrasound, and/or endoscopic retrograde cholangiopancreatography with biopsy. The population variables analyzed were: age, sex, body mass index, and comorbidities (Table 1). The preoperative variables were: tumor location, tumor marker measurement, liver function tests, and if warranted, bypass prior to the procedure. The oncologic variables were: histologic type, differentiation grade, number of positive lymph nodes, lymphovascular invasion, perineural invasion, surgical margins, adjuvant therapy, recurrence, disease-free period, and survival.
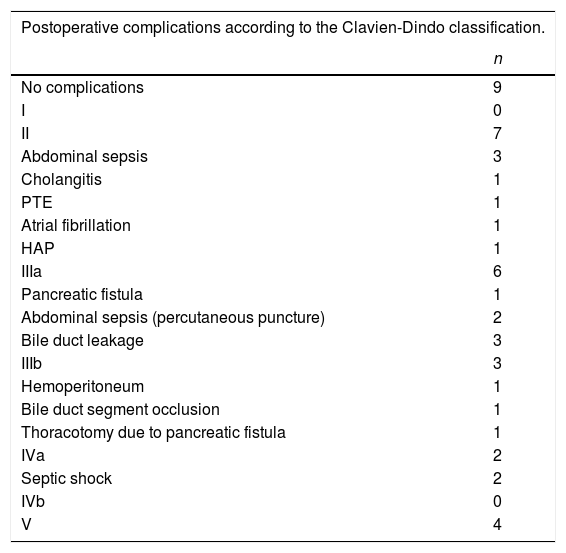
A total of 16 hepatectomies, 10 Whipple procedures, and 5 bypass surgeries were performed. All were open procedures and had oncologic and surgical follow-up. Any adverse event that occurred within the first 30 days after the procedure was considered a postoperative complication and was classified based on the Clavien-Dindo scale.10
Statistical analysisAll the statistical tests were 2-tailed and statistical significance was set at a p value less than 0.05. The continuous variables, expressed as median and standard deviation, were analyzed using the chi-square test. Overall survival was evaluated through the Kaplan-Meier method. The data analysis was carried out using the IBM Statistical Package for the Social Sciences, version 16.0, for Mac (IBM SPSS Inc., Chicago, IL, USA) software.
Ethical considerationsThe present study was approved by the intrahospital ethics committee for its execution and diffusion, registered as SCI-2647-18-18-1. The authors declare that they have followed the protocols of their work center with respect to the publication of patient data. Informed consent was not required for the publication of the present article because it contains no personal data that could identify the patients.
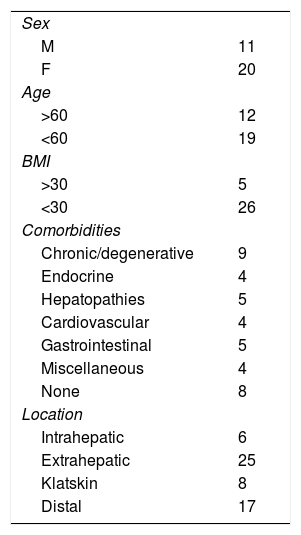
ResultsSurgical resection could only be performed in 31 of the 36 patients included in the study. Twenty-five of the patients presented with extrahepatic cholangiocarcinoma, 17 patients had the distal location, and 8 had Klatskin tumors. Only 6 patients had intrahepatic disease. The remaining 5 patients were excluded due to tumor unresectability (2 extrahepatic tumors, 2 Klatskin tumors, and 1 intrahepatic tumor). The median age of the patients was 56.87 ± 13.38 and 61.2% of the population was over 60 years of age.
The most frequent comorbidity was the chronic/degenerative type and presented in 29% (n=9) of the patients. Only 16% had a history of some type of hepatopathy and none had a history of hepatolithiasis. Eight individuals (26%) were previously healthy. Table 1 shows the population characteristics.
A total of 83.8% (n=26) of the patients had more than one preoperative imaging study.
Ten of the study subjects had a CA 19-9 level greater than 129 IU/ml and another 14 had levels under that value. Tumor marker measurement was not carried out prior to surgery in only 3 patients. Of the patients that presented with a high tumor marker value, 4 had disease recurrence. Of the patients that did not reach the 129 IU/ml value, 5 had disease recurrence, with a p=0.94 (Table 2).
Preoperative variables.
| Intrahepatic | Extrahepatic | |||
|---|---|---|---|---|
| Klatskin | Distal | Overall | ||
| (n = 6) | (n = 8) | (n = 17) | ||
| CA 19-9 | ||||
| >129 | 3 | 2 | 5 | 10 |
| <129 | 2 | 4 | 7 | 13 |
| CEA | 5.3±4.03 | 3.39 | 2.1±1.3 | 1.2±3.9 |
| AFP | 4.6±4.1 | 7.8±7.5 | 9.4±11.9 | 7.8±9.5 |
| TB | 2.94±2.51 | 8.3±7.7 | 7.27±8.5 | 6.04±7.02 |
| Diagnostic imaging | ||||
| MRI | 3 | 4 | 11 | 18 |
| Triphasic CAT | 6 | 5 | 14 | 25 |
| ERCP | 2 | 4 | 14 | 20 |
| TEUS | 1 | 3 | 8 | 12 |
| More than 1 | 6 | 5 | 15 | 26 |
| Preoperative bypass | 2 | 6 | 11 | 19 |
AFP: alpha-fetoprotein; CAT: computed axial tomography; CEA: carcinoembryonic antigen; ERCP: endoscopic retrograde cholangiopancreatography; MRI: magnetic resonance imaging; TB: total bilirubin; TEUS: transendoscopic ultrasound.
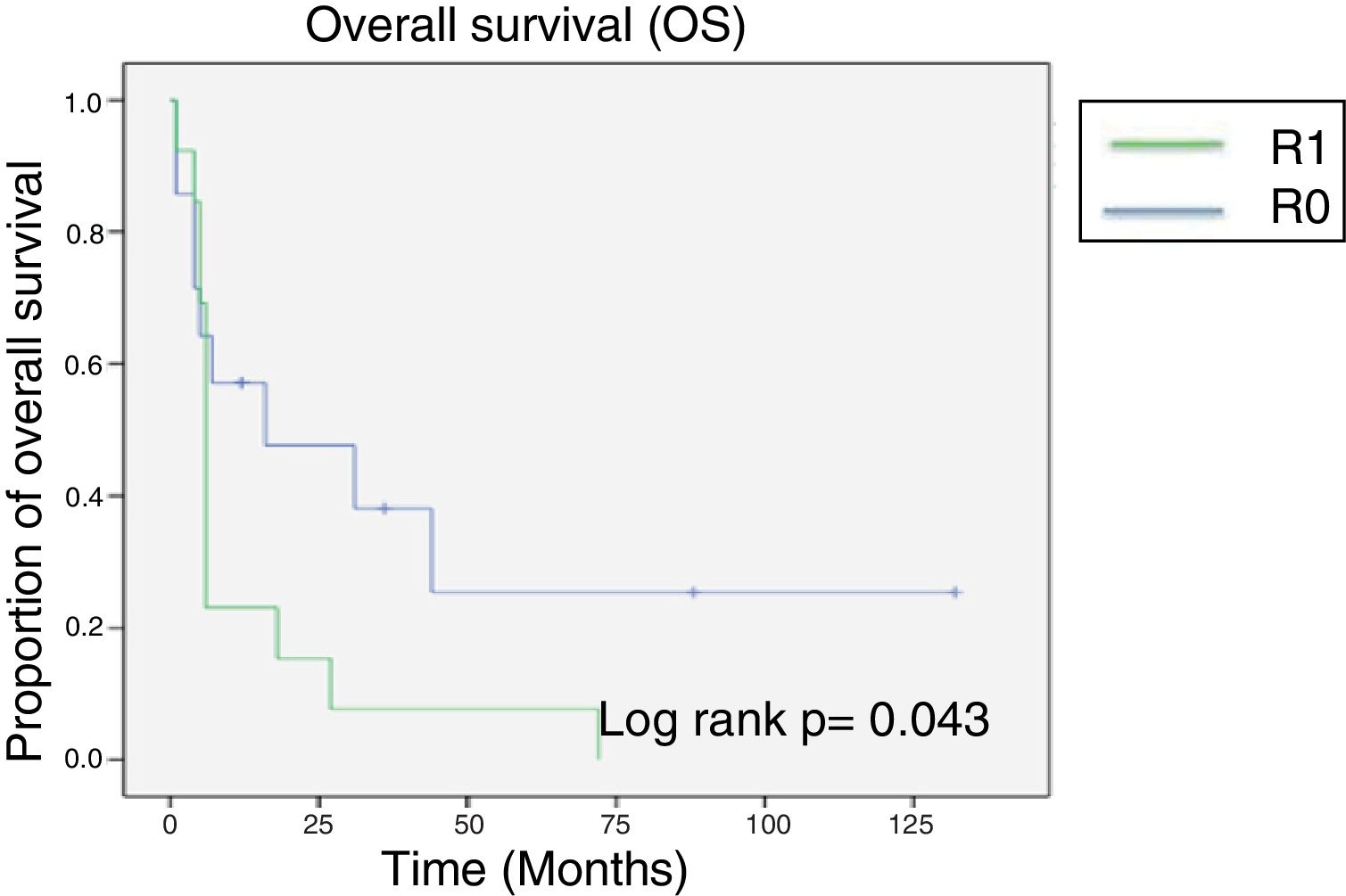
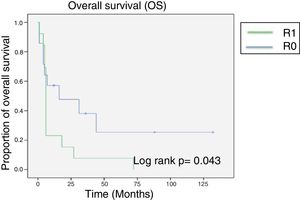
After a mean follow-up of 14 months (minimum of 4 months and maximum of 132 months) the median overall survival attained was 24 months. At the end of the oncologic follow-up, only 6 patients were living, all of whom initially had negative margins (fig. 1).
The statistical significance of survival between the patients with a positive surgical margin and those with a negative surgical margin was calculated using the Mann-Whitney U test, resulting in a p > 0.05 and a U of 40.5. However, median survival in the group with R0 resection was 45 months, which was noticeably higher than the 12 months of survival of the R1 patients.
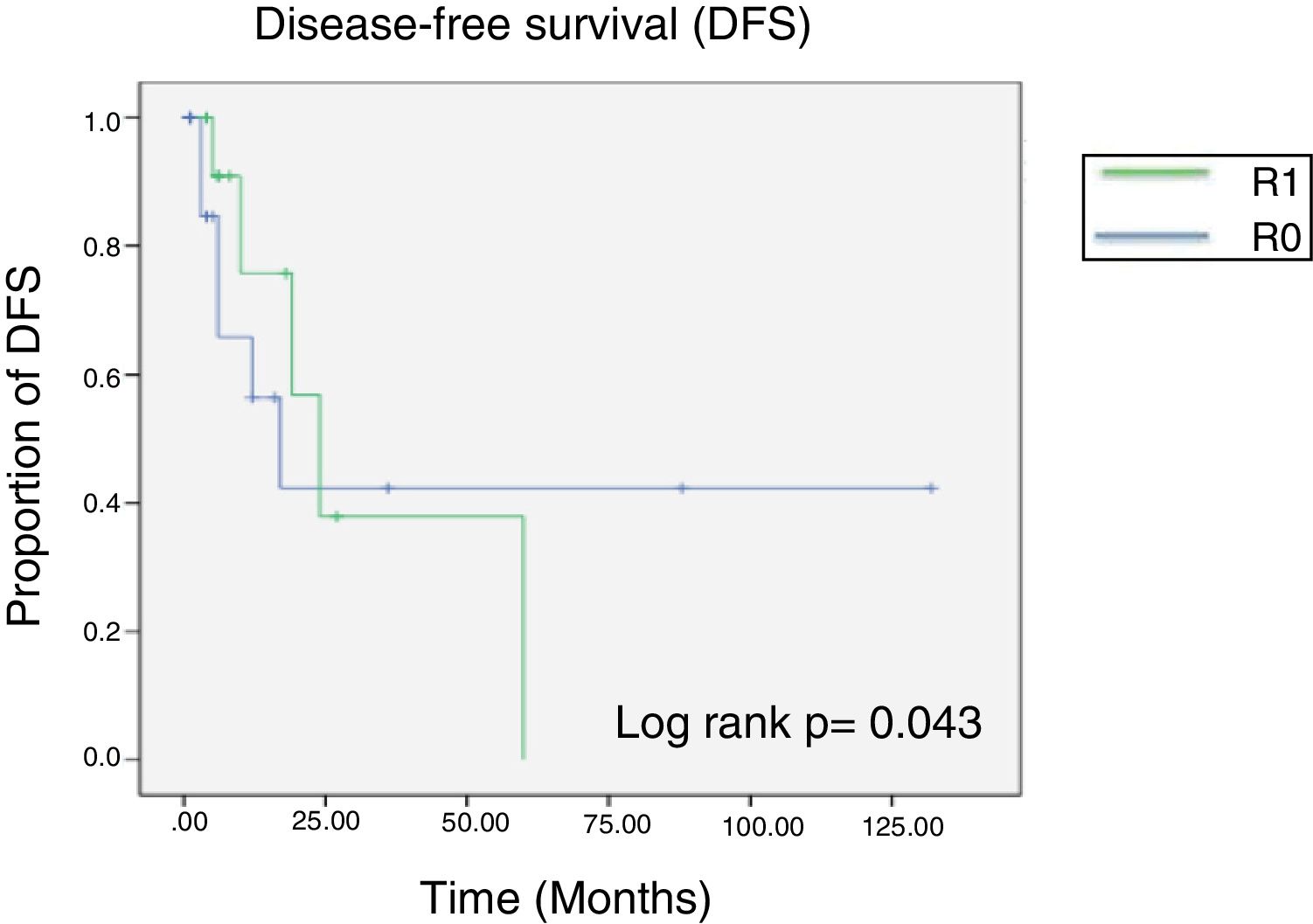
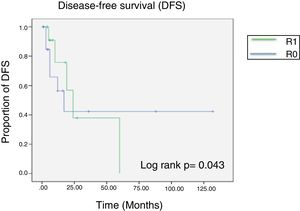
Eleven patients (35.4%) had disease recurrence and intrahepatic lesions were the most prevalent. Mean recurrence time was 12 months.
The statistical impact of the surgical margins was calculated using the chi-square test, obtaining a nonsignificant p value of 0.89 (fig. 2).
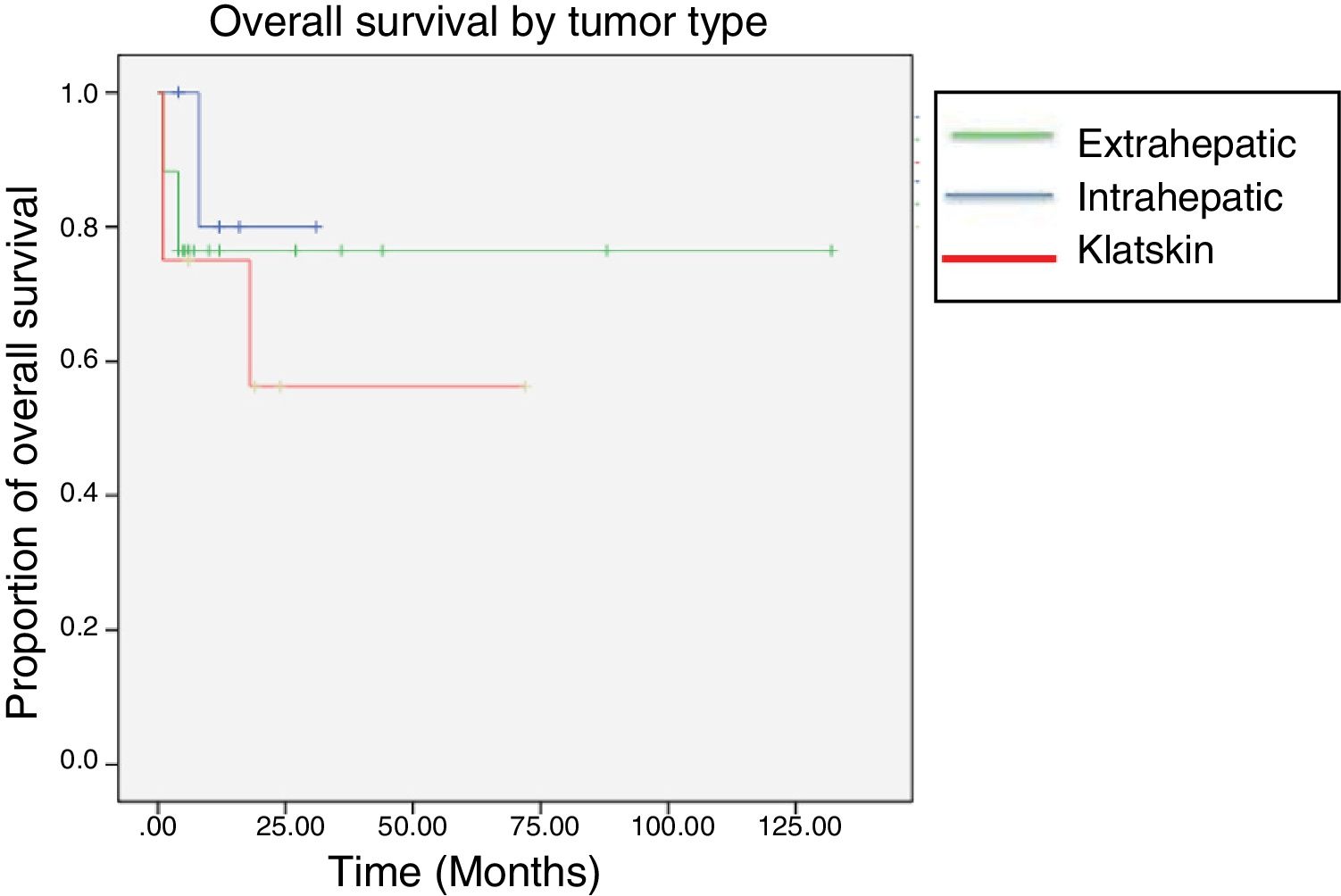
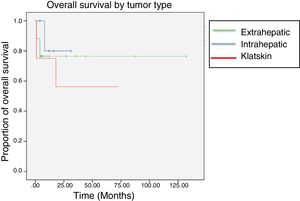
In the lesion location sub-analysis, the longest survival was found in the patients with intrahepatic tumors, with a median of 85 months, followed by the Klatskin tumors, with 44 months, and then the extrahepatic lesions, with only 25 months. Five-year survival was 11.76% for the intrahepatic tumors, 12.5% for the Klatskin tumors, and 0% for the extrahepatic lesions (fig. 3).
Likewise, a statistically significant difference was looked for when having received adjuvant treatment or not was added to the surgical margin variable, but no epidemiologic impact was found.
Overall morbidity was 58.06% (n=18), whereas mortality was 12.9% (n=4). A total of 45.1% (n=14) of the patients presented with a complication equal to or greater than Clavien-Dindo classification III (Table 3). The mortality rate for each group was 25% (n=2) in the patients with Klatskin tumors, 11% (n=2) in those with extrahepatic tumors, and 0% in the patients with intrahepatic tumors. In the patients that died, right hepatectomies were performed, one extended and one not. Whipple procedures were carried out in the two patients with extrahepatic tumors. The final causes of death were septic shock due to cholangitis prior to the surgery in 3 patients and gastrointestinal bleeding of variceal origin in one patient. All deaths occurred within the first 30 postoperative days. The earliest death took place within the first 24h and the latest at day 20. No other deaths were registered 60 or 90 days after the procedure. The most frequent complication was abdominal sepsis, presenting in 7 patients (22.6%), 2 of whom required percutaneous puncture for adequate focal control. The second associated complication was the presence of bile duct leaks in 3 patients, 2 of whom needed percutaneous drain placement. The only documented reintervention was due to hemoperitoneum secondary to superior mesenteric artery injury and the repair was successful (Table 3).
Morbidity and mortality after resection of cholangiocarcinoma based on the Clavien-Dindo classification.
| Postoperative complications according to the Clavien-Dindo classification. | |
|---|---|
| n | |
| No complications | 9 |
| I | 0 |
| II | 7 |
| Abdominal sepsis | 3 |
| Cholangitis | 1 |
| PTE | 1 |
| Atrial fibrillation | 1 |
| HAP | 1 |
| IIIa | 6 |
| Pancreatic fistula | 1 |
| Abdominal sepsis (percutaneous puncture) | 2 |
| Bile duct leakage | 3 |
| IIIb | 3 |
| Hemoperitoneum | 1 |
| Bile duct segment occlusion | 1 |
| Thoracotomy due to pancreatic fistula | 1 |
| IVa | 2 |
| Septic shock | 2 |
| IVb | 0 |
| V | 4 |
HAP: hospital-acquired pneumonia; PTE: pulmonary thromboembolism.
No consensus on the criteria for resectability of cholangiocarcinoma has yet been achieved, but the fact that its feasibility depends on numerous conditions has been well-described and they are: the patient's general state of health, comorbidities, type of tumor, adequate functioning of the hepatic remnant, the skill of the surgeon, and the volume of cases treated at the hospital center.2
Computed tomography is extremely useful for predicting the success of the surgical procedure in 90% of the patients, and therefore is the most widely employed. There are different classification and staging systems for cholangiocarcinoma: The Bismuth-Corlette classification is the most well-known and is useful for defining the longitudinal extension of the tumor, but not for predicting its resectability.11 The TNM system facilitates preoperative staging but does not evaluate the feasibility of the surgical procedure. The Jarnagin-Blumgart (Memorial Sloan Kettering Cancer Center [MSKCC]) classification has demonstrated great accuracy in predicting surgical outcome by taking into account factors associated with local tumor extension.12
Different factors for unfavorable prognosis in patients with hilar cholangiocarcinoma that will undergo hepatectomy have been described: tumor biology, the presence of vascular invasion, metastasis to lymph nodes, elevated CA 19-9 levels, and positive surgical margins. That last factor is the only one that can be modified by the surgeon.
The adequate distance of resection margins is not well-defined. In the meta-analysis conducted by Tang et al., they state that it is ideal to obtain a margin of at least 10mm.5 In other studies, a clear association between margin length and survival has not been observed. Surgical resection continues to be the only intention-to-cure option and the French Association Francophone de Chirurgie–Intrahepatic Cholangiocarcinoma Study Group states there is better patient survival, the wider the resection. In their case series, they reported survival of 17.5 months in the patients with positive margins, versus 25.1 months in those with negative margins, identifying the length of the margin as an independent predictive factor.13
The incidence of positive surgical margins varies from 15-68%, given that large tumor size, the presence of hepatolithiasis, advanced T stage, and infiltrative periductal growth compromise the integrity of the other hilar structures.14
The fact that patients with a microscopically positive resection have better survival than those in whom macroscopic disease remains has been analyzed in different studies. That occurs more frequently in the liver, in which the main problem is maintaining adequate liver reserve. The influence of surgical margins on disease prognosis is still controversial, albeit adequate oncologic resection (R0) should always be striven for. In their study, Tamandl et al. described the low impact of R1 margins on survival, whereas Hemming et al. reported that R1 margins were more beneficial, compared with unresectable disease.12
In the 2010 study by Shingu et al., they analyzed surgical margins of the proximal bile duct after resection of hilar cholangiocarcinoma. Seventeen cases had positive margins and a new surgical procedure was proposed to widen the resection. Only 12 patients were included in the second analysis. The results were worse than those for the patients that had presented with negative margins and they were the same as those for the patients that had presented with R1.15 Lee et al. showed that 5-year survival after resections with microscopically positive margins was not significantly lower than in the patients that had R0 results.16 Surgical resection should be attempted, even if it does not appear possible to obtain negative margins.17
Some researchers have suggested that hepatectomy, itself, induces a state of immunosuppression, resulting in Kupffer cell and T cell dysfunction, which could be thought to have an impact on perioperative complications and oncologic results.18
Serum CA 19-9 levels appeared to have a prognostic impact on the natural history of the cholangiocarcinoma, given that depending on the titers we found prior to the surgical procedure, tumor resectability could be inferred. However, those results are a subject of debate:19 patients with primary sclerosing cholangitis, considered a predisposing condition to cholangiocarcinoma, tend to have elevated CA 19-6 levels, making it necessary to adjust the cohort, because the universally accepted value is 129 IU/ml. In addition, patients that do not have the Lewis antigen (7% of the general population) do not have high levels of CA 19-9, which should be taken into account.20
Despite the use of different diagnostic tools, 50% of patients turn out to be inoperable at the time of laparotomy and there is still no sufficiently sensitive method enabling us to know that ahead of time.
The advances in surgical techniques and perioperative management of the patient with cholangiocarcinoma have resulted in a decrease in the mortality rate, whereas morbidity rates continue to be high.
The results of the present study did not show a difference in the relation of disease recurrence or disease-free survival to the presence of microscopically positive surgical margins (R1) after resection. However, there was a trend toward longer disease-free survival when R0 resection was attained. Sample size and the retrospective design were among the most important limitations of our study, which can explain the lack of statistical significance and the overestimation of mortality. Nevertheless, few hospital centers in Mexico treat cholangiocarcinoma and few studies have been conducted on it in Latin America, motivating us to describe the prevalence of the disease in Mexico, as well as its treatment. The lack of a standardized oncologic follow-up regimen complicates the surveillance of those patients.
Financial disclosureNo financial support was received in relation to this study.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Morales-Cruz M, Armillas-Canseco F, Carpinteyro-Espín P, Domínguez-Rosado I, Mercado MA. Valor pronóstico del margen quirúrgico positivo tras la resección de un colangiocarcinoma. Experiencia en un centro de alto volumen en cirugía hepatopancreatobiliar. Revista de Gastroenterología de México. 2020;85:18–24.