Clostridium difficile is a Gram-positive bacillus that has become one of the main hospital-acquired human gastrointestinal infections in recent years. Its incidence is on the rise, involving more virulent strains, affecting new and previously uncontemplated groups of patients, and producing changes in clinical presentation and treatment response that influence disease outcome. Early diagnosis and disease stratification based on the severity of C.difficile infection are essential for therapeutic management and the implementation of containment measures. However, the speed at which new strains with greater pathogenicity are developing is surpassing that of the development of new drugs, making it necessary to validate other therapeutic options. The present article is a review of the epidemiologic, pathophysiologic, diagnostic, and therapeutic aspects of C.difficile infection, from its first isolation to the present date, that aims to contribute to the preparation of general physicians and specialists, so that patients with this infection receive opportune and quality medical attention.
Clostridium difficile es un bacilo grampositivo que durante los últimos años se ha convertido en una de las principales infecciones gastrointestinales adquiridas en el hospital para el ser humano. Recientemente su incidencia se ha incrementado, implicando cepas más virulentas que afectan nuevos grupos de pacientes que antes no se tenían contemplados, generado cambios en la presentación clínica y en la respuesta al tratamiento que influyen en el pronóstico de la enfermedad. El diagnóstico precoz y la estratificación de la enfermedad con base en la gravedad de la infección por C.difficile es fundamental para el manejo terapéutico y para la implementación de medidas de contención. Sin embargo, la velocidad con la que se desarrollan nuevas cepas con mayor patogenicidad se encuentra por encima de aquella con la que se desarrollan nuevos fármacos, siendo necesaria la validación de otras opciones terapéuticas. En el presente artículo revisamos los aspectos epidemiológicos, fisiopatológicos, diagnósticos y terapéuticos de la infección por C.difficile desde su primer aislamiento hasta la fecha, con el objetivo de contribuir en la preparación de médicos generales y especialistas para que proporcionen atención oportuna y de calidad a quienes la padezcan.
Clostridium difficile is a Gram-positive bacillus that has become one of the main hospital-acquired human gastrointestinal infections over the last 40 years.1 It has numerous virulence factors that contribute to intestinal colonization and facilitate the development of a pathologic imbalance within the resident microbiota.2 It is a significant public health system burden in terms of outbreaks, implementation of containment measures, and increased morbidity and mortality in the general population, with an estimated mean cost of $9,197.00 USD per hospital stay and an approximate yearly expense of 800 million dollars in the intensive care units of the United States.3
The aims of the present literature review were to provide information on the epidemiologic, pathophysiologic, diagnostic, and therapeutic aspects of C. difficile infection, from its first isolation to the present day, to contribute to the medical knowledge of general practitioners and specialists so they can give opportune and quality care to patients that present with the infectious disease.
Materials and methodsA review of the medical literature was carried out using the DynaMed, EBSCOhost, Google Scholar, and PubMed databases to search for systematic reviews, clinical practice guidelines, and randomized controlled trials in Spanish and English with the words: “Clostridium difficile”, “pseudomembranous colitis”, and “toxic megacolon” within the time frame of 2005 and 2015. The abstracts included were manually searched for and the articles selected were based on their accommodation to the review aims. Historic and recent articles that complemented or enriched the review were then located.
Characteristics of Clostridium difficileC. difficile is a strict anaerobic, spore-forming Gram-positive bacillus that may or may not produce toxins, depending on the strain. The majority of strains produce two toxins (TcdA and TcdB), whereas others produce only one (TcdB). However, the strains that produce only the one toxin can produce the same disease spectrum as those that produce the two. In addition, some strains produce no toxins, and others have been described that produce a binary toxin whose role in human disease is still unclear.4,5C. difficile grows in cycloserine-cefoxitin-fructose agar, forming yellowish rhizoid colonies that have birefringent internal structures with a characteristic odor similar to that of horse manure.6
Historic perspectivesIn 1935, Hall and O’Toole7 isolated a new microorganism in the stools of neonates. It was considered part of the normal bacterial flora and given the name Bacillus difficilis. In 1977, because of the difficulty involved in isolating the bacillus, given that its growth is relatively slow compared with most of the other members of the genus Clostridium, it was renamed Clostridium difficile.4 That same year, Larson et al.8 found that antibiotic-induced colitis was caused by C. difficile, and in 1978, Bartlett et al.9 demonstrated that it was also a causal agent of pseudomembranous colitis (PMC). The first anatomopathologic studies of PMC were conducted by Finney, and in 1893 he reported postoperative pseudomembranous changes in the intestinal tract of a 22-year-old patient.10 Finally, during the twentieth century, cases of PMC were found to present after the administration of antibiotics, such as aureomycin, chloramphenicol, and clindamycin,11,12 establishing a strong correlation between them in the following years.
EpidemiologyThe incidence of C. difficile infection (CDI) has increased over the last decade, involving more virulent strains that affect new groups of previously uncontemplated patients,5 producing changes in clinical presentation and treatment response and influencing disease outcome.
C. difficile affects up to 8% of hospitalized patients and is the most common cause of nosocomial diarrhea worldwide. It is the causal agent of 15-25% of the cases of antibiotic-associated diarrhea, 50-75% of antibiotic-associated colitis, and 90-100% of antibiotic-associated PMC.13,14 In addition, it is estimated that 20 to 28% of CDIs are community-acquired, with an incidence of 20-50 cases per every 100,000 inhabitants in the United States, Sweden, and England.15 These infections are less severe and more frequently present in women 50 years of age, compared with the hospital-acquired infections showing a greater frequency in 72-year-old women.16
PathophysiologyC. difficile is propagated via the oral-fecal route and can be ingested in its vegetative form or its spore form, which can survive long periods in the environment outside the body and resist stomach acidity.17 In the healthy individual, the gut microbiota acts as a protective barrier against CDI. In contrast, in individuals with altered gut microbiota, the spores germinate into their vegetative form in the small bowel and consequently are established in the colon. After colonization, C. difficile produces its main virulence factors, enterotoxin (TcdA) and cytotoxin (TcdB), encoded by the tcdA and tcdB genes in the chromosomal 19.6kb pathogenicity locus (PaLoc). The PaLoc encodes 2 regulatory genes, tcdC (which negatively regulates tcdA and tcdB) and tcdD (which positively regulates tcdA and tcdB), and a porin gene, tcdE (which codifies a protein [holin] that is in charge of making pores in the cell membrane to facilitate the release of the toxins).18–21 TcdA is responsible for the activation and recruitment of the inflammatory mediators, such as IL-1, IL-6, IL-8, and TNF-α,22 whereas TcdB is essential as a virulence factor. Both bind to the surface of the colonic epithelium to be internalized and to catalyze the glycosylation of the cytoplasmic proteins and the consequent inactivation of the Rho-GTPases (Rho, Rac, and Cdc42), causing inflammation, neutrophil infiltration, reactive oxygen species production, mast cell activation, substance P production, and damage to the intestinal mucosa.21,23 Some C. difficile strains are carriers of a transferase (CDT), called the binary toxin (formed by 2 subunits, CDTa and CDTb), whose genes are found in the CDT locus (CDTLoc) and whose expression is capable of producing major toxicity. The pathogenic mechanism of said toxin is not yet completely understood, but it appears to increase the adhesiveness of C. difficile and alter the cell cytoskeleton, allowing a greater loss of fluids.24
The host's immune system response to the C. difficile toxins determines whether symptoms develop after exposure to the microorganism. High anti-TcdA antibody titers are related to protection, whereas low titers are related to a greater propensity for developing diarrhea.25 Paradoxically, the local cytokine response contributes to the formation of a pseudomembrane composed of neutrophils, fibrin, mucin, and cell detritus.18 Its formation is less common in patients that are under immunosuppressive therapy.26
One of the most problematic aspects of CDI is recurrence and its pathogenesis is not completely understood. The presence of spores that persist after the initial infection and the inability of the immune system to respond to the C. difficile toxins are among the theories that have been postulated.4,27
Risk factorsThe main risk factors that are associated with CDI are: advanced age (≥ 64 years), prolonged hospitalization, treatment with proton pump inhibitors and/or H2 receptor antagonists, and antibiotic treatment with penicillins, cephalosporins (especially third generation), lincosamides (clindamycin), and fluoroquinolones, albeit any antibiotic is theoretically a potential risk factor for developing CDI.4,13
Their use induces detrimental effects in the intestine, causing dysbiosis of the microbiome, with the potential absence of the commensal species and the indirect rupture of mutualist interactions.28 Recent studies have shown that low levels of Bacteroidetes are significantly involved in C. difficile-associated diarrhea14 and that the reduction of Firmicutes and Bacteroidetes and an increase in facultative anaerobes facilitates colonization.15 Knecht et al.29 showed that this is due to the consequent reduction in the metabolite production of those microorganisms, rather than the overall gut microbial compositional level.
Other risk factors involved in CDI are: prolonged parenteral nutrition, the presence of a nasogastric tube and/or endotracheal tube, kidney failure, chemotherapy, immunosuppression, malnutrition, hypoalbuminemia, and gastrointestinal surgery.17,30
Clinical manifestationsCDI has a broad clinical spectrum, producing symptoms that range from asymptomatic colonization to PMC, intestinal perforation, fulminant colitis, toxic megacolon, septic shock, and death.21
A considerable percentage of patients are asymptomatic and diarrhea is the most common sign in the symptomatic patients. In patients with mild-to-moderate infection, diarrhea is watery, fetid, and not bloody, but ileus can present, with its respective absence of bowel movements.21 PMC presents with pain and abdominal hypersensitivity, fever, and severe diarrhea that can become bloody. Fulminant colitis presents with signs of systemic toxicity, with possible ileus and toxic megacolon (transverse diameter of the colon ≥ 6cm).31 Extra-intestinal symptoms are rare, and cases of bacteremia and reactive arthritis are exceptional.5
DiagnosisCDI diagnosis should be based on the combination of clinical, laboratory, and imaging findings.5 The clinical findings should guide the decision to order laboratory tests, given that those tests alone cannot distinguish an asymptomatic carrier from one with CDI. They should not be done on asymptomatic patients and the sample should be taken from diarrhetic stools. In addition, imaging studies should never be carried out in an isolated manner because their findings are nonspecific. They can be used as a diagnostic complement, and more frequently, to rule out pathologies in the differential diagnosis.
Today there are a diversity of laboratory tests with different sensitivities and specificities, that include the following:
- -
Cell cytotoxicity assay. This test identifies the cytopathic effect induced by TcdB in stool samples in a fibroblast culture. A halo is observed around the cell that is neutralized by the administration of antisera. It has 64-76% sensitivity and 99-100% specificity. Response time is longer than 48h and it does not detect toxin-producing strains, because they are gradually degraded by the effects of pH.6,21
- -
Cytotoxigenic culture in stool. The sample is cultured in a selective chromogenic medium (cycloserine-cefoxitin-fructose agar) or a non-selective medium (Brucella or Schaedler agar enriched with 5% sheep blood, vitamin K, and hemin) in anaerobiosis, isolating the C. difficile strains to detect toxin production. It has 94-100% sensitivity and 84-100% specificity, response time is 48-72h, and has replaced the cell cytotoxicity assay as the preferred method, becoming the criterion standard. In addition, it enables the characterization of the isolated microorganism for epidemiologic studies and the antimicrobial susceptibility profile, as well as the management of patients with recurrent or refractory disease.6,21,24
- -
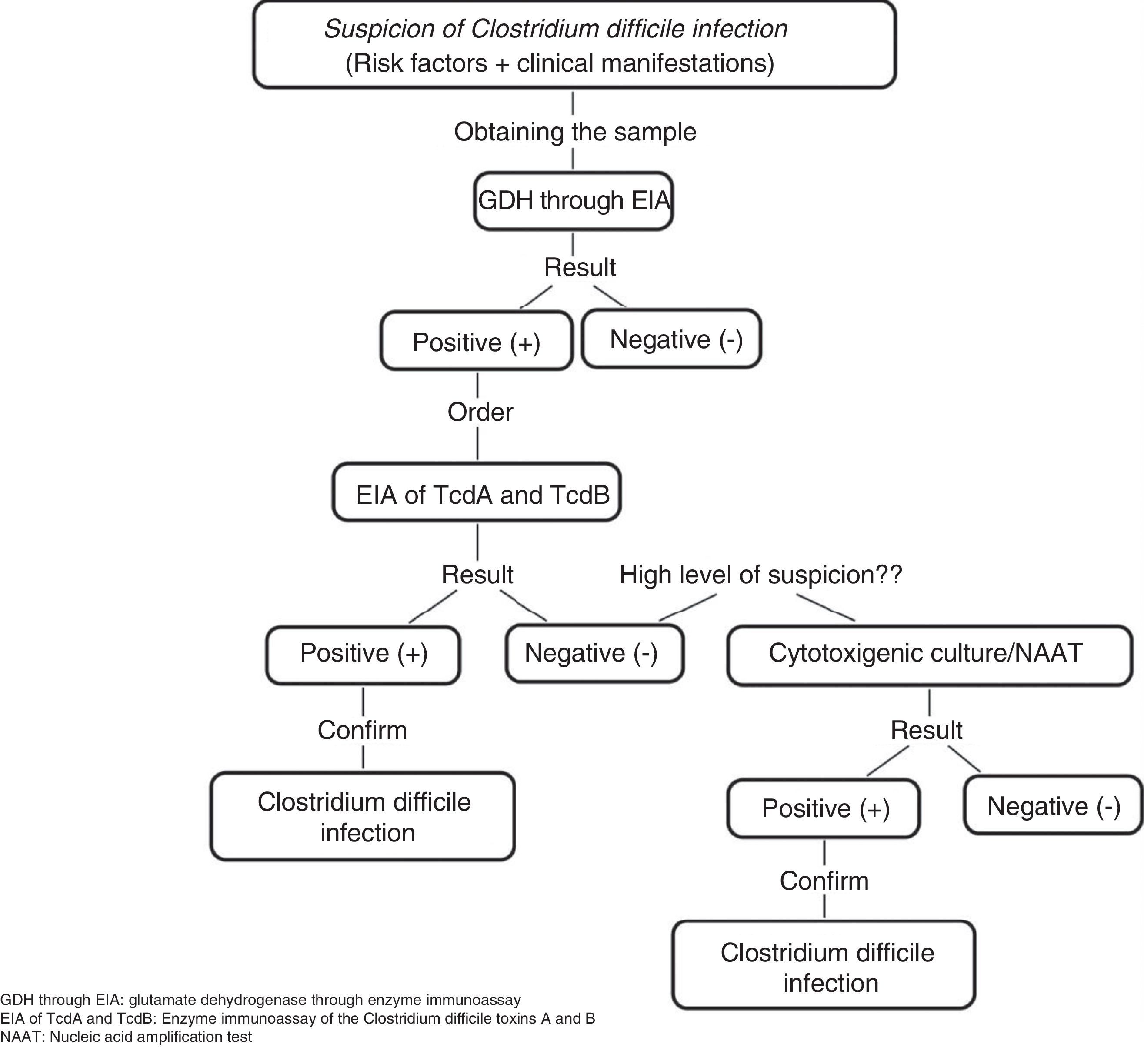
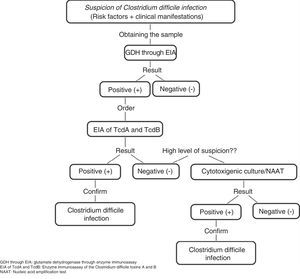
C. difficile common antigen test (glutamate dehydrogenase [GDH]) through enzyme immunoassay (EIA). This test identifies the presence of GDH, a cell wall protein produced by C. difficile through EIA. It has 85-95% sensitivity and 89-99% specificity and its response time is 15-45min. It has the disadvantage of not differentiating between toxigenic and non-toxigenic strains, thus requiring the subsequent determination of toxins through the cytotoxicity assay in cell culture or cytotoxigenic culture in stool in cases of positive GDH (fig. 1).5,6,21
Figure 1.Diagnostic algorithm for confirming Clostridium difficile infection
Adapted from Meyer et al.21.
(0.28MB). - -
EIA of TcdA or TcdA and TcdB. This assay utilizes monoclonal or polyclonal antibodies to identify free TcdA or TcdA and TcdB in stool. It has 48-96% sensitivity and 75-99% specificity, its response time is 15-30min, and it is the most widely used method for diagnosing CDI. However, it cannot be recommended as the only diagnostic method due to the emergence of new virulent TcdA-negative strains.6,21
- -
Nucleic Acid Amplification Tests. These tests detect toxin-producing genes, but not toxin-free ones. There are two types of molecular assays: polymerase chain reaction (PCR) that identifies the TcdB gene that encodes for TcdB with sensitivity of 84-96% and specificity of 96-99% and loop-mediated isothermal amplification that consists of an isothermal reaction for detecting the PaLoc TcdA gene with sensitivity of 92-96% and specificity of 98%. The response time of the nucleic acid amplification tests is 45-180min and they been evaluated as a single step method, as well as a confirmation method for detecting C. difficile in GDH-positive specimens. Nevertheless, due to their high cost and limited availability, they are not considered a method of choice.6,21
Accurate diagnosis can be made through clinical findings and their combination with the tests identifying the presence of C. difficile, its toxin production, and the organic damage the toxins cause. In our environment, some experts recommend the simultaneous determination of GDH and the TcdA and TcdB toxins, observing the concordance, or lack thereof, between them and making a decision based on those results or performing nucleic acid amplification tests, mainly PCR, if available, given that it is a rapid and highly sensitive and specific test for detecting CDI.32
With respect to the different imaging studies, each one has a distinct usefulness, as described below:
- -
Abdominal x-rays. They provide nonspecific data that can correspond to any type of colitis, and they are used exclusively for making the differential diagnosis with other intra-abdominal pathologies and for ruling out complications, such as bowel obstruction or perforation and toxic megacolon. The radiologic findings associated with CDI include: thickening of the haustra with thumbprinting suggestive of submucosal edema or distension of the colon.33
- -
Abdominal and pelvic helical computed tomography. This study has been employed to diagnose colitis caused by C. difficile and to make the differential diagnosis with other intra-abdominal pathologies. The tomographic findings related to CDI include: parietal thickening and dilation of the colonic wall, pericolonic fat striation, the “accordion sign” (administration of oral contrast medium [PO] with high attenuation in the lumen of the colon alternating with low attenuation of the inflamed mucosa due to contrast medium entrapment between the thickened haustra), the “double halo” or “target” sign (intravenous administration [IV] of contrast medium with the presence of concentric rings with different degrees of attenuation caused by mucosal hyperemia and submucosal inflammation), and ascites.6,34
- -
Abdominal ultrasound. This study can be useful for the diagnosis and management of critical patients that cannot be moved to the imaging study area. Ultrasound findings include: thickening of the colonic wall with heterogeneous echogenicity and intraluminal narrowing, the presence of pseudomembranes that can be viewed as hyperechoic lines that cover the mucosa, and free intraperitoneal fluid seen in more than 70% of the cases.33,35
- -
Colonoscopy. This examination should be used in moderation to confirm the diagnosis of colitis caused by C. difficile, given that diagnosis can generally be made through clinical findings, laboratory tests, and imaging studies. Colonoscopy is indicated when there is a high degree of suspicion of CDI and negative microbiologic tests, when a rapid diagnosis is a priority due to severe clinical symptoms, when other concomitant diseases are suspected (inflammatory bowel disease and ischemic colitis), especially in immunosuppressed patients, and when there is a lack of response to antimicrobial agents. It is contraindicated when there is suspicion of toxic megacolon or fulminant colitis. Colonoscopic findings related to CDI include: edema, erythema, mucosal erosions, and the presence of whitish-yellowish plaques that vary in size, are raised and adhered to the mucosa, are unmovable and sometimes converge, and are seen in 50-60% of CDIs. This procedure has the risk for bowel perforation, with the consequent development of sepsis, septic shock, and death, if not diagnosed and treated opportunely.6,21
The goals of treatment are to stabilize the patient, prevent the development of complications, and eliminate the CDI. The first step is to suspend the precipitating antibiotic and implement support measures to correct the concomitant fluid and electrolyte imbalance. Antiperistaltic agents should not be used because they can mask symptoms and accelerate the appearance of toxic megacolon. Antimicrobial agents should then be given to eliminate the CDI.5,36
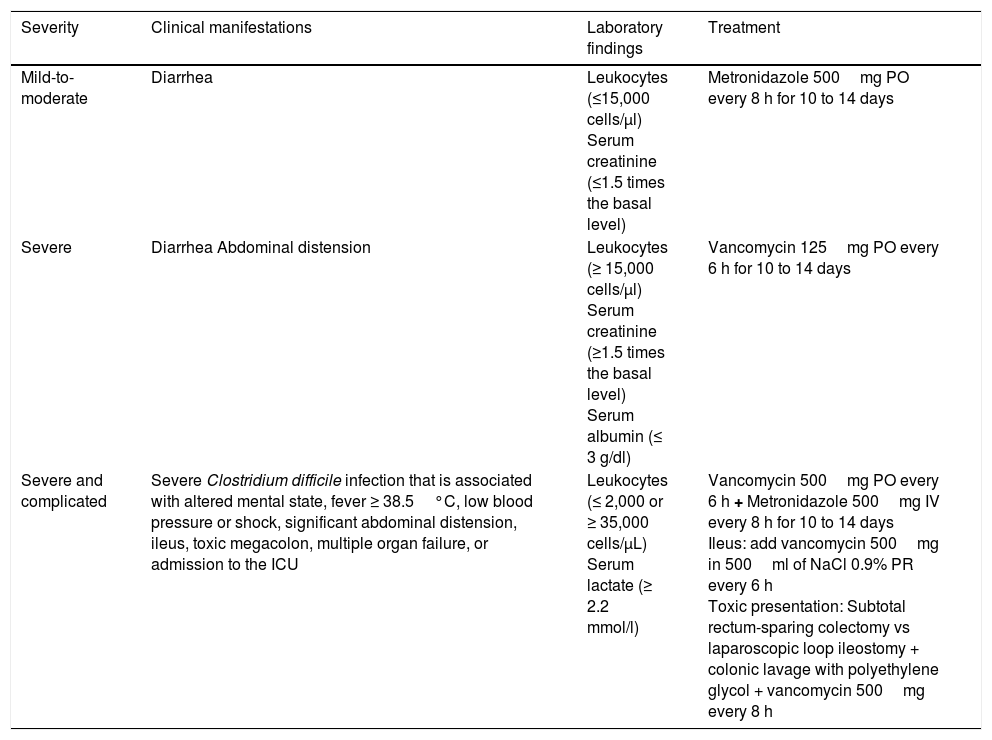
The practical guidelines for CDI in adults –the 2010 update from the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA)– and the 2013 Guidelines for the Diagnosis, Treatment and Prevention of Clostridium difficile Infections published by the American College of Gastroenterology (ACG), stratify antimicrobial treatment based on CDI severity (Table 1).
Stratification and treatment of Clostridium difficile infection.
| Severity | Clinical manifestations | Laboratory findings | Treatment |
|---|---|---|---|
| Mild-to-moderate | Diarrhea | Leukocytes (≤15,000 cells/μl) Serum creatinine (≤1.5 times the basal level) | Metronidazole 500mg PO every 8 h for 10 to 14 days |
| Severe | Diarrhea Abdominal distension | Leukocytes (≥ 15,000 cells/μl) Serum creatinine (≥1.5 times the basal level) Serum albumin (≤ 3 g/dl) | Vancomycin 125mg PO every 6 h for 10 to 14 days |
| Severe and complicated | Severe Clostridium difficile infection that is associated with altered mental state, fever ≥ 38.5°C, low blood pressure or shock, significant abdominal distension, ileus, toxic megacolon, multiple organ failure, or admission to the ICU | Leukocytes (≤ 2,000 or ≥ 35,000 cells/μL) Serum lactate (≥ 2.2 mmol/l) | Vancomycin 500mg PO every 6 h + Metronidazole 500mg IV every 8 h for 10 to 14 days Ileus: add vancomycin 500mg in 500ml of NaCl 0.9% PR every 6 h Toxic presentation: Subtotal rectum-sparing colectomy vs laparoscopic loop ileostomy + colonic lavage with polyethylene glycol + vancomycin 500mg every 8 h |
ICU: Intensive care unit; PO: Oral administration; IV: Intravenous administration PR: Rectal route administration.
Adapted from the clinical practice guidelines for Clostridium difficile infection in adults: 2010 update carried out by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA), and the Guidelines for the Diagnosis, Treatment and Prevention of Clostridium difficile infections published in 2013 by the American College of Gastroenterology (ACG).
Because vancomycin capsules for PO administration are not available in Mexico, it is prepared in a solution from vancomycin in vials. The preparation process consists of a vial of 500mg of vancomycin plus 10ml of injectable water to obtain a concentration of 50mg/ml. To administer a 125-mg dose, a 2.5ml solution is needed. The dose can then be diluted in 30ml of water for PO or nasogastric tube administration. The preparation can be stored in refrigeration for only 24h, and therefore the date and hour of preparation must be included, in addition to the patient's name and administration route.37
Fidaxomicin is a macrocyclic antibiotic derived from actinomycete fermentation that inhibits RNA synthesis by interfering with the formation of the polymerase DNA-RNA complex. It is recommended as an alternative to vancomycin in patients with mild-to moderate CDI. Its administration regimen is fidaxomicin 200mg PO every 12h for 10 days. It has been shown to be superior to vancomycin by presenting a similar clinical cure rate, a higher sustained cure rate, and fewer recurrence episodes. It is poorly absorbed in the gastrointestinal tract and therefore does not cause systemic adverse effects. However, its high cost has limited its routine use and it is not available in Mexico.32,38
Surgical treatment is reserved for those patients in a deteriorated state of health (a state of shock at the time of presentation, or low blood pressure requiring vasopressor treatment, colonic perforation, toxic megacolon, or refractory ileus). Two procedures are frequently performed: rectum-sparing subtotal colectomy and laparoscopic loop ileostomy, together with anterograde colonic lavage with polyethylene glycol or electrolyte solution, and vancomycin instillation. Postoperative mortality rates are 35-80% and 19%, respectively. In addition to the lower mortality rate, laparoscopic loop ileostomy enables colonic preservation in 93% of the cases, increasing the acceptance rates of the patients that benefit from an opportune intervention.21
After initially successful treatment with metronidazole or vancomycin, close to 30% of patients experience another episode of disease (first recurrence), for which the same treatment as the first is recommended, stratifying it in accordance with the severity of the episode. If there is a second recurrence, metronidazole should not be given, because of its potential accumulative neurotoxicity. Vancomycin at a gradually reduced regimen can be administered. For example, 125mg PO every 6h for 10 to 14 days, followed by 125mg PO every 12h for 7 days, 125mg PO every 24h for 7 days, 125mg PO every 48h for 8 days, and 125mg PO every 72h for 15 days.5,21,39,40 If there is a third recurrence, a vancomycin regimen followed by rifaximin as an adjuvant can be administered or fecal microbiota transplant can be carried out.
- -
Rifaximin is a semi-synthetic analogue of rifampicin that inhibits RNA synthesis upon binding to the ≡ subunit of polymerase RNA. It is recommended as an alternative to fecal microbiota transplant in patients with three or more CDI recurrences. Its administration regimen is: vancomycin 125mg PO every 6h for 14 days, followed by rifaximin 400mg PO every 8h for 28 days. It is poorly absorbed in the gastrointestinal tract and therefore does not cause adverse systemic effects. However, its profile of resistance to C. difficile strains has limited its routine use.41,42
- -
Fecal microbiota transplant consists of the infusion of a fecal suspension from a healthy donor to reestablish the microbiota of the recipient. It is an alternative to vancomycin/rifaximin that is recommended in patients with three or more CDI recurrences and even in patients with one recurrence, if the episode is moderate and does not respond to a week of standard treatment, or if the episode is severe and does not respond to 2 days of standard treatment. The transplant can be administered PO in capsules, through a nasogastric tube, nasoduodenal tube, or through gastroscopy, and it can be administered via the rectal route through retention enemas or colonoscopy, with the latter having higher cure rates. Even though it has been demonstrated to be superior to all other available treatments, its main limitations are the fact that both physicians and patients are reticent to use stool transplant, due to the inherent characteristics of the procedure and the difficulty in finding fecal donors.32,40,43
Currently under study are the selective inhibitors of the C. difficile dehydroquinate dehydratase and the anti-TcdA and anti-TcdB monoclonal antibodies as adjuvant therapies, as well as vaccines that contain the C. difficile toxoid. Other therapeutic options, such as nitazoxanide, ramoplanin, teicoplanin, and tigecycline must be evaluated in broader studies to compare their efficacy. At present, there is not enough evidence to recommend their routine use.44,45
Control and preventionThere are numerous guidelines for CDI prevention and control. In the United Kingdom in 2007, the “High Impact Intervention No. 7” care bundle came out and its recommendations were later reiterated in the European and United States guidelines. The measures to consider are the following:
- -
Rational use of antimicrobials and proton pump inhibitors. The rational use of narrow-spectrum antibiotics for short periods of time and of proton pump inhibitors should be encouraged, preventing their ever-increasing unnecessary prescription.5,36,46
- -
The use of probiotics. Probiotics are cultures of living microorganisms that inhibit the adhesion of C. difficile and modulate the response of the host and the specific stimulation of the IgA antitoxin.32 They have been used in patients on a low regimen of antimicrobials to reduce the risk for C. difficile-associated diarrhea, employing combinations of different strains of lactobacilli (L. acidophilus, L. casei, L. rhamnosus) and bifidobacteria (Bifidobacterium bifidum, B. lactis), as well as yeasts (Saccharomyces boulardii), among others.47 The Mexican Consensus on Probiotics in Gastroenterology recommends the use of probiotics for the prevention of antibiotic-associated diarrhea and for CDI recurrence in the adult and pediatric populations.48
- -
Contact precautions. Wearing gloves can reduce the incidence of CDI, but cannot completely prevent contamination, nor is there an antiseptic agent that has reliable sporicidal activity. Therefore, hand-washing with soap and water is the method of choice, and routinely wearing a lab coat is also indicated.5,36,46
- -
Isolation. The isolation of patients with infectious diarrhea limits the environmental contamination of a part of the hospital and optimizes the care and management of a multidisciplinary team. Confirmed cases should remain isolated until there has been no diarrhea for 48h.5,36,46
- -
Decontamination. There must be effective decontamination of the environment, because the C. difficile spores are resistant to conventional cleaning agents. Sodium hypochlorite and hydrogen peroxide vapor are recommended. In addition, the decontamination of medical equipment should also be considered, given that outbreaks have been associated with instruments, such as rectal thermometers. Therefore, the use of disposable equipment is recommended.5,36,46
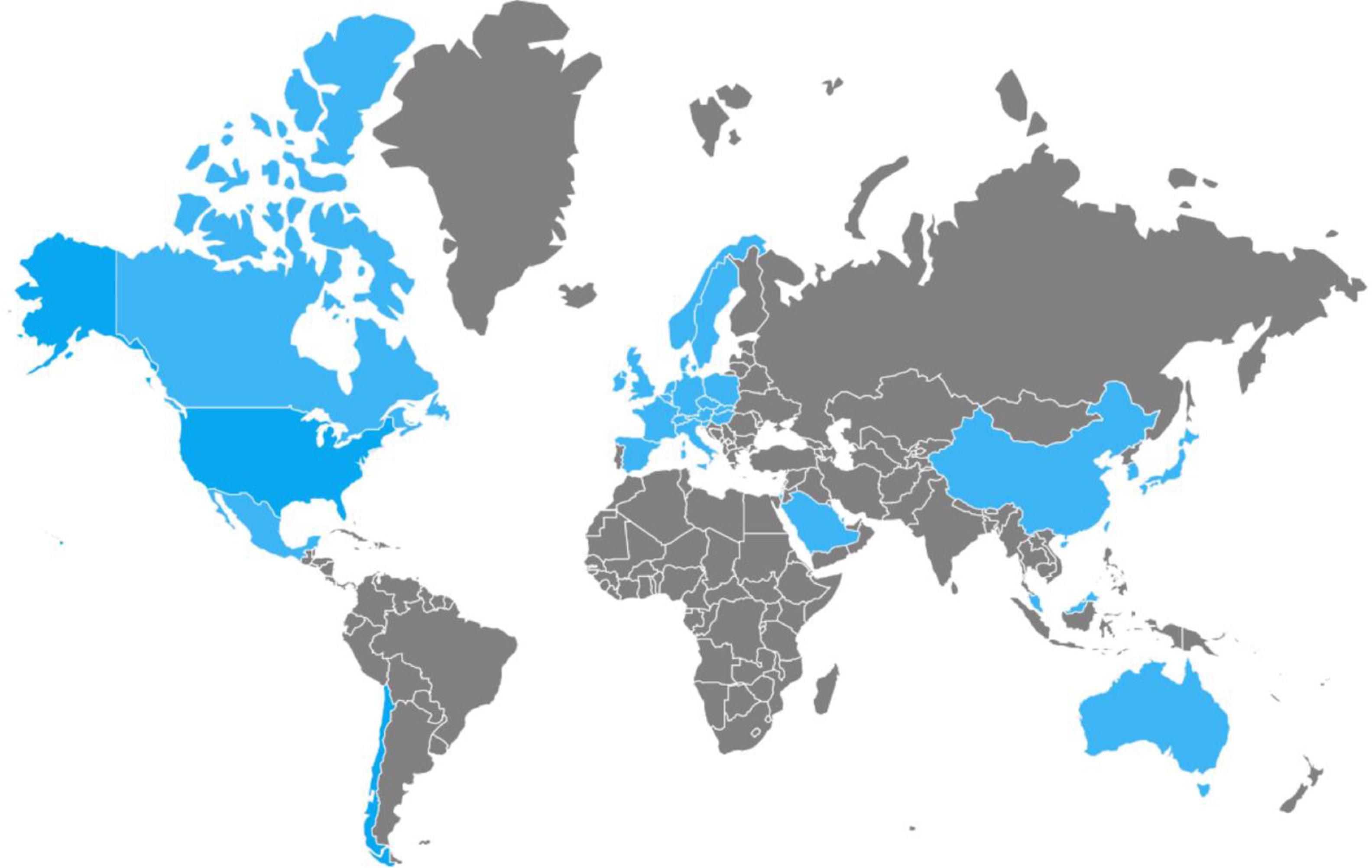
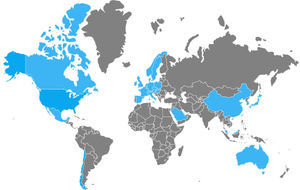
Between 2002 and 2006, numerous nosocomial CDI outbreaks were reported in the United States and Canada that presented with greater severity and recurrence. Isolation of the strain named “NAP1/B1/027”: North American pulsed-field type 1 (NAP1) for its pulsed-field gel electrophoresis pattern, B1 for the restriction endonuclease analysis pattern, and 027 for its PCR ribotype, was achieved. It has propagated through the Americas, Asia, Europe, and Oceania (fig. 2).5,21,24,49,50 It has a more efficacious transmission pattern than other C. difficile strains and is characterized by a deletion in the tcdC gene, the negative regulator of tcdA and tcdB that enables the production of elevated concentrations of TcdA and TcdB (16 and 23 times higher than the rest of the C. difficile strains, respectively), the expression of a binary toxin (CDT), and increased resistance to certain fluoroquinolones (ciprofloxacin, levofloxacin, and moxifloxacin).5,6 That resistance is probably due to the concomitant increase in their indiscriminate use, which has produced similar effects in other microorganisms, such as Escherichia coli, Salmonella typhi, and Salmonella enterica, among others.51 Recently, the NAP1/B1/027 strain has become highly prevalent and has conditioned an increase in the number of therapeutic failures that require emergency colectomies secondary to fulminant colitis, presenting a mortality rate at 30 days that varies from 6 to 30%. In 2014, Camacho-Ortiz et al.49 first reported the isolation of the NAP1/B1/027 strain in Mexico, and even though it is the current focus of attention, it is very likely that hypervirulent strains will continue to emerge, making continuous epidemiologic surveillance a necessity for the early identification of new cases.
On the other hand, the first reports on CA-CDI in Australia were carried out in 1986,52 and recently there have been more reports on the presence of cases worldwide, demonstrating that its incidence continues to be on the rise.53 At present, cases have been reported in the Americas, Asia, Europe, and Oceania.52–54 The IDSA defines CA-CDI as CDI in which symptom onset occurs in the community, within the first 48h of hospital admission, or within the first 4 weeks after hospital release.5,55 It has been described in populations that were previously considered low-risk, including healthy post-delivery women, children, young adults, and patients with no recent exposure to antimicrobials or hospitalization. Thus, an attempt has been made to identify other risk factors, such as: antibiotic prescription to outpatients, increased use of proton pump inhibitors, an increase in the number of asymptomatic carriers with the consequent increase in person-to-person transmission, the contamination of water and food with C. difficile, and the presence of hypervirulent strains like NAP1/B1/027. Generally, patients with CA-CDI are characterized by presenting with light-to-moderate CDI, but they can develop severe CDI and poor-outcome complications, requiring hospitalization, continuous monitoring, and intensive treatment. Therefore, in the face of the recent increment of cases and their apparent under-diagnosis, physicians must begin to consider CA-CDI within the differential diagnosis of patients that present with diarrhea outside the hospital setting, for opportune intervention. In addition, studies should be conducted to aid in clarifying other possible transmission mechanisms, risk factors, and measures of control, in the presence of this situation that is becoming a public health problem.53,54
ConclusionsC. difficile is an anaerobic, spore-forming Gram-positive bacillus that may or may not produce toxins, depending on the strain.4,5 The host immune system response to C. difficile toxins determines the development of symptoms after exposure that range from asymptomatic colonization to PMC, intestinal perforation, toxic megacolon, septic shock, and death.21,25 Opportune CDI diagnosis is essential for therapeutic management and the implementation of containment measures. Diagnosis is made through clinical, laboratory, and/or imaging findings. Conventional antimicrobial treatment is limited to metronidazole and vancomycin, with the possibility of using fidaxomicin in cases of moderate-to-severe CDI.5 However, new strains with greater pathogenicity are developing faster than new drugs, making it necessary to validate other therapeutic options. The treatment of recurrence continues to be a challenge for the specialist, despite the fact that fecal microbiota transplant still shows promising results and has gradually been gaining more supporters within the medical community. C. difficile expansion is a problem concerning the public health systems, given that its presence has been demonstrated in the community environment, and neither its transmission mechanisms nor its risk factors are fully understood. Hospital policies should promote the typing of isolated specimens and adherence to the clinical practice guidelines to reduce the indiscriminate use of antimicrobial agents, especially the fluoroquinolones. Such measures can aid in limiting the appearance of outbreaks of hypervirulent strains.
Ethical responsibilitiesProtection of persons and animalsThe authors declare that no experiments were performed on humans or animals for this study.
Data confidentialityThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Financial disclosureNo financial support was received in relation to this study/article.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Álvarez-Hernández DA, González-Chávez AM, González-Hermosillo-Cornejo D, Franyuti-Kelly GA, Díaz-Girón-Gidi A, Vázquez-López R. Perspectivas históricas y vigentes sobre la infección por Clostridium difficile. Revista de Gastroenterología de México. 2018;83:41–50.