Antimicrobial resistance has become a worldwide problem due to its excessive increase in recent years. The aim of the present review was to bring together data from different articles describing the levels of antimicrobial resistance in the most common gastrointestinal infections reported across the globe.
The literature search was carried out in Google Scholar, Medline, Embase, and Pubmed, with the terms “antimicrobial resistance”, “resistance in gastrointestinal disorders”, and “resistance in amoebiasis”, in Spanish and English. Mexican treatment guidelines and consensuses from 2017 to the present were utilized. Publications from the last ten years were chosen to describe the level of resistance. They had adequate sample sizes, the Material and Methods sections were precise, and they included multicenter studies, national and international consensuses, meta-analyses, systematic reviews, and extensive texts. The final number of articles was 51.
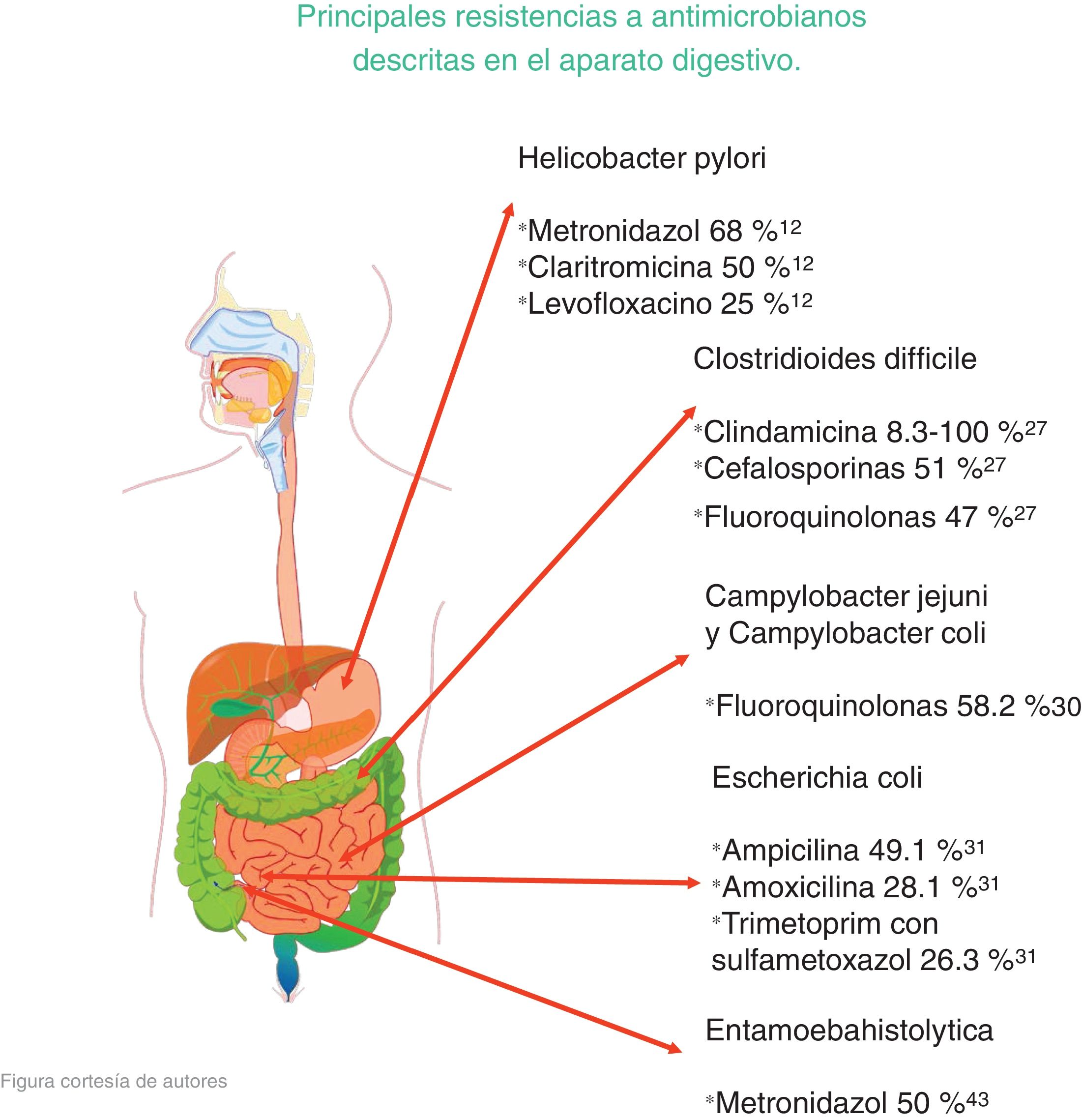
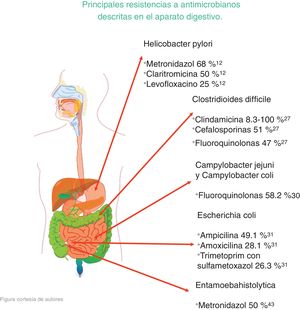
The microorganisms that demonstrated the highest percentage of resistance were Helicobacter pylori (metronidazole 50%-80%, clarithromycin 20%-40%, and levofloxacin 30%-35%), Clostridioides difficile (clindamycin 8.3%-100%, cephalosporines 51%), Campylobacter jejuni and Campylobacter coli (fluoroquinolones 85%), Escherichia coli (ampicillin 76.5%), Entamoeba histolytica (metronidazole 50%), and bacterial peritonitis (third-generation cephalosporines 40%, methicillin 85%).
Antimicrobial resistance is reaching elevated percentages, making it necessary to evaluate the situation of each patient, to successfully treat gastrointestinal infections.
La resistencia antimicrobiana se ha convertido en un problema a nivel mundial debido a su incremento excesivo en los últimos años. El objetivo de esta revisión narrativa es conjuntar los datos obtenidos de distintos artículos que demuestran grados de resistencia a antimicrobianos en las infecciones más comunes del tracto digestivo reportadas en el mundo.
La búsqueda de la literatura se realizó a través de las plataformas Google Académico, Medline, Embase y Pubmed con los términos resistencia antimicrobiana, resistencia en padecimientos gastrointestinales y resistencia en amibiasis, tanto en español como en inglés. Se utilizaron guías de tratamiento y consensos mexicanos sobre el tratamiento actual de las infecciones desde 2017 a la fecha. Se incluyeron publicaciones de los últimos 10 años para describir el grado de resistencia, con adecuado número de muestra, descripción precisa de material y métodos, estudios multicéntricos, consensos nacionales e internacionales, metaanálisis, revisiones sistemáticas y textos en extenso con un total de 51 artículos finales.
Los microorganismos que demostraron mayor porcentaje de resistencia son Helicobacter pylori (metronidazol 50-80%, claritromicina 20-40% y levofloxacino 30-35%), Clostridioides difficile (clindamicina 8.3-100%, cefalosporinas 51%), Campylobacter jejuni y coli (fluoroquinolonas 85%), Escherichia coli (ampicilina 76.5%), Entamoeba histolytica (metronidazol 50%) y peritonitis bacteriana (cefalosporinas de tercera generación 40%, meticilina 85%).
La resistencia a antimicrobianos está alcanzando porcentajes elevados, por lo que es necesario evaluar la situación de cada paciente para tener éxito en los tratamientos de infecciones gastrointestinales.
Infectious diseases are responsible for high morbidity and mortality, worldwide. Antibiotics changed the perspective on and prognosis of those types of diseases, resulting in the golden age of antibiotics that was in place from 1930 to 1960. In 1945, Alexander Fleming and Howard Walter Florey, with their discovery of penicillin, were the first to warn about and study resistance to antibiotics.1 Microorganisms mutate faster than new antibiotics are developed, thus antibiotic use itself propels the appearance, amplification, and dissemination of resistance, consequently shortening the susceptibility intervals. For antibiotics to continue to be useful, fewer antibiotics, or new antibiotics, must be used.2 We are currently facing new mutations that arise not only from antibiotic use, but from a process called “natural transformation”. A study published in the journal, Diagnostic Microbiology and Infectious Disease, describes said process, stating that bacteria target the resistance genes of other bacteria, incorporating them into their genomes when those bacteria, upon dying, release their DNA.
The growing resistance to antibiotics in recent years is due to their inadequate use and abuse, and is a problem that, if not dealt with in time, could compromise the lives of future generations, with an estimated 10 million annual deaths by 2050.3 Given that situation, on May 27, 2010, a law was published in the Mexican Diario Oficial de la Federación stating that “antibiotics can be administered only when they are backed by a written prescription from a licensed healthcare professional, to control their use and abuse, limiting the negative consequences of inadequate prescription and contributing to preserving the health of the Mexican people”.4 Antibiotic resistance will still exist, despite limiting their inappropriate use, but it will be less frequent. Importantly, inadequate antibiotic use also affects the organism’s microbiota that plays a vital role in maintaining the functions of the human body. It must be kept in mind that antibiotics equally attack both the physiologic biota and the microorganisms causing the disease.
Antimicrobial resistance not only arises directly from the use of antibiotics in treatments for diseases, but also from their use in agriculture and livestock farming, the quality in their manufacturing as medicine, and the instability of the environment. Those are all factors that indirectly affect health.4 Thus, it is essential to create awareness of the current, continuously developing problem. It is one of the most important public health challenges and must be dealt with, as stated above, to prevent more severe repercussions in the next generations.
The aim of the present review was to demonstrate the impact of antimicrobial resistance in gastroenterology, describing the pathogens that have developed resistance to specific antibiotics, the possible causes, and how said resistance is affecting those suffering from infectious diseases, as well as delineating the challenges faced by all healthcare personnel in treating those infections.
Helicobacter pyloriHelicobacter pylori (H. pylori) infection is one of the most prevalent worldwide. It is estimated that 80% of the population has been infected by that microorganism, mainly in developing countries. Its eradication is important, given that it has been catalogued as a type 1 carcinogen since 1994 by the World Health Organization.5 It is related to stomach cancer and other diseases, such as mucosa-associated lymphoid tissue lymphoma, peptic ulcer disease, and chronic gastritis. For a treatment regimen to be considered effective, it must result in an eradication rate of 80%. That success rate has been affected by antimicrobial resistance in recent years. The IV Mexican consensus on H. pylori states that the triple eradication therapy of amoxicillin, clarithromycin, and a proton pump inhibitor (PPI) should no longer be considered first-line treatment and proposes 2 treatment options: quadruple therapy with bismuth (PPI, bismuth subcitrate, tetracycline, and metronidazole) and quadruple therapy without bismuth (PPI, amoxicillin, clarithromycin, and metronidazole).6
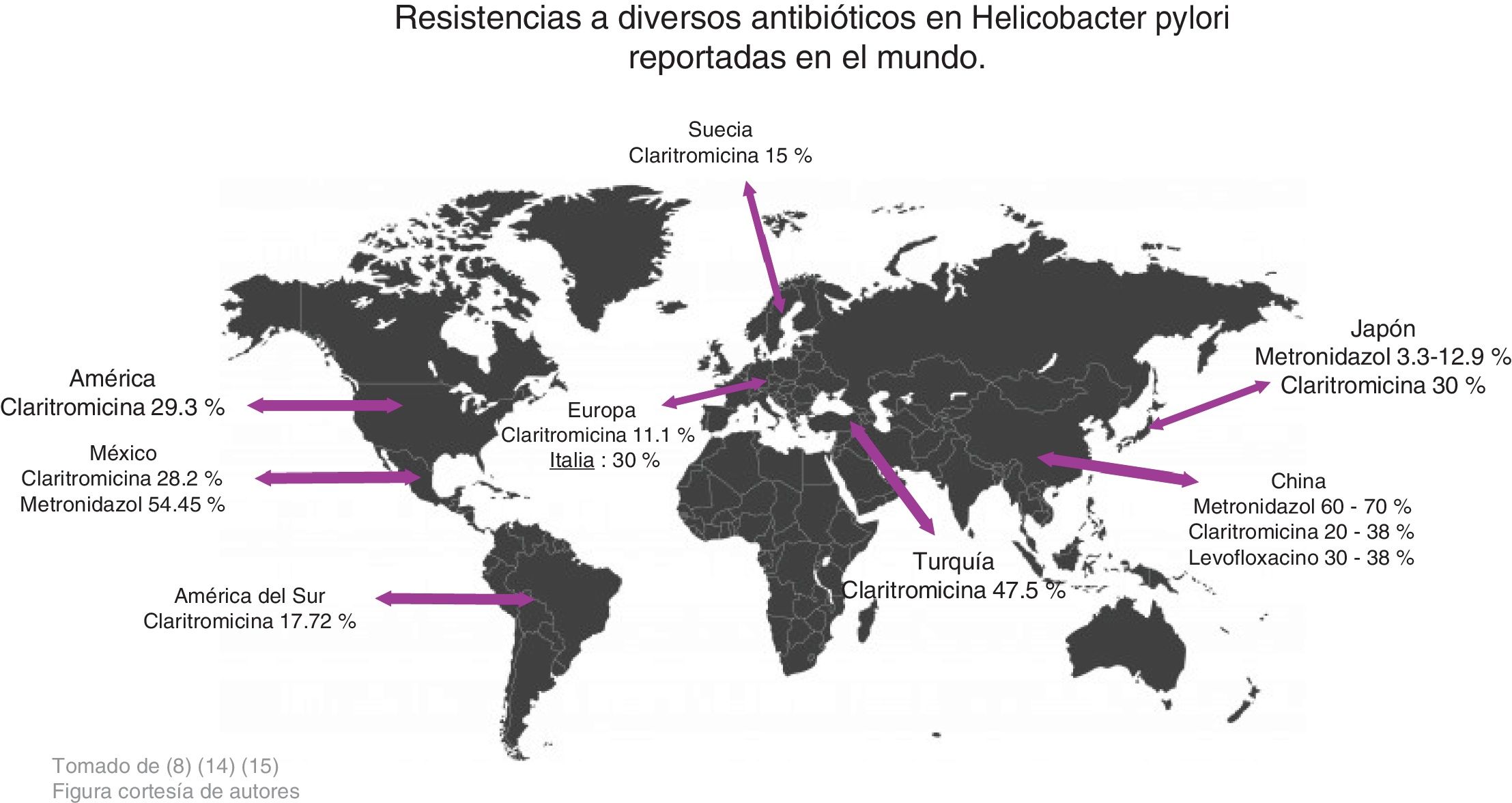
In a study conducted in Mexico by Cano Contreras et al., they evaluated the knowledge of general practitioners about the diagnosis and treatment of H. pylori. The results showed that 31% of the physicians used more than one test to make the diagnosis, and the most utilized was the serologic test. The most widely used treatment regimen was clarithromycin plus amoxicillin (63.8%), followed by metronidazole plus tetracycline (16%). Thus, it is important to be aware of resistance to different antibiotics and prescribe the most adequate regimen for eradicating the infection7 (Fig. 1).
A study conducted in Mexico at the Centro Médico Issemym described the following results: The conventional triple regimen (amoxicillin 1 g bid every 12 h, clarithromycin 500 mg bid every 12 h, and a PPI [omeprazole 20 mg bid every12 h for 14 days]) had an eradication frequency of 65.50% and the quadruple regimen (tetracycline 500 mg qid every 6 h, metronidazole 500 mg bid every 12 h, bismuth subsalicylate 525 mg qid every 6 h, and omeprazole 20 mg bid every 12 h for 14 days) eradicated the infection in 45.45% of the cases, for a total eradication rate of 67.70%8 (Fig. 2).
In Mexico, Ladrón de Guevara et al. compared the regimen based on levofloxacin (levofloxacin 500 mg, pantoprazole 80 mg, and azithromycin 500 mg) with the standard triple therapy (clarithromycin 500 mg bid every 12 h, lansoprazole 30 mg, and amoxicillin 1 g), both for 10 days. The results were an eradication rate of 63% with the treatment with levofloxacin and of 58.5% with the standard triple therapy. Resistance to clarithromycin was 28.2% and there were more adverse events in the standard triple therapy group.9
In another study by Selgrad et al., those authors compared antibiotic resistance, according to how many treatment regimens the patients received to eradicate infection, with the following general results: of 66 patients, none demonstrated resistance to amoxicillin or the tetracyclines, 34 patients (51.5%) had resistance to metronidazole, 29 (43.9%) to clarithromycin, 15 (22.7%) to levofloxacin, and 3 patients (4.5%) had resistance to rifabutin. Results varied in the patients that were receiving antibiotic therapy for the first time: of 29 patients, 6.9% had resistance to clarithromycin, 17.2% to metronidazole, and 13.8% to levofloxacin. In the patients that had already received therapy once: of 13 patients, 69.2% had resistance to metronidazole, 53.8% to clarithromycin, and 23.1% to levofloxacin. In the 24 patients that had already been treated with antibiotics two or more times, resistance increased considerably: 83.3% to clarithromycin and metronidazole, 33.3% to levofloxacin, and 12.5% to rifabutin.10
A study conducted by Silveira Vianna et al. analyzed antimicrobial resistance, giving importance to the different H. pylori mutations: cagA, 23S rRNA, and gyrA. Of the 80 samples collected for the study, 7 had mutations in 23S rRNA related to resistance to clarithromycin (8.7%). Mutations in gyrA related to resistance to levofloxacin were found in 18 samples (22.5%).11
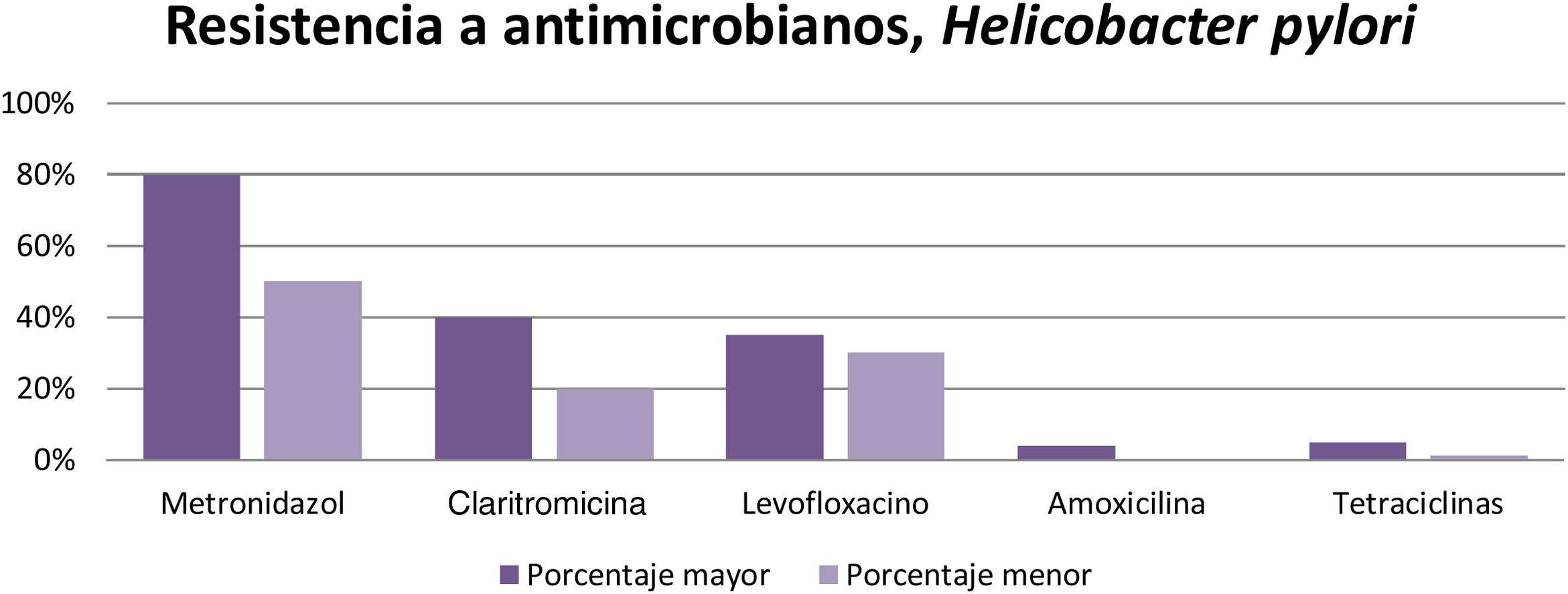
In their published study on patients with gastritis, Mascellino et al. studied resistance to the following antibiotics: metronidazole, levofloxacin, tetracycline, clarithromycin, and amoxicillin. Other environmental factors were taken into account for each person, such as the use of alcohol, tobacco, and nonsteroidal anti-inflammatory drugs (NSAIDs), among others. After corroborating the presence of H. pylori infection, the patients were given treatment, with the following results: In 30 patients, resistance to clarithromycin was 50% and resistance to metronidazole was 68%. Amoxicillin was the most effective, with only 4% resistance, resistance to tetracycline was 6%, and resistance to levofloxacin was 25%.12
Antibiotic resistance was evaluated in Latin America by Camargo et al., obtaining the following results: 12% resistance to clarithromycin, 53% to metronidazole, 4% to amoxicillin, 6% to tetracycline, 3% to furazolidone, 15% to fluoroquinolones, and 8% to dual therapy with clarithromycin and metronidazole.13
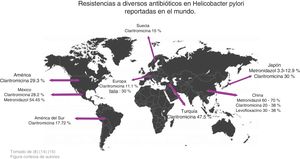
Zhang conducted a study comparing antimicrobial resistance in different parts of the world and found the following data: in China, resistance to metronidazole was 60-70%, to clarithromycin 20-38%, and to levofloxacin 30-38%, whereas resistance percentages to amoxicillin, furazolidone, and tetracycline were very low, at 1-5%. With respect to clarithromycin, resistance in American and European populations was 29.3% and 11.1%, respectively, in Turkey it was 47.5%, and in South America 17.72%. Resistance to metronidazole in China ranged from 75.6% to 95.4% in certain regions and was very low in Japan, from 3.3% to 12.9%. Resistance increased when a therapy with more than one antibiotic was evaluated, and in general, resistance was 34.5%. Resistance to the combination of levofloxacin with metronidazole was 16.9% and to clarithromycin with metronidazole was 7%14 (Fig. 2).
In a study carried out by Malfertheiner et al., they reported that resistance to clarithromycin was 30% in Italy and Japan, 40% in Turkey, 50% in China, and 15% in Taiwan and Sweden. Thus, they proposed not administering conventional triple therapy in regions where resistance to clarithromycin was > 15%, recommending in its place, quadruple therapy, with or without bismuth, for 14 days, to increase treatment efficacy15 (Fig. 2).
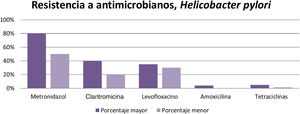
According to the results obtained, it can be concluded that resistance to metronidazole (50-80%), clarithromycin ( 20-40% ), and levofloxacin ( 30-35%) is high in different parts of the world, resulting in inefficient infection eradication. In contrast, the percentage of resistance to amoxicillin (4%), furazolidone (1-5%), and tetracyclines (1-5%) is low, and so they can continue to be used for eradicating said infection that affects a large part of the world population (Fig. 3).
Clostridioides difficileClostridioides difficile (C. difficile), formerly called Clostridium difficile, is a Gram-positive bacillus that in recent years has become one of the main pathogens infecting the gastrointestinal tract, in the hospital environment. It is the causal agent of 15-25% of the cases of diarrhea associated with antibiotics, 50-75% of the cases of colitis associated with antibiotics, and 90-100% of the cases of pseudomembranous colitis associated with antibiotics. The main risk factors for acquiring infection include advanced age, combined with prolonged hospitalization;16 the use of H2 blockers; previous use of cephalosporines and fluoroquinolones; intensive care unit stay; and the use of antibiotics before the diagnosis.17 Chronic kidney disease has been reported as a risk factor, given that those patients are more susceptible to infections and complications.18 Morfin-Otero et al. analyzed the association of C. difficile in surgical patients in Mexico and found that the majority of cases with the infection were in patients in the neurosurgery, heart surgery, orthopedic surgery, and general surgery services. Fifty-three percent of the cases were associated with the NAP1/027 C. difficile strain and the common factors of those patients were leukocytosis (> 12,000 cell/mm), albumin < 3 g/dl, hospitalization above 7 days within the past 12 weeks, and the use of immunosuppressants and antibiotics (meropenem and fluconazole).19
In a study conducted by Rodríguez et al., they reported an increase in the number of cases with the NAP/BI/027 C. difficile strain. The patients were treated with ceftriaxone (70.3%), oral vancomycin and intravenous metronidazole (44.4%), and oral vancomycin alone (37%). A total of 77.7% of the patients had clinical cure, 14.8% died, and 7.4% developed recurrent infection. However, there was no increase in the complications associated with said strain, unlike that reported in different articles.20
Treatment of C. difficile infection is based on eliminating the precipitating antibiotic and implementing measures to correct the fluid and electrolyte imbalance, after which an antibiotic should be administered to eradicate the infection. For mild cases, 500 mg oral metronidazole every 8 h for 10 to 14 days is indicated; for severe cases, 125 mg oral vancomycin every 6 h for 10 to 14 days; and for severe and complicated cases the combination of both antibiotics is indicated.21
In the consensus on the prevention, diagnosis, and treatment of C. difficile infection carried out by Abreu y Abreu et al., they recommend that once the diagnosis is made, all antimicrobial treatment should be eliminated and medical treatment should only be indicated if there are symptoms: 125 mg vancomycin every 6 h (alternative: metronidazole) in mild-to-moderate cases; vancomycin for 14 days in cases of severe infection; and oral vancomycin, associated with intravenous metronidazole, in severe, complicated cases. In cases of first recurrence, if the patient had been treated with metronidazole, it should be changed for vancomycin, and if vancomycin was the first treatment, that same drug should be administered, at decreasing and pulsed doses at the end. Fecal microbiota transplantation is a safe and effective option in patients with two recurrent infections or with severe episodes, in whom antibiotic treatment has failed.22 Importantly, 28-30% of patients will experience recurrence, once again requiring antibiotic treatment. One alternative is to administer 400 mg of rifaximin 3 times a day for 20 days, after completing retreatment with oral vancomycin.23
The inhibitory effect of fidaxomicin on the biofilm of C. difficile was studied by Masakaze et al. and they found that its effect was dose-dependent, unlike that of vancomycin, which does not have that mechanism of action.24 Srisharan compared the different antimicrobials utilized in C. difficile infection and reported that teicoplanin and fidaxomicin were superior to vancomycin, metronidazole, and fusidic acid.25
A study conducted by Johnson et al. compared the effectiveness of treatments with vancomycin and metronidazole in C. difficile infection, with the following results: treatment success was 66.3% in patients treated with metronidazole, whereas success was 78.5% in the patients treated with vancomycin.26
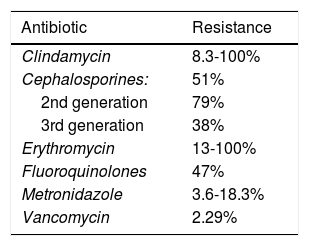
Research carried out by Peng et al. emphasized the spore formation that characterizes C. difficile. Those spores survive certain antibiotics. Between 2012 and 2015, resistance to clindamycin was 8.3% to 100%; to cephalosporines, in general, it was 51%; to second-generation cephalosporines, such as cefotetan and cefoxitin, it was 79%; to third-generation cephalosporines, such as ceftriaxone and cefotaxime, it was 38%; to erythromycin it was 13% to 100%; and to fluoroquinolones it was 47% (Table 1). With respect to first-line treatment for C. difficile, different levels of resistance to metronidazole and vancomycin are reported worldwide. Resistance to metronidazole of 0.11% is reported in Europe, 5.3% in Iran, 15.6% in China, 18.3% in Israel, and 3.6% in the United States. Resistance to vancomycin is reported at 47% in Israel and 2.29% in Europe. And finally, resistance to treatment alternatives have been reported. Resistance to rifampicin was 57% in Italy, the Czech Republic, Denmark, and Hungary and 7.9% in North America; resistance to tetracyclines was 2.4% to 41.67%; and resistance to chloramphenicol was 3.7%.27
A study by Martínez et al. described 2 highly antimicrobial-resistant ribotypes, 027 and 001. Those authors found reduced susceptibility to vancomycin (40.3%) and to fidaxomicin (3.2%).28
Despite the levels of resistance to the antibiotics of first-line treatment for C. difficile (metronidazole with 3.6-18.3% and vancomycin with 2.29%), those drugs have better eradication success rate. Nevertheless, the fact that resistance is on the rise in different parts of the world must be kept in mind, and so treatment must be chosen, according to the relation to resistance for the geographic zone.
Campylobacter jejuni and Campylobacter coliThose microorganisms have become very important from a public health perspective, given that they cause infectious diarrhea in humans. Campylobacter jejuni (C. jejuni) has been more frequently isolated in developing and developed countries, whereas Campylobacter coli (C. coli) has been isolated in 25% of the cases of infectious diarrhea in South America. The treatment of choice for that infection is erythromycin and fluoroquinolones.
A study conducted by Simaluiza et al. showed the level of resistance to different antibiotics for C. jejuni and C. coli. Both strains were susceptible to gentamycin and amoxicillin in all cases; the resistance to ampicillin by C. jejuni was 7.7%; a high resistance to ciprofloxacin of 76.9% in C. jejuni and 100% in C. coli was found; and finally, resistance to erythromycin was lower for C. jejuni, at 7.7%, whereas it was 33.2% for C. coli.29
Research has shown that fluoroquinolone resistance is related to the ingestion of foods of animal origin, given that those foods are reservoirs of C. jejuni and C. coli and the fact that fluoroquinolones have veterinary applications. Fernández et al. studied the different levels of resistance in South America, with the following results: in Chile, C. jejuni showed resistance to ciprofloxacin of 50%; in Argentina resistance to ciprofloxacin and norfloxacin was reported at 59.9% for C. jejuni and 49.1% for C. coli; in Peru, resistance to ciprofloxacin was 89.9% for C. jejuni; in Brazil, resistance to norfloxacin was 25% and resistance to ciprofloxacin was 18.2% for the strains; in Mexico, C. jejuni showed resistance to ciprofloxacin of 58.2%.30
Resistance to fluoroquinolones is very high in Latin America (85-100%), as reported in the two studies previously described. Therefore, treatment with other antibiotics, such as amoxicillin with clavulanic acid and erythromycin (7.7-36%), would be more efficacious, given that both drugs have been reported to have efficient susceptibility and low grades of resistance.
Escherichia coliEscherichia coli (E. coli) is responsible for cases of acute diarrhea in developing countries, where there is a lack of hygiene, together with poor access to basic sanitation. Children are primarily affected and said infection is estimated to cause 10% of deaths in those under 5 years of age.
Different E. coli pathotypes have been identified, the most common of which are enterotoxigenic E. coli (ETEC) and enteropathogenic E. coli (EPEC). The former causes watery diarrhea, colonizes in the epithelium of the small bowel, and produces enterotoxins that interfere with the processes of absorption and intestinal secretion, whereas the latter causes distinctive lesions in the intestinal epithelium (adhesion and elimination), destroying the microvilli and also causing watery diarrhea. Treatment is based on hydration and antibiotic use, but antibiotic therapy depends on different factors, such as symptom severity, the immunologic condition of the patient, and infection transmission. Resistance to the antibiotics used to treat said infection has increased in recent years, as it has with respect to other infections.
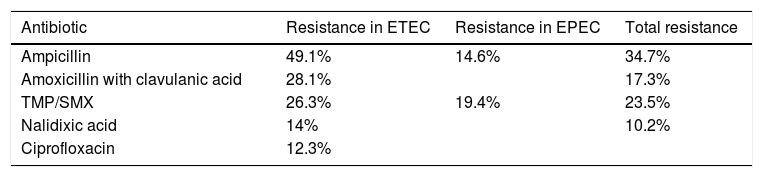
A study by Oliveira et al. analyzed the levels of resistance to different antibiotics utilized in treating E. coli infection. In infections due to ETEC: resistance to ampicillin was 49.1%, to amoxicillin with clavulanic acid was 28.1%, to trimethoprim with sulfamethoxazole was 26.3%, to nalidixic acid was 14%, and to ciprofloxacin was 12.3%. In infections caused by EPEC, the highest resistance percentages were seen with the use of trimethoprim with sulfamethoxazole (19.4%) and ampicillin (14.6%). Considering all the samples, in general, the following resistance percentages were reported: ampicillin 34.7%; trimethoprim with sulfamethoxazole 23.5%; amoxicillin with clavulanic acid 17.3%; and nalidixic acid 10.2%. All the samples were susceptible to cefotaxime and ceftriaxone31 (Table 2).
Antibiotic resistance of Escherichia coli.
| Antibiotic | Resistance in ETEC | Resistance in EPEC | Total resistance |
|---|---|---|---|
| Ampicillin | 49.1% | 14.6% | 34.7% |
| Amoxicillin with clavulanic acid | 28.1% | 17.3% | |
| TMP/SMX | 26.3% | 19.4% | 23.5% |
| Nalidixic acid | 14% | 10.2% | |
| Ciprofloxacin | 12.3% |
EPEC: enteropathogenic Escherichia coli; ETEC: enterotoxigenic Escherichia coli. TMP/SMX: trimethoprim with sulfamethoxazole.
In another study conducted by Uddin Rasheed et al., those authors demonstrated resistance to different antibiotics: ampicillin and amoxicillin 13.3%, tetracyclines 12.6%, trimethoprim with sulfamethoxazole 11.3%, streptomycin 8%, ciprofloxacin and ofloxacin 6.6%, cefotaxime 5.3%, and gentamycin 4.6%.32
Miranda et al. studied food contamination by E. coli and its antibiotic resistance. Vegetables and poultry meat had higher levels of contamination and the reported resistance to ampicillin was 76.5%, doxycycline 56.3%, sulfisoxazole and ciprofloxacin 77.3%, and chloramphenicol 58%.33
Ampicillin is no longer a useful antibiotic for eradicating E. coli. Its resistance ranges from 13.3-76.5% and utilizing antibiotics with lower resistance percentages is suggested, such as ciprofloxacin (6.6-12.3%), amoxicillin (13.3-28.1%), cefotaxime (5.3%), or gentamycin (4.6%).
Blastocystis sp.Blastocystis sp. is a protozoan parasite of worldwide distribution. Over one billion persons are colonized by it, with greater prevalence in developing countries, as opposed to developed countries. Blastocystis sp. produces a series of nonspecific gastrointestinal symptoms, such as diarrhea, flatulence, abdominal cramps, and even iron deficiency anemia and urticaria.34 First-line treatment is 250-800 mg of metronidazole, 3 times a day for 10 days, and in pediatric patients, the weight-based dose is 20-30 mg/kg/day.35
In their study, Rajamanikam et al. reported certain constant levels of resistance to different doses of metronidazole in Blastocystis sp. cultures.36
Batista et al. showed low efficacy of metronidazole in eradicating Blastocystis sp. A total of 79.5% of the patients treated had a clinical response and 48.4% had a microbiologic response. Response to metronidazole is variable.37
Entamoeba histolyticaEntamoeba histolytica (E. histolytica) is a protozoan with worldwide distribution, particularly affecting developing countries. It infects approximately 500 million persons and causes 110,000 deaths per year due to complications, situating it in third place, with respect to lethal parasitoses.38 Ninety percent of all persons that acquire the infection can be asymptomatic. Some of the factors favoring its transmission are poverty, overpopulation, poor hygiene, and malnutrition.39 Two strains have been identified: pathogenic E. histolytica and nonpathogenic E. dispar. The latter is responsible for asymptomatic infections and does not require antibiotic therapy. In contrast, pathogenic E. histolytica requires antimicrobial treatment and can cause fulminant colitis with manifestations that may include dysentery, toxic megacolon, and peritonitis. Extra-intestinally, it is related to liver abscess or hepatic amoebiasis.40
Providing treatment, even for asymptomatic infections, can lead to an increase in antimicrobial resistance. Metronidazole is the first choice of treatment but tinidazole, secnidazole, and ornidazole can also be used.
Recent studies have reported differences in the sensitivity of E. histolytica to different medications, showing that there is a small percentage of resistance to certain antimicrobials.41
Bansal et al. compared different studies on the in vitro susceptibility to antimicrobials used against E. histolytica, with different results. A minimum inhibitory concentration of metronidazole ranged from 12.5-15µm, whereas another study reported that 50% of the minimum inhibitory concentration of metronidazole was 18.47 µm for the most susceptible cultures, with up to >30 µm as the cutoff value for resistance. Higher minimum inhibitory concentrations have also been reported for chloroquine, tinidazole, and emetine.42
Wassmann et al. carried out in vitro cultures of E. histolytica, evaluating its susceptibility to metronidazole. In the culture with 5-7% of oxygen, the concentration of 12 µm of metronidazole eliminated 50% of the cells at 24 h and eliminated all of the cells at 72 h. In contrast, the anaerobic cultures at the same dose were less susceptible to metronidazole, with only 28% of the cells eliminated within the first 24 h and reaching only 50% at 72 h.43
Rossignol et al. studied the effect of nitazoxanide on E. histolytica infection, with the following results: there was clinical response and microbiologic response in 94% of the patients and only one patient (2%) presented with reinfection on day 14, according to the stool sample.44
Metronidazole resistance in intestinal amoebiasis is still reported as low, but in certain regions can reach 50%. Thus, having alternative treatments, such as nitazoxanide, is recommendable. Good results have been reported with nitazoxanide, and its 3 days of administration are fewer than the 7 days with metronidazole.
Bacterial peritonitisSpontaneous bacterial peritonitis is the most frequent infectious complication in cirrhotic patients, causing a 32% mortality rate. Variations in the gut microbiota have currently been observed in those patients and the prevalence of said infection has increased. The frequent use of quinolones as prophylaxis in bacterial peritonitis, together with the modifications in the gut microbiota, have led to the development of bacterial resistance.
The use of third-generation cephalosporines has been implemented as empiric treatment for bacterial peritonitis.45 Alves de Mattos et al. studied the efficacy of different antimicrobials for treating bacterial peritonitis, emphasizing the community-acquired or hospital-acquired origin of the infection. In the hospital-acquired infections, the classically recommended therapies had only 40% efficacy, complete resolution of the infections caused by multiresistant bacteria was 72%, and infections caused by other types of bacteria were resolved in 90% of the cases. In community-acquired infections, the use of beta-lactams as first-choice therapy is suggested. If there is no favorable response at 48 h, carbapenems and piperacillin-tazobactam are options, and their use is recommended as the first choice in hospital-acquired infections.46
Lutz et al. studied antimicrobial resistance in hospital-acquired bacterial peritonitis and obtained the following results: the isolated germs were E. coli (14% vs 11%), Klebsiella species (14% vs 8%), enterococci (14% vs 5%), and streptococci (10% vs 6%), the first percentage corresponding to peritonitis associated with healthcare and the second with hospital care. Likewise, antibiotic resistance varied according to etiology, with the first percentage corresponding to infections associated with healthcare and the second with hospital care: quinolones 50% vs 18%, piperacillin-tazobactam 30% vs 11%, and third- generation cephalosporines 30% vs 33%. The only microorganisms that were not sensitive to therapy with carbapenems were Enterococcus faecium and Candida albicans in hospital-acquired bacterial peritonitis.47
Fiore et al. carried out a review of treatment for hospital-acquired bacterial peritonitis caused by multiresistant pathogens and found that etiology due to Gram-positive bacteria has increased from 29.3% to 62.5%.48 Only 60% of the Gram-negative bacteria are susceptible to third-generation cephalosporines, and enterococci (24%) and staphylococci (19%) are the Gram-positive bacteria that have most commonly been isolated.49 The prevalence of methicillin-resistant Staphylococcus aureus (MRSA) was 85.7%, resistance of Enterococcus spp. to ampicillin varied from 37.5% to 75%, whereas the prevalence of Enterobacteriaceae varied from 28% to 51.2% and the prevalence of E. coli was 66.7%, both associated with extended-spectrum beta-lactamases.50
Determining the etiology of bacterial peritonitis is very important, given that both treatment and its efficacy are dependent on it. Resistance to different antibiotics, such as third-generation cephalosporines (40%), quinolones (50%), and methicillin (85%), is higher in hospital-acquired infections than in community-acquired infections. Establishing empiric antibiotic therapy with high doses of daptomycin, 8-12 mg/kg/24 h, plus meropenem 1 g every 8 h, plus an anti-MRSA beta-lactam is recommended.51
ConclusionsAccording to the present narrative review, the microorganisms that showed the highest resistance were Helicobacter pylori, with levels of resistance to metronidazole of 50-80%, to clarithromycin of 20-40%, and to levofloxacin of 30-35%; Clostridioides difficile, with levels of resistance to clindamycin of 8.3-100% and to cephalosporines of 51%; Campylobacter jejuni and Campylobacter coli with levels of resistance to fluoroquinolones of 85%; Escherichia coli with levels of resistance to ampicillin of 76.5%; Entamoeba histolytica with levels of resistance to metronidazole of 50%; and bacterial peritonitis with levels of resistance to third-generation cephalosporines of 40% and to methicillin of 85%.
In recent years, antimicrobial resistance has risen exponentially, with increasingly fewer antibiotics that are efficacious against gastrointestinal infections (Fig. 4). A continuous analysis of infected patients is required to prevent treatment failure, reducing unnecessary exposure to antibiotics and the consequent resistance to them.
To preserve the future use and function of antibiotics, new medications must be created that act against now-resistant microorganisms and are successful in reducing the speed at which antimicrobial resistance is developing. Treatment guidelines must be continuously updated, emphasizing the proper prescription of antibiotics and the specific criteria involved.
The correct use of antibiotics will always result in the adequate treatment of gastrointestinal infections, decreasing the impact on the microbiota, with a minimum of adverse effects, and in turn, reducing antimicrobial resistance.
Financial disclosureNo specific grants were received from public sector agencies, the business sector, or non-profit organizations in relation to this study.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Contreras-Omaña R, Escorcia-Saucedo AE, Velarde-Ruiz Velasco JA. Prevalencia e impacto de resistencias a antimicrobianos en infecciones gastrointestinales: una revision. Revista de Gastroenterología de México. 2021;86:265–275.