Laparoscopic Nissen fundoplication is currently considered the surgical treatment of choice for gastroesophageal reflux disease (GERD) and its long-term effectiveness is above 90%. Adequate patient selection and the experience of the surgeon are among the predictive factors of good clinical response. However, there can be new, persistent, and recurrent symptoms after the antireflux procedure in up to 30% of the cases. There are numerous causes, but in general, they are due to one or more anatomic abnormalities and esophageal and gastric function alterations. When there are persistent symptoms after the surgical procedure, the surgery should be described as “failed”. In the case of a patient that initially manifests symptom control, but the symptoms then reappear, the term “dysfunction” could be used. When symptoms worsen, or when symptoms or clinical situations appear that did not exist before the surgery, this should be considered a “complication”. Postoperative dysphagia and dyspeptic symptoms are very frequent and require an integrated approach to determine the best possible treatment. This review details the pathophysiologic aspects, diagnostic approach, and treatment of the symptoms and complications after fundoplication for the management of GERD.
En la actualidad la funduplicatura laparoscópica tipo Nissen se considera el tratamiento quirúrgico de elección para la enfermedad por reflujo gastroesofágico (ERGE) y su efectividad a largo plazo es mayor del 90%. Dentro de los factores predictores de buena respuesta clínica están la adecuada selección del paciente y la experiencia del cirujano. Sin embargo, la prevalencia de síntomas nuevos, persistentes y recurrentes posteriores al procedimiento antirreflujo puede ser de hasta un 30%. Las causas son múltiples pero en general se deben a una o más alteraciones en la anatomía y en la función esofagogástrica. Ante la persistencia de los síntomas posterior al procedimiento quirúrgico se debería de utilizar el término «falla». En el caso de que un paciente inicialmente manifieste control de sus síntomas y posteriormente estos reaparezcan, se pudiera emplear el término «disfunción». Por otra parte, ante el empeoramiento de los síntomas o la aparición de síntomas o situaciones clínicas que no existían antes de la cirugía, debe de considerarse una «complicación». La disfagia postoperatoria y los síntomas dispépticos son muy frecuentes y requieren un abordaje integral para poder determinar el mejor tratamiento posible. En esta revisión se detallan los aspectos fisiopatológicos, de diagnóstico y tratamiento de los síntomas y las complicaciones posteriores a la funduplicatura para el manejo de la ERGE.
Current indications for the surgical treatment of gastroesophageal reflux disease (GERD) include at least some of the following situations: a) symptomatic erosive GERD in young patients with adequate response to proton pump inhibitors (PPIs), b) evidence of large hiatal hernia or lower esophageal sphincter (LES) dysfunction, c) patients that wish to suspend treatment due to cost or convenience, d) intolerance to medical treatment, and d) severe symptoms, especially with nocturnal reflux and regurgitation.1
It is also recognized that before undergoing surgery, subjects must be thoroughly and adequately evaluated to corroborate that their symptoms are related to GERD. This is done through endoscopic studies, esophagram, outpatient pH study, and esophageal manometry. This preoperative approach is essential and can confirm the surgical indication or not, and in some cases, predict response.
Among all the surgical procedures, the laparoscopic Nissen fundoplication is considered the procedure of choice and its long-term effectiveness is above 90%, similar to that of the PPIs.2–5 Adequate patient selection and the experience of the surgeon are the predictive factors of good clinical response. However, symptom control generally decreases over time (90% at 3 years vs 67% at 7 years),4,5 even though this response expectedly varies, depending on the type of fundoplication (360o, 270 o, or 180o).
The main pathophysiologic mechanism for GERD is an increase in the transient LES relaxations. Only 10% of the patients have sphincter hypotonia and in some patients the length of the abdominal LES segment is short. The barrier function of the LES depends on its basal pressure that confers valve competence and on the concordance between the LES and the impingement with the diaphragmatic crura; the LES undergoes an opening during the phases of the respiratory cycle and when it is subjected to tension from gastric distension. The resulting incompetence exposes the esophageal mucosa to acid.6,7 The fundoplication (360o) around the distal esophagus strengthens the valve, preventing reflux, and promotes the correction of the short intra-abdominal segment and the concordance of the diaphragmatic impingement on the esophageal hiatus.7
Once a patient has undergone fundoplication, possible clinical outcomes are:
- a.
Complete response: there are no residual symptoms of GERD
- b.
Partial response: mild residual symptoms or symptoms of less magnitude
- c.
Appearance of “new” symptoms
- d.
Absence of clinical response
Even through there is no current standardized definition, patients that do not respond or that have de novo symptoms could be said to have failed antireflux surgery.8
As mentioned above, when symptoms persist after the surgical procedure, the term “failed” should be used. In a patient that initially has symptom control, but the symptoms then reappear, the term “dysfunction” can be used. The worsening of symptoms or the appearance of symptoms or clinical situations that did not exist before the surgery should be considered a “complication”.
Why does a patient that underwent antireflux surgery present with failure, dysfunction, or complications? It appears that poor patient selection and/or failure of the surgical technique are the most satisfying answers to this unsettling question.
PPI response does not define the GERD phenotype, given that the therapeutic test has very low specificity and the patients with acid-related diseases can respond satisfactorily.9–12 The PPI only changes the pH of the reflux material, but has no direct effect on the reflux.10 Likewise, the therapeutic gain with a PPI is greater in erosive reflux disease (ERD), than in non-erosive reflux disease (NERD) (48% [95% CI: 24.6-93.8] vs 27.2% [95% CI: 20.9-35.3]).13 Similarly, the clinical response to surgery is suggested to be different between the two diseases, given that in addition to different grades of acid exposure, there can also be associations with distinct pathophysiologic mechanisms. Whereas the size of the hiatal hernia, the supra-diaphragmatic residual acid pouch, the low basal LES pressure, and esophageal emptying alteration13 are the principal mechanisms in ERD, the transient LES relaxations and esophageal hypersensitivity are the main mechanisms in NERD.13,14 The latter disease is a very heterogeneous, more refractory entity, and abnormal esophageal acid exposure is present in only 35 to 42% of the cases.9,10,14,15 Physiologic studies (24-h pH study and esophageal manometry) performed during patient selection for surgery, can reduce the risk of overlooking non-visible preexisting diseases whose symptoms can be exacerbated in the postoperative period.
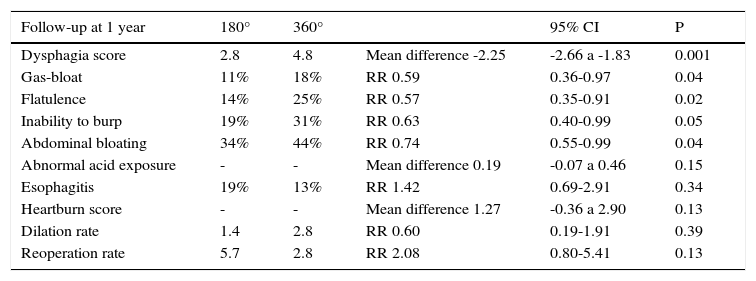
Anti-reflux techniques include partial fundoplication (anterior [Dor 180°] or posterior [Toupet 270°]) and total fundoplication (Nissen 360°) with crural re-approximation16 (Table 1). Dysphagia and gas-bloat syndrome are the main discomforts associated with the 360° wrap. For the purpose of reducing their incidence, techniques such as the Toupet and Dor fundoplications, which are efficacious for controlling reflux and regurgitation, have been proposed.17 The incidence of postoperative dysphagia (Nissen 360° wrap) is significantly reduced when the esophagus is calibrated (52 French bougie) during the construction of the wrap, when the esophageal wall is not anchored, and when the short vessels are cut for the adequate mobilization of the esophagus (tension-free fundoplication).17
Efficacy and safety of the two antireflux surgery methods.
| Follow-up at 1 year | 180° | 360° | 95% CI | P | |
|---|---|---|---|---|---|
| Dysphagia score | 2.8 | 4.8 | Mean difference -2.25 | -2.66 a -1.83 | 0.001 |
| Gas-bloat | 11% | 18% | RR 0.59 | 0.36-0.97 | 0.04 |
| Flatulence | 14% | 25% | RR 0.57 | 0.35-0.91 | 0.02 |
| Inability to burp | 19% | 31% | RR 0.63 | 0.40-0.99 | 0.05 |
| Abdominal bloating | 34% | 44% | RR 0.74 | 0.55-0.99 | 0.04 |
| Abnormal acid exposure | - | - | Mean difference 0.19 | -0.07 a 0.46 | 0.15 |
| Esophagitis | 19% | 13% | RR 1.42 | 0.69-2.91 | 0.34 |
| Heartburn score | - | - | Mean difference 1.27 | -0.36 a 2.90 | 0.13 |
| Dilation rate | 1.4 | 2.8 | RR 0.60 | 0.19-1.91 | 0.39 |
| Reoperation rate | 5.7 | 2.8 | RR 2.08 | 0.80-5.41 | 0.13 |
Taken from Pennathur et al.19
The prevalence of persistent and recurrent new postoperative symptoms is from 2 to 20%.17,18 The causes are multiple, but in general they are due to one or more abnormalities in the anatomy and esophagogastric function.19 Prevalence of reflux persistence of 8.2 and 10.1% and dysphagia of 7.5 and 5.1% at 2 and 5 years, respectively, after antireflux surgery, has been reported.20
These clinical manifestations can be immediate or early (within the first 4 weeks), or they can be late (more than 4 weeks).
- 1.
Early manifestations. Postoperative dysphagia is the most common manifestation and its incidence is quite variable, depending on the experience of the surgeon with the technique, the type of technique, and the follow-up time. This common manifestation can be temporary, due to edema and inflammation of the tissue involved in the procedure and can resolve in 2 to 4 weeks. Immediate severe complications are rare. Pneumothorax and emphysema are related to excessive hiatal dissection. Fundoplication disruption and/or perforation (esophagus or stomach) can be suspected when there is very intense pain, persistent vomiting, fever, tachycardia, and leukocytosis.5,20
- 2.
Late manifestations. The appearance of gas-bloat syndrome is a rare complication of unknown pathophysiology characterized by fullness and/or abdominal pain (sensation of intestinal gas), difficulty to burp, aerophagia, and delayed gastric emptying. Management should include dietary modifications, such as decreasing or suspending carbonated beverages, fermented foods, and foods that delay gastric emptying. If symptoms are mild, simethicone and prokinetics can be employed. Severe cases may require surgery and dismantling of the fundoplication and/or conversion of the 360o fundoplication to a 180o wrap.21
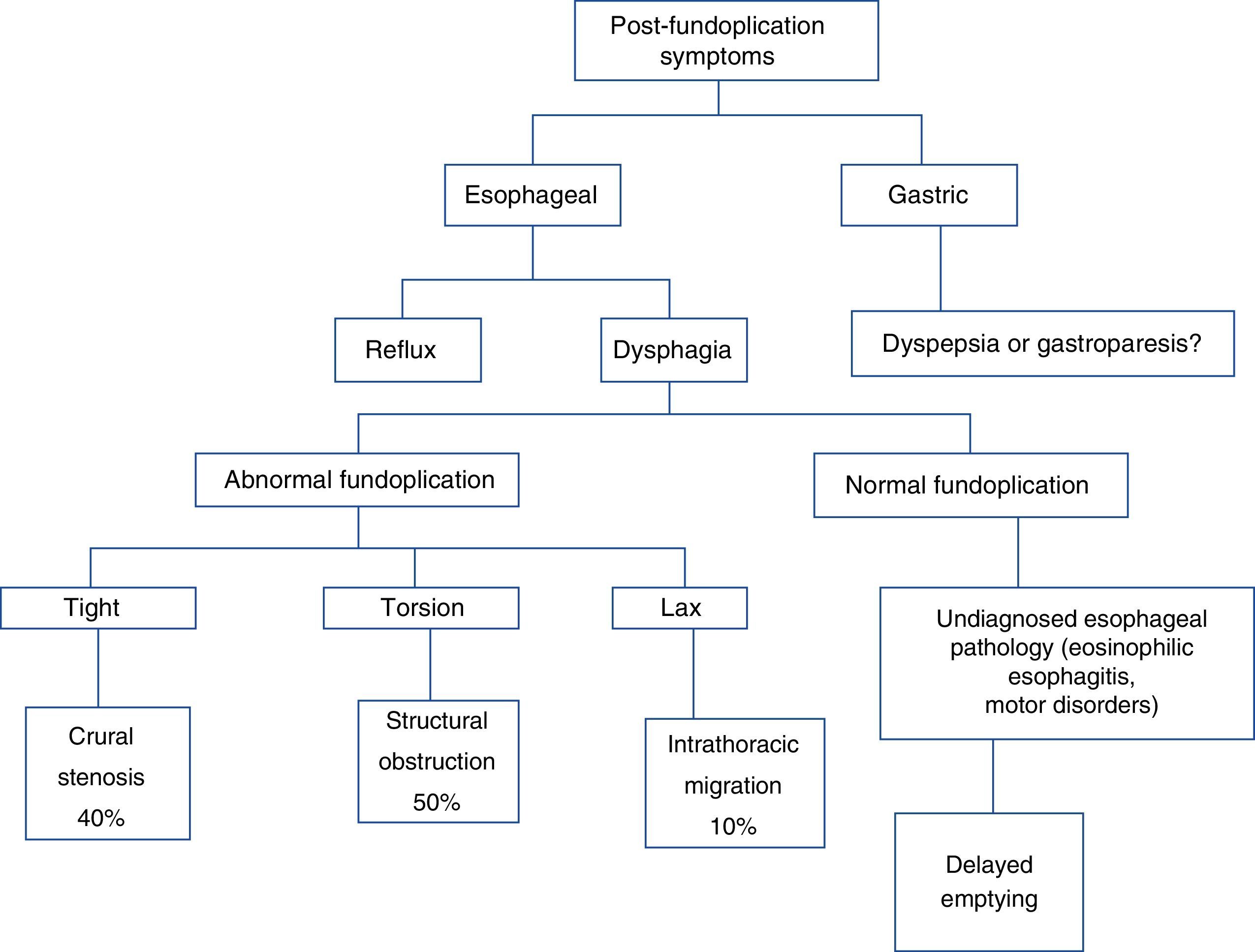
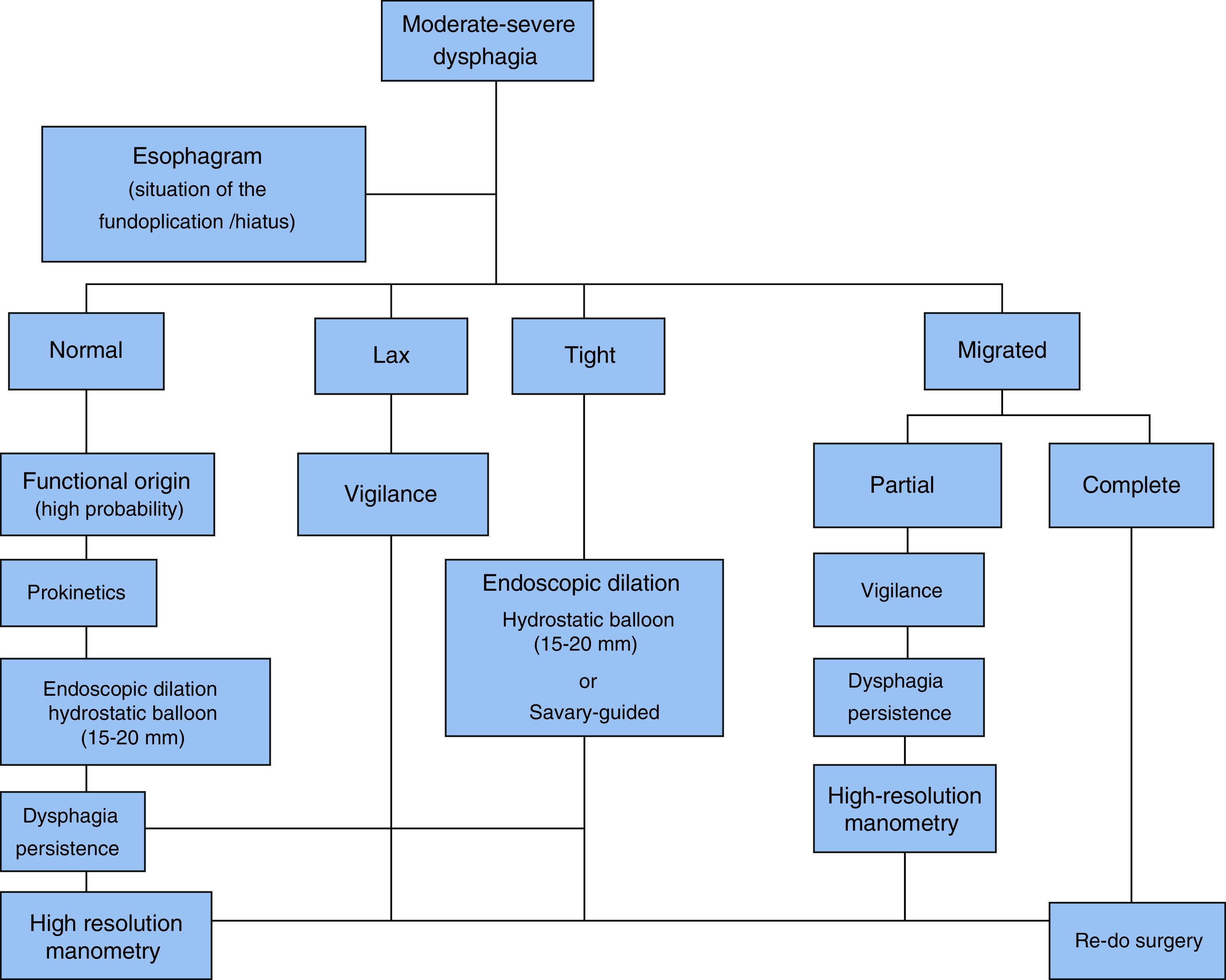
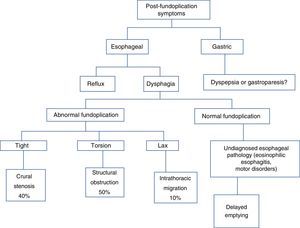
Figure 1 shows a proposed diagnostic algorithm based on the appearance of symptoms after fundoplication.
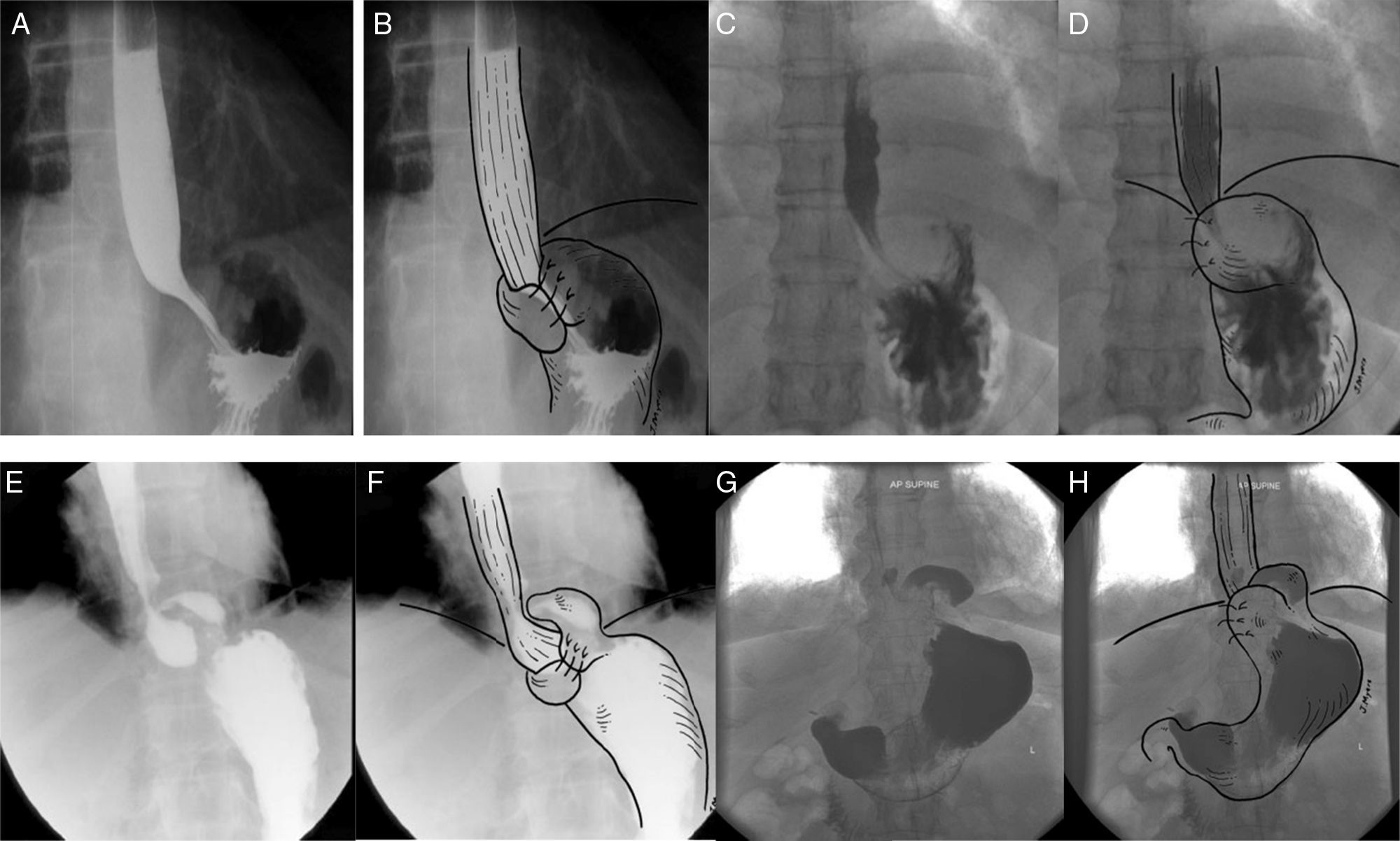
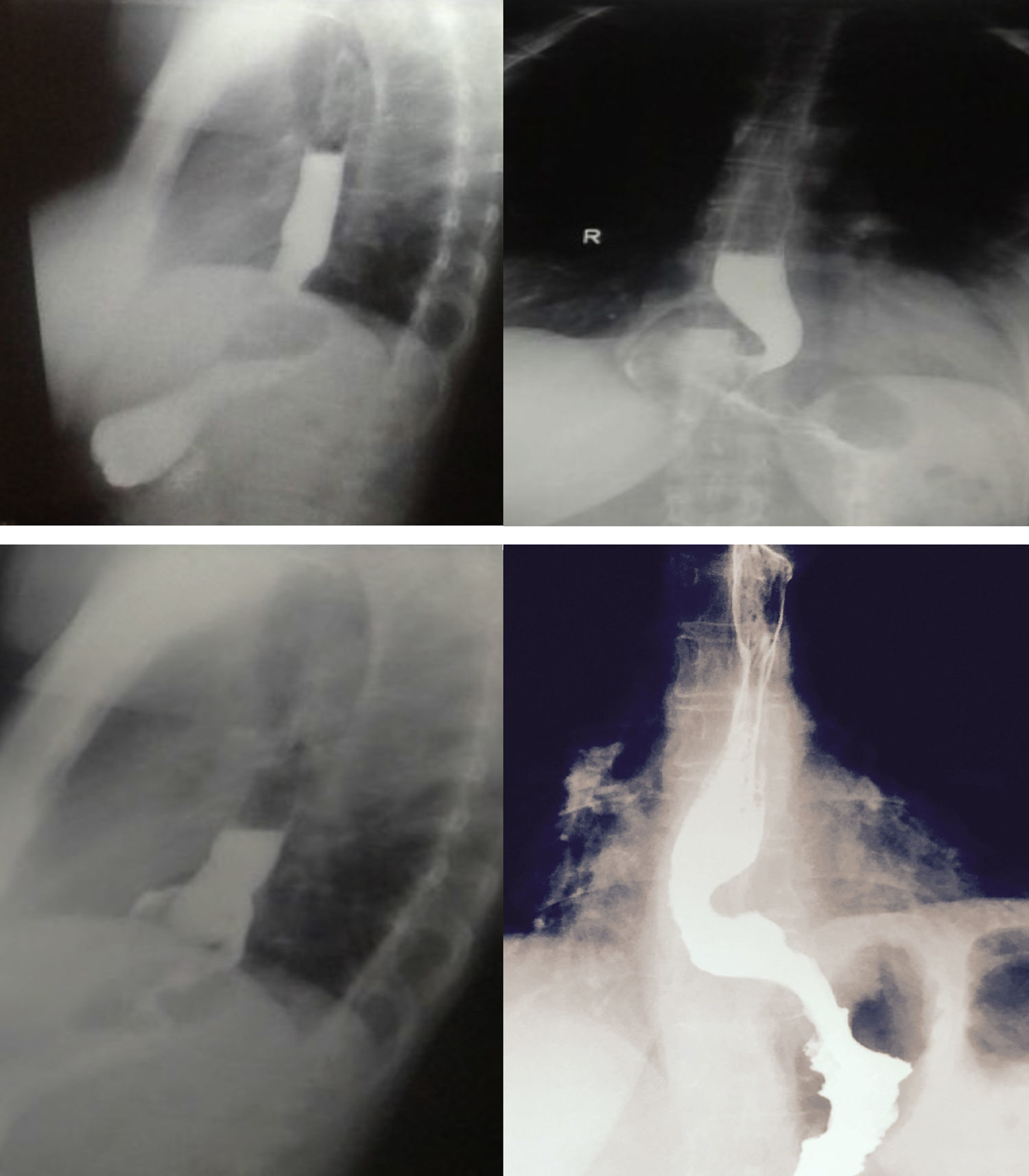
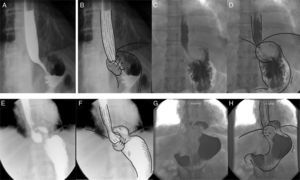
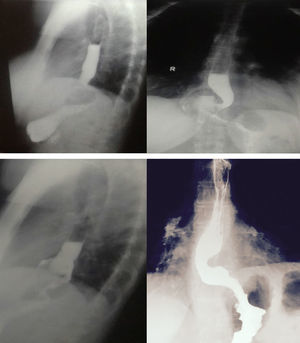
Esophageal symptomsA. Dysphagia. The incidence of postoperative dysphagia is high (76%). In general, its intensity is mild and resolves within the first weeks. Up to 20% of cases persist for one year and from 5 to 8% for a longer period of time. If dysphagia is persistent, a contrast esophagram is recommended5 (fig. 2). Preoperative dysphagia, delayed gastric emptying, lower amplitude pressure in the distal esophagus, hiatal hernia, and greater basal pressure in the gastroesophageal junction (GEJ) are predictors of postoperative dysphagia.21,22
Contrast radiologic evaluation in different fundoplication scenarios. A) Barium swallow with anteroposterior projection of a Nissen 360o fundoplication, in which the fundus of the stomach is completely wrapped around the distal esophagus. B) Barium swallow with artistic overlay. C) Barium swallow with anteroposterior projection of a Nissen 180o fundoplication, in which the fundus is partially wrapped around the distal esophagus. D) Barium swallow with artistic overlay. E) Barium swallow with anteroposterior projection of a Nissen 360o fundoplication with type I migration, showing the fundoplication herniation above the diaphragm, and the GEJ below the diaphragm. F) Barium swallow with artistic overlay. G) Barium swallow with anteroposterior projection of a Nissen 180o fundoplication with type I migration, showing the fundoplication herniation above the diaphragm, and the GEJ below the diaphragm. H) Barium swallow with artistic overlay.
Taken from Raeside et al.34
Post-fundoplication dysphagia can be related to inefficient peristalsis or to abnormal esophageal bolus transit, especially when the esophageal smooth muscle does not have the necessary peristaltic reserve to overcome the obstructive effect of the fundoplication.23 Patients with ineffective esophageal motility can have different post-fundoplication behavior patterns (persistence, modification, or resolution). Distal obstruction created by the surgery can improve the contraction amplitude of the esophageal body, particularly in the S2 segment that has been related to late smooth muscle contraction (“proximal latency”), and be a determining factor in postoperative dysphagia.24–26
Postoperative dysphagia can be due to:
- a.
Incorrect diagnosis: An undiagnosed, preexisting disease must always be considered. High-resolution manometry (HRM) is the reference standard for identifying esophageal motility disorders (ineffective motility and other primary motor disorders, including esophageal hypercontractility and achalasia).23 All patients being considered for antireflux surgery are evaluated through manometry to rule out any of those disorders, specifically achalasia.
- b.
Functional alterations: Functional outlet obstruction of the GEJ is secondary to crural stenosis caused by a tight wrap that sometimes is not evident in the esophagram and/or endoscopy. HRM is the reference standard for making this diagnosis.23,27
- c.
Structural alterations. Three well-defined alterations in this context are: 1) migration of the fundoplication (50%) due to laxity of the repair, 2) obstruction at or above the GEJ (40%) secondary to torsion, and c) stenosis (10%) due to a tight fundoplication (fig. 1).28 A tight or long wrap produces distal narrowing, proximal dilation, and delayed emptying of the radiologic contrast material. Figure 3 summarizes the diagnostic methods to be employed in patients with post-fundoplication dysphagia.6
Various classifications in relation to fundoplication dysfunction and/or failure have been described. Two of the most widely used follow below:
- 1.
Types of fundoplication dysfunction (Hinder):29
- -
Associated with hiatal hernia recurrence. A small fundic defect can be observed in the esophagram and endoscopy that is due to herniation. It can be caused by inadequate suture material or increased intra-abdominal pressure from extreme physical effort or from intragastric pressure due to persistent nausea and vomiting.
- -
Poor positioning. The fundoplication is intact with no supra-diaphragmatic stomach slippage. An hourglass image is observed in the esophagram and endoscopy.
- -
Formation of a pouch under the crura. Herniation of the fundoplication through the diaphragm is observed, showing deformation of the region, pronounced angulation of the elements, difficulty passing the endoscope, and food retention in the esophagus.
- -
- 2.
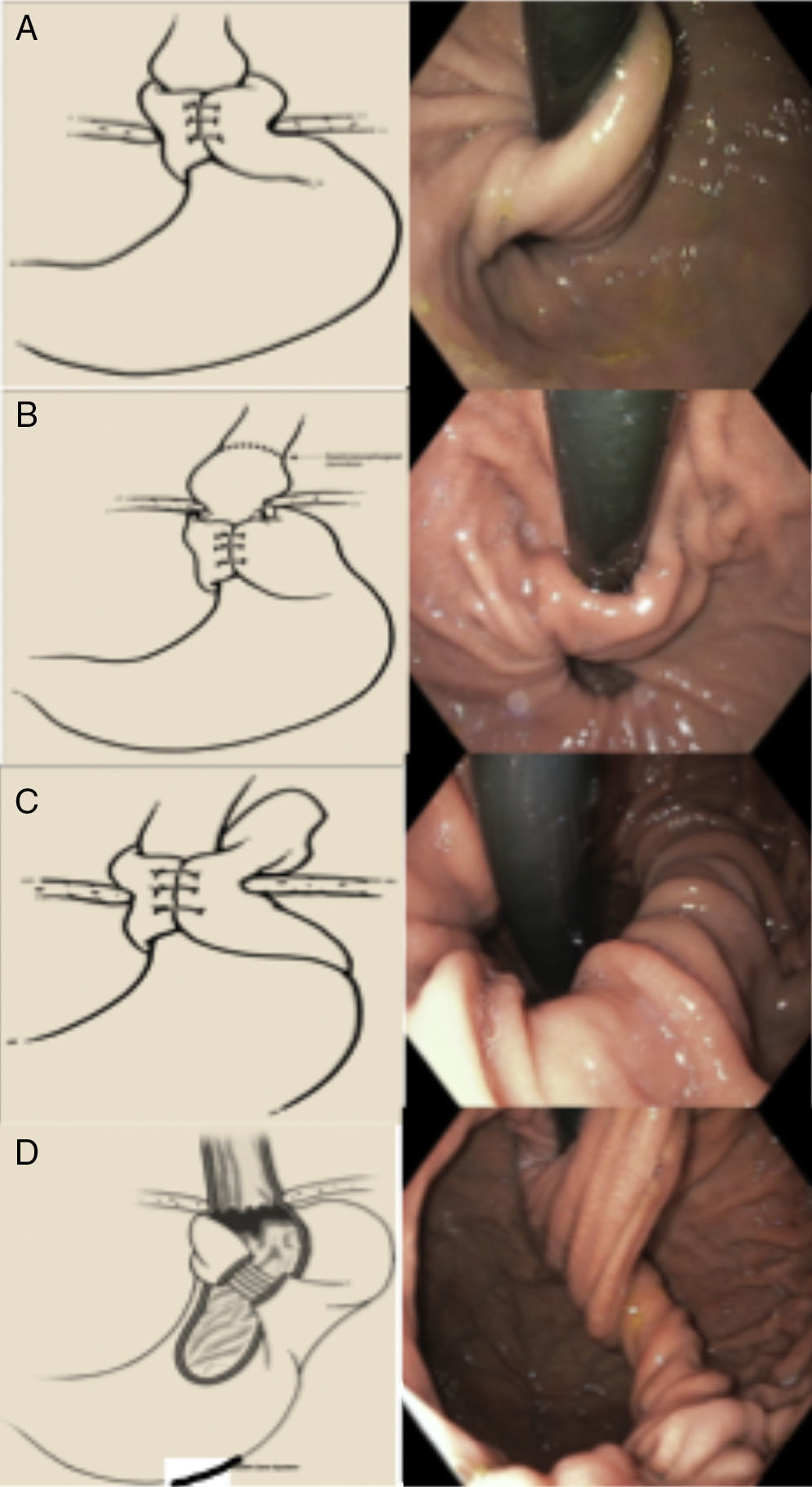
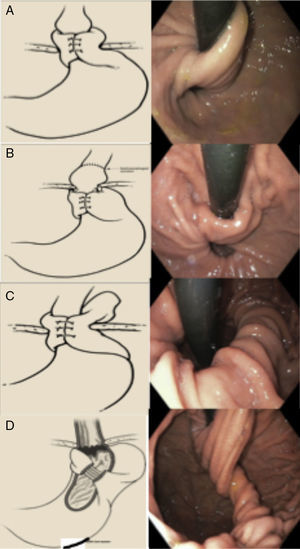
Horgan classification for failed fundoplication30 (fig. 4):
- -
Type I: GEJ herniated through the hiatus, with the fundoplication (IA) or without the fundoplication (IB).
- -
Type II: involves a paraesophageal component resulting from a redundant fundoplication.
- -
Type III: malformation (defect in the position or construction) of the fundoplication.
Figure 4.Types of failed fundoplication (Horgan classification). A) Type IA: hernia with no fundoplication herniation. B) Type IB: with fundoplication herniation. C) With paraesophageal displacement. D) Defect in the formation of the fundoplication.
Taken from Horgan and Pellegrini.30
(0.16MB). - -
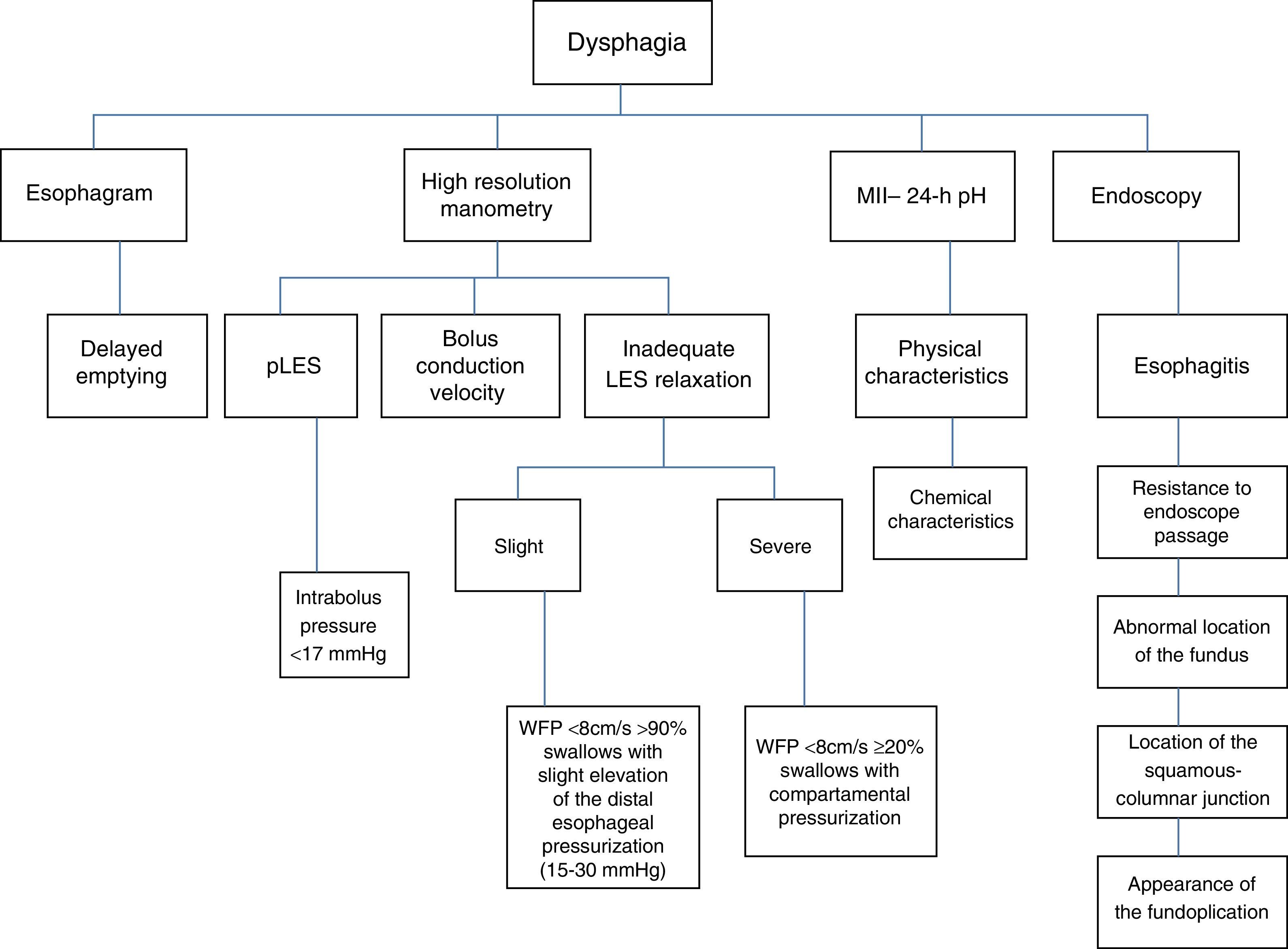
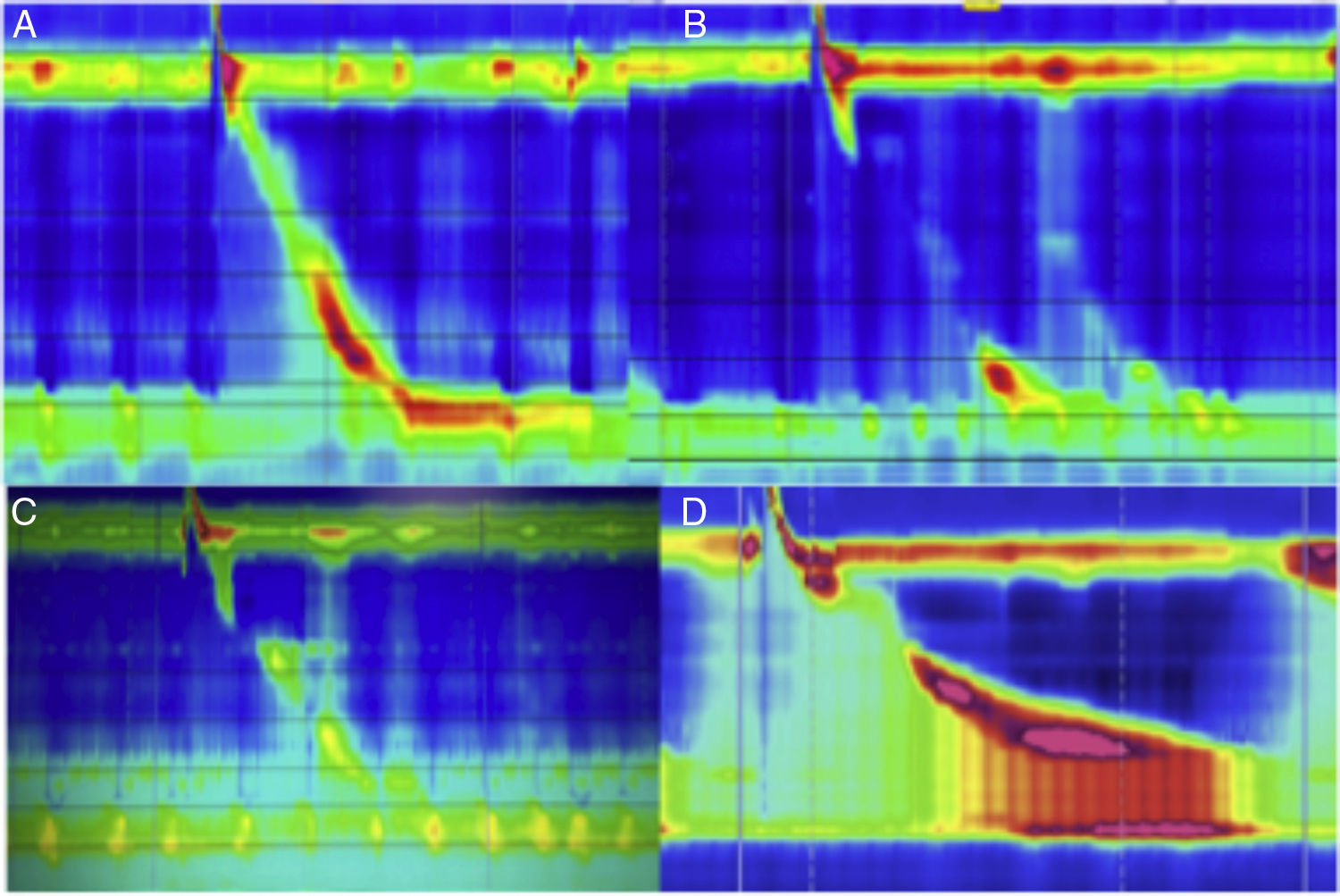
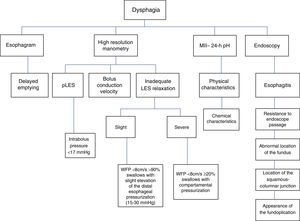
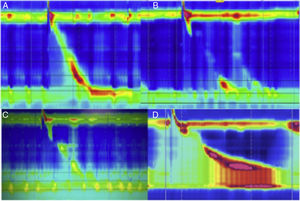
Dysphagia can be persistent if the fundoplication is very tight or long, or if the closure of the hiatal pillars is very close-fitting. Other causes are excessive GEJ angulation, intraluminal penetration of the prosthetic material (sutures), torsion of the abdominal esophagus due to poor spatial positioning of the fundoplication, longitudinal gastric volvulus, pseudo-tumoral fibrosis around the GEJ, etc. (fig. 5). HRM has become a very useful tool for the evaluation of postoperative dysphagia (fig. 6), because it enables the assessment of outlet tract obstruction or of inadequate esophageal clearance (if it is carried out with impedance study). Neither of these situations is perceived or evident with the conventional studies of endoscopy or esophagram.
A) Normal swallowing, normal peristalsis with competent LES in patient with fundoplication. B) Normal swallowing, failed peristalsis with incompetent LES in patient with fundoplication. C) Normal swallowing, reduced amplitude, lack of LES relaxation in patient with fundoplication. D) Normal swallowing, increased amplitude, lack of LES relaxation, and obvious outlet tract obstruction with increased intrabolus pressure (frontalization).
Postoperative dysphagia presents more in laparoscopic fundoplication than in the open procedure.31 Between 6 and 12% of cases require endoscopic dilation to palliate the dysphagia.32 In one case series, 12.4% (29/233) of the patients required from 1 to 5 dilations (mean 1.5) over a period of 3 to 330 days (mean 72 days), achieving a mean diameter of 18.6mm with a very variable clinical response, depending on the cause, and without severe complications. Dysphagia was resolved in 67% of the cases (12 out of 18) and 6 patients had reoperation with symptom resolution in half of those cases. No case showed improvement when dilation indication was other than dysphagia.32
Even though endoscopic dilations are the most widely used treatment of outlet tract obstruction and stricture, there are no clinical guidelines on endoscopic dilation in post-fundoplication dysphagia. There are no consensuses on the type of dilators, the number and periodicity of sessions, maximum optimal diameter, or especially, a universal definition of clinical success. In general, guidelines are based on the management guidelines for benign strictures. Dysphagia secondary to tight fundoplication can improve after endoscopic dilations in the majority of cases. Persistence can be due to: 1) decreased peristalsis secondary to the effect of esophageal distension, and 2) contraction defects during swallowing. Failure can result from a problem not only in the axial shortening of the external longitudinal muscle layer, but also in the contraction of the internal circular muscle layer.26
In a retrospective cases series, good clinical response was reported in 9 out of 14 (64%) dysphagia patients after balloon pneumatic dilation (30-40mm). The lowest residual LES pressure was the only predictor of success and was higher among the responders (mean 10 vs 5mmHg). The authors concluded that pneumatic dilation is safe and effective in postoperative dysphagia.33
Excessive cicatrization, intrathoracic migration, disruption of the fundoplication or hiatus, and stricture or angulation are structural causes that must be resolved as quickly as possible, given that they affect quality of life and increase the risk for severe complications.8,34 Endoscopic dilation is not beneficial in these cases.
The decision to perform a new surgery due to mechanical obstruction depends on the intensity of the dysphagia, because deferring it can cause esophageal dilation, retention esophagitis, chest pain, and pulmonary aspiration. However, there is no established waiting time. It depends on the cause, degree of discomfort, quality of life, or risk for severe complications, and thus each case must be individualized.
The incidence of postoperative failure is 2 to 30%. The frequency of post-fundoplication paraesophageal hernia is 7%35–36 and reoperation may be necessary if clinical response is not good. Reoperation frequency is 4 to 9%, but the fact that postoperative dysphagia is very high (33%) must be taken into consideration.37–39 Ascending migration of the fundoplication towards the thorax can be progressive and life-threatening for the patient. Other authors have reported reintervention rates of 1.8 to 10.8% for persistent dysphagia.40–42
Other case series state that herniation and migration are dependent on the type of surgical technique employed, and are reported at rates of 0.8 and 26%, respectively.39 The most frequent indications for surgical examination (“redo” fundoplication or reoperation) were:41 dysphagia (48%), reflux (33%), paraesophageal hernia (15%), and atypical symptoms (4%). Reoperation failure following Nissen fundoplication is approximately 10%.
Dismantling a fundoplication and re-doing it is indicated if the symptoms are associated with physiologic abnormalities or anatomic defects. Likewise, patients that do not adequately respond to endoscopic dilations should be considered candidates.38 Up to 46.8% of the patients with failed fundoplication (hernia recurrence, 71.1%) require a new surgery or fundoplication reconstruction and the success rate can be as high as 78 to 96%. However, the associated morbidity rate can reach 38%.43
The principles of a reconstructed fundoplication include GEJ identification, adequate esophageal mobilization, protection of the vagal nerves, complete reduction of the hernial content, and adequate dissection of the hiatal pillars, sparing the muscle fascia.44 It is essential that the surgeon be highly experienced in surgical reintervention and in the assessment or knowledge of esophageal pathophysiology.
Whether surgical failure was due to a relatively “anticipated” complication after the surgical procedure, or if it resulted from a deficient surgical technique from the beginning due to inexperience of the surgeon (360°, 270°, or 180°), should be discerned early, in the mediate postoperative period (6 months after surgery). With this in mind, it is recommended to carry out a contrast esophagram in all patients with fundoplication, with at least 50ml of water-soluble material 72h after the surgical procedure to detect any emptying delay. If there is none, then a barium esophagram should be performed, because at this period the inflammation of the tissue involved is sufficiently reduced and the functionality and technical pulchritude of the surgery can be objectively documented in this simple manner. Likewise, it is recommended to repeat this procedure at 6 months after surgery to evaluate and document the status and functional viability of the procedure. This simple recommendation lets the evaluating physician determine whether the patient under study has incident symptoms or if their persistence has actually been due to a technical deficiency.
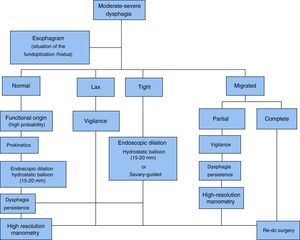
Figure 7 shows a proposed algorithm for moderate-to-severe post-fundoplication dysphagia management.
B. Regurgitation. Antireflux surgery significantly improves regurgitation (87 to 97%).6 However, recurrence of acid or non-acid regurgitation can reach 23%.5,7 In one case series, regurgitation was the most common preoperative symptom associated with medical treatment failure (54%).45 This symptom should cause suspicion of dysfunction and/or failure secondary to the structural alterations described in the section above.46–52
C. Heartburn. Antireflux surgery should be considered only if there is a clear association between heartburn and acid exposure prior to the intervention.43,44 In one case series, acid-associated GERD symptoms were the most common indication (88%) for antireflux surgery, with improvement of 82% and patient satisfaction with the surgery in 94% of the cases.45 Despite the significant improvement in heartburn, the recurrence rate is above 10%.53 A positive symptom index through MII-pH monitoring in patients with PPI has been suggested to predict a favorable response to medical or surgical treatment. Weakly acidic reflux events containing gas can produce distension of the proximal esophagus, generating symptoms in patients with fundoplication. The proximal esophagus is more sensitive to chemical and mechanical stimulation, compared with the distal esophagus. Woodland and Sifrim54 reported that the afferent nerves of the proximal mucosa are more superficial, which can have physiologic (prevention of gastroesophageal reflux aspiration) and pathologic (perception of reflux or dysphagia) implications. Pure gas reflux associated with pH descent (“acid vapor”) can be perceived as heartburn and regurgitation. PPI failure, like surgery failure, can be due to poor disease classification, even though approximately 20% (15-27%) of correctly diagnosed patients do not respond to PPI or surgery.2,51,52,55
D. Chest pain. This is one of the most frequent symptoms of post-fundoplication dysfunction. It is indistinguishable from ischemic heart pain and requires a reasonable differential diagnosis excluding a cardiac cause. In non-operated patients, the origin can be GERD, dysmotility, or hypersensitivity. The esophageal pain that presents after surgery may be induced by mechanical distension, acid exposure, temperature, and stimuli related to osmolarity. In the absence of acid, possible symptom explanations are: a) chemical stimuli (weakly acid reflux, bile, and proteolytic enzymes) that can activate the chemoreceptors of the mucosa, b) mechanical stimuli (distension or contraction) due to the activation of the mechanoreceptors in the esophageal wall because of fluid or gas that produce reactive changes in the circular (internal) and longitudinal (external) muscle layers, and c) peripheral and central hypersensitivity.55–59
E. Gas-bloat syndrome. This syndrome includes a poorly defined group of physical discomforts associated with the inability to vent gas from the stomach into the esophagus after fundoplication. The symptoms are abdominal bloating (subjective), inability to burp, postprandial fullness, nausea, flatulence, inability to vomit, and epigastric pain.
Gas bloat is more prevalent in the complete Nissen fundoplication than in the partial Toupet type (31.19% vs 23.91%, RR: 1.31, 95% CI: [1.05, 1.65], p = 0.02). The inability to burp is more prevalent in the complete fundoplication (14.9% vs 8.4%; RR: 1.79, 95% CI: [1.06, 3.03], p = 0.03). Gas-bloat syndrome has been reported in 18.64%/10.34%, flatulence 74.58%/67.24%, and epigastralgia in 25.42%/31.03% after complete and partial antireflux surgery, respectively.60
In a case series of 1,063 operated patients, 101 (9.5%) stated they were unsatisfied after the procedure, with gas-bloat syndrome being the most frequent cause of discomfort one year after surgery (59%).61
The cause of the syndrome is not very clear, but the proposed mechanisms include: 1) the inability of the GEJ to relax in response to gastric distension, 2) aerophagia, a frequent habit in patients with severe GERD, which becomes problematic after fundoplication when the air cannot be vented, 3) the alteration in receptive relaxation and accommodation in response to food with rapid gastric emptying, and 4) surgical injury to the vagal nerve that delays gastric emptying and interferes with transient relaxation.
The recommended therapies, albeit with no convincing evidence of their efficacy, include: 1) dietary modifications, avoiding gas-producing foods and carbonated beverages, 2) eating more slowly to prevent aerophagia, 3) suspending tobacco smoking, 4) using gas-reducing agents, such as simethicone, and 5) using prokinetic drugs.62
Gastroduodenal symptomsA. Dyspeptic symptoms. The majority of patients with fundoplication have dyspeptic symptoms. After surgery, fullness, abdominal bloating, and early satiety will develop in more than 30% of the patients and only some patients will develop severe gastric dysfunction (gastroparesis).63 Post-fundoplication dyspeptic symptoms are the result of changes in the sensory and motor function of the proximal stomach, but they may also result from the fundoplication technique employed and not necessarily from vagal nerve injury.64,65 The accommodation reflex is an important mechanism of gastric physiology.66 In functional dyspepsia, it is the pathophysiologic mechanism responsible for symptoms in 40% of the cases. It is also the cause of symptoms in upper gastrointestinal disorders, such as diabetic gastropathy and post-fundoplication syndrome. The gastric accommodation reflex enables the temporary storage of food before its controlled passage into the intestine. This reflex consists of a reduction in tone and an increase in gastric capacitance in response to food ingestion, which increases the volume without increasing the intragastric pressure. This adaptive relaxation is not perceived under physiologic conditions.67 Altered accommodation produces autonomous nervous system dysfunction. The afferent dysfunction increases the perception of the visceral stimulus (physiologic or noxious), due to the vagal impulses that stimulate the nitrergic neurons.68–70 Nissen fundoplication has been associated with more alterations in gastric accommodation. There is greater symptom intensity with the 360o fundoplication than with the 180o procedure. Lindeboom et al.,67 demonstrated that maximum fundic relaxation was significantly reduced in patients that underwent complete fundoplication (p < 0.01), resulting in a positive correlation between surgery duration and the degree of fundic relaxation (r = 0.67; p < 0.001). Diabetic patients and those with fundoplication will have more gastric accommodation alterations.68 This altered accommodation (“diastolic dysfunction”) can be associated with autonomous nervous system dysfunction. Afferent dysfunction or “irritable stomach” increases the perception of the visceral stimulus (physiologic or noxious) due to vagal impulses that stimulate the nitrergic neurons.69,70
On the other hand, when delayed gastric emptying is documented, the most likely pathophysiologic mechanism causing the dyspeptic symptoms is vagal nerve injury.
B. Gastroparesis. From the clinical perspective, it is sometimes quite difficult to distinguish dyspepsia from gastroparesis. The latter could be considered the final stage or the maximum expression of dyspeptic symptoms and it is clinically characterized by very-difficult-to-manage nausea and vomiting. Diagnosis requires the documentation of delayed gastric emptying through one of the available techniques, such as 4-h radionuclide scintigraphy, the Smart Pill, or breath tests.71 Post-fundoplication gastroparesis is secondary to vagotomy or vagal nerve injury. Early satiety in 88% of the patients and abdominal bloating/flatulence in 64% are the most common symptoms in the first 3 months following surgery, with a resolution rate above 90% at one year.72–75 Initial medical treatment should be directed toward fluid and electrolyte resuscitation, nutritional support, and weight loss prevention. If oral ingestion is insufficient, enteral feeding through a jejunostomy catheter should be used.72 Even though prokinetics can be used (as in the case of diabetic or idiopathic gastroparesis), there is very little evidence in relation to symptom resolution. The prokinetics to be used should be selected based on their safety profile and interaction with other medications, and they include: domperidone, itopride, metoclopramide, levosulpiride, and erythromycin, among others.76–79 Even though botulinum toxin has been described in the treatment of diabetic and idiopathic gastroparesis, its effectiveness in cases of post-fundoplication gastroparesis is not known. The same holds true for gastric pacemakers (Enterra therapy, Medtronic Inc, Minneapolis, MN). Sub-total gastrectomy may be indicated in extreme cases, especially if there are refractory symptoms and weight loss conditioning severe malnutrition.80–83
Extra-esophageal manifestationsThe frequency of extra-esophageal manifestations (cough, laryngitis, asthma, anxiety attacks, sleep alterations) after fundoplication is not known.84,85 Nevertheless, it is assumed that the appearance or reappearance of these, as well as heartburn and regurgitation, are indicators of procedure failure or dysfunction. When extra-esophageal symptoms appear de novo in the postoperative period, impedance-24-h pH monitoring is suggested for documenting symptom presentation and their association with reflux (acid or non-acid).
In addition to laryngeal manifestations, a wide range of symptoms (arrhythmia, sleep disorders, anxiety attacks, etc.) can present with varied frequency throughout the postoperative period, without an obvious cause to explain them. These symptoms can be associated with undocumented preexisting dysautonomia, or simply with the fact that the esophageal innervation and the heart have the same embryonal origin. These manifestations are generally underestimated and their etiology is attributed to primary gastrointestinal problems. Cardiac rhythm alterations related to swallowing are very frequent in patients with GERD, dyspepsia, or in those that have undergone antireflux surgery. In postoperative esophageal dysfunction, with no evidence of previous esophageal motor disorder, arrhythmias can be triggered by esophageal distension during dry or voluminous food ingestion or cold and/or carbonated drinks, or even after burping, probably secondary to the functional obstruction to the bolus. Arrhythmia can be experimentally reproduced during the swallowing of food or by insufflating a balloon inside the esophagus.86–88
The mechanism behind post-swallow syncope is the stimulation of the hypersensitive mechanoreceptors of the esophageal wall that causes distension through an autonomic vasovagal reflex resulting in a parasympathetically mediated negative chronotropic effect. Another proposed mechanism is the sympathetic stimulation followed by cholinergic vasodilation or the release of vasodilators that initially increase blood pressure, followed by deep hypotension and finally, bradycardia. The triggering mechanism of the tachyarrhythmias caused by swallowing is less clear. However, it is thought that atrial ectopic beats can trigger atrioventricular re-entry tachycardias. When they occur in the presence of atrioventricular block, the enhanced automaticity may be the mechanism behind the arrhythmia.86–88
The most accepted hypothesis is that the initiating mechanism is a neural reflex resulting from autonomic stimulation. The increase in vagal tone may paradoxically cause tachycardia. It is plausible that the mechanisms mediated by the parasympathetic nervous system are involved in all arrhythmias associated with swallowing, causing bradycardia in some cases and tachycardia in others.88
ConclusionsComplications associated with fundoplication for the treatment of GERD are frequent, albeit the majority of them are minor and transitory. A small group of patients will present with symptom persistence, recurrence, or the appearance of new symptoms. The frequency of incident symptoms will not be the same, due to the different fundoplication techniques (Nissen 360o, 180o, Toupet, Hill, etc.), resulting in a variable durability of its effectiveness over time. The appearance of new symptoms can be triggered by the surgery in cases of existing pathologies not detected beforehand. Thus, preoperative evaluation is essential in deciding the fundoplication technique to employ. Follow-up should be systematic with contrast radiology at specific times, performing these studies in all cases at the period of edema resolution, and in individualized cases according to intervention durability (effectiveness). Adequate diagnostic approach and treatment will improve quality of life in the patient with incident symptoms after surgery. We believe more studies are required that describe the behavior and pathophysiologic mechanisms of the different extra-esophageal complications related to fundoplication failure. In addition, the formulation of guidelines for follow-up and for defining the response time to a given treatment are necessary, so that decisions can be made in relation to surgical reintervention.
Financial disclosureNo financial support was received in relation to this study.
Conflict of interestSergio Sobrino-Cossío is an Advisory Board Member of Takeda Pharmaceuticals and a Speaker for Takeda.
Julio César Soto-Pérez is a Speaker for Takeda.
Gualberto Mateos-Pérez is an Advisory Board Member of Takeda Pharmaceuticals and a Speaker for Takeda.
Oscar Teramoto-Matsubara is an Advisory Board Member of Takeda Pharmaceuticals and a Speaker for Takeda.
Miguel Morales-Arámbula is an Advisory Board Member of Takeda Pharmaceuticals and a Speaker for Takeda.
Adolfo Sáez-Ríos works at Takeda Pharmaceuticals (Medical Marketing Manager).
José Antonio Vargas-Romero works at Endo Pharmaceuticals-SOMAR Group (Medical Director).
Enrique Coss-Adame is a speaker at Laboratorios Takeda de México and has been a Consultant for and collaborates with Laboratorios Asofarma de México.
José Tawil, Manuel Vallejo-Soto, Ángel Mario Zárate-Guzmán, Elymir Galvis-García, José Antonio Carrasco-Rojas, and Oscar Quiroz-Castro declare that they have no conflict of interest.
José María Remes-Troche is an Advisory Board Member of Takeda Pharmaceuticals, Alfa-Wassermann, Asofarma, and Almirall. He is a Speaker for Takeda, Asofarma, Alfa-Wassermann, Almirall, and Astra-Zeneca.
Please cite this article as: Sobrino-Cossío S, Soto-Pérez JC, Coss-Adame E, Mateos-Pérez G, Teramoto Matsubara O, Tawil J, et al. Síntomas y complicaciones posfunduplicatura: abordaje diagnóstico y tratamiento. Revista de Gastroenterología de México. 2017;82:234–247.