Pancreatic cancer is considered one of the most aggressive solid tumors. In Mexico, it is the twelfth cause of cancer, with 4,489 cases diagnosed annually, and accounts for 4.9% of oncologic deaths.
AimThe aim of our study was to describe the clinical and epidemiologic characteristics of the patients diagnosed with pancreatic cancer spanning an 11-year period at the Instituto Nacional de Ciencias Médicas y Nutrición “Salvador Zubirán”.
MethodsA retrospective, cross-sectional study was conducted that included 479 patients diagnosed with pancreatic cancer, within the time frame of 2003-2013. The documented findings were summarized through descriptive statistics.
ResultsOf the patients with pancreatic ductal adenocarcinoma, 50.9% were women, and the mean patient age at diagnosis was 61.5 years. A total of 48.4% of the cases were diagnosed at clinical stage IV, 12.9% presented with clinical stage III, and 25.0% had localized disease. Surgery was performed on 37.5% of the patients, the most frequent of which was pancreatoduodenectomy. The surgical mortality rate was 5.5%.
ConclusionThe clinical characteristics in our study group were similar to those described in the literature. However, the number of candidates for surgical treatment was higher than that reported in other hospitals and the percentage of borderline tumors was lower. Those differences, respectively, are possibly associated with the nature of our referral center and the prolonged intervals between diagnosis and treatment that result in the loss of potential surgical patients.
El cáncer de páncreas es considerado uno de los tumores sólidos más agresivos. En México representa la doceava causa de cáncer con 4,489 casos diagnosticados por año y representa el 4.9% de las defunciones oncológicas. El objetivo del estudio es describir las características epidemiológicas y clínicas de los pacientes con diagnóstico de cáncer de páncreas en un periodo de once años del Instituto Nacional de Ciencias Médicas y Nutrición “Salvador Zubirán”.
MétodosEstudio retrospectivo – transversal que incluyó 479 pacientes con diagnóstico de cáncer de páncreas en el periodo 2003 - 2013. Se incluyó estadística descriptiva para resumir los hallazgos documentados.
ResultadosDe los pacientes con adenocarcinoma ductal de páncreas el 50.9% fueron mujeres, la edad promedio al diagnóstico fue de 61.5 años. Se diagnosticaron en un estadio clínico IV el 48.4 % de los casos, mientras que el 12.9% se presentaron como estadio clínico III y el 25.0% como enfermedad localizada. El 37.5% de los pacientes fueron sometidos a cirugía, siendo la pancreatoduodenectomía el procedimiento más frecuentemente realizado. La mortalidad quirúrgica fue del 5.5%.
ConclusiónLas características clínicas en nuestro grupo de estudio muestran similitud con la literatura, sin embargo, el número de candidatos a un tratamiento quirúrgico fue superior a las cifras reportadas en otros hospitales, no obstante, la cifra de tumores limítrofes fue menor; probablemente asociado con la naturaleza de centro de referencia que representa nuestra institución, así como a la pérdida de pacientes potencialmente quirúrgicos debido a un periodo de ventana prolongado entre diagnóstico y tratamiento.
Pancreatic cancer is considered one of the most aggressive solid tumors and is one of the main causes of oncologic mortality in Western countries. Pancreatic ductal adenocarcinoma (PDAC) is the most common histologic variant in 85% of cases.1–3 It frequently presents in patients above 70 years of age as locally advanced or metastatic disease.4
According to 2018 data from the World Health Organization, PDAC is the fifteenth cause of cancer worldwide, with an incidence of 4.8 cases per 100,000 inhabitants and a mortality rate of 4.4 cases per 100,000 inhabitants.5
In 2018, in the United States, 55,440 new cases were diagnosed, and 43,330 deaths were reported, making it the fourth cause of oncologic mortality in that country.6 An incidence of 6.9 cases per 100,000 inhabitants has been estimated, according to the latest 2012 data. Incidence has risen, compared with the 6.6 and 5.7 cases per 100,000 inhabitants in 2008 and 1999, respectively. That increase is most likely associated with the risk factors of obesity and population aging. In contrast, there have been no significant changes in the mortality rate over time,7–9 showing the trend for an increase in frequency of the disease and its poor survival rate. Currently, overall 5-year survival is estimated at less than 5%,5 which can increase to 15-30% with surgical treatment. However, only 15-20% of patients present with resectable disease at diagnosis, based on the degree of contact between the tumor and certain vessels.10,11
In Mexico, PDAC is the twelfth cause of cancer, with 4,489 cases diagnosed annually, and is the cause of 4.9% of oncologic deaths, according to 2015 data from the Instituto Nacional de Estadística y Geografía (INEGI), figures that demonstrate an area of opportunity in the prevention, diagnosis, and treatment of PDAC.12
The aim of the present study was to describe the epidemiologic and clinical characteristics of the patients diagnosed with pancreatic cancer spanning an 11-year period at the Instituto Nacional de Ciencias Médicas y Nutrición “Salvador Zubirán”.
MethodsA total of 479 patients were included that had a histopathologic diagnosis of PDAC, documented within the time frame of 2003 and 2013. Electronic and physical case records were reviewed to obtain the clinical and epidemiologic characteristics.
Statistical analysis. Through descriptive statistics, the quantitative variables were expressed as mean and range and the qualitative variables as frequency and percentage. Electronic and physical case records were reviewed for the postoperative follow-up. The patients whose records did not contain the information required were called by telephone if a current number was available.
Ethical considerationsThe present study was approved by the research and ethics committee of the Instituto Nacional de Ciencias Médicas y Nutrición “Salvador Zubirán” and the authors declare that it contains no personal information through which the patients could be identified.
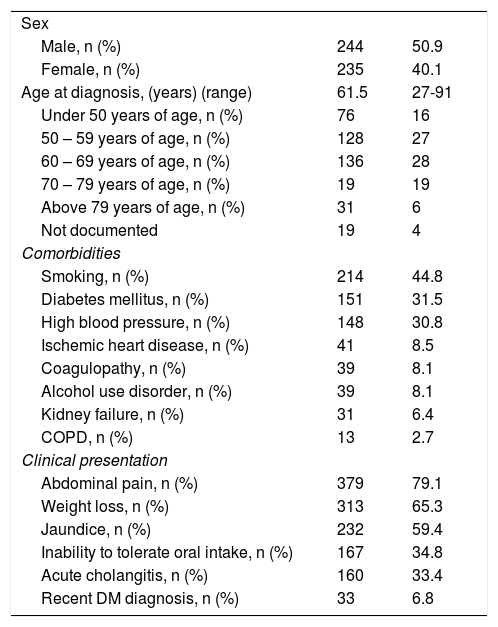
ResultsThe data from 479 patients with a histopathologic diagnosis of PDAC were obtained. Of those patients, 244 (50.9%) were women, 235 (49.1%) were men, and the mean patient age at diagnosis was 61.5 years (range 27-91). According to age distribution, 53% of the cases were reported in patients above 60 years of age and 16% of the cases were diagnosed in patients under 50 years of age. The most frequent associated comorbidities were smoking (44.8%, with a mean smoking index of 0.45), diabetes mellitus (DM) in 151 cases (31.5), and high blood pressure in 148 (30.8). Abdominal pain was the most frequent symptom in 379 patients (79.1%), followed by weight loss in 313 (65.3%) and jaundice in 232 (59.4% n=232). Other clinical presentations were inability to tolerate oral intake, acute cholangitis, and recently diagnosed DM (defined as DM diagnosed within the 6 months prior to PDAC diagnosis) (Table 1).
Clinical characteristics of the patients diagnosed with PDAC.
| Sex | ||
| Male, n (%) | 244 | 50.9 |
| Female, n (%) | 235 | 40.1 |
| Age at diagnosis, (years) (range) | 61.5 | 27-91 |
| Under 50 years of age, n (%) | 76 | 16 |
| 50 – 59 years of age, n (%) | 128 | 27 |
| 60 – 69 years of age, n (%) | 136 | 28 |
| 70 – 79 years of age, n (%) | 19 | 19 |
| Above 79 years of age, n (%) | 31 | 6 |
| Not documented | 19 | 4 |
| Comorbidities | ||
| Smoking, n (%) | 214 | 44.8 |
| Diabetes mellitus, n (%) | 151 | 31.5 |
| High blood pressure, n (%) | 148 | 30.8 |
| Ischemic heart disease, n (%) | 41 | 8.5 |
| Coagulopathy, n (%) | 39 | 8.1 |
| Alcohol use disorder, n (%) | 39 | 8.1 |
| Kidney failure, n (%) | 31 | 6.4 |
| COPD, n (%) | 13 | 2.7 |
| Clinical presentation | ||
| Abdominal pain, n (%) | 379 | 79.1 |
| Weight loss, n (%) | 313 | 65.3 |
| Jaundice, n (%) | 232 | 59.4 |
| Inability to tolerate oral intake, n (%) | 167 | 34.8 |
| Acute cholangitis, n (%) | 160 | 33.4 |
| Recent DM diagnosis, n (%) | 33 | 6.8 |
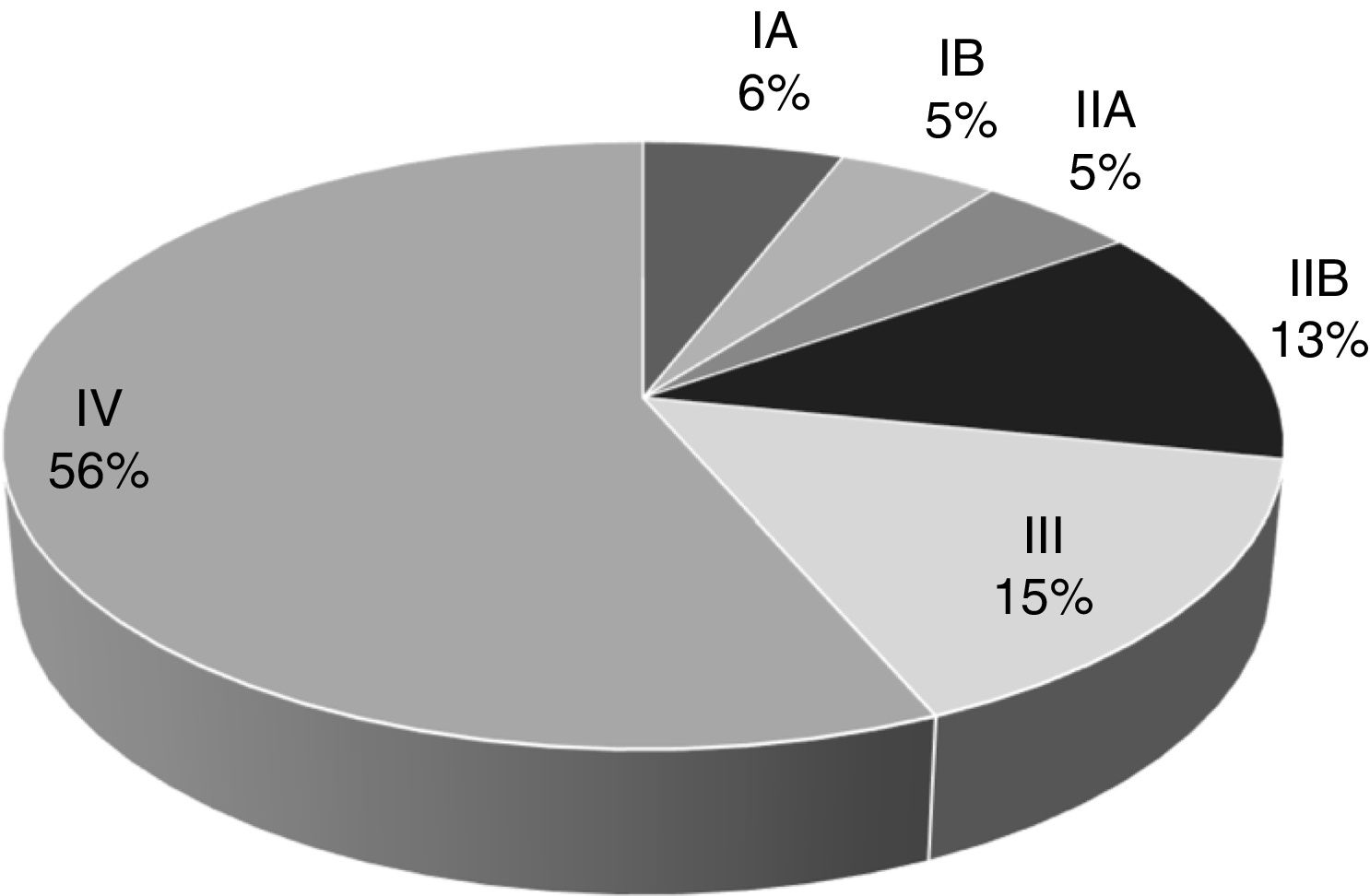
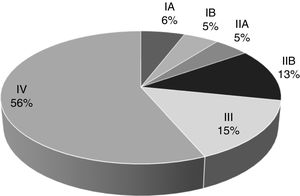
Over the span of the eleven years analyzed, 232 patients (48.4%) were diagnosed with clinical stage IV metastatic disease, 62 cases (12.9%) presented with clinical stage III locally advanced disease, and 121 patients (25.0%) had localized disease, the most favorable scenario. Clinical stage was not determined at diagnosis in 64 cases (14.4%) because those patients were diagnosed outside of our hospital center or their case records contained inaccurately recorded data (Fig. 1).
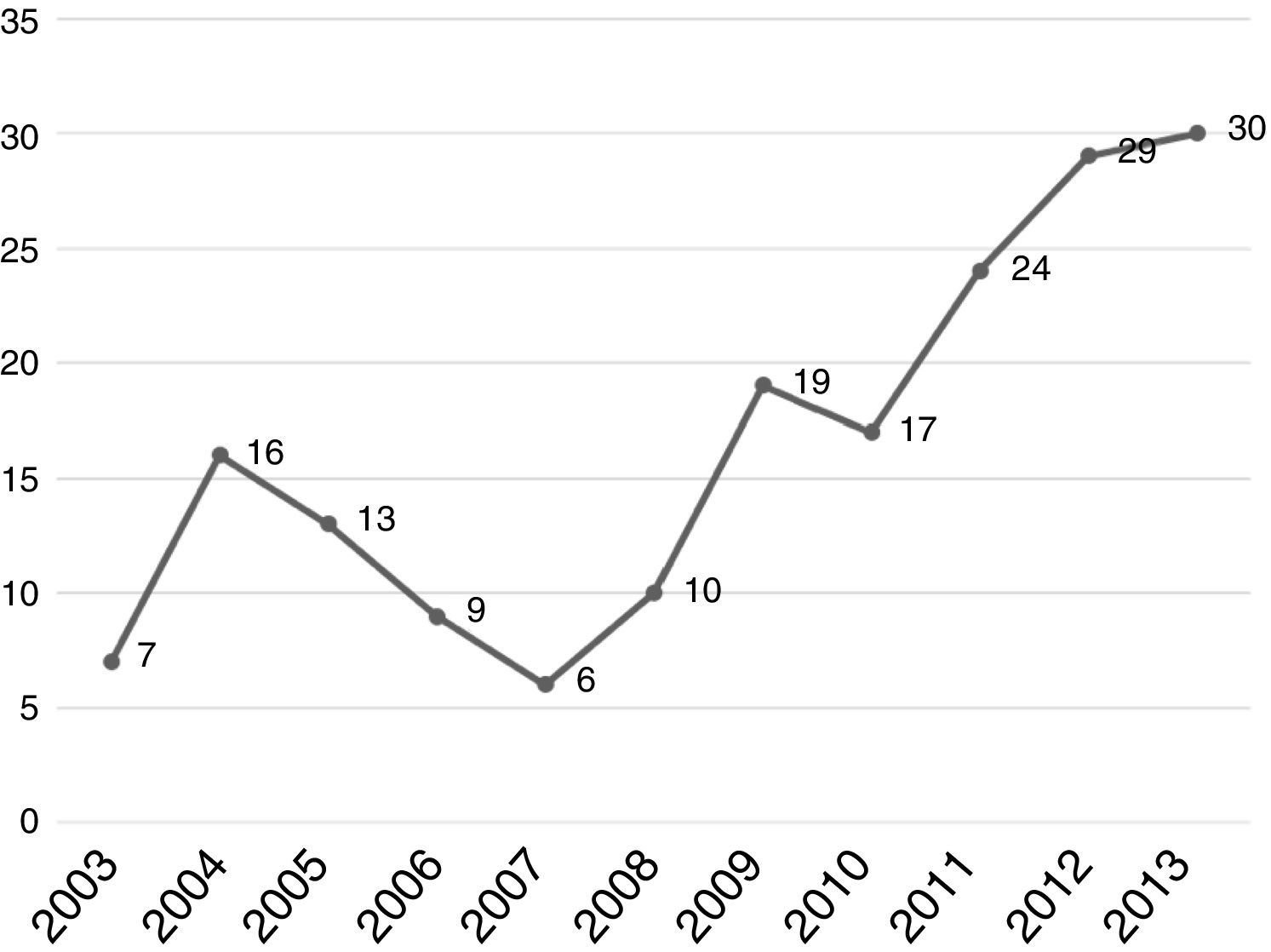
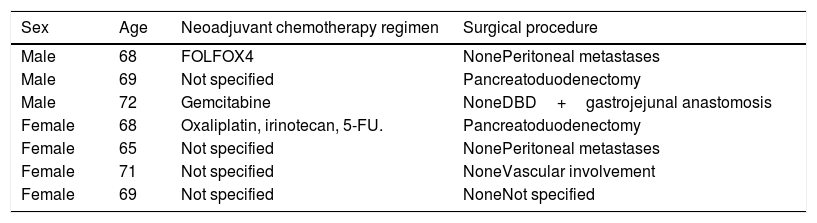
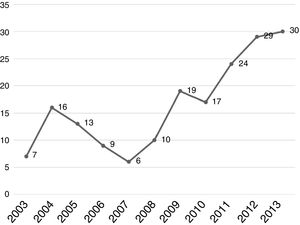
Regarding tumor characteristics, 353 cases (73.6%) had tumors located at the head of the pancreas, 54 cases (11.2%) at the neck-body of the pancreas, and only 28 cases (5.8%) at the tail of the pancreas. Specific location was not recorded for 44 patients (9.1%). All patients diagnosed at our hospital center were evaluated through tomography, revealing resectable tumor in 178 patients (37.1%). Seven patients (1.6%) had borderline tumors, according to the definition provided by the National Comprehensive Cancer Network and they received neoadjuvant chemotherapy (Table 2). A total of 180 patients (37.5%) underwent surgery: 178 patients with resectable PDAC at diagnosis and 2 patients (28.5%) with borderline PDAC that responded to neoadjuvant chemotherapy. A total of 128 patients (71.1%) received adjuvant chemotherapy. The analysis of the yearly number of cases that were candidates for surgery showed an increase over time and 2011-2013 was the most representative period of time at our hospital center (Fig. 2).
Patients diagnosed with pancreatic ductal adenocarcinoma that received neoadjuvant chemotherapy.
| Sex | Age | Neoadjuvant chemotherapy regimen | Surgical procedure |
|---|---|---|---|
| Male | 68 | FOLFOX4 | NonePeritoneal metastases |
| Male | 69 | Not specified | Pancreatoduodenectomy |
| Male | 72 | Gemcitabine | NoneDBD+gastrojejunal anastomosis |
| Female | 68 | Oxaliplatin, irinotecan, 5-FU. | Pancreatoduodenectomy |
| Female | 65 | Not specified | NonePeritoneal metastases |
| Female | 71 | Not specified | NoneVascular involvement |
| Female | 69 | Not specified | NoneNot specified |
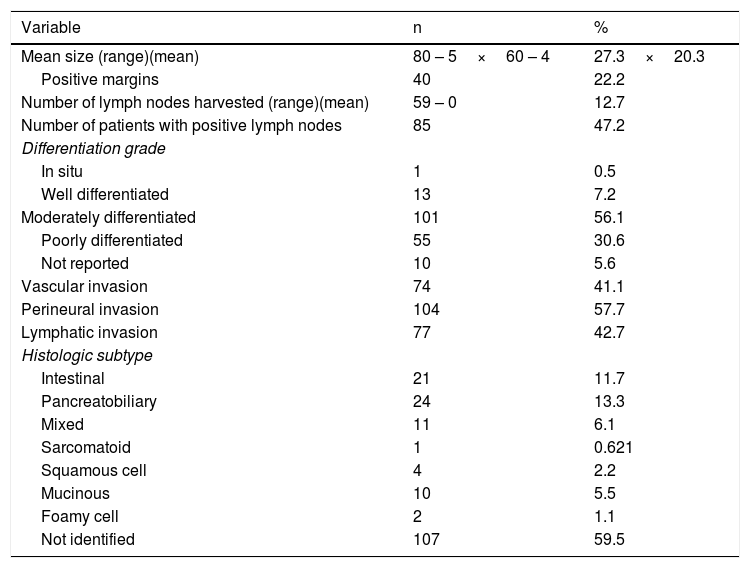
Two main surgical procedures were carried out. The Whipple procedure (pancreatoduodenectomy) was the most frequent. It was performed on 176 patients (97.7%) and 37 cases (21.0%) required portal vein reconstruction. Distal pancreatectomy was performed on the rest of the patients (2.3%). Mean tumor size was 27.3×20.3mm, the majority of the patients presented with moderately differentiated lesions (56.1%) and the pancreatobiliary subtype (13.3%). The pathologic evaluation, showing other characteristics, as well, is summarized in Table 3.
Pathologic characteristics of the patients with pancreatic resection.
| Variable | n | % |
|---|---|---|
| Mean size (range)(mean) | 80 – 5×60 – 4 | 27.3×20.3 |
| Positive margins | 40 | 22.2 |
| Number of lymph nodes harvested (range)(mean) | 59 – 0 | 12.7 |
| Number of patients with positive lymph nodes | 85 | 47.2 |
| Differentiation grade | ||
| In situ | 1 | 0.5 |
| Well differentiated | 13 | 7.2 |
| Moderately differentiated | 101 | 56.1 |
| Poorly differentiated | 55 | 30.6 |
| Not reported | 10 | 5.6 |
| Vascular invasion | 74 | 41.1 |
| Perineural invasion | 104 | 57.7 |
| Lymphatic invasion | 77 | 42.7 |
| Histologic subtype | ||
| Intestinal | 21 | 11.7 |
| Pancreatobiliary | 24 | 13.3 |
| Mixed | 11 | 6.1 |
| Sarcomatoid | 1 | 0.621 |
| Squamous cell | 4 | 2.2 |
| Mucinous | 10 | 5.5 |
| Foamy cell | 2 | 1.1 |
| Not identified | 107 | 59.5 |
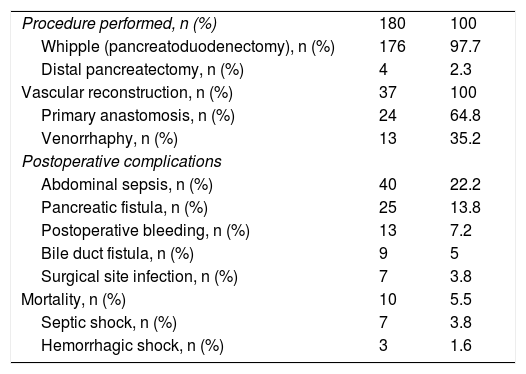
Abdominal sepsis and pancreatic fistula were among the postoperative complications, presenting in 40 and 25 patients, respectively (22.2 and 13.8%). Less than 10% of the cases presented with postoperative bleeding, bile duct fistula, or surgical site infection. The mortality rate was 5.5% (10 patients), 7 cases of which were secondary to septic shock and 3 to hemorrhagic shock, which presented within the first 24h after surgery (Table 4).
Surgical characteristics of the patients diagnosed with PDAC.
| Procedure performed, n (%) | 180 | 100 |
| Whipple (pancreatoduodenectomy), n (%) | 176 | 97.7 |
| Distal pancreatectomy, n (%) | 4 | 2.3 |
| Vascular reconstruction, n (%) | 37 | 100 |
| Primary anastomosis, n (%) | 24 | 64.8 |
| Venorrhaphy, n (%) | 13 | 35.2 |
| Postoperative complications | ||
| Abdominal sepsis, n (%) | 40 | 22.2 |
| Pancreatic fistula, n (%) | 25 | 13.8 |
| Postoperative bleeding, n (%) | 13 | 7.2 |
| Bile duct fistula, n (%) | 9 | 5 |
| Surgical site infection, n (%) | 7 | 3.8 |
| Mortality, n (%) | 10 | 5.5 |
| Septic shock, n (%) | 7 | 3.8 |
| Hemorrhagic shock, n (%) | 3 | 1.6 |
The mean follow-up for the patients that underwent surgery was 28 months (range 0-137) in 58% of the cases. The remaining 42% were lost to surgical follow-up and could not be reached by telephone. Fifty percent of the cases that underwent surgery (52 patients) had disease recurrence at a mean of 22 months (range 1-114). Recurrence was local in 26.0% (14 patients) and systemic in the remaining 38 patients. Overall postoperative 3-year survival was 13.6%.
Discussion and conclusionThe Instituto Nacional de Ciencias Médicas y Nutrición “Salvador Zubirán” is a tertiary care hospital center in Mexico City that focuses on different surgical areas, including hepatopancreatobiliary surgery and pancreatic cancer surgery. The population treated at the center is made up of patients diagnosed there, as well as a majority of patients that are referred there for multidisciplinary treatment. Said population has specific characteristics, given that the hospital is a referral center, and represents a small sample of pancreatic cancer in Mexico. Among the limitations of our study, are those inherent in its retrospective design, which include a lack of data availability, loss of information, and loss of long-term follow-up. Nevertheless, it provides valuable information on the PDAC population at our hospital center.
We know that the incidence of PDAC varies across the globe and that the highest incidence is in developed regions, such as the United States and the European countries. However, there is a worldwide trend toward an increase in the number of cases, associated with a longer-living population and better diagnosis and treatment of oncologic tumors.13 A higher incidence has also been reported in male patients in certain regions of Armenia, the Czech Republic, and Hungary, but sex distribution is the same in high incidence regions, such as North America and Western Europe.14 In addition, age at diagnosis in those areas tends to be above 70 years and only 5-10% of the cases present in patients under 50 years of age.15 Sex distribution was the same in our patients, showing a similar behavior in relation to developed regions. However, the mean age at diagnosis was 61 years, with 16% of the patients under 50 years of age, revealing a younger age at diagnosis. That could be related to the presence of risk factors in our society, such as smoking, obesity, and DM, which are comorbidities that are strongly associated with the development of PDAC.16–19 In our study patients, 44.8% were smokers and 31.5% presented with DM, but given the limitations of our study, we could not establish a cause-and-effect relationship.
With respect to the clinical stage of presentation, worldwide statistics show that more than 50% of the patients are diagnosed with metastatic disease, whereas 30-35% and 15-20% present with locally advanced disease and localized disease, respectively. An effort has been made to improve those figures through new tools for disease prevention and diagnosis in patients with risk factors.20–22 Over the 11 years analyzed herein, the percentage of patients with localized disease that were candidates for surgical treatment was 25%, slightly higher than the figures reported by other hospitals, which is most likely associated with the fact that our hospital is a referral center.
The clinical presentation of PDAC varies, depending on its location and the time of disease progression. It tends to manifest nonspecifically, and the most frequent symptoms are weight loss in 92% of cases, jaundice in 80%, and abdominal pain in 70%, according to reports in the literature.23,24 Other symptoms are anorexia, choluria, acholia, nausea, vomiting, and weakness. We found similar figures at our hospital center. The most frequent symptom was abdominal pain in 79.1% of the patients, weight loss in 65.3%, and jaundice in 59.4%. Those data were strongly associated with tumor location, with 84.8% at the head of the pancreas and 5.8% at the tail of the pancreas.
Surgery is currently the only curative treatment for PDAC, improving 3-year survival from 5-6% to 35-40% in patients with clinical stage I and from 3-4% to 15-25% in patients with clinical stage II.20 In the subgroup of patients with borderline disease, response to neoadjuvant treatment enables them to undergo surgical treatment at high-volume centers, with the same survival rates.25 In our study, 28.3% of the patients were found to have resectable tumors at diagnosis, through tomographic evaluation, compared with the 10-20% reported in the majority of studies in the literature,26 and 13.9% of the cases fit the criteria for borderline tumor, in contrast to the 30-40% reported in other studies.26,27 That low figure could be associated with the prolonged time interval between diagnostic suspicion and diagnostic approach, in which patients that are candidates for potentially curative treatment are lost.
A total of one hundred and eighty patients underwent surgery and the Whipple procedure was performed in 97.7%. In the 11-year evaluation, the number of annual cases were shown to increase at our hospital center up to a mean 25-30 cases in recent years. In addition, 21% of the surgical cases required vascular reconstruction, which is a lower percentage than that reported at other hospital centers.28,29 Those findings are associated with the higher level of experience attained in pancreatic surgery at our hospital, as well as the emergence of greater scientific evidence related to vascular reconstruction techniques. In the postoperative evaluation, abdominal sepsis and the presence of pancreatic fistula were the most common complications, at 22.2 and 13.8%, respectively. The postoperative mortality rate was 5.5%, comparable to that of other high-volume referral centers.30 Importantly, our hospital center now has the protocols for postoperative serial drain amylase measurement, something that was not adequately established during part of our study period, resulting in bias related to the diagnosis of pancreatic fistulas, especially grade A fistulas (“biochemical leaks”).
Mean follow-up was 28.06 months in 58% of the postoperative patients. There was disease recurrence in 50% of the cases at a mean 22.06 months and systemic recurrence was the most frequent. The general 3-year mortality rate was 13.6%, similar to that reported in the literature for patients with clinical stage III, but below that expected for earlier stages.
In conclusion, our study group had clinical characteristics similar to the etiology reported in developed countries, but there was a higher number of patients under 50 years of age. On the other hand, the number of candidates for surgical treatment was higher than the figures described at other hospitals, but the number of borderline tumors was lower. Respectively, those differences are possibly associated with the fact that our hospital is a referral center, and with the prolonged time interval between diagnostic suspicion and diagnostic approach, resulting in the loss of patients that could have been possible surgical candidates. Despite the limitations inherent in the retrospective design of our study, the data we found show areas of opportunity for improving PDAC statistics in Mexico: establishing measures for improving modifiable risk factors, carrying out opportune screening in high-risk patients, and improving the time intervals involved in the care given patients suspected of PDAC.
Financial disclosureNo financial support was received in relation to this article.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Sánchez Morales GE, Moguel Valladares RA, Flores Maza J, Clemente Gutiérrez U, Sánchez-García Ramos E, Domínguez Rosado I, et al. Adenocarcinoma ductal de páncreas. Experiencia de 11 años en un centro de tercer nivel. Revista de Gastroenterología de México. 2021;86:118–124.