Crohn’s disease (CD) is a subtype of chronic and incurable inflammatory bowel disease. It can affect the entire gastrointestinal tract and its etiology is unknown.
ObjectiveThe aim of this consensus was to establish the most relevant aspects related to definitions, diagnosis, follow-up, medical treatment, and surgical treatment of Crohn’s disease in Mexico.
Material and methodsMexican specialists in the areas of gastroenterology and inflammatory bowel disease were summoned. The consensus was divided into five modules, with 69 statements. Applying the Delphi panel method, the pre-meeting questions were sent to the participants, to be edited and weighted. At the face-to-face meeting, all the selected articles were shown, underlining their level of clinical evidence; all the statements were discussed, and a final vote was carried out, determining the percentage of agreement for each statement.
ResultsThe first Mexican consensus on Crohn’s disease was produced, in which recommendations for definitions, classifications, diagnostic aspects, follow-up, medical treatment, and surgical treatment were established.
ConclusionsUpdated recommendations are provided that focus on definitions, classifications, diagnostic criteria, follow-up, and guidelines for conventional medical treatment, biologic therapy, and small molecule treatment, as well as surgical management.
La enfermedad de Crohn (EC) es un subtipo de la enfermedad inflamatoria intestinal crónica e incurable que puede afectar a todo el tracto gastrointestinal y cuya etiología es desconocida.
ObjetivoEstablecer los aspectos más relevantes relacionados a las definiciones, diagnóstico, seguimiento, tratamiento médico y quirúrgico de la enfermedad de Crohn en nuestro país.
Material y métodosSe invitaron a especialistas de la República Mexicana de las áreas de Gastroenterología y Enfermedad Inflamatoria Intestinal. Se dividió el consenso en 5 módulos, con 69 enunciados. Se aplicó el método de panel Delphi, para ello se envió las preguntas previó a la reunión a todos los participantes para que fueran editadas y ponderadas. Durante la reunión presencial se mostraron los artículos seleccionados al nivel de evidencia clínica y se llevó a cabo la discusión y votación final del grado de acuerdo en todos los enunciados.
ResultadosEs el primer consenso mexicano de la enfermedad de Crohn en donde se establecen las recomendaciones para las definiciones, clasificaciones, aspectos diagnósticos, seguimiento, tratamiento médico y quirúrgico.
ConclusionesSe dan recomendaciones actualizadas enfocadas a las definiciones, clasificaciones, los criterios diagnósticos, seguimiento y pautas del tratamiento médico convencional, biológico y moléculas pequeñas, así como el manejo quirúrgico.
Crohn’s disease (CD) is a subtype of chronic and incurable inflammatory bowel disease that can affect the entire gastrointestinal tract, from the mouth to the anal region. It is characterized by flare-ups and remissions and its etiology is unknown.1
The EPIMEX study included patients from all of the Mexican States and showed a four-fold increase in incidence and prevalence in the 15-year period from 2000 to 2015.2 The disease is characterized by chronic inflammatory diarrhea, with the presence of mucus and microscopic blood, as well as abdominal pain, fever, and weight loss.
Diagnosis is made through the correlation of clinical, biochemical, endoscopic, radiologic and/or histopathologic findings.3 Once diagnosed, the disease should be classified according to the Montreal classification, which includes age at diagnosis, location, and phenotype. Age categories are (A1) under 16 years of age, (A2) between 17 and 40 years of age, and (A3) above 40 years of age; location can be in the terminal ileum (L1), the colon (L2), the ileum and colon (L3), and the upper gastrointestinal tract (L4); and phenotype can be classified as inflammatory (B1), stricturing (B2), or fistulizing (B3).4 According to the EPIMEX study2 the most frequent location was ileocolonic, presenting in 50% of patients, followed by colonic (25%), ileal (23%), and upper gastrointestinal (3%) location.
The ideal medication choice for a patient is dependent on the phenotype of the disease, degree of disease activity, and comorbidities; evaluating long-term medication-related adverse effects and complications is important. The main treatment goal is to achieve clinical remission, reduce relapse, lower complication rates, and achieve mucosal healing (MH), which is associated with a decrease in relapses, hospitalizations, and surgeries. There are two treatment strategies for CD. The step-up protocol is to start conventional therapy, mainly steroids and immunomodulators, in patients that do not have poor prognosis factors. The top-down strategy, utilized in patients with the poor prognosis factors of age at diagnosis under 40 years, stricturing and fistulizing phenotype, perianal disease, upper gastrointestinal tract involvement, and extensive disease of the intestine, is to start biologic therapy and finish with steroid use.3
Definitions of characteristics of CD follow below:
Degree of activity: Crohn’s Disease Activity Index (CDAI)
- i
Mild: 150-220 points
- ii
Moderate: 220-450 points
- iii
Severe: > 450 points
- i
Remission: clinical remission is a CDAI score < 150.
Treatment response: a decrease of > 100 points in the CDAI score.
Relapse: symptom flare-up in a patient with CD that had been in clinical remission, either spontaneously or after medical treatment, and with an increase of 70 points in the CDAI score.
Early relapse: symptom flare-up in a patient with CD that was in remission for fewer than three months with medical treatment.
Relapse pattern: infrequent: ≤ 1 time per year; frequent: ≥ 2 times per year; and continuous: persistent symptoms of active CD with no period of remission.
Steroid-refractory disease: patients with disease activity despite the administration of up to 0.75 mg/kg/day for a period of four weeks.
Steroid-dependent disease: patients that cannot reduce a steroid dose equivalent to 10 mg/day of prednisone (budesonide below 3 mg/day), within the first three months of having received steroids, with no recurrent active disease, or patients with relapse in the first three months after having interrupted steroid use. The total duration of steroid use cannot exceed three months before reaching the limit of 10 mg/day of prednisolone, or the equivalent, to be considered steroid-dependent disease.
Recurrence: reappearance of lesions after having undergone surgical resection.
Clinical recurrence: reappearance of symptoms after macroscopic resection of the disease, once lesion recurrence has been confirmed.
Endoscopic recurrence: Rutgeerts criteria:
- -
i0: no lesions seen
- -
i1: fewer than five aphthous ulcers
- -
i2: more than five aphthous ulcers with normal mucosa between lesions
- -
i3: diffuse aphthous ileitis with diffusely inflamed mucosa
- -
i4: ileal inflammation with nodules, ulcers, narrowing
Localized disease: intestinal involvement of CD, whose extension does not go beyond 30 cm.
Extensive disease: intestinal involvement of CD, whose extension goes beyond 100 cm, regardless of its location. It includes the sum of the alternate inflamed zones.
New or first-time patient: patient with CD that presents with disease activity at the time of diagnosis, or shortly after it, with no previous treatment of CD.
AimOur aim was to establish the most relevant aspects of CD in Mexico, with respect to definitions, diagnosis, follow-up, medical treatment, and surgical treatment.
MethodologyPanelists were summoned from the entire country of Mexico, according to their career record and experience in treating CD in the areas of gastroenterology and advanced specialty in inflammatory bowel disease (IBD). To organize this first Mexican consensus on Crohn’s disease, the general coordinator, Dr. Jesús Kazuo Yamamoto Furusho, divided it into five modules that were then distributed as follows:
Module 1. Definitions, epidemiology, classifications, and indexes. Coordinator: Dr. López.
Module 2. Diagnosis (clinical, biochemical, endoscopic, radiologic, and histopathologic aspects) Coordinator: Dr. Bosques.
Module 3. Monitoring and follow-up of the disease through established tools, including treat-to-target strategies and the measurement of drug levels and anti-drug antibodies. Coordinator: Dr. Martínez.
Module 4. Conventional and biologic treatment. Coordinator: Dr. Yamamoto.
Module 5. Surgical treatment, including perianal disease. Coordinator: Dr. de León.
Importantly, the remaining experts cast their votes and made suggestions on each of the statements, based on the available scientific evidence.
A systematic search of the literature in English and Spanish was carried out for each statement formulated by the coordinators, utilizing Medline/PubMed, the Cochrane Database, EMBASE (Ovid), and LILACS. The search strategy included the following MeSH terms: Crohn’s disease, definitions, epidemiology, diagnosis, fecal calprotectin, mucosal healing, colonoscopy, magnetic resonance enterography, 5-aminosalicylates, thiopurines, immunomodulators, biologic therapy, anti-TNF-alpha, infliximab, adalimumab, certolizumab pegol, vedolizumab, ustekinumab, risankizumab, JAK inhibitors, upadacitinib, surgical treatment, intestinal resection, postoperative recurrence, strictureplasty. All randomized clinical trials, meta-analyses, systematic reviews, cohort studies, and case-control studies published within the last 25 years (1998-2023) were included.
The First Mexican Consensus on Crohn’s Disease Working Group was made up of 16 participants (gastroenterologists and IBD specialists). The coordinators of each module as described above were in charge of developing the initial statements and documenting the scientific evidence. An online platform (Survey Monkey) was utilized to survey the participants and make modifications to the statements. Importantly, the sponsors only provided support for the travel expenses of the consensus participants and did not intervene in the content and development of the statements; in addition, no one received honoraria. A first round of voting was carried out on the online platform to determine the level of agreement on the statements, utilizing the Delphi method, and to comment on specific references or suggested modifications for the statements. The statements were then voted on and the final modifications were made.
The concluding face-to-face meeting took place in the city of Monterrey, at which the final 69 statements were accepted when > 75% of the participants voted 4 or 5, on a scale of 1 to 5.
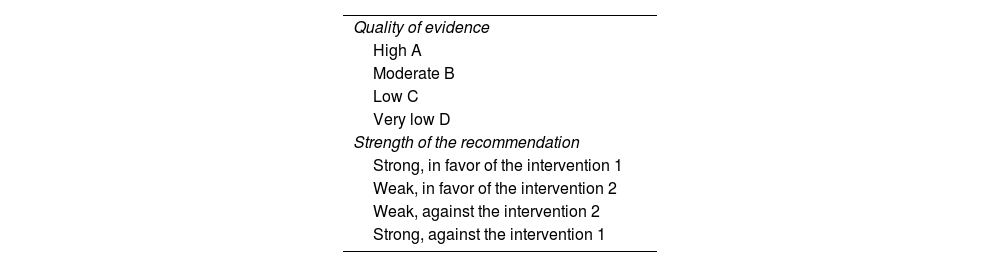
The recommendations were based on the level of available evidence, according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system classification: grade A, level of evidence 1, corresponding to randomized clinical trials; grade B, corresponding to level of evidence 2 or 3 in cohort studies or case-control studies; grade C, recommendations based on level of evidence 4, i.e., poor quality case series or cohort studies; and grade D, recommendations based on level of evidence 5, corresponding to expert opinion, as shown in Table 1.5 The quality of evidence was classified as high, moderate, low, or very low. The grade of each recommendation was assigned as strong (recommended) or weak (suggested). The strength of the recommendation consists of four aspects: the risk/benefit balance, patient preference and values, resource availability, and quality of evidence. The final manuscript was drafted by the coordinators of each module and approved by all the consensus authors.
Classification of the quality of evidence and strength of recommendations5
| Quality of evidence |
| High A |
| Moderate B |
| Low C |
| Very low D |
| Strength of the recommendation |
| Strong, in favor of the intervention 1 |
| Weak, in favor of the intervention 2 |
| Weak, against the intervention 2 |
| Strong, against the intervention 1 |
1. CD is a chronic and incurable IBD with an unpredictable clinical course and characterized by discontinuous and transmural involvement from the mouth to the anus. Agreement percentage: 100%. Quality of evidence: B. Weak, in favor of the intervention: 2.
CD is an immune-mediated chronic inflammatory disease that can affect any part of the digestive tract; its most frequent location is the terminal ileum and the proximal colon.6 Presentation symptoms tend to be heterogeneous and insidious. Clinical presentation depends on the location of the disease, the severity of inflammation, and the behavior of the disease. The most common clinical setting is a young patient that presents with abdominal pain in the right lower quadrant, altered bowel habits, and weight loss. In patients with colonic involvement, rectal bleeding or bloody diarrhea can be the main symptoms.7
The inflammatory process is segmental and transmural, as well as progressive, leading to intestinal injury and disability. The majority of patients present with an inflammatory phenotype at the time of diagnosis, but over time, will develop complications, such as strictures, fistulas, and abscesses in half of the patients, that often result in surgery.6–8
2. Early CD is that which is diagnosed within the first 18 months from symptom onset, with no prior exposure to immunomodulators and/or biologic treatment. Agreement percentage: 100%. Quality of evidence: B. Weak, in favor of the intervention: 2.
Evidence based on the natural history of CD and clinical studies suggests the advisability of utilizing CD-modifying drugs during a period of the disease, so that said intervention can improve prognosis.9
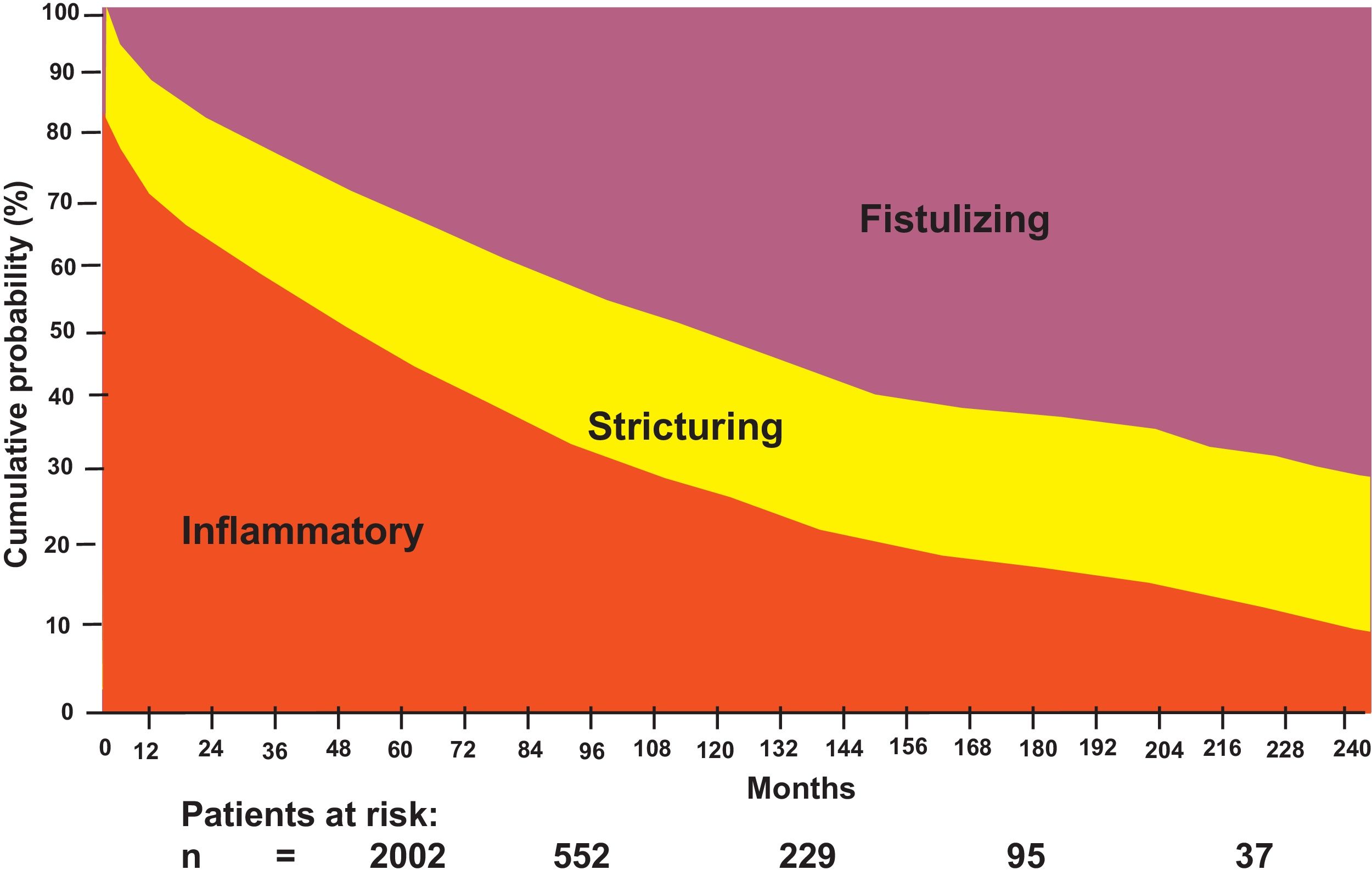
A number of patients present with disease progression. Louis et al. reported that, in 125 patients followed for at least 10 years, 77% had disease with the inflammatory pattern (uncomplicated with stricture or fistula) at diagnosis, whereas 11% had stricture and 16% presented with fistula. At the 10-year follow-up, 46% had experienced a change in behavior, with inflammatory, stricturing, and fistulizing patterns of 30.6%, 32.6%, and 37.6%, respectively. There was a change in progression from inflammatory to stricturing disease in 27.1% of patients and to fistulizing disease in 29.4% (p < 0.0001, in both cases).10
Strictures, fistulas, and abscesses are the main indications for surgery in CD and population-based cohort studies describe a cumulative risk for surgery of 40% to 71% in a 10-year time frame after diagnosis. Thus, the surgical requirement at a mean of 13.2 years in Omstem County between 1940 and 2001 (n = 314) was 58% and half of the patients had two resections. In a Danish study, Munkholm et al. reported that out of 373 patients, 70% underwent surgery after 15 years. In addition, one-third of the cases (36%) required two or more surgeries, and 22% of the patients underwent three or more operations.8 In the EPIMEX study,2 the frequency of surgical treatment in Mexican patients with CD was 19%.
An increase was observed in the risk for intestinal resection throughout the disease progression of patients with CD from 25% at diagnosis to 80% in 20 years,8 as well as fistulizing disease progression up to 45%, as shown in Fig. 1.
Different definitions for early CD have been proposed for the purpose of being used in clinical trials of drugs that modify the disease. An expert consensus at the international IBD meeting in Paris established the current definition of early CD as disease of ≤ 18 months of duration in patients with no previous use of disease-modifying immunomodulators and/or biologics.11
3. The etiopathogenesis of CD is unknown but is considered to have a multifactorial origin in which environmental, genetic, and immunologic factors intervene in its development. Agreement percentage: 100%. Quality of evidence: B. Weak, in favor of the intervention: 2.
The etiopathogenesis of CD is unknown but could be understood as a multidirectional relation between genetic factors, innate as well as adaptive immune responses, microbial factors, and certain environmental factors.12
CD results from many factors that end up having an influence on a genetically susceptible host. Studies on monozygotic twins have shown a 40 to 50% concordance in CD, resulting in two observations: environmental factors continue to be determining factors in the pathogenesis of CD, but genetic factors play an important role in disease onset.13
Studies on the association of the genome have shown the presence of genetic polymorphisms in different molecules and genes related to the development, susceptibility, and progression of CD. Mutations in the nucleotide-binding oligomerization domain 2 (NOD2) genes, situated on chromosome 16, the DLG5 gene on chromosome 10, and the IBD5 gene on chromosome 5, predispose to the development of the disease and determine some of the phenotypic characteristics, such as ileal location, age at earliest onset, and fibrostricturing behavior.14
4. NOD2/CARD15 gene mutations are associated with an increase in the risk for developing CD, as well as for ileal location, age under 40 years at diagnosis, intestinal resection, and the stricturing and fistulizing phenotypes. Agreement percentage: 100%. Quality of evidence: B. Weak, in favor of the intervention: 2.
The first region of the genetic association in CD, located on chromosome 16, was described in 2001. The NOD2 gene, with three possible polymorphisms, is found on that chromosome.15 NOD2/CARD15 gene alterations are associated with toll-like receptor alterations, related to the innate immune system, whose function is to start the activation of the nuclear kappa B factor that is involved in the inhibition of immune system cell apoptosis. This is why there is a large number of receptors and environmental stimuli that act as risk factors in patients with a genetic component, enabling the development of the disease.16
Age at early diagnosis is a risk factor associated with complications and reduced quality of life in CD. In that regard, the rs2076756 polymorphism of the NOD2 gene is associated with age under 25 years at diagnosis in those patients.17 The 3020insC polymorphism of the NOD2 gene is associated with the need for multiple surgeries and with a shorter interval between diagnosis and surgery.18
The contribution of genetic risk to the pathogenesis of extraintestinal manifestations (EIMs) is illustrated by a significant overlapping of the genetic risk loci for EIMs and IBD. The first risk variant identified in patients with CD, NOD2/CARD15, has also been associated with sacroiliitis and uveitis.19
5. Smoking is the most important modifiable environmental risk factor in CD, given that it has been associated with the appearance of fistulas, a greater risk of relapses, and treatment refractoriness, as well as an increased number of intestinal resections and a higher risk of postoperative recurrence. Agreement percentage: 100%. Quality of evidence: B. Weak, in favor of the intervention: 2.
Smoking increases the risk for developing CD and aggravates disease course in patients, compared with non-smokers.14 Smokers have a greater risk for developing CD, close to two-times higher than that of non-smokers. The meta-analyses by Calkins and Logan showed a relative risk (RR) of 2.0 (95% confidence interval [CI]: 1.7-2.5) and 2.4 (95% CI: 2.0-2.9) for CD. There was a higher risk for CD related to smoking in women (odds ratio [OR] = 4.1; 95% CI: 2.0-4.2) than in men (OR = 1.5; 95% CI: 0.8-6.0).20
Part of the process of pathogenesis is linked to alterations in the microcirculation of the intestinal mucosa and smoking can also affect the innate immune pathways. An increase in enterocyte autophagy, as a response to oxidative damage associated with tobacco, was shown in a study.21
Smoking increases the risk for the appearance of fistulas, as well as the risk for at least one surgery at some time in the course of the disease.22
In a prospective study, smokers had more years of active disease, regardless of the number of cigarettes smoked or treatment. Kane et al., evaluated 59 patients that underwent surgery due to the disease and found that smoking tobacco doubled the clinical recurrence risk (50% vs 25%), and in addition, the recurrence occurred earlier (130 vs 234 weeks; p < 0.001).23
6. The incidence and prevalence of CD, as well as the number of hospitalizations, has increased in Mexico. Agreement percentage: 100%. Quality of evidence: B. Weak, in favor of the intervention: 2.
Epidemiologic studies on IBD in Latin America are heterogeneous. A systematic review of 25 studies published between 2002 and 2015 reported an incidence for CD of 0.24 to 3.50 and a prevalence of 0.24 to 14.90 per 100,000 inhabitants.24
In Mexico, a study by Yamamoto et al. found that, for 2015, prevalence of CD ranged from 8.1 to 8.4, the total number of cases (prevalence of treated cases) of CD in women was 5,009 (8.1) and was 4,944 (8.4) in men. There were 1,097 hospitalized CD cases for that year; patients ≥ 50 years of age accounted for 43.7% of all patients. Regarding deaths from CD (specific death rate), 32 women (0.52) and 36 men (0.50) died, representing a 2.44-fold increase in the death rate of CD, in a period of 10 to 25 years.25
7. The Montreal classification is utilized for evaluating the key phenotypic characteristics of patients with CD, such as age at diagnosis and the location and phenotype of the disease, for the purpose of registering change over time in the location and behavior of CD. Considering the presence of growth failure in the pediatric population with IBD, the Paris classification was developed as a modification of the Montreal classification. Agreement percentage: 100%. Quality of evidence: D. Weak, in favor of the intervention: 2.
The Montreal classification is widely used for classifying the key phenotypic characteristics of patients with CD. It categorizes the disease into phenotypes according to the following features: 1) age at diagnosis: A1 (under 16 years), A2 (17-40 years), and A3 (over 40 years); 2) location of the lesions: L1 (ileum), L2 (colon), L3 (ileum + colon), and L4 (upper gastrointestinal tract); and 3) behavior: B1 (inflammatory or nonfistulizing/nonstricturing), B2 (stricturing), and B3 (fistulizing).4
The important modifications developed in the Paris classification included classifying age at diagnosis as A1a (0 to < 10 years), A1b (10 to < 17 years), A2 (17 to 40 years), and A3 (> 40 years); distinguishing disease above the distal ileum as L4a (proximal to the ligament of Treitz) and L4b (ligament of Treitz above the distal ileum), enabling the classification of both stricturing and penetrating disease in the same patient (B2B3); and classifying the presence of growth failure in the pediatric patient as G(1) vs G(0), in which there was no growth failure.26
8. The CDAI has been used in the past in clinical trials but has a series of limitations that includes the parameters utilized for defining remission (CDAI < 150) and contemporary trial design, no longer favoring its use. Agreement percentage: 87.5%. Quality of evidence: C. Weak, in favor of the intervention: 2.
The CDAI was developed and validated at the end of the 1970s,27 and was used in the past in clinical trials but it has a series of limitations that includes the parameters utilized for defining remission (CDAI < 150).
CD is a complex entity that can be modified by complications of the disease, factors influenced by subjectivity, and functional symptoms. The heterogeneity of the disease hinders accurate activity index quantification, when comparing the presence and magnitude of structural lesions. In clinical practice, the evaluation criteria of the CDAI are extensive and the calculation is complex. In addition, it is not validated in postoperative patients nor is it applicable in patients with stoma, tending to limit its use in clinical practice.28
9. The Harvey Bradshaw index (HBI) is easy to calculate and make measurements with and is less susceptible to confounding factors solely dependent on clinical parameters but is strongly weighted for diarrhea. An HBI score ≤ 4 is often utilized to define clinical remission. Agreement percentage: 93.8%. Quality of evidence: C. Weak, in favor of the intervention: 2.
The HBI was developed at the beginning of the 1980s and provides a simple and practical tool for measuring the clinical activity of CD.29
The HBI is correlated with the CDAI but is less complex. It is considered adequate for the long-term follow-up of patients and for use in clinical practice because it is simpler to implement. Said index consists of five clinical parameters: general patient status, abdominal pain, palpable mass, the presence of complications, and the number of liquid or soft stools per day. However, it is also weighted heavily by the number of stools. In general, an HBI score ≤ 4 is considered clinical remission and a decrease in the HBI ≥ 3 is considered clinical response.30
10. The two most widely used endoscopic indexes for evaluating endoscopic activity are the CD endoscopic index of severity (CDEIS) and the simple endoscopic score for CD (SES-CD). The CDEIS is complex to calculate, whereas the SES-CD is a simple, reproducible, and reliable endoscopic score. Agreement percentage: 100%. Quality of evidence: C. Weak, in favor of the intervention: 2.
The objective measurement of CD activity includes the endoscopic inflammation indexes, which are useful as treatment goals in clinical trials and can drive decision-making in clinical practice.
The SES-CD was proposed to simplify the endoscopic classification of CD and is based on four endoscopic variables on a scale of 0-3, in the same five segments considered in the CDEIS. The two scoring systems are utilized to assess complete MH as an evaluation criterion in clinical trials.31
With an interobserver reproducibility index of 0.87 and an intraobserver reproducibility index of 0.91, the SES-CD is a highly reliable tool in the evaluation of inflammatory activity in CD.32
11. The Rutgeerts score is specifically used for evaluating the recurrence of CD in the neoterminal ileum after surgery. Agreement percentage: 100%. Quality of evidence: B. Weak, in favor of the intervention: 2.
Patients with CD often require surgical resection due to the complications of strictures and abscesses or when disease is refractory to medical treatment. The risk for surgery after diagnosis is 16.3% at one year, 33.3% at five years, and 46.6% at 10 years.33 In the evolving management of patients with CD after surgery, risk factors for postoperative recurrence must be identified and postoperative endoscopic evaluation performed.
In the study by Rutgeerts et al. at the end of the 1990s, they endoscopically observed lesions in the neoterminal ileum within one year after surgery in 73% of the patients, even though only 20% of the patients presented with symptoms. Three years after surgery, the endoscopic recurrence rate had increased to 85% and symptomatic recurrence presented in 34%. Final disease course was better predicted by the severity of early postoperative lesions seen at ileoscopy.34
Even though the Rutgeerts score has not been validated, it is accepted that patients with a Rutgeerts score of ≥ i2 or a modified score of ≥ i2b should receive immunomodulator treatment or biologic therapy, even if they are asymptomatic, given that the possibilities of presenting with clinical recurrence and complications in the short term are high.35
Module 2. Diagnosis of Crohn’s disease12. An appropriate clinical history and physical examination are required, as well as the correlation of the results of laboratory tests, ileocolonoscopy, small bowel radiologic imaging studies and/or histology for making the diagnosis. Agreement percentage: 100%. Quality of evidence: D. Weak, in favor of the intervention: 2.
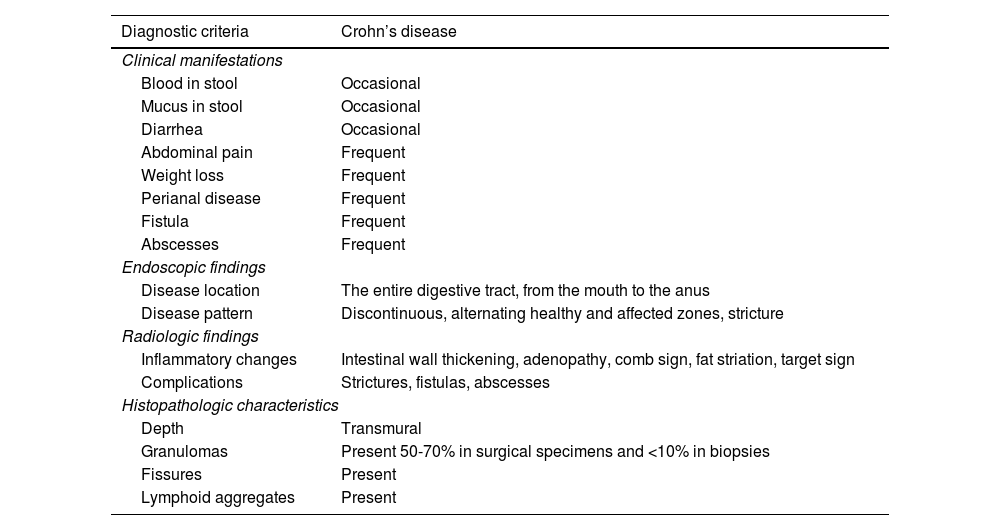
The diagnosis of CD should include a complete anamnesis and physical examination, serum tests, stool tests, imaging studies, and endoscopy, with a histopathologic report of the small intestine and large intestine. In the approach, Clostridioides difficile infection should be ruled out in patients with high clinical suspicion and normal endoscopy. Capsule endoscopy should be considered for evaluating the entire small bowel.36Table 2 illustrates the diagnostic criteria for CD.
Diagnostic criteria of Crohn’s disease.
| Diagnostic criteria | Crohn’s disease |
|---|---|
| Clinical manifestations | |
| Blood in stool | Occasional |
| Mucus in stool | Occasional |
| Diarrhea | Occasional |
| Abdominal pain | Frequent |
| Weight loss | Frequent |
| Perianal disease | Frequent |
| Fistula | Frequent |
| Abscesses | Frequent |
| Endoscopic findings | |
| Disease location | The entire digestive tract, from the mouth to the anus |
| Disease pattern | Discontinuous, alternating healthy and affected zones, stricture |
| Radiologic findings | |
| Inflammatory changes | Intestinal wall thickening, adenopathy, comb sign, fat striation, target sign |
| Complications | Strictures, fistulas, abscesses |
| Histopathologic characteristics | |
| Depth | Transmural |
| Granulomas | Present 50-70% in surgical specimens and <10% in biopsies |
| Fissures | Present |
| Lymphoid aggregates | Present |
13. The suspicion of Crohn’s disease should be studied through ileocolonoscopy with biopsies of the mucosa, even when it appears normal, in the six segments (rectum, sigmoid colon, descending colon, transverse colon, ascending colon, and ileum), to look for microscopic disease, and through imaging studies to evaluate the location and extension of the disease in the small bowel. Agreement percentage: 100%. Quality of evidence: D. Weak, in favor of the intervention: 2.
When IBD is suspected, ileocolonoscopy should be performed with biopsies of the mucosa from both inflamed and noninflamed segments, except in the case of severe acute colitis due to the high risk of intestinal perforation. Upper gastrointestinal endoscopy is recommended in patients with upper gastrointestinal symptoms suggestive of CD. Although no endoscopic characteristic is specific of CD, the diagnosis should be made when there are at least three of the following histologic findings36:
- o
The presence of epithelioid granuloma
- o
Focal architectonic crypt abnormalities
- o
Mucin preservation in the active sites
- o
Focal chronic inflammation without the presence of crypt atrophy
14. Transverse images, specifically magnetic resonance imaging (MRI), computed axial tomography (CAT), and ultrasound (US) are suggested to have largely replaced the conventional techniques of nuclear medicine and barium fluoroscopy and have the advantage of evaluating luminal and extraluminal disease. US and MRE are two accurate procedures for diagnosing Crohn’s disease, with the advantage of not exposing the patient to ionizing radiation. Agreement percentage: 100%. Quality of evidence: B. Weak, in favor of the intervention: 2.
Numerous recent studies have shown that noninvasive procedures, such as CAT, MRI, and US have high diagnostic accuracy, given that they can establish wall thickness and enhancement, for evaluating patients with IBD.37–40 However, expert opinion recommends initial evaluation of CD through US or MRE because of the lack of exposure to radiation.32 Two systematic reviews showed no significant difference between the two procedures, with respect to sensitivity and specificity.41,42 Several limitations of ultrasound imaging should be taken into account, such as the relatively long duration of the exploration that largely depends on the skill and experience of the sonographer/radiologist.43
15. The radiologic signs of disease activity include increased thickness and vascularization of the intestinal wall and mesentery, contrast enhancement in T2, diffusion-weighted imaging (for MRE), and the identification of ulceration and acute extraluminal complications. Agreement percentage: 100%. Quality of evidence: B. Weak, in favor of the intervention: 2.
The diagnosis of CD is predominantly based on the measurement of the intestinal wall. A thickness greater than or equal to 4 mm is considered pathologic in the literature.42 There is a high correlation between intestinal lesion severity evaluated through endoscopy and the intensity of changes seen in US, MRI, and computed tomography (CT) studies.42
US, CT, and MRI have high sensitivity and specificity for the diagnosis of intra-abdominal fistulas and abscesses, with similar diagnostic accuracy.42
16. Capsule endoscopy should be restricted to patients in whom there is a high index of suspicion of CD, including having a clinical picture suggestive of the disease, elevated fecal calprotectin (FC), normal ileocolonoscopy, and imaging studies that show no alterations or are inconclusive. Agreement percentage: 100%. Quality of evidence: C. Weak, in favor of the intervention: 2.
Expert opinion recommends using capsule endoscopy in patients in whom there is a high index of clinical and paraclinical suspicion of CD, with normal or inconclusive imaging studies. Contraindications for capsule endoscopy are gastrointestinal obstruction, stricture, and swallowing disorders.40
17. Capsule endoscopy is suggested to have greater sensitivity for detecting mucosal involvement of the small bowel in CD, compared with radiologic imaging techniques, and can be carried out when inflammatory disease of the small bowel is suspected, despite normal or equivocal transverse images. Agreement percentage: 100%. Quality of evidence: C. Weak, in favor of the intervention: 2.
Patients with clinical suspicion of CD and normal endoscopy should be considered for undergoing capsule endoscopy, given that it is a sensitive tool for detecting abnormalities in small bowel mucosa. It is also useful for disease prognosis because involvement in said segment is associated with a higher risk of surgery.40
18. Balloon-assisted enteroscopy can visualize small bowel mucosa beyond the reach of panendoscopy and ileocolonoscopy, enabling tissue biopsies to be taken for histologic evaluation. Agreement percentage: 100%. Quality of evidence: D. Weak, in favor of the intervention: 2.
The entire mucosa of the small bowel can be visualized through balloon-assisted enteroscopy. In addition, biopsies can be taken, and interventions for controlling bleeding or dilating stricture zones can be carried out.44
19. Balloon-assisted enteroscopy should be reserved for patients in whom there is high clinical suspicion of CD, despite a negative ileocolonoscopy, in patients that have suspicious, but not diagnostic, transverse images or capsule endoscopy, especially if the findings would alter the therapeutic strategy, or in patients in whom a strong histologic diagnosis would alter management. Agreement percentage: 100%. Quality of evidence: C. Weak, in favor of the intervention: 2.
Despite being an excellent diagnostic study that can also be therapeutic, balloon-assisted enteroscopy is not a routine diagnostic study in patients suspected of having CD because it is an invasive procedure that requires sedation and there is an almost 2% risk for perforation (in the majority of cases, the perforation is secondary to stricture dilation). However, it is useful in cases that require visualizing the small bowel mucosa and taking biopsies for making the definitive diagnosis, in turn providing a guide for a targeted treatment.44,45
20. Balloon-assisted enteroscopy has an established role in CD when therapeutic intervention is required, such as stricture dilation. Agreement percentage: 93.8%. Quality of evidence: B. Weak, in favor of the intervention: 2.
Balloon-assisted enteroscopy for the dilation of small bowel strictures associated with CD has a short-term clinical efficacy of 82% and low complication rates. Nevertheless, at follow-up, 48% of patients present with obstructive symptom recurrence, 39% require repeat stricture dilations, and 27% require surgery.46
21. Histologic findings in patients with CD are inconsistent but focal or irregular inflammation and/or crypt distortion tend to be seen in endoscopic biopsies or surgical resections. Discontinuous segments (“patchy lesions”), ileal involvement, transmural inflammation, and granulomatous inflammation are suggestive of CD. Agreement percentage: 100%. Quality of evidence: D. Weak, in favor of the intervention: 2.
CD is identified as presenting with ileal, ileocolonic, or colonic involvement in approximately the same number of patients each, and < 15% of patients will have a change in disease location over time.45
To accurately diagnose CD, ileocolonoscopy should be performed, taking ≥ 2 biopsies at inflammation zones. Taking biopsies from zones with no macroscopic inflammation, as well as from each colonic segment, is also useful for making the diagnosis. In biopsies from patients with CD, a discontinuous inflammation pattern -diseased segments separated by unaffected areas of the intestine (patchy lesions)- are normally observed in the macroscopic examination, and in the histologic findings, irregular villous architecture of the ileal mucosa secondary to the discontinuous inflammatory infiltrate is the most frequent and characteristic result in the histologic evaluation. Other characteristic findings of greater diagnostic value in CD are discontinuous chronic inflammation (in patches) not confined to the superficial mucosa, focal crypt architectonic distortion, and granulomas unrelated to crypt lesion. Said lesions may be found along the small intestine and large intestine, as well as in the esophagus, stomach, and duodenum.47
Importantly, crypt architecture can be preserved at a very early stage of the disease, but infectious colitis should be ruled out. The presence of granulomas and focal architectonic crypt abnormalities accompanied by chronic or irregular inflammation, with lymphocytes and plasma cells and conserved mucin at active disease sites, are findings consistent with CD. Inflammation in patches is typical in untreated patients.44
Despite the fact that the presence of granulomas is one of the characteristic findings in CD, they are present in only approximately 40 to 60% of surgical specimens and in 15 to 36% of endoscopic biopsy samples.48
22. Distinguishing IBD from non-IBD is easier for pathologists than separating CD from ulcerative colitis (UC) and diagnosis is largely enriched by discussions at multidisciplinary team meetings, at which both physicians and pathologists are present. Studies have shown that around 3% of patients with UC are reclassified as having colonic CD, and inversely, a small number (0.6-3%) will be reclassified as having UC, after an initial diagnosis of CD. Agreement percentage: 100%. Quality of evidence: D. Weak, in favor of the intervention: 2.
In approximately 5% of patients with clinical suspicion of IBD, a definitive diagnosis of UC or CD cannot be made due to a lack of clinical, radiologic, or endoscopic information, as well as to an overlapping of symptoms of the two diseases; in such cases, the term inflammatory bowel disease unclassified can be used. Epidemiologic studies indicate that the majority of these patients end up having a diagnosis of UC.
Sending the biopsies to a pathologist who is a specialist in the gastrointestinal tract will help reduce the percentage of errors, given that the majority of pathologists at hospital units, especially those at secondary care hospitals, are not familiar with IBD images.
Module 3. Disease monitoring and follow-up with established tools including treat-to-target strategies and the measurement of biologic drug levels and anti-drug antibodies23. Clinical response is an immediate treatment goal defined as a decrease of at least 50% in the patient-reported outcome 2 (PRO2) score, regarding abdominal pain and stool frequency. Agreement percentage: 100%. Quality of evidence: C. Weak, in favor of the intervention: 2.
Clinical symptoms correlate poorly with the degree of mucosal inflammation in CD, and it is not uncommon to discover significant inflammation of the mucosa during complete clinical remission. The PRO2 is useful only as a measure of clinical response to initial management, making it possible to identify whether the patient’s level of improvement could be sustained while waiting for clinical remission. The expert group voted in favor of utilizing this clinical measure as part of the evaluation.49
24. Clinical remission is an intermediate (i.e., medium term) treatment goal that can be defined as: PRO2 (abdominal pain 1 and stool frequency 3) or HBI < 5. Agreement percentage: 100%. Quality of evidence: C. Weak, in favor of the intervention: 2.
Even when there is a desirable response, the level of evidence comes from expert recommendations, and so this measure should correlate with the most significant indexes.49
25. The normalization of C-reactive protein (to values below the upper limit of normal) and FC (100-250 mg/g) is an intermediate treatment goal in CD. Treatment optimization or a treatment change should be considered if said goal has not been achieved. Agreement percentage: 100%. Quality of evidence: C. Weak, in favor of the intervention: 2.
In retrospective analyses of patients with CD, FC measurements carried out during routine control have been found to identify patients at risk for disease progression, regardless of symptoms or disease location.50 Thus, it is important to evaluate asymptomatic patients for mucosal inflammation and attempt to achieve complete resolution of the inflammation.
Even when more accurate studies are required for establishing the exact level of the marker, the evidence comes from a first meta-analysis that evaluated FC levels, obtaining an excellent cutoff value for determining whether patients will relapse in the near future, which is more useful in clinical practice. We suggest a FC cutoff value of 150 µg/g is associated with optimal diagnostic accuracy for predicting postoperative endoscopic recurrence in CD.51 The consensus panel decided to leave a range based on more studies, modifying the original recommendation of 152 µg/g.
26. Endoscopic healing is a long-term goal defined as a SES-CD < 3 points or the absence of ulcerations, which should be evaluated through ileocolonoscopy; treatment optimization or change should be considered if said goal has not been achieved. Agreement percentage: 100% Quality of evidence: A. Strong, in favor of the intervention: 1.
Objective tests for CD activity have displaced symptom-based evaluations. Despite its limitation, MH has become an important treatment goal associated with better long-term results. MH can be evaluated through ileocolonoscopy in the majority of patients. Nevertheless, noninvasive methods, albeit utilized clinically, have not yet been shown to be reliable for evaluating MH, especially for small bowel CD, and more studies are needed. Although targeting MH may be the most important goal in evaluating therapeutic options in CD, it may not be achievable in clinical practice for many patients.52
27. Transmural remission (evaluated by CT enterography, MRE, or intestinal US) is not yet a treatment goal in CD. However, it should be used in CD as a complement to endoscopic remission, as a measure of deep remission. Agreement percentage: 93.8%. Quality of evidence: C. Weak, in favor of the intervention: 2.
A retrospective, observational study on 214 patients with CD found that those with transmural remission (defined as endoscopic and MRE remission together) had lower rates of surgery, hospitalization, and therapy escalation at 12 months, compared with patients with only MH (15.2% vs 44.2%, p = 0.005) and with no healing (15.2% vs 63.6%, p < 0.001).53 On the other hand, a retrospective multicenter study that included 404 patients evaluated the outcomes of hospitalization, surgery, steroid use, and treatment escalation at five years in patients with transmural remission (previously defined), compared with patients with only radiologic or endoscopic remission or no remission, and reported that the patients with transmural remission had a lower risk of hospitalization (OR 0.244 [0.111-0.538], p < 0.001), surgery (OR 0.132 [0.030-0.585], p < 0.001), steroid use (OR 0.283 [0.159-0.505], p < 0.001), and treatment escalation (OR 0.088 [0.044-0.176], p < 0.001).54
28. Histologic remission is not a treatment goal in CD. Agreement percentage: 81.3%. Quality of evidence: C. Weak, in favor of the intervention: 2.
In retrospective studies, approximately 61% of patients with active CD that achieve clinical and endoscopic remission with treatment optimization, simultaneously achieve histologic remission, which was associated with a lower risk for treatment failure. Evaluations carried out in randomized trials are necessary to determine whether histologic remission should be a treatment goal in CD.55
29. Reactive therapeutic drug monitoring (TDM) should be carried out in patients that have no primary response and a confirmed loss of response to biologic therapy (anti-tumor necrosis factor agents [anti-TNFs], vedolizumab, ustekinumab, or other mechanism of action). It is more cost-effective than empiric anti-TNF therapy. Agreement percentage: 93.6%. Quality of evidence: C. Weak, in favor of the intervention: 2.
Higher MH or deep remission rates have been shown with infliximab levels of 4.4 μg/mL with an interquartile range (IQR) of 3.3-6.5 and adalimumab levels of 6.3 μg/mL with an IQR of 4.2-8.2 μg/mL, compared with lower levels of infliximab of 2.3 μg/mL (IQR: 1.1-4.2 μg/mL) and adalimumab of 3.9 μg/mL (IQR: 2.4-5.5 μg/mL) that have also shown higher intestinal resection rates.56
Levels above 5.0 µg/mL for infliximab (area under the curve: 0.92; 95% CI: 0.82-1.00) and above 5.9 µg/mL for adalimumab (area under the curve: 0.89; 95% CI: 0.71-1.00) are associated with fistula closure in patients with perianal CD.57
Early infliximab levels below 6.8 μg/mL (area under the curve = 0.68, p = 0.002, 50% sensitivity, 86% specificity) and anti-infliximab antibodies > 4.3 μg/mL (area under the curve = 0.78, p = 0.0004, 77% sensitivity, 71% specificity), measured at week two after the first infliximab infusion, were primary nonresponse predictors.58
In a multivariate analysis, post-induction monitoring showed that the only factor associated with the lack of primary response at week 14 was a low anti-TNF concentration (OR: 0.35 [95% CI 0.20-0.62], p = 0.00038 for infliximab and OR: 0.13 [95% CI: 0.06-0.28], p < 0.0001) for adalimumab). The optimum concentration at week 14 associated with remission was 7 μg/mL for infliximab and 12 μg/mL for adalimumab.59
Importantly, proactive monitoring of anti-TNF therapy drug levels cannot be done in Mexico due to a lack of routine measuring of biologic (mainly anti-TNF) levels, resulting in a reactive behavior, i.e., when a patient presents with secondary response loss.
As expert opinion: reactive monitoring for vedolizumab and ustekinumab in patients with suspected primary or secondary failure. The conditional recommendation of the American Gastroenterological Association (AGA): in patients treated with anti-TNF therapy, reactive TDM could be beneficial for changing treatment, avoiding empiric escalation or switching.56
30. The increase in eliminating anti-TNF therapy is associated with anti-drug antibodies, male sex, low albumin, high initial CRP, and high body mass index (BMI). Agreement percentage: 100%. Quality of evidence: D. Weak, in favor of the intervention: 2.
This information has come from different retrospective studies or prospective studies, such as those on adalimumab that support treat-to-target. A criticism by the panel is that randomized studies avoid patients that have a high inflammatory burden, making the recommendation strong, and that said burdens are important factors to be taken into account.36 Other factors that can modify anti-TNF therapy levels are the patient-related factors of smoking, Hispanic origin, and age under 40 years; disease-related factors of high CRP levels and leukocytosis; and treatment-related factors of “drug holidays” of more than 11 weeks, no concomitant immunomodulator use, initial infliximab dose below 7.5 μg/mL, concomitant antibiotic use (cephalosporines, penicillin), and immunogenicity prior to the use of other anti-TNFs.60
31. Patients with secondary response loss to anti-TNF therapy due to the development of high-titer anti-drug antibodies should not have an increase in dose but should be switched to a therapy with a different mechanism of action. However, when considering the switch within the drug class, in the case of secondary response loss to a first anti-TNF drug because of the development of anti-drug antibodies, an immunomodulator should be added to the anti-TNF therapy. Agreement percentage: 100%. Quality of evidence: A. Strong, in favor of the intervention: 1.
In secondary response loss due to antibodies, the change of mechanism is accepted as adequate conduct. However, the panel accepts the possibility that some patients will have to maintain a second anti-TNF drug. There is more evidence from expert opinion than from studies that supports the conduct of anti-TNF use and the addition of an immunomodulator. The REACT study supports immunomodulator use and we take certain evidence from there in the context of CD.61
32. Low titers of anti-drug antibodies can be overcome through treatment optimization (increasing the dose, shortening dose intervals and/or adding an immunomodulator). Agreement percentage: 100%. Quality of evidence: B. Weak, in favor of the intervention: 2.
Concomitant therapy with immunomodulators reduces the formation of antibodies to infliximab (ATIs) associated with treatment with the drug and improves its pharmacokinetics. There is no difference between methotrexate and azathioprine in reducing those risks. ATIs have a profound influence on the pharmacokinetics of infliximab. The formation of ATIs > 8 microg/mL is associated with lower serum infliximab levels from four weeks after its first administration.62
In a meta-analysis, the addition of immunomodulators to TNF-α inhibitors was shown to restore clinical response in 74% of patients, by reducing or completely eliminating anti-drug antibody levels.63 More long-term multicenter studies are needed to validate those findings.
33. HLA-DQA1*05 is associated with a higher risk for developing antibodies to infliximab and adalimumab. The formation of antibodies to infliximab or adalimumab can be reduced through immunomodulator use. Agreement percentage: 100%. Quality of evidence: B. Weak, in favor of the intervention: 2.
In an observational study, there was a significant association in the entire genome between HLA-DQA1*05 and the development of antibodies to anti-TNF agents (infliximab and adalimumab).64 However, even when other retrospective studies have found evidence, it is not a medical recommendation as a prediction, because at present, there are no prospective studies that validate such a statement.
Module 4. Medical treatment of Crohn’s disease34. In ileocecal CD with mild activity, the use of ileal-release budesonide is recommended at a dose of 9 mg/day. Agreement percentage: 100% Quality of evidence: A. Strong, in favor of the intervention: 1.
Clinical studies have shown that budesonide is superior to placebo (RR = 1.96, 95% CI: 1.19-3.23) and mesalazine (RR 1.63, 95% CI: 1.23-2.16). Budesonide is preferred to prednisolone because it is associated with minor adverse events (RR 0.64, 95% CI: 0.28-0.95). The remission rate with budesonide is 51-60% in 8-10 weeks, according to various studies.65,66
35. The use of sulfasalazine or mesalazine has been shown to be efficacious as induction therapy and maintenance in patients with mild colonic CD. Agreement percentage: 88% Quality of evidence: B. Weak, in favor of the recommendation: 2.
A meta-analysis of three large clinical trials found significant clinical efficacy with mesalazine in patients with ileocecal CD of mild-to-moderate activity.63 A more recent meta-analysis found a trend toward a beneficial effect related to the use of sulfasalazine, compared with placebo (two studies), with a RR of failure to achieve remission of 0.83 (95% CI: 0.69-1.00). A systematic review and meta-analysis of randomized clinical trials reported no benefit with mesalazine (four studies) (RR: 0.91; 95% CI: 0.77-1.06). In moderate-to-severe CD, sulfasalazine was more effective than placebo (RR 0.78, 95% CI: 0.65-0.93), but regarding corticosteroid-free maintenance, it was not superior to placebo (RR 0.98, 95% CI: 0.82-1.17). The efficacy of mesalazine in remission induction in CD is uncertain, and most likely, it is not effective in remission maintenance (sulfasalazine: RR = 0.98; 95% CI: 0.82-1.17 and 5-aminosalicylates: RR = 1.02; IC 95% CI: 0.92-1.16).67,68
36. Oral systemic corticosteroid use is recommended for remission induction in patients with moderate-to-severe active CD at any location. Corticosteroid use is not recommended as remission maintenance therapy. Agreement percentage: 100% Quality of evidence: A. Strong, in favor of the intervention: 1.
Prednisolone is an appropriate option for mild-to-severe CD. A Cochrane systematic review with two studies showed that corticosteroids were more effective than placebo for remission induction, with a RR of 1.99 (95% CI: 1.51-2.64, p < 0.00001).69 The idea is to minimize prolonged exposure with steroids in patients with CD, given the lack of efficacy in remission maintenance.
37. In patients with CD that achieve remission with systemic corticosteroids, thiopurine use is recommended. Agreement percentage: 100% Quality of evidence: A. Strong, in favor of the intervention: 1.
A meta-analysis of six clinical studies compared azathioprine with placebo and found a remission rate of 71% vs 52%, respectively (OR = 2.32; 95% CI: 1.55-3.49, and number-needed-to-treat [NNT]: 6, to prevent relapse), with a response effect to a dose of 1 mg/kg/day (OR: 1.2; 95% CI: 0.60-2.41), 2 mg/kg/day (OR = 3.01; 95% CI: 1.66-5.45) and 2.5 mg/kg/day (OR = 4.13; 95% CI: 1.59-10.71).70
38. Methotrexate use is efficacious in remission induction and maintenance in patients with CD. Agreement percentage: 88% Quality of evidence: C. Weak, in favor of the intervention: 2.
In a meta-analysis that included seven studies (four controlled clinical trials) and a total of 495 patients, one study utilized intramuscular methotrexate at a dose of 25 mg that significantly induced remission in 39% of the patients, compared with 19% in the placebo group (RR = 0.75, 95% CI: 0.61-0.93; NNT = 5),67 whereas weekly oral doses of 12.5, 15, and 20 mg showed no significant differences with the placebo group.71
Another study compared intramuscular methotrexate at a weekly dose of 15 mg with placebo, for remission maintenance in patients with CD. At 40 weeks, the remission rates were 65% vs 39% of the placebo group (p = 0.04).72
39. Biologic therapy with anti-TNF-alpha agents, such as infliximab, adalimumab, and certolizumab pegol, is recommended in patients with moderate-to-severe CD in whom there has been refractoriness or intolerance to steroid and immunomodulator-based conventional treatment. Agreement percentage: 100% Quality of evidence: A. Strong, in favor of the intervention: 1.
The ACCENT 1 study evaluated the efficacy and safety of infliximab in patients with CD, through a multicenter, randomized, double-blind, placebo-controlled trial lasting 54 weeks that involved 573 patients with a CDAI score > 220. A total of 335 patients that responded to an induction dose were randomized into three groups: group I with placebo; group II with infliximab, with a maintenance dose of 5 mg/kg; and group III with infliximab, with a maintenance dose of 10 mg/kg. At weeks 30 and 54, the number of patients in remission was higher in the infliximab groups (39% group II and 45% group III), compared with placebo (21% group I) (p = 0.002). No difference was found in the remission rates between the groups receiving 5 mg/kg and 10 mg/kg of infliximab. At week 54, 29% of the patients in the infliximab groups were in clinical remission and had discontinued their treatment with corticosteroids, compared with 9% in the placebo group (p = 0.004). Those results showed that infliximab administration was safe and effective in patients with CD.73
The CLASSIC 1 study was a multicenter, randomized, double-blind, placebo-controlled trial with different induction dose ranges. Patients with moderate-to-severe CD and no previous anti-TNF therapy received induction therapy at weeks zero and two with adalimumab 40/20 mg, 80/40 mg, 160/80 mg, or placebo and had follow-up at week four. The primary outcome measured in the study was the efficacy of induction therapy with adalimumab in patients with CD. A total of 299 patients were randomized at week zero to receive induction with placebo, adalimumab 40 mg/20 mg, adalimumab 80 mg/40 mg, or adalimumab 160 mg/80 mg. The remission rates between the patient groups that received adalimumab 80/40 mg (24%), adalimumab 160/80 mg (36%), or placebo (12%) at week four were significantly different (p = 0.004). Those study results showed that the induction dose of 160/80 mg of adalimumab was superior to the other adalimumab doses and placebo in patients with moderate-to-severe CD activity.74
The CLASSIC II study included patients that achieved remission after four weeks of induction in the CLASSIC I study and that also maintained remission for four additional weeks (CLASSIC II weeks zero and four), with an adalimumab dose of 40 mg every two weeks, in the open label phase. The patients were subsequently randomized to receive adalimumab or placebo for another 56 weeks in a double-blind trial. Of the 256 patient total, there was a significant difference in the remission rate between the groups treated with 40 mg of adalimumab every two weeks (79%) and 40 mg of adalimumab every week (83%), compared with placebo (44%) (p < 0.05).
Those study results support the efficacy of adalimumab in remission induction and maintenance in patients with moderate-to-severe CD with no prior infliximab therapy. There was an increase in clinical remission in 46% of patients at week 56 with adalimumab therapy.75
The CHARM study, a phase 3, randomized, double-blind placebo-controlled trial lasting 56 weeks evaluated the safety and efficacy of adalimumab in response maintenance, as well as remission maintenance, in patients with moderate-to-severe CD. At the first visit, all the patients received an open label dose of adalimumab of 80 mg, followed by a dose of 40 mg at week two. At that week, 778 patients were randomized into three groups: adalimumab 40 mg every two weeks, adalimumab 40 mg every week, or placebo, until reaching week 56. The percentage of patients that responded at week four and were in remission at week 56 was: 36% adalimumab 40 mg every two weeks, 41% adalimumab 40 mg every week, and 12% placebo (p < 0.001). The difference between the adalimumab groups was not statistically significant (p = 0.34). The results of the trial confirmed that adalimumab was more effective than placebo in long-term remission maintenance in patients with moderate-to-severe CD that initially responded to adalimumab.76
The aim of the ADHERE study was to evaluate the long-term effect of adalimumab therapy at two years in an open trial, as an extension of the CHARM trial, and it included 467 patients. At week 60, clinical remission was 37.6, 41.9, and 49.8% in patients that received placebo, adalimumab 40 mg every two weeks, and adalimumab 40 mg every week, respectively. A total of 84.1% of the patients that received adalimumab in the CHARM trial, maintained remission up to the end of the ADHERE study. Those study results showed that adalimumab therapy could maintain long-term remission and reduce the number of hospitalizations in patients with moderate-to-severe CD.77
The aim of the GAIN study was to determine the efficacy of adalimumab in patients with CD that did not improve or that lost the capacity to respond to infliximab. The trial included 325 patients with moderate-to-severe CD that were randomized to receive an induction dose of adalimumab 160/80 mg at week zero and week two, or placebo, for four weeks. At the end of week four, 21% of the adalimumab group achieved clinical remission, compared with 7% of the patients in the placebo group (p < 0.001). Adalimumab therapy was superior to placebo for inducing remission and response in patients with moderate-to-severe CD that did not tolerate infliximab or that lost the capacity to respond to infliximab.78
The PRECISE 1 study was a 26-week, multicenter, randomized, double-blind, placebo-controlled trial. A total of 662 patients with CD were divided into two groups 1) certolizumab pegol 400 mg, and 2) placebo. In patients with a baseline CRP level ≥ 10 mg/L, 22% of the certolizumab pegol group had a reduction of at least 100 points on the CDAI at week 26, compared with 12% in the placebo group (p = 0.05). Treatment with certolizumab pegol was associated with a modest benefit in response rates but there was no improvement in remission rates, compared with placebo in patients with moderate-to-severe CD.79
The PRECISE 2 study was designed as a multicenter, randomized, double-blind, placebo-controlled study. It evaluated the efficacy of certolizumab pegol as maintenance therapy in patients with moderate-to-severe CD. The patients received an open label induction dose of 400 mg at weeks zero, two, and four. The patients that responded to induction therapy at week six were randomized to receive 400 mg of certolizumab pegol or placebo. Follow-up was conducted at week 26. In total, 213 patients had baseline CRP levels ≥ 10 mg/L. Sixty-two percent of patients in the certolizumab pegol group had clinical response, compared with 34% in the placebo group (p < 0.001). The clinical remission rate was 48% in the certolizumab pegol group, compared with 29% in the placebo group (p < 0.001). The study results showed that the continuous administration of certolizumab pegol was superior to the administration of placebo un 64% of the patients with moderate-to-severe CD.80
40. Anti-TNF therapy based on infliximab and adalimumab has been shown to be effective in fistula closure and its maintenance in patients with CD. Agreement percentage: 100% Quality of evidence: A. Strong, in favor of the intervention: 1.
The ACCENT II study evaluated the efficacy of infliximab in the treatment of fistulizing CD. Compared with placebo, the majority of patients that received infliximab 5 mg/kg responded to treatment, defined as a 50% decrease, or higher, in fistula drainage (p = 0.002). There was complete response (fistula closure) in 55% of the patients that received infliximab 5 mg/kg, compared with 13% of the patients in the placebo group (p = 0.001). The ACCENT II study included patients with CD and simple or complex fistula. At week 54, 23% of the patients in the placebo group had response (defined as a 50% decrease in fistula drainage), compared with 46% of the patients that received infliximab (p = 0.001).81
In a substudy of the ACCENT II study82 that evaluated patients with rectovaginal fistula, 71.4% of the patients that received infliximab achieved fistula healing, compared with 54.5% in the placebo group. The French GETAID232 group conducted a retrospective multicenter study on patients with enterocutaneous fistulas that received anti-TNF treatment. It showed that only 33% of the patients analyzed achieved complete healing of the enterocutaneous fistula. In the multivariate analysis, lack of healing was associated with multiple fistula tracts (risk quotient [RQ]: 5.80, 95% CI: 1.07-31.5, p = 0.04) and the presence of intestinal stricture (RQ: 4.67, 95% CI: 1.05-20.82, p = 0.04). Adalimumab therapy has been shown to be effective in closure induction and maintenance of perianal fistulas for a period longer than two years.76,77,83
A systematic review of the literature79 showed that the combination of pharmacologic therapy (anti-TNF and immunomodulators) and surgery is the strategy with the best healing rates, compared with pharmacologic therapy or surgery by themselves.
41. Infliximab and azathioprine combination therapy is superior to monotherapy with an anti-TNF agent or azathioprine in patients with CD, regarding response and clinical remission, MH, and steroid-free remission. Agreement percentage: 100% Quality of evidence: A. Strong, in favor of the intervention: 1.
The 30-week, multicenter, randomized, double-blind SONIC study,84 recruited a total of 508 patients diagnosed with CD. The patients were divided into three groups according to the therapy administered: 1) azathioprine monotherapy; 2) infliximab monotherapy, and 3) infliximab and azathioprine combination therapy. At week 26, 56.8% of the patients that received the combination therapy had steroid-free remission, compared with the patients that received azathioprine monotherapy (30%, p < 0.001) and the patients that received infliximab monotherapy (44.4%, p = 0.02).
There was also a statistically significant difference in MH between patients that received combination therapy versus azathioprine (p < 0.001) and infliximab (p = 0.06). The incidence of adverse events was similar between the three groups, with no significant difference.
42. In patients with CD and poor prognosis predictors, the recommendation is to start intensive top-down therapy, which consists of starting first-line biologic therapy. Agreement percentage: 100% Quality of evidence: A. Strong, in favor of the intervention: 1.
Poor prognosis factors have been identified in patients with CD, such as young age at diagnosis (< 40 years of age), stricturing and fistulizing pattern that includes perianal disease, upper gastrointestinal tract involvement, extensive disease (more than 70 cm), and active smoking.85,86
In the Top-Down study,87 133 patients newly diagnosed with CD were randomized into two groups: the first group received early treatment with the combination of immunosuppressants (infliximab and thiopurines) and the second group received conventional treatment with corticosteroids. At week 52, 61.5% of the patients that received early treatment with immunosuppression were in clinical remission, compared with 42.2% of the patients that received conventional treatment (p = 0.0278, 95% CI: 2.4-36.3). After week 52, there were no differences between the two groups. At week 104, 73.1% of the patients with the Top-Down approach had MH, compared with 30.4% in the Step-Up protocol (p = 0.002). The study showed that early therapy with the combination of immunosuppressant agents produced a larger percentage of patients in clinical remission, faster normalization of CRP levels, and remission induction in patients with CD not previously treated with corticosteroids, thiopurines, or biologic drugs, compared with patients that received conventional treatment.
43. Vedolizumab is an efficacious therapy in the induction and maintenance of clinical remission in patients with moderate-to-severe CD that is refractory to conventional treatment and anti-TNF therapy. Agreement percentage: 100% Quality of evidence: A. Strong, in favor of the intervention: 1.
The GEMINI 2 study demonstrated the efficacy of vedolizumab in CD, with clinical remission achieved in 15% of the patients receiving vedolizumab, compared with 7% receiving placebo (p = 0.02) during induction at week six. In the maintenance phase (300 mg every four or eight weeks), of the patients in the two groups that responded to induction therapy, 39% that received vedolizumab every eight weeks and 36% that received the drug every four weeks were in clinical remission at week 52 (p < 0.001 and p = 0.004, respectively), compared with 22% that received placebo.88 The GEMINI 3 study evaluated the efficacy of vedolizumab in patients with moderate-to-severe CD that had experienced failure to at least one anti-TNF-alpha agent that were randomized to receive placebo or vedolizumab at a dose of 300 mg/day at weeks zero, two, and six. The primary outcome was clinical remission and was reported in 15.2% of the vedolizumab group, compared with 12.1% of the placebo group (p = 0.433). However, as the secondary outcome measure, clinical remission at week 10 was evaluated and there was a statistically significant difference, in favor of the vedolizumab group in 26.6% of the patients versus 12.1% in the placebo group (p = 0.001).89
44. First-line vedolizumab therapy is equally as effective as first-line anti-TNF therapy in patients with CD. Agreement percentage: 100% Quality of evidence: B. Weak, in favor of the intervention: 1.
The 24-month retrospective EVOLVE cohort study included a total of 1,095 patients with CD (n = 491) and UC (n = 604) that had received first-line therapy with either vedolizumab or an anti-TNF agent. Results showed that the persistence rate to treatment with vedolizumab in patients with CD was comparable to anti-TNF therapy (67.2% vs 67.2%) at 24 months and CD flare-up rates were lower for the vedolizumab group, compared with anti-TNF therapy. No statistically significant difference was reached (risk ratio = 0.84, 95% CI: 0.59-1.19) and the conclusion was that both treatments are equally effective.90
45. Treatment with vedolizumab is a very safe therapy in patients with CD, given that it does not increase infectious or neoplastic adverse events. At present, no case of progressive focal leukoencephalopathy has been documented. Agreement percentage: 100% Quality of evidence: A. Strong, in favor of the intervention: 1.
The main adverse effects described are nasopharyngitis, headache, arthralgias, and upper respiratory tract infections. The chief difference between natalizumab and vedolizumab is that natalizumab inhibits leukocyte trafficking in numerous organs, including the brain, whereas vedolizumab acts specifically with α4β7 intestinal heterodimers, thus selectively inhibiting lymphocyte trafficking in the intestine with no complications, as of yet, affecting the central nervous system. Because vedolizumab acts selectively in the intestine, it has no systemic effect, signifying fewer adverse events.88,89
46. Ustekinumab therapy is efficacious in the induction and maintenance of response and clinical remission in patients with moderate-to-severe CD that is refractory to conventional therapy and/or anti-TNF therapy. Agreement percentage: 100% Quality of evidence: A. Strong, in favor of the intervention: 1.
Ustekinumab was evaluated in anti-TNF-alpha-refractory CD with moderate-to-severe activity. During the induction phase, 526 individuals were randomly assigned to receive intravenous ustekinumab at a dose of 1, 3, or 6 mg/kg, respectively, or placebo. During the maintenance phase, 145 patients that had treatment response at six weeks entered a random phase, in which they received subcutaneous injections of ustekinumab (90 mg) or placebo at week eight and 16. Clinical response at week six was 36.6, 34.1, and 39.7% for doses of 1, 3, and 6 mg/kg of ustekinumab, respectively, compared with 23.5% of the patients that received placebo (p = 0.005, compared with the group that received 6 mg/kg). Maintenance therapy with ustekinumab, compared with placebo, showed statistically significant clinical remission (41.7% vs 27.4%, p = 0.03) and clinical response (69.4% vs 42.5%, p < 0.001) at 22 weeks, signifying that ustekinumab in anti-TNF-alpha-refractory CD with moderate-to-severe activity was effective, compared with placebo. A total of 34.3% patients with moderate-to-severe CD and a history of anti-TNF-alpha treatment failure had clinical response at week six with ustekinumab. A total of 55.5% of patients that were anti-TNF-alpha-naïve had clinical response at week six. Interestingly, ustekinumab started to act rapidly from week three and clinical remission was maintained in 53.1% of patients at week 44.91,92
47. Ustekinumab therapy can be considered first-line in patients with CD that have a contraindication for anti-TNF therapy. Agreement percentage: 94% Quality of evidence: C. Weak, in favor of the intervention: 2.
A retrospective real-life study included 156 biologic therapy-naïve patients with CD that received anti-TNF therapy as first treatment and 50 biologic therapy-naïve patients with CD that received ustekinumab. Clinical response was 64% and 86% in the ustekinumab and anti-TNF groups, respectively, at three months (p = 0.01). In the multivariate analysis adjusted by disease duration, location, and immunomodulator and steroid use, clinical remission was independently associated with the type of biologic therapy received (OR = 2.6 for anti-TNF vs ustekinumab; p = 0.02), with no differences in treatment abandonment or safety.93
48. Treatment with ustekinumab is very safe, given that it does not increase infectious or neoplastic adverse events throughout five years of treatment in patients with CD. Agreement percentage: 100% Quality of evidence: A. Strong, in favor of the intervention: 1.
The most frequent adverse events caused by ustekinumab are vomiting, nasopharyngitis, erythema at the injection site, candidiasis vulvovaginal, bronchitis, pruritus, urinary tract infections, and sinusitis. The drug does not increase the risk of neoplasia, nor has it been associated with deaths at five years of treatment.94
49. Risankizumab therapy is efficacious and safe in remission induction and maintenance in patients with moderate-to-severe CD that has been refractory to conventional and biologic therapy. Agreement percentage: 100% Quality of evidence: A. Strong, in favor of the intervention: 1.
A phase 3 controlled clinical trial consisting of two cohorts (ADVANCE and MOTIVATE) included patients with moderate-to-severe CD refractory to conventional therapy and biologic therapy of any mechanism of action. In the induction phase, the patients were randomized to receive risankizumab at doses of 600 mg and 1,200 mg, and placebo. The results showed that in the ADVANCE cohort, clinical remission, as defined by the CDAI, was 45% with risankizumab 600 mg and 42% with risankizumab 1,200 mg vs 25% of the placebo group. Stool frequency and abdominal pain improved in 43% of the patients with risankizumab 600 mg and 41% with risankizumab 1,200 mg, compared with 22% (38/175) of the placebo group. Endoscopic improvement was 40% with risankizumab 600 mg and 32% with risankizumab 1,200 mg vs 12% with placebo. In the MOTIVATE cohort, CDAI-defined clinical remission was 42% with risankizumab 600 mg and 40% with risankizumab 1,200 mg, compared with 20% of the placebo group. Stool frequency and abdominal pain improved in 35% of the patients with risankizumab 600 mg and 40% with risankizumab 1,200 mg vs 19% of the patients in the placebo group. Lastly, endoscopic response was 29% with risankizumab 600 mg, 34% with risankizumab 1,200 mg, and 11% with placebo. The differences in all the comparisons of the risankizumab groups with placebo were statistically significant.95
The FORTIFY maintenance trial with risankizumab in patients with CD reported better CDAI-defined clinical remission in 52% of the patients and endoscopic improvement in 47% in the risankizumab 360 mg group, compared with placebo. There were better clinical remission and endoscopic response rates with the 180 mg dose administered subcutaneously but there was no improvement in stool frequency or abdominal pain, with respect to the placebo group (p = 0.124). Adverse events were similar in all groups.96
50. Upadacitinib is an efficacious and safe therapy in patients with moderate-to-severe CD activity that have had refractoriness or intolerance to conventional and biologic therapy, including anti-TNFs, anti-integrins, and anti-interleukins. Agreement percentage: 100% Quality of evidence: A. Strong, in favor of the intervention: 1.
Two induction trials (U-EXCEL and U-EXCEED) and a maintenance trial (U-ENDURE) made up a phase 3 clinical program. The patients presented with moderate-to-severe CD and received 45 mg of upadacitinib, administered orally, or placebo. There was significant improvement in clinical remission in the patients that received the 45 mg of upadacitinib, compared with placebo (in U-EXCEL, 49.5% vs 29.1%; in U-EXCEED, 38.9% vs 21.1%), as well as in endoscopic response (in U-EXCEL, 45.5% vs 13.1%; in U-EXCEED, 34.6% vs 3.5%) (p < 0.001 for all comparisons). At week 52 in the U-ENDURE cohort, a high percentage of patients had clinical remission with 15 mg of upadacitinib (37.3%) vs 30 mg of upadacitinib (47.6%) vs placebo (15.1%), as well as a high percentage of endoscopic response with 15 mg of upadacitinib (27.6%), 30 mg of upadacitinib (40.1%), and placebo (7.3%) (p < 0.001 for all comparisons). Adverse events were similar in the three groups, with the exception of herpes zoster infection, which was more frequent in the two upadacitinib groups of 15 mg and 30 mg, and altered liver function and neutropenia, which were more frequent in the 30 mg upadacitinib group.97
Module 5. Surgical treatment including perianal disease51. Various factors are associated with surgical treatment, such as early age at diagnosis, ileal location, perianal disease, stricturing or penetrating disease, current smoking, treatment with corticosteroids at diagnosis or corticosteroid dependence, and extensive disease. Agreement percentage: 100%. Quality of evidence: B. Weak, in favor of the intervention: 2.
CD can require surgical treatment in some cases. Various factors have been associated with the need for surgery in patients with CD and they include:
- 1
Early age at diagnosis: patients that have been diagnosed with CD at an early age have been described to have a greater risk of requiring surgery, compared with those diagnosed later in life.98
- 2
Ileal location: small bowel involvement, specifically ileal location, has been related to a greater risk for needing surgery, in patients with CD.99
- 3
Perianal disease: the presence of perianal disease, including anal fissures, fistulas, and abscesses, has been associated with a higher risk of requiring surgery, in patients with CD.100
- 4
Stricturing or penetrating phenotype: patients with CD that present with a stricturing phenotype (narrowing of the intestine) or a penetrating phenotype (fistula formation) have a greater risk for needing surgery.
- 5
Current smoker label: tobacco smoking has been identified as a risk factor for disease progression and the need for surgery, in patients with CD.100
- 6
Treatment with corticosteroids at diagnosis or corticosteroid dependence: prolonged corticosteroid use for the treatment of CD, especially at diagnosis or as long-term dependence, has been associated with a greater risk for requiring surgery.101
- 7
Extensive disease: the involvement of multiple segments of the gastrointestinal tract has been related to a greater risk for needing surgery, in patients with CD.102
These factors can aid in identifying patients with CD that could have a higher risk for requiring surgical treatment. However, it is important to have in mind that each patient is unique and the decision to perform surgery should be based on a comprehensive evaluation of the condition of each individual.
52. Emergency surgery is indicated in complete bowel obstruction or obstruction that does not respond to conservative management, when intestinal ischemia or peritonitis is suspected. Surgery is the preferred option in patients with localized ileocecal CD (short strictures that are not amenable to endoscopic treatment), with obstructive symptoms but no significant signs of active inflammation. Agreement percentage: 100% Quality of evidence: D. Weak, in favor of the intervention: 2.
Intestinal stricture frequently occurs in the course of CD. Acute small bowel obstruction generally presents with uncontrollable nausea/vomiting, abdominal distension, and the absence of gas or stool canalization. In the absence of peritonitis, conservative management is the preferred option and includes intestinal rest, gastric decompression, and intravenous fluid therapy. In the presence of active inflammatory disease, intravenous steroid use should be considered.103,104 Primary conservative management enables the nutritional and immunosuppressant status to be optimized before potential surgery.105
On the other hand, when clinical or radiologic signs indicate intestinal perforation, emergency surgery, in which the affected intestinal segment is resected, is required. Early surgical evaluation, assessing the surgical indication and jointly monitoring the clinical course of the patient receiving conservative treatment is highly recommended. Subacute episodes of small bowel obstruction tend to recur over time. Therefore, surgical assessment is important in the context of interdisciplinary care and the discussion of treatment options.103
The guidelines of the European Crohn’s and Colitis Organisation (ECCO) have stated that (laparoscopic) resection is the preferred option in patients with localized ileocecal CD with obstructive symptoms but without active inflammation.103
A multicenter trial compared 143 patients with non-stricturing active CD involving fewer than 40 cm of the terminal ileum, in whom conventional therapy had failed. They were randomly assigned to receive infliximab or undergo laparoscopic ileocecal resection. There was no difference in the primary quality-of-life result, according to the Inflammatory Bowel Disease Questionnaire (IBDQ) at 12 months, nor in general quality of life measured by the SF-36 quality-of-life questionnaire. Nevertheless, the patients that underwent surgery had a 3.1-point better score (95% CI: 4.2-6.0) on the physical subscale of that questionnaire. There were no differences regarding severe complications between the medical and surgical groups. During a four-year follow-up, 37% of the patients treated with infliximab required resection, whereas 26% of the patients that initially underwent resection received infliximab. Thus, laparoscopic resection in stricturing and fibrous disease of the terminal ileum, as well as in a terminal ileum with active disease (< 40 cm) can be offered as a solid therapeutic option in an interdisciplinary context, with a risk-benefit profile comparable to that of medical treatment.106
53. Preoperative control of sepsis before abdominal surgery for CD is recommended. Pelvic sepsis and perianal CD that are refractory to medical or surgical interventions can be controlled through a diverting stoma. However, the fistula healing rate and stoma closure rate are limited. Agreement percentage: 100%. Quality of evidence: C. Weak, in favor of the intervention: 2.
The quality of evidence for the use of a diverting stoma in perianal CD is low and there are no randomized controlled trials that compare diverting stomas with other surgical or medical interventions. There are several small and heterogeneous case series with different types of stomas and definitions of success.107–109 A meta-analysis that included 556 patients reported clinical response in 63.8% of patients.110 Clinical response was similar in the eras before and after biologic therapy, in patients that did not respond to biologic therapy, as well as in those that did not receive it.105,106 Intestinal transit restoration was attempted in 34.5% of patients but was successful in only 16.6%. The absence of rectal involvement was consistently associated with a greater possibility for restoring intestinal transit. In addition, approximately one-fourth of the patients with stoma reversal required a new diversion due to severe disease recurrence. Proctectomy, as a last resort, was required in 41.6% of patients that had a failed temporary diversion. Similar results have been reported in a later single center study that included 77 patients, 57 of whom received concomitant treatment with biologic therapy. Successful restoration of intestinal transit was somewhat higher (27%) and reached 48% in the absence of active perianal disease. Quality of life was not discussed in any of the studies. Despite the low level of evidence and low fistula healing rate, diverting stoma can offer an alternative to extensive resection or proctectomy and allow time for the patient to adjust to or assimilate the construction of a permanent stoma.
Pelvic sepsis control is multidisciplinary and requires the intervention of the interventional radiologist, infectiologist, gastroenterologist, and colorectal surgeon. Nutritional support is key for obtaining maximum results in this context, especially if a stoma is created. Imaging studies (pelvic MRI or endosonography), rapid draining through seton placement, the start of antimicrobial therapy, and intensified medical treatment for disease control are the treatment cornerstones. In cases of deficient sepsis control, a diverting stoma can provide relief and enable clinical optimization before performing a surgical procedure.111
54. Imaging-guided percutaneous drainage of well-defined and accessible intra-abdominal abscesses is recommended as first-line treatment. After successful percutaneous drainage, medical management without surgery can be considered. If medical management is not successful, surgical treatment should not be delayed. Agreement percentage: 100%. Quality of evidence: B. Weak, in favor of the intervention: 2.
Percutaneous drainage (PD) is recommended as principal treatment for well-defined unilocular abscesses when they are accessible through interventional radiology, with reported successful drainage rates from 74% to 100%.112 Ultrasound or tomography-guided PD is a safe procedure with a low complication rate. When PD is successful, later emergency surgery can be avoided in 14% to 85% of patients with intra-abdominal abscesses related to CD.113,114 There is limited evidence on the optimum management of patients with CD that have intra-abdominal abscesses and have undergone PD. In particular, the ideal time for surgical intervention after the draining of the abscess is not known. Surgery can be avoided after successful PD in up to 30% of patients.110 Identifying the patients that can be treated without additional surgery is a challenge and is currently based on clinical judgement rather than evidence. However, elective surgery should be considered after controlling or resolving sepsis through PD and antimicrobial therapy, given that the recurrence of the abscess is up to 6.5-times higher after PD as the only therapy, compared with PD followed by surgical resection. Medical treatment-refractory disease and the presence of stricture or enterocutaneous fistula, whether a primary entity or a consequence of PD, increase the probability of surgery. On the other hand, emergency surgery without previous PD and sepsis control is associated with a higher complication and stoma rate, compared with initial PD followed by surgery.115 Successful PD can be considered a bridge to elective surgery, enabling nutritional and medical optimization, thus improving postoperative results.111,112
Intra-abdominal abscess control resembles the approach to pelvic sepsis, involving interventional radiology, infectiology, gastroenterology, and colorectal surgery, together with nutritional support. Frequent monitoring and surgical consultation are critical. Fortunately, surgery can be postponed in the majority of cases. Nonsurgical definitive management can be successful but must be carefully evaluated and discussed with the patient on an individual basis.116
55. Preoperative corticosteroid use is associated with a higher risk of postoperative complications. The preoperative decrease in corticosteroid doses can reduce postoperative complications, but it must be carefully controlled to prevent disease relapse. The construction of a temporary stoma should be considered if steroids cannot be withdrawn or significantly reduced before surgery. Agreement percentage: 100%. Quality of evidence: C. Weak, in favor of the intervention: 2.
Treatment with 20 mg of prednisolone daily, or its equivalent, for more than six weeks, is a recognized risk factor for surgical complications and hyperglycemia, according to the ECCO guidelines.103,117 This has been widely documented, albeit large randomized clinical trials specifically dedicated to the theme have not been conducted. Two meta-analyses of prospective and retrospective studies included 1,714 patients with IBD118 and 3,807 patients with CD.119 Up to a doubled increase in surgical site wounds in patients that received steroids was reported, as well as an increase in surgical complications when doses between 10 mg and 40 mg of prednisolone daily were used for more than three to six weeks. Gradually reducing the steroids whenever possible before surgery was recommended. On the other hand, thiopurines can safely be continued perioperatively.103,117–122 A surgical procedure in stages with a temporary stoma can be considered when high doses of steroids cannot be reduced (emergency surgery) and/or when other risk factors are present (e.g., sepsis, malnutrition, smoking). Lastly, there is little evidence supporting the common practice of perioperatively administering stress doses of steroids for patients with long-term corticosteroid use, rather that continuing with the preoperative dose, converted into intravenous equivalents when necessary.123 Two small randomized clinical trials (37 patients) and five cohort studies (462 patients) showed no benefit in stress dose steroid administration. The evaluation of the hypothalamic-pituitary-adrenal axis can be considered individually for assessing adrenal suppression.124
56. A preoperative nutritional evaluation should be carried out on all patients with CD that require surgical treatment. Nutritional optimization prior to surgery, with enteral or parenteral nutrition, is recommended in patients in whom nutritional deficiencies have been identified. Agreement percentage: 100%. Quality of evidence: C Weak, in favor of the intervention: 2.
Nutritional deficiencies are common in patients with CD that require surgery. Persistent or recurrent inflammation of the intestinal mucosa, enteric fistulas or strictures, chronic diarrhea, and medication adverse effects put nutritional status at risk, which in turn, is an important factor in surgical and medical outcomes.125,126 Even though randomized clinical trials are lacking, IBD referral centers have long integrated nutritional support into multidisciplinary teams.
Observational studies have shown that preoperative optimization in malnourished patients improves results. In a meta-analysis that included 1,111 patients with CD that received preoperative enteral or parenteral supplementation, compared with standard care, preoperative nutritional supplementation reduced postoperative complications (20% compared with 61.3%, OR 0.26, 95% CI 0.07-0.99; p < 0.001). In particular, enteral nutrition led to notably reduced postoperative morbidity and mortality (21.9% compared with 73.2%, OR 0.09, 95% CI 0.06-0.13, p < 0.01), with a NNT of 2. Goal-based parenteral nutrition should be considered whenever enteral nutrition is hindered.127
57. Current evidence suggests that preoperative treatment with anti-TNF therapy, vedolizumab, or ustekinumab does not increase the risk for postoperative complications in patients with CD undergoing abdominal surgery. Suspending biologic therapy before surgery is not obligatory. Agreement percentage: 100%. Quality of evidence: B. Weak, in favor of the intervention: 2.
Anti-TNF therapyThe use of biologic therapy in patients with CD programmed for surgery has been a subject of debate. Concern was expressed that by modulating the immune response, biologic medications could increase surgical site infections and morbidity. Recent guidelines have warned against the use of anti-TNF therapy in that context but the safest period for its suspension is not known.103
The most recent meta-analysis on the theme included 18 nonrandomized controlled studies and identified 1,407 patients that received infliximab and 4,589 that did not.128 No differences were found with respect to the appearance of complications between the patients that received infliximab and those that did not: the OR for major complications was 1.41, 95% CI 0.85-2.34; the OR for minor complications was 1.14, 95% CI 0.81-1.61; the OR for infectious complications was 1.23, 95% CI 0.87-1.74; the OR for noninfectious complications was 1.06, 95% CI 0.88-1.28; and the OR for hospital readmission was 1.46, 95% CI 0.8-2.66. This was also applicable to the need for surgical reintervention and mortality considered as separate events or included as major complications.
The results of the prospective PUCCINI study, that included 955 patients with IBD, showed that exposure to anti-TNF therapy, including drug level measurement, had no effect on the appearance of any surgical site infection or anastomotic leak.129
VedolizumabInitial data, included in a multicenter retrospective analysis that compared the postoperative results of 146 patients that received vedolizumab, compared with 289 patients that received anti-TNF therapy, revealed a significantly higher surgical site infection rate after abdominal surgery in patients that received vedolizumab.130 However, the most recent meta-analysis, that compared 307 patients with IBD treated with vedolizumab versus 490 patients treated with anti-TNF therapy and 535 patients that were not exposed to preoperative biologic therapy, found no differences regarding postoperative infectious complications and overall postoperative complications (RR 0.99 and 1.00, respectively) between the patients treated with vedolizumab and those with no biologic therapy. A similar result was found upon comparing the patients that received vedolizumab with those treated with anti-TNF therapy, with respect to postoperative infectious complication and overall postoperative complications (RR 0.99 and 0.92, respectively).131 Although larger studies that are randomized and include perioperative medication monitoring are needed, treatment with vedolizumab appears to be safe in the surgical context.
UstekinumabTwo retrospective multicenter cohort studies compared patients with CD that were preoperatively exposed to ustekinumab (for three to six months) with patients that received anti-TNF therapy (up to six months of follow-up after surgery). In the univariate analysis, the patients treated with ustekinumab had more probabilities of requiring the construction of a stoma (70% compared with 12.5%; p < 0.001), receiving combination therapy (25% compared with 2.5%; p = 0.01), and undergoing reoperations (16% compared with 5%; p = 0.01).132,133 Nevertheless, there were no increases in early or late postoperative complications upon comparing the surgical results of the 60 patients that received ustekinumab with the 209 patients treated with anti-TNF therapy.132,133 Once again, better designed studies with a larger number of patients are needed to confirm those results.
58. Endoscopic balloon dilation or surgery are viable treatment options in patients with CD that have short fibrotic strictures (< 5 cm) at the level of the terminal ileum. Agreement percentage: 100%. Quality of evidence: B Weak, in favor of the intervention: 2.
Even though symptomatic short strictures are frequent in patients with CD, no randomized clinical trial has been conducted that compares surgery with endoscopic balloon dilation. The largest study that has addressed the risks and benefits of balloon dilation is a joint analysis by Bettenworth et al., published in 2017, that included 1,493 patients that underwent a combined total of 3,213 endoscopic balloon dilations.134 A total of 98.6% of the strictures were ileal strictures and 62% were anastomotic strictures. The primary technical success rate (passage of the endoscope through the stricture) was 89.1% and clinical efficacy (absence of symptoms at the end of follow-up) was 80.8%. Complications (perforation and/or bleeding) presented in 2.8% of the procedures. Despite the initial high success rate, 73.5% of patients required a new dilation within 24 months and 42.9% needed surgical resection. Similar results were reported in a systematic review by Morar et al., analyzing 1,089 patients and 2,664 dilations and reporting a technical success rate of 90.6% and a clinical success rate of 70.2%. Complications occurred in 6.4% of the balloon dilations. At five years of follow-up, 75% of the patients had undergone surgery.135 There were no differences in results between the dilation of primary strictures and anastomotic strictures. Recent observational studies have shown comparable results.136–138 Therefore, the endoscopic balloon dilation of short primary strictures and anastomotic strictures in CD appears to be safe and effective in the short term. However, recurrence is common and the need for surgery is frequent in the following five years.
59. Strictureplasties are a safe option for treating small bowel strictures related to CD. Strictureplasty can be preferable to the resection of long segments of the intestine, with a potential reduction in surgical recurrence rates. Agreement percentage: 100%. Quality of evidence: B. Weak, in favor of the intervention: 2.
Strictureplasty is a safe and established surgical option for treating CD-related strictures and is an alternative to intestinal resection.139,140 Strictureplasty is recommended whenever it is reasonable and technically feasible, especially in cases of multiple fibrous strictures that would require a more extensive intestinal resection.103,141 A meta-analysis that included 1,112 patients that underwent a combined total of 3,259 strictureplasties (81% with the Heineke-Mikulicz technique, 10% with the Finney technique, and 5% with side-to-side isoperistaltic anastomosis), before the era of biologic therapy, revealed a recurrence rate at five years of 28%.142 The Heineke-Mikulicz technique is preferable for strictured segments up to 6-8 cm, whereas the Finney and side-to-side isoperistaltic techniques are employed for treating larger or multiple strictures.143 Surgical morbidity and mortality ranges from 8% to 15% and is not related to stricture length.143,144 Long-term favorable results have been reported,140,143,144 suggesting better results with strictureplasty, compared with intestinal resection. A large Japanese series included 526 patients, 435 of whom underwent intestinal resection alone and 91 of whom had a combined total of 199 strictureplasties. At 10 years, the cumulative surgical reintervention rate at the anastomosis site was 18%, compared with 7% at the strictureplasty site (p < 0.01).145
Whenever possible, elective surgery is preferable to an emergency procedure in cases of acute small bowel obstruction due to stricture caused by CD. It can be achieved in the majority of cases with primary conservative management, such as endovenous hydration and nasogastric decompression. Treatment options should be discussed in an interdisciplinary manner and include the opinions of the patient. When surgery becomes necessary, it is important to thoroughly evaluate the intestine, ideally through MRE before performing the surgical procedure. MRE can reveal a distinction between inflammatory strictures (susceptible to treatment with intensified medical therapy) and fibrous strictures. The evaluation of the intestine during surgery can be very useful for identifying stricture sites. To maximize the preservation of the intestine, the surgeon specialized in IBD should be familiar with the different types of strictureplasty, including nonconventional ones. Be that as it may, strictureplasty of the colon is not recommended.103,146
60. Laparoscopic resection in patients with non-stricturing limited ileocecal CD (that involves < 40 cm of the terminal ileum) is a reasonable alternative to anti-TNF therapy, especially with infliximab. Agreement percentage: 100%. Quality of evidence: A. Strong, in favor of the intervention: 1.
As described above, a clinical trial conducted at numerous European centers compared 143 patients that had non-stricturing active CD that affected fewer than 40 cm of the terminal ileum and had not responded to conventional treatments. The patients were randomly divided into two groups: one that received infliximab and one that underwent laparoscopic ileocecal resection. The main study results that included the evaluation of quality of life at 12 months through the IBDQ and general quality of life through the SF-36 questionnaire found no significant differences between the two groups. However, the patients that underwent surgery obtained a 3.1-point higher score on the physical subscale of the questionnaire, with a 95% CI of 4.2 to 6.0 points. In addition, there were no differences in the severe complication rate between the medical and surgical groups. During the follow-up with a mean of four years, 37% of the patients treated with infliximab ended up having to undergo resection, whereas 26% of the patients that initially underwent resection received infliximab at some time.106
The primary aim of a randomized clinical trial conducted at 29 centers in the Netherlands and the United Kingdom was to determine the cost-effectiveness of laparoscopic ileocecal resection, compared with treatment with infliximab, in adult patients with CD in the terminal ileum that had not adequately responded to conventional treatment with immunomodulators or steroids for more than three months and that showed no signs of critical strictures. The patients were randomly assigned to laparoscopic ileocecal resection or to receive infliximab. The results were measured as quality-adjusted life years (QALYs), utilizing the EuroQol (EQ) 5D-3L and the IBDQ. The main results of the cost-effectiveness study showed that the direct costs of medical care per patient with CD during the first year were significantly lower in the laparoscopic ileocecal resection group than in the infliximab group, with a mean difference of Є-8,931 (95% CI Є-12,087 to Є-5,097). Furthermore, even though the difference was not statistically significant, the total costs from a societal perspective tended to be lower in the resection group, with a mean difference of Є-5,729 (95% CI Є-10,606 to Є172). In terms of cost-effectiveness, the probability that resection would be a viable option, compared with infliximab, was high at different levels of willingness to pay for improvements in quality of life. Said probability was 0.96, with a willingness to pay of Є0 per QALY gained and per improvement in the IBDQ score, and increased to 0.98, with a willingness to pay of Є20,000 per QALY gained and to 0.99, with a willingness to pay of Є500 per point of improvement in the IBDQ score.147
In a retrospective follow-up study, the long-term consequences were evaluated of two treatment modalities for patients with non-stricturing ileocecal CD that was refractory to immunomodulators: laparoscopic ileocecal resection and treatment with infliximab. The analysis was based on a set of data from 134 patients, 69 of whom were assigned to the resection group and 65 to the infliximab group, with a mean follow-up period of 63.5 months. Twenty-six percent of the patients in the resection group required starting anti-TNF therapy, but none of them needed a second resection during the follow-up period. Interestingly, 42% of the patients of that group continued to have no need for additional medications related to CD, albeit 48% received prophylactic immunomodulator therapy. In contrast, 48% of the patients in the infliximab group had to undergo resection related to CD, whereas the rest of them maintained, modified, or intensified their treatment with infliximab. Treatment effect duration was comparable in the two groups, with a mean time with no need for additional treatment related to CD of 33.0 months in the resection group and 34.0 months in the infliximab group, with no statistically significant differences (log-rank p = 0.52). An important finding was that the use of an immunomodulator added to the assigned treatment was associated with a significant prolongation in the duration of the therapeutic effect, in both groups. In the resection group, the RQ was 0.34, whereas it was 0.49 in the infliximab group, indicating that the coadjuvant therapy had a positive influence on the long-term clinical result.148
61. Segmental colectomy is the appropriate surgical treatment for patients with only one affected colonic segment in CD. Agreement percentage: 100%. Quality of evidence: C. Weak, in favor of the intervention: 2.
When only one segment of the colon is affected, performing a segmental colectomy is recommended. However, when several segments of the colon are compromised, generally the preferred option is subtotal colectomy. A meta-analysis by Tekkis et al.149 compared the results of 223 subtotal/total colectomies with ileorectal anastomosis and 265 segmental colectomies for treating colonic CD. Even though there were no significant differences in recurrence rates, complications, or the need for a permanent stoma, recurrence occurred at a mean 4.4 years later in the subtotal/total colectomy cases, with statistical significance (p < 0.001).
In another more recent meta-analysis by Angriman et al., 1,436 patients that underwent different surgical procedures for treating CD in the colon were evaluated. Complications were more frequent after segmental colectomy, compared with subtotal colectomy, suggesting that, in terms of safety, subtotal colectomy could be the preferred option. Nevertheless, segmental colectomy had a lower probability of requiring a permanent stoma, compared with subtotal colectomy.150 With respect to CD recurrence, subtotal colectomy had a higher risk for recurrence and need for repeat surgical treatment, compared with total proctocolectomy. However, there were no significant differences regarding recurrence between segmental colectomy and subtotal colectomy.151
In exceptional situations in which two distinct segments of the colon are affected, the possibility of performing two segmental resections could be considered, instead of opting for a subtotal colectomy.103 Determining the extension of the resection of the colon is based on the clinical situation, which can be a planned surgery or an emergency surgery, as well as on the number of affected segments of the colon. To the degree it is feasible, segmental colectomy is preferred.146
62. Ileocolic or small bowel side-to-side mechanical anastomoses are associated with lower postoperative complication rates, compared with end-to-end anastomoses in CD. Kono-S anastomosis and extended resection of the mesocolon are promising surgical techniques for preventing postoperative recurrence in CD. Agreement percentage: 100%. Quality of evidence: A. Strong, in favor of the intervention: 1.
The technical aspects in surgery are fundamental and can be affected by different factors, such as the previous training of the surgeon, his/her experience, resource availability, and the clinical situation of the patient. The choice of the optimum technique in anastomosis for small bowel and ileocolic resections has been a subject of debate. In the past 10 years, evidence that increasingly supports the preference for a side-to-side anastomosis has appeared and been consolidated over time.
A meta-analysis by Simillis et al. included 661 patients and showed that the anastomotic leak rate was significantly higher in end-to-end anastomoses, compared with side-to-side anastomoses (OR 4.37; p = 0.02), even when the focus was on the subgroup of ileocolic anastomoses (OR 3.8; p = 0.05). As a result of that difference, postoperative complications in general were also more frequent (OR 2.64; p < 0.001) and hospital stay duration was considerably longer (2.81 additional days; p = 0.007) when an end-to-end anastomosis was performed.151
In a later meta-analysis led by Guo et al., the superiority of side-to-side anastomosis was reaffirmed, compared with other configurations in terms of general postoperative complications (RM 0.6; p = 0.01). However, there were no statistically significant differences in anastomotic leak rates, endoscopic and symptomatic recurrence, or in the need for reintervention due to recurrence.152
Another meta-analysis conducted by He et al. compared 396 cases of mechanical side-to-side anastomosis and 425 cases of manual end-to-end anastomosis. The results showed that the mechanical side-to-side anastomoses were superior in all aspects analyzed: postoperative complications in general (OR 0.54, 95% CI 0.32-0.93), anastomotic leak rate (OR 0.45, 95% CI 0.20-1.00), recurrence (OR 0.20, 95% CI 0.07-0.55) and need for reintervention due to recurrence (OR 0.18, 95% CI 0.07-0.45).153
A network meta-analysis that included data from 11 clinical trials and 1,113 patients confirmed the superiority of mechanical side-to-side anastomosis regarding postoperative complications in general, clinical recurrence, and the need for reintervention due to recurrence. There were no significant differences in anastomotic leak rates, surgical site infections, mortality, and hospital stay duration associated with the choice of anastomosis technique.154
Recently, the mesentery has been recognized as an active immune organ that can play a pathophysiologic role in CD.155 Two techniques have been developed for the purpose of reducing the influence of the mesentery on luminal CD. Kono-S anastomosis (KSA), as described by Kono et al. in 2011, is a manual side-to-side anastomosis with a wide intestinal lumen.156,157 A recent clinical trial (CD-SuPREMe) showed that, compared with mechanical side-to-side anastomosis, the KSA was associated with a lower risk for endoscopic recurrence after surgery, with severe recurrence (Rutgeerts ≥ i2) occurring in 18% of patients, compared with 30%, after 2 years.153 Later, Coffey et al.158 described extended mesenteric extirpation (EME). In a prospective case series, compared with historical controls, they found that surgical recurrence was lower after EME (2.9% vs 40%).159
A meta-analysis focused on evaluating the two innovative techniques of KSA and EME, with the aim of reducing recurrence in patients undergoing surgery for CD. After reviewing nine studies that included a total of 896 patients, KSA was found to be associated with a lower incidence of endoscopic (0% vs 3.4%) and surgical (15% vs 24.4%) recurrence, as well as lower complication rates, especially in the anastomotic leak rate (1.8% vs 9.3%). Nevertheless, it is important to point out that the level of evidence in general was limited (grade IV), underlining the need for additional studies. In addition, the preservation of the mesentery, a component of the Kono-S technique, was identified as possibly having an impact on disease recurrence, emphasizing the importance of considering both the anastomosis technique and mesenteric extirpation in future studies.160
The choice of anastomosis technique in surgery for CD, particularly in small bowel and ileocolonic resections, is a critical aspect that can significantly affect postoperative results. Studies support the preference for mechanical side-to-side anastomosis in intestinal resection surgeries, given that the technique is associated with fewer postoperative complications and lower anastomotic leak rates. Innovative surgical techniques, such as KSA and EME, are promising with respect to reduced disease recurrence but more scientific validation is needed for confirming these results.
63. Restorative proctocolectomy with ileoanal pouch anastomosis can be considered in selected patients with refractory pancolonic CD with no history of perianal disease, taking into account the high risk of pouch failure. Agreement percentage: 100%. Quality of evidence: C. Weak, in favor of the intervention: 2.
Several specialized centers have shared their experiences in the use of restorative proctocolectomy and ileal pouch anal anastomosis (IPAA) in patients with refractory pancolonic CD. The ECCO guidelines emphasize the higher complication and failure rates associated with IPAA in cases of CD and recommend reserving this option for highly motivated/empowered patients and performing it under the supervision of multidisciplinary teams, preferably when there are no concurrent diseases in the small bowel or perianal involvement.103
Reese et al.161 carried out a meta-analysis that included 3,103 patients, 225 of whom underwent IPAA for CD. Compared with the other patients, those that had IPAA for CD presented with double the number of strictures at the anastomotic site and six-times more pouch failures (32% vs 4.8%, p < 0.01). However, in patients with CD confined to the colon, there were no significant differences in postoperative complications or the pouch failure rate (8% in patients with IPAA for CD vs 7.1% in patients with IPAA for UC). Importantly, the patients with colonic CD did not present with more complications nor with a higher rate of pouch failures, compared with the patients with UC. Nevertheless, in the patients with CD, the pouch was inferior (with double the cases of incontinence and urgency), although stool frequency was similar. In an extensive case series, there were no differences in quality-of-life scores, regardless of the reason for performing IPAA.162
64. Regarding minimally invasive surgery in CD, surgeons must not only have experience in the treatment of CD in open surgery, but also advanced laparoscopic/robotic surgical skills. Agreement percentage: 100%. Quality of evidence: C. Weak, in favor of the intervention: 2.
A meta-analysis and Cochrane review that involved randomized clinical trials,163,164 found no statistically significant differences in the results between laparoscopic surgery and open surgery for treating CD in the small bowel. However, a more recent meta-analysis that included randomized clinical trials, such as observational studies, revealed that the laparoscopic approach resulted in fewer complications and fewer cases of incisional hernias.165 Another joint review evaluated laparoscopic resection in cases of recurrent CD, confirming that the technique is safe and feasible when performed by adequately experienced surgeons.166 In that context, conversion to open surgery was 2.5-times more frequent, although there was no increase in complications. Therefore, patients with CD in the small bowel, in both its primary and recurrent forms, benefit from the laparoscopic approach because it involves fewer complications after surgery and fewer cases of incisional hernias. In situations in which surgeons experienced in laparoscopic surgery are not available, emergency surgical treatment should not be delayed.
The use of the robotic-assisted surgical platform enables better visualization, greater control of the surgical field, and greater surgical skill. Better vision contributes to eliminating adhesions or resecting the affected intestine without injuring adjacent organs. In addition, the application of a robotic-assisted approach can potentially tackle the visual and ergonomic limitations of laparoscopic surgery, especially in the context of complex diseases and in narrow spaces, such as the pelvis. When performing procedures, such as intramesorectal exeresis or total exeresis of the mesorectum, the use of robotic technology can aid in sparing the nearby nerves along the entire surgical plane.167,168 At present, there are only a few small case series describing the use of robotic-assisted surgery for CD. Aydinli et al. reported on robotic-assisted ileocolic resection, with a shorter intestinal function recovery time, compared with standard laparoscopy.169 Robotic surgery requires a completely different set of skills and learning curve from open/laparoscopic surgery and significantly increases costs for the patient. With greater experience, surgical skill, and continuous technological advances, these problems associated with robotic surgery could be mitigated. Consequently, robotic-assisted surgery could become a valuable instrument for surgeons to efficaciously manage the complications related to CD. Under certain unique circumstances, a combination of robotic and laparoscopic techniques can simplify complex surgical procedures.168
Despite the difficulties in the surgical management of CD, minimally invasive surgery has become an increasingly popular option. The appearance of CD surgery as a specialized field, with surgeons that focus on the surgical management of IBD, has contributed to establishing the optimum role of minimally invasive surgery in the treatment of CD. Given the complex characteristics of CD, the laparoscopic approach should be utilized carefully by highly experienced surgeons. A progressive approach can be carried out in the surgical management of CD, starting with less complex cases, such as ileal CD with short strictures, to then tackle more challenging cases, such as resection of the colon, and finally treating advanced forms of CD, such as recurrent or penetrating disease. The decision on which minimally invasive surgical procedure to adopt, should be made case by case and depend on the experience of the surgeon and the characteristics of the patient.
65. In patients that undergo a surgical intervention, it is important to stratify the postoperative recurrence risk based on their history and pertinently set up the postoperative treatment and follow-up. Agreement percentage: 100%. Quality of evidence: B. Weak, in favor of the intervention: 2.
Despite the fact that a considerable number of patients with CD require surgery at some time in their lives, it is important to understand that surgery is not a cure for this disease and that it is common for symptoms to reappear after surgery.7 Up to 93% of patients can show signs of endoscopic recurrence within the first year after surgery and around 20% to 30% experience recurrent symptoms.34,169–172 The need for additional surgery, called surgical recurrence, has been seen in 25% to 45% of patients within the 10 years after their first intestinal surgery.173
The Rutgeerts scoring system has been developed for evaluating the endoscopic findings in the area of the intestine that has undergone surgery. This score helps predict the probability of clinical recurrence. A low score, such as i0 or i1, indicates endoscopic remission and is associated with a low probability of clinical recurrence within the next three years. On the other hand, higher scores, such as i2, i3, and i4, indicate endoscopic recurrence and are associated with a greater probability of clinical recurrence.174 Different studies have analyzed the factors that predict recurrence after surgery for CD. These factors include smoking, previous intestinal surgeries, more aggressive disease (penetrating-fistulizing), and perianal involvement.175–178 Smoking is the most important of these factors and can significantly increase the risk for recurrence. Giving up smoking can reduce said risk.179 Myenteric plexitis, a histologic characteristic, has also been associated with recurrent disease in patients with CD.180
Postoperative recurrence rates vary depending on whether clinical, endoscopic, or surgical recurrence is considered. Endoscopic recurrence precedes clinical recurrence and is indicative of the clinical course of CD. Severe endoscopic recurrence predicts an unfavorable prognosis.34,181
Given the high level of evidence regarding the postoperative recurrence risk in smokers, abstinence from tobacco should always be promoted, with counseling and dedicated support, before surgical resection. In addition, prophylactic therapy should always be considered for CD after surgery and starting treatment in patients with at least one recurrence risk factor within four weeks after surgery is recommended.117,181 Numerous drugs have been studied for preventing postoperative clinical or endoscopic recurrence in CD, with different efficacy profiles.
The most recent guidelines of the ECCO,117 the American Gastroenterological Association (AGA),34 and the American College of Gastroenterology (ACG)45 were published in 2016, 2017, and 2018, respectively. All these organizations agree on the importance of patients giving up smoking. However, there are differences in practical orientation and levels of evidence regarding the recommendations on the use of medications. In patients with remission induced by surgery for CD, the AGA suggest not using 5-aminosalicylates. High-dose mesalazine is an option in patients with isolated ileal resection, according to the ECCO, and is also an option in patients with isolated ileal resection and no risk factors for recurrence in the ACG guidelines.35,45,117 Both the AGA and ECCO suggest that anti-TNF medications and/or thiopurines are the most adequate for preventing recurrence after surgery in patients with at least one risk factor.34,45 Nevertheless, the ACG puts forward anti-TNF agents over thiopurines in high-risk patients178 and states that imidazole antibiotics can also be effective after ileocolic resection, although their tolerance can be a problem, according to the ECCO. The ACG considers that those antibiotics can be used after small bowel resection to prevent recurrence and the AGA states that patients with a lower risk for recurrence or wish to avoid the risk for adverse events with thiopurines and/or anti-TNF treatment can opt for nitroimidazole antibiotics for a specific period of time.35,45,117
Lastly, the guidelines of the National Institute for Health and Care Excellence (NICE) of the United Kingdom suggest the use of azathioprine in combination with metronidazole for maintaining remission in patients with ileocolonic CD that have undergone complete resection in the last three months and advise against the use of biologic treatments in that situation. They also underline the cost-effectiveness of thiopurines, albeit pointing out the need for long-term analyses for evaluating the costs of rehospitalization and recurrent surgery.182
Due to the efficacy anti-TNF agents, their use is increasing in the prevention of recurrence after surgery in CD. In a proposed clinical algorithm, patients are divided into groups, according to risk factors and response prior to anti-TNF use. Anti-TNFs could be an option after surgery. If there was no prior success with anti-TNFs, despite adequate management, other options, such as vedolizumab or ustekinumab, could be considered. Given that there is scant information on new biologic medications, more research on them is needed. In general, a personalized approach based on an endoscopic evaluation and early FC measurement is recommended. In the future, with more common use of biologics after surgery, we could consider “top-down” treatment strategies in patients in remission, but this still has to be supported by clinical studies.183
66. The combination of medical and surgical treatment for treating complex perianal CD is advisable for controlling the septic focus and luminal activity of the disease. Agreement percentage: 100%. Quality of evidence: A. Strong, in favor of the intervention: 1.
A heterogeneous group of retrospective studies that compares treatment with anti-TNF agents with different surgical approximations was combined in a meta-analysis published in 2014. The results suggested that combination treatment could produce additional benefits, compared with surgical treatment or medical treatment separately. However, the heterogeneity of the studies included, the retrospective nature of the analysis, and the low quality of the studies hinder reaching conclusions or making solid recommendations.83
In the PISA study, high-risk patients with perianal fistulas due to CD and a single initially drained fistulous orifice were randomly assigned to one of the following: a drainage seton; anti-TNF therapy for one year; or advancement plasty under anti-TNF therapy for four months. The primary result was fistula-related reintervention (surgery and/or restarting of anti-TNF therapy). Said randomized clinical trial was stopped after having included 44 of the 126 planned patients, based on a futility analysis. The use of a drainage seton was associated with the highest reintervention rate in 1.5 years (10/15 patients vs 6/15 patients with anti-TNF therapy and 3/14 patients with advancement plasty + anti-TNF therapy; p = 0.02). There were no differences in quality of life or in perianal disease activity index. Those authors concluded that treatment with drainage seton should not be recommended as the sole or superior treatment for perianal fistula due to CD.184
Thorough evaluations of different biologic therapies in patients with fistulizing CD were carried out in a systematic review and network meta-analysis. Regarding response induction, infliximab surpassed adalimumab (OR = 0.24; 95% CI 0.06-0.99), but no significant differences were found in relation to remission induction between the two drugs (OR = 0.31; 95% CI 0.04-2.27). The TNF antagonists were superior to placebo in both response induction (OR = 0.51; 95% CI 0.35-0.75) and remission induction (OR = 0.36; 95% CI 0.22-0.58). Infliximab was also superior to placebo in response induction (OR = 0.36; 95% CI 0.17-0.75) and remission induction (OR = 0.17; 95% CI 0.03-0.87). Ustekinumab was more effective than placebo in response induction (OR = 0.48; 95% CI 0.26-0.86), but not in remission induction (OR = 0.50; 95% CI 0.13-1.93). In the different biologic therapy comparisons, there were no statistically significant differences in remission induction. On the other hand, vedolizumab was not superior to placebo in induction remission (OR = 0.32; 95% CI 0.04-2.29). Lastly, certolizumab was not superior to placebo in response induction (OR = 0.78; 95% CI 0.40-1.55) or in remission induction (OR = 0.78; 95% CI 0.40-1.55). Said study concluded that TNF antagonists were effective in response and remission induction in patients with fistulizing CD. Infliximab stood out as the preferred option for response induction. Nevertheless, more research and specialized clinical trials are needed to thoroughly evaluate the efficacy of those therapies in this patient population.185
In clinical practice, management decisions should be made by the gastroenterologist and the colorectal surgeon, taking into account the clinical information and available resources. Surgery plays an important role in perianal sepsis control through the exploration under anesthesia and adequate draining through seton placement. Most essentially, any sign of infection must be handled rapidly because the use of anti-TNF drugs is contraindicated in the presence of active sepsis or infection.
67. Advancement flaps, fibrin glues, and ligation of the intersphincteric fistula tract are a therapeutic option for selected patients with CD and complex perianal fistulas, although their efficacy is limited. Plugs for anal fistulas should not be routinely considered for perianal fistula closure in CD, given that seton use is equally efficacious. Agreement percentage: 100%. Quality of evidence: A. Strong, in favor of the intervention: 1.
A meta-analysis brought together data from 135 patients with perianal fistula due to CD treated with advancement flap in 11 retrospective studies,186 reaching a joint success rate of 66%. However, the definitions of success and the periods of follow-up varied considerably in the studies, leading to heterogeneous results and a low general level of evidence.
A more recent analysis conducted by Stellingwerf et al. evaluated 35 patients with perianal fistula due to CD and found a 61% success rate utilizing flap advancement. There were no significant differences in that rate when compared with ligation of the intersphincteric fistula tract (LIFT), which had a success rate of 53%. However, it is important to keep in mind that incontinence rates were significantly higher in the patients that had advancement flap plasty, compared with LIFT, with 7.8% vs 1.6%, respectively.187 Because conducting a randomized clinical trial directly comparing advancement flap plasty with the absence of surgery would be ethically questionable, the need for collaboration to bring together a higher number of cases treated with advancement flap in patients with perianal CD is to be emphasized. Those cases should be backed by clear definitions of results and adequate follow-up to establish the role of that technique more accurately in the treatment of perianal CD.
A study was conducted to evaluate the efficacy of fibrin glue in the treatment of perianal fistulas in patients with CD.188 It was an open label clinical trial that included 71 patients with CD. The patients were randomly assigned to receive the instillation of fibrin glue in the fistulas or no additional treatment after seton removal. After eight weeks of follow-up, 38% of the patients treated with fibrin glue achieved clinical remission, compared with only 16% in the observation group (p = 0.04). This suggests that the use of fibrin glue was significantly more effective for remission induction of the perianal fistulas in patients with CD. Importantly, the follow-up period of the study was not sufficiently long for arriving at a definitive conclusion about the true success rate of said therapy in patients with CD. In addition, several cohort studies with a limited number of patients with CD reported variable success rates in the use of fibrin glue for treating perianal fistulas. Nevertheless, the fact that this technique had an acceptable safety profile consistently stood out, given that no lesions in the sphincteric muscles resulting from the treatment with fibrin glue were reported.189
LIFT is an option in the surgical treatment arsenal for perianal fistulas. Sirany et al. conducted a systematic review of the literature and identified 26 studies that included a total of 713 patients, 13 of whom had CD.190 There was only one randomized clinical trial among the studies, but it did not include patients with CD and the other 25 studies were case series or cohort studies. The studies included were heterogeneous, with a wide variety of result measures and follow-up times. The techniques utilized were only partially described and included seven technical variations. The primary healing rates ranged from 47% to 95%. Therefore, even the lower extreme of said range appears promising, compared with other therapeutic options. There were very few complications associated with classic LIFT or any of its variations (three complications were reported in six studies) and they were minor. Nevertheless, due to the lack of data, the role of LIFT in the treatment of perianal fistulas in CD is not clear, but the complication rate appears to be relatively low. To clarify the role of LIFT in fistulas in CD, randomized clinical trials are required, possibly comparing the procedure with advancement flap plasty as the control group.
A randomized clinical trial was conducted to evaluate the use of collagen anal fistula plugs (AFPs) in patients with perianal fistulas due to CD. The study compared two groups: in one group, the setons were removed and AFPs were placed in the tract of the fistula, and in the other group, the setons were removed and the patients were only observed. A total of 106 patients with CD participated in the study. After 12 weeks, the fistula closure rate in the AFP group was 33.3% in patients with complex fistulas and 30.7% in patients with simple fistulas, compared with 15.4% and 25.6%, respectively in the group that only had seton removal. The differences were not statistically significant, possibly due to the limited size of the study. Importantly, there was a trend toward a grater incidence of adverse events at 12 weeks in the AFP group, compared with the seton group (17% vs 8%), but that difference was not statistically significant either (p = 0.07). Nevertheless, at the follow-up at 12 months, the cumulative adverse event rates were similar in the two groups.191 A systematic review was carried out that involved 12 observational studies and included 84 patients with a nine-month follow-up (range of three to 24 months). In general, around 58.3% of the anal fistulas closed satisfactorily but when a smaller subgroup of patients with recurrent anal fistulas after previous treatments was examined, the success rate was 40%. Importantly, the definition of fistula closure was not uniform, and the follow-up regimens varied widely among the studies. Furthermore, the quality of evidence of the systematic review was considered low due to concerns of bias and the lack of accuracy in the data.192 The use of AFPs in patients with CD appears to be relatively safe and can be considered for selected patients that are aware of the low success rate.
In patients with CD and complex perianal fistulas, options such as advancement flaps, fibrin glues, and LIFT, can be considered but their efficacy is limited. AFPs are not routinely recommended. LIFT is promising but more clinical trials are needed to confirm its efficacy. Fibrin glue shows positive results for remission induction in perianal fistulas in patients with CD, but long-term follow-up is needed to evaluate success and complications. Further research is required to better understand these options in patients with CD and perianal fistulas.
68. Allogenic therapy with stem cells derived from adipose tissue could be a safe and efficacious treatment for complex perianal fistulas in patients with CD. Stem cells derived from autologous adipose tissue could have a positive effect on patients with CD and complex perianal fistulas, with good tolerance and safety. Agreement percentage: 93.8%. Quality of evidence: A. Strong, in favor of the intervention: 1.
ADMIRE CD is a crucial phase 3 study evaluating the use of allogenic stem cells derived from adipose tissue in patients with perianal fistulas due to CD, in which 212 patients participated.193–195 All the patients underwent curettage of the fistula tract and closure of the internal opening in the fistula (IOF) and were then randomly assigned to receive the application of stem cells or placebo around the IOF and along the fistulous tracts. Patients with more than two IOFs or three external openings of the fistula (EOFs), patients with rectovaginal fistula, and patients with rectal or anal stricture or proctitis were excluded from the study. At 12 months, there was significantly higher combined remission (defined as closure of the EOF in the physical examination and the absence of abscesses in the MRE) in the patients treated with the stem cells, compared with placebo (56.3% vs 38.6%; p = 0.010).
A meta-analysis of 11 studies that included three randomized clinical trials, of which the ADMIRE CD was the largest, showed improved healing rates, compared with the control groups.196 Importantly, none of the studies compared the stem cell administration mode and technique.
According to the report by Dozois et al., the highest healing rates were produced when the stem cells were combined with fibrin glue or were impregnated in a Gore Bio-A® fistula plug, compared with direct application (71% and 83%, respectively, vs 50%).197
Allogenic stem cell therapy can be a safe and effective focus for complex perianal fistulas in patients with CD. However, additional studies are needed to determine patient selection, the optimum administration mode, dose, and frequency of applications.195
69. Anal and rectogenital fistulas related to CD are very complex and rare, and so should be treated by an experienced multidisciplinary team. Agreement percentage: 100%. Quality of evidence: C. Weak, in favor of the intervention: 2.
A systematic review encompassing a total of 23 studies on rectovaginal fistulas associated with CD was conducted. The studies included one randomized clinical trial, six prospective studies, and 16 retrospective studies, for a total of 137 cases of rectovaginal fistulas in patients with CD. Three of the studies focused on combination medical and surgical treatment and reported a healing rate of 44.2%.198
On the other hand, Hotouras et al. conducted a review that evaluated 17 studies, with a total of 106 patients that underwent a surgical intervention known as the gracilis muscle interposition for the treatment of rectovaginal fistulas. The majority of the studies were retrospective and nonrandomized. Of those patients, only 34 had fistulas due to CD. After a mean follow-up time of 21 months, 50% of the CD fistulas that had undergone gracilis muscle interposition had healed, compared with the healing rate of 60 to 90% of rectovaginal fistulas that were not related to CD.199
The treatment of rectovaginal fistulas in patients with CD presents significant challenges and the choice of medical and surgical options should be carefully considered, often in the context of a multidisciplinary team of experts in CD.
Financial disclosureThe AbbVie, Janssen, and Takeda laboratories provided financial support for the travel and hotel expenses of the participants in the first Mexican consensus on Crohn’s disease and were not present during the development of the consensus.
Conflict of interestJesús Kazuo Yamamoto Furusho is an advisory board member, opinion leader, and speaker for Abbvie Laboratories de México, Abbvie Internacional, Takeda Internacional, Takeda México, Pfizer Internacional y Regional, Janssen Cilag Internacional y México. He is an opinion leader and speaker for Alfasigma, Celltrion, Ferring, and Farmasa Schwabe and a research advisor for UCB México. He has received financial support for research studies from the Shire, Bristol Myers Squib, Pfizer, Takeda, and Celgene laboratories.
Francisco Bosques Padilla is a speaker for Abbvie, Janssen, Takeda, and Ferring.
Jesús Gerardo López Gómez has been a speaker for Abbvie, Janssen, and Takeda.
Manuel Martínez Vázquez is a speaker for Abbvie, Janssen, and Takeda.
Jorge Luis de León Rendón has been a speaker for Janssen, Schwabe Pharma, and Takeda.
First Mexican Consensus on Crohn’s Disease Working GroupAzucena Casanova Lara has been a speaker for Abbvie and Takeda.
Carlos Manuel Del Real Calzada has been a speaker for Abbvie.
Fabiola Maely González Ortiz has been a speaker for Abbvie.
Raquel Yazmín López Pérez has been a Speaker for Janssen y Schwabe Pharma.
Francisca Martínez has been a speaker for Abbvie.
Arturo Mayoral Zavala has been a speaker for Abbvie and Janssen.
Rosa María Miranda Cordero has been a speaker for Abbvie, Janssen, and Takeda.
Laura Ofelia Olivares Guzmán has been a speaker for Pfizer and Takeda.
Azalia Ruiz Flores has been a speaker for Janssen and Takeda.
The remaining authors declare they have no conflict of interest.
Ethical considerationsNo patients participated in the present study nor were their data used, eliminating the need for obtaining informed consent. Likewise, because no interventions, maneuvers, or information management were carried out, the study is considered low risk and no review or approval by a local ethics committee is required. Even so, the present document meets the current research regulations and the confidentiality of identification and personal data and the anonymity of the participants (all healthcare workers that voluntarily participated) are guaranteed. The present article contains no personal information that could identify the participants.
Azucena Casanova Lara. Hospital Regional del Instituto Mexicano del Seguro Social (IMSS), Cancún, Mexico.
Carlos Manuel Del Real Calzada. Centro Médico Nacional La Raza, IMSS, Mexico City, Mexico.
Fabiola Maely González Ortiz. Piedras Negras, Mexico.
Raquel Yazmín López Pérez. Servicio de endoscopia, Hospital General de México, Mexico City, México.
Francisca Martínez. Centro Médico Nacional La Raza, IMSS, Mexico City, Mexico.
Arturo Mayoral Zavala. Centro Médico Nacional Siglo XXI, IMSS, Mexico City, Mexico.
Rosa María Miranda. Centro Médico Instituto de Seguridad Social del Estado de México y Municipios (ISSEMYM), Toluca, Estado de México, Mexico.
Laura Ofelia Olivares Guzmán. Hospital General de Culiacán, Sinaloa, Mexico.
Ariadnee Irma Reyna Aréchiga. Departamento de Gastroenterología, Hospital Universitario de la Universidad Autónoma de Nuevo León, Monterrey, Nuevo León, Mexico.
Azalia Ruiz Flores. Hospital de Alta Especialidad del IMSS, Monterrey, Mexico.
Luis Salgado. Hospital Zambrano Hellion Tec Salud, Monterrey, Nuevo León, Mexico.
Leticia Santoyo Fexas. Departamento de Gastroenterología, Hospital Universitario de la Universidad Autónoma de Nuevo León, Monterrey, Nuevo León, Mexico.
Juan Antonio Villanueva Herrero. Servicio de Coloproctología, Hospital General de México, Mexico City, Mexico.