There has been an increase in the incidence of hepatocellular carcinoma (HCC) worldwide and information on this disease is limited in Mexico.
AimsTo analyze the available evidence on the diagnosis and treatment of HCC in the Mexican population.
Material and methodsThe Mexican Association of Hepatology organized a meeting that 24 medical specialists interested in HCC attended. An electronic database search was carried out to identify documents published from 2000 with the keywords «Hepatocellular carcinoma» and «Mexico», «epidemiology», «diagnosis», and «treatment».
ResultsThe incidence of HCC in Mexico has increased over the last few decades. The mean age of disease presentation is in patients from 60 to 70 years old, and the man:woman ratio appears to be equal. HCC is frequently associated with underlying hepatopathy and the primary cause reported in our country is chronic hepatitis C virus) infection. Surveillance is recommended for high-risk groups in Child-Pugh stages A and B, and for those in stage C if the patient is on a waiting list or regarded as a candidate for liver transplantation. HCC should be evaluated by a multidisciplinary team of experts in the field.
ConclusionsHCC is a neoplasia that is on the rise in Mexico, with epidemiologic characteristics similar to those of other populations. Diagnosis and treatment should be individualized in accordance with these Consensus guidelines.
La incidencia del carcinoma hepatocelular (CHC) ha presentado un aumento a nivel global y en México existe información limitada sobre la enfermedad.
ObjetivoAnalizar la evidencia disponible en población mexicana sobre el diagnóstico y tratamiento del CHC.
Material y métodosLa Asociación Mexicana de Hepatología convocó a una reunión donde participaron 24 médicos especialistas con interés en CHC. Se realizó una búsqueda en bases de datos electrónicas para identificar documentos publicados a partir del 2000 con los términos «Carcinoma hepatocelular» y «México» agregando además términos como: epidemiología, diagnóstico y tratamiento.
ResultadosLa incidencia de CHC en México se ha incrementado en las últimas décadas. En México la edad promedio de presentación se sitúa en la década de los sesenta y la relación femenino:masculino parece ser igual. El CHC se asocia frecuentemente a hepatopatía subyacente y la principal causa reportada en nuestro país es la infección crónica por el virus de la hepatitis C. La vigilancia se recomienda a grupos de alto riesgo en estadios A y B de Child-Pugh, y en estadio C solo si se encuentra en lista de espera o se considera candidato a trasplante hepático. El CHC debe ser evaluado por un equipo multidisciplinario de expertos en el área.
ConclusionesEl CHC representa una neoplasia que va en aumento en nuestro país con características epidemiológicas similares a otras poblaciones. El diagnóstico y el tratamiento deben de individualizarse de acuerdo a lo mostrado en estas guías.
The incidence of hepatocellular carcinoma (HCC) has increased worldwide and in Mexico there is limited information about this disease, its survival rates, and its treatment. The aim of this work was to analyze the available evidence on the Mexican population in relation to the diagnosis and treatment of HCC within the framework established by the current international clinical and therapeutic guidelines for this pathology. The Mexican Association of Hepatology organized a meeting in November 2012 in Merida, Yucatan, that was attended by 24 specialized physicians with a specific interest in HCC. The physicians were invited through their respective medical associations to participate in the discussion of the different disciplines involved in the diagnosis and management of HCC that included: gastroenterology, hepatology, radiology, pathology, medical oncology, and liver surgery and transplantation.
MethodologyDocuments published from 2000 were identified through electronic database searches using the keywords «hepatocellular carcinoma» and «Mexico» and adding terms such as: epidemiology, diagnosis, and treatment. Earlier documents were included if they were considered valuable for the current analysis, as well as abstracts presented in national medical congresses that provided data of interest for the present review. The international clinical and therapeutic guidelines published by the American and European associations on this subject were added to the bibliography, along with articles that were regarded as useful for the elaboration of the present consensus. The bibliographic information was sent to the participants to be reviewed prior to the meeting.
The consensus panel was divided into the following 4 working groups made up of the different specialists in each of the topics:
- I
Epidemiology and risk groups
- II
Surveillance and diagnosis
- III
Curative treatment
- IV
Non-curative treatment
A document was produced for each topic according to the available evidence and contributions from each of the disciplines, with their respective references. Each working group presented its assessment to the entire panel for discussion, after which key statements for each theme were elaborated. These statements were then put to the consideration of the participants through a Delphi panel, grading them as follows:
- -
6 pts. In complete agreement
- -
5 pts. In agreement
- -
4 pts. In partial agreement
- -
3 pts. In partial disagreement
- -
2 pts. In disagreement
- -
1 pt. In complete disagreement
When a statement was graded as disagreement (1 to 3 points), an explanatory motive was requested.
Agreement > 70% in the responses was regarded as consensus. Twenty-three of the 24 reunion participants answered the questionnaire and the omission of a statement response was considered disagreement. The document with the key statements and their levels of agreement in number and percentage is presented below.
I. Epidemiology and risk groupsM.S. González-Huezo, C. Hernández Hernández, R. Malé Velázquez, N. Méndez- Sánchez, R. Moreno Alcántar, M. Ramos Gómez
EpidemiologyPrimary liver cancer represents approximately 4% of all new cancers diagnosed worldwide, and of all the neoplasias originating in the liver, approximately 90% correspond to hepatocellular carcinoma.1
There has been a global increase in hepatocellular carcinoma incidence over the last few decades; it is the fifth most frequent neoplasia and the third cause of cancer-related death. The greatest prevalence is in Asia and Africa, whereas prevalence is much lower in America and Europe.2 In the United States, the Hispanic population has experienced the greatest annual percentage increase in the last decade. Compared with other ethnic groups, this population presented an important increase and according to a study by El-Serag et al. encompassing the decade of 1992 to 2002, women predominated at 63%, compared with 31% for men.3 This was confirmed by Alterkruse et al.4 in their analysis of death certificates in the U.S. Hispanic population carried out from 1975 to 2005. They also reported a triplication of hepatocellular carcinoma in this population, finding an annual percentage increase of 4% within their study time frame. It is striking that 40% of the Hispanics included in the analysis were born outside of the United States and that the increase in this disease was greater in native Hispanics than in their immigrant peers.
A rise in incidence of this pathology has been observed in Mexico for several decades. Cortes-Espinosa et al.5 demonstrated that incidence doubled within a 25-year period (1965-1990). They obtained this datum through a necropsy analysis (n = 12,556) at a referral hospital in Mexico City (0.35% for 1965-1969 vs 0.69% in 1985-1989). More recently, Méndez-Sánchez et al.6 analyzed official death certificates in Mexico from the year 2000 to 2006 and reported a national increase of 14% in the death rate from HHC (4.16 deaths per 100,000 inhabitants in the year 2000 vs 4.74 in 2005). Women experienced the greatest change, with a 15% increase, compared with the 12.5% increase in men. And finally, the Mexican National Health Information System (available at www.sinais.salud.gob.mx) analyzed causes of death from 1979 to 2008 in the population and corroborated an increase in mortality from this disease from 0.4% in the 1980s to 1.3% for 2008. According to the available information, the age groups above 55 years are the most affected, with an equal mortality rate for men and women.
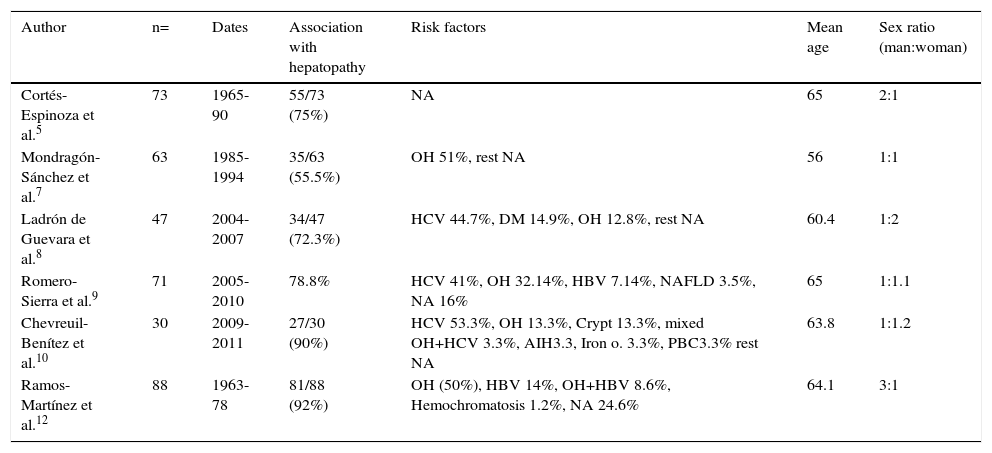
Risk GroupsTable 1 presents the data from the published series in both the texts and abstracts on patients with HCC and its primary epidemiologic characteristics. In relation to sex, the international literature generally describes a predominance in men; according to the national information presented by different authors6–10 and recent international epidemiologic reports,3,4 predominance has been equaling out over the last decade and may possibly increase for women in the future. According to the international literature, the most affected age groups are persons in the seventh decade of life in regions with low prevalence and from the sixth decade of life in regions with high prevalence. In Mexico, as shown in table 1, hepatocellular carcinoma presents mainly in the sixth decade of life. The main risk factor for 70 to 90% of the patients with HCC is cirrhosis of the liver. Currently, the two main causes of cirrhosis of the liver in Mexico are alcoholic liver disease (39.5%) and hepatitis C virus infection (36.6%); to a lesser degree are cryptogenic cirrhosis (10.4%), primary biliary cirrhosis (5.7%), hepatitis B virus (5%), and other reported causes (2.8%).11 It is noteworthy that cryptogenic cirrhosis has been catalogued as the third cause in relation to cirrhosis frequency, which could correspond to nonalcoholic fatty liver disease, given its strong association with obesity, diabetes mellitus, and dyslipidemia.
Epidemiologic characteristics of different reported case series on hepatocellular carcinoma in Mexico.
| Author | n= | Dates | Association with hepatopathy | Risk factors | Mean age | Sex ratio (man:woman) |
|---|---|---|---|---|---|---|
| Cortés-Espinoza et al.5 | 73 | 1965-90 | 55/73 (75%) | NA | 65 | 2:1 |
| Mondragón-Sánchez et al.7 | 63 | 1985-1994 | 35/63 (55.5%) | OH 51%, rest NA | 56 | 1:1 |
| Ladrón de Guevara et al.8 | 47 | 2004-2007 | 34/47 (72.3%) | HCV 44.7%, DM 14.9%, OH 12.8%, rest NA | 60.4 | 1:2 |
| Romero-Sierra et al.9 | 71 | 2005-2010 | 78.8% | HCV 41%, OH 32.14%, HBV 7.14%, NAFLD 3.5%, NA 16% | 65 | 1:1.1 |
| Chevreuil-Benítez et al.10 | 30 | 2009-2011 | 27/30 (90%) | HCV 53.3%, OH 13.3%, Crypt 13.3%, mixed OH+HCV 3.3%, AIH3.3, Iron o. 3.3%, PBC3.3% rest NA | 63.8 | 1:1.2 |
| Ramos-Martínez et al.12 | 88 | 1963-78 | 81/88 (92%) | OH (50%), HBV 14%, OH+HBV 8.6%, Hemochromatosis 1.2%, NA 24.6% | 64.1 | 3:1 |
AIH: Autoimmune hepatitis; Crypt: Cryptogenic; DM: Diabetes mellitus; HBV: Hepatitis B virus; HCV: Hepatitis C virus; Iron o.: Iron overload; NA: Not available; NAFLD: Non-alcoholic fatty liver disease; OH: Alcohol; PBC: Primary biliary cirrhosis.
Before the discovery of the hepatitis C virus, 2 case series published in Mexico described alcoholic liver disease as the main risk factor for HCC.7,12 After its identification, different case series described this virus as the main risk factor associated with hepatocellular carcinoma. Table 1 describes these and other epidemiologic characteristics related to this pathology. The presence of underlying hepatopathy in the cases of HCC varies from 55%7 to 92%,12 a figure in accordance with internationally published figures.
The presence of advanced fibrosis without cirrhosis associated with hepatitis B and C virus infections has been reported to be a possible etiologic factor for developing HCC.13 It should be underlined that there is a higher risk associated with inflammatory activity and a greater viral burden in the presence of chronic virus B infection. However, this does not appear to be a frequent cause of HCC in Mexico, where the prevalence of this infection is considered low (HBsAg < 2% in the general population) according to the World Health Organization. In addition, universal hepatitis B vaccination was implemented in Mexico in 1998, and infants born in that year and those that followed have been vaccinated, resulting in coverage above 80% in the infant population for the year 2007, according to the official website of that organization.
Obesity is another recognized factor that predisposes to an increase in the incidence of different cancers, including HCC. This tumor is 4.5% more frequent in men with a BMI above 35 and 1.7% more frequent in women with a BMI above 35, compared with normal-weight subjects.1 The presence of diabetes mellitus is a frequent finding, particularly in cases of cryptogenic cirrhosis, which in turn, can be secondary to nonalcoholic fatty liver disease, and therefore its causal role in HCC is not clearly defined. Other risk factors described for the development of HCC include iron toxicity, aflatoxin exposure, Wilson disease, alpha-1-antitrypsin deficiency, and other metabolic disorders whose prevalence in Mexico is unknown.
Recommendations:
- -
HCC incidence in Mexico has increased over the last few decades (23/24 = 95.8%).
- -
The mean age for HCC presentation in Mexico is in the sixth decade of life and the woman:man ratio appears to be equal (22/24 = 91.6%).
- -
HCC is frequently associated with underlying hepatopathy (23/24 = 95.8%).
- -
Chronic hepatitis C virus infection is the main cause of HCC reported in Mexico (22/24 = 91.6%).
- -
Chronic hepatitis B virus infection does not appear to be a frequent cause of HCC in Mexico, whereas cirrhosis due to nonalcoholic fatty liver disease may emerge in the near future as an important cause of HCC in Mexico (23/24 = 95.8%).
- -
Hereditary metabolic diseases are a very infrequent cause of HCC in Mexico (23/24 = 95.8%).
- -
There is no available data on aflatoxin exposure in the Mexican population (23/24= 95.8%).
J. Aguirre García, M. Castillo Barradas, R. Contreras Omaña, L. Ladrón de Guevara, J.M. Remes-Troche, M.C. Zepeda-Florencio
SurveillanceScreening consists of the application of minimally invasive tests that enable early-stage disease detection, allowing curative treatment to be offered and thus improving the survival rate. In the high-risk population for HCC, lesions < 2cm are considered potentially curable. Surveillance is the programmed application of these screening tests to this risk group.
Surveillance is carried out by the attending physician of patients with chronic liver disease (cirrhosis), a high-risk group for developing HCC.14,15
HCC surveillance requires a multidisciplinary team that includes hepatologists, radiologists, and pathologists specifically trained in the detection and study of space-occupying lesions of the liver.
The most widely used screening study is transabdominal ultrasound. It is a simple, relatively inexpensive, and minimally invasive study with approximately 65-80% sensitivity and over 90% specificity. Despite its being operator-dependent, it is the screening study recommended by the current international guidelines,16–18 in the absence of a more effective biomarker,19,20 and approach algorithms are available in the event of an abnormal study. According to a recent meta-analysis carried out by Singal et al.,21 the addition of alpha-fetoprotein to this strategy is of minimal benefit and raises costs. Abdominal ultrasound is recommended every 6 months, even though the ideal interval is unknown. It is believed that a period of 4 to 12 months is needed for an undetectable lesion to become identifiable (2cm) by this method and undergo curative treatment. In contrast, performing the study at shorter intervals (every three months) increases the frequency of abnormal findings, without modifying the survival rate of those patients with confirmed and treated tumors.22
A frequent ultrasound finding is an echogenic nodule surrounded by a hypoechogenic halo, and when Doppler color ultrasound is applied, aberrant intratumor vascularity can be observed.19,23,24 However, it should be emphasized that any recently appearing or not previously identified nodule merits evaluation. Routine AFP determination is not recommended as an isolated screening and surveillance test because it is not cost-effective.19,25–27
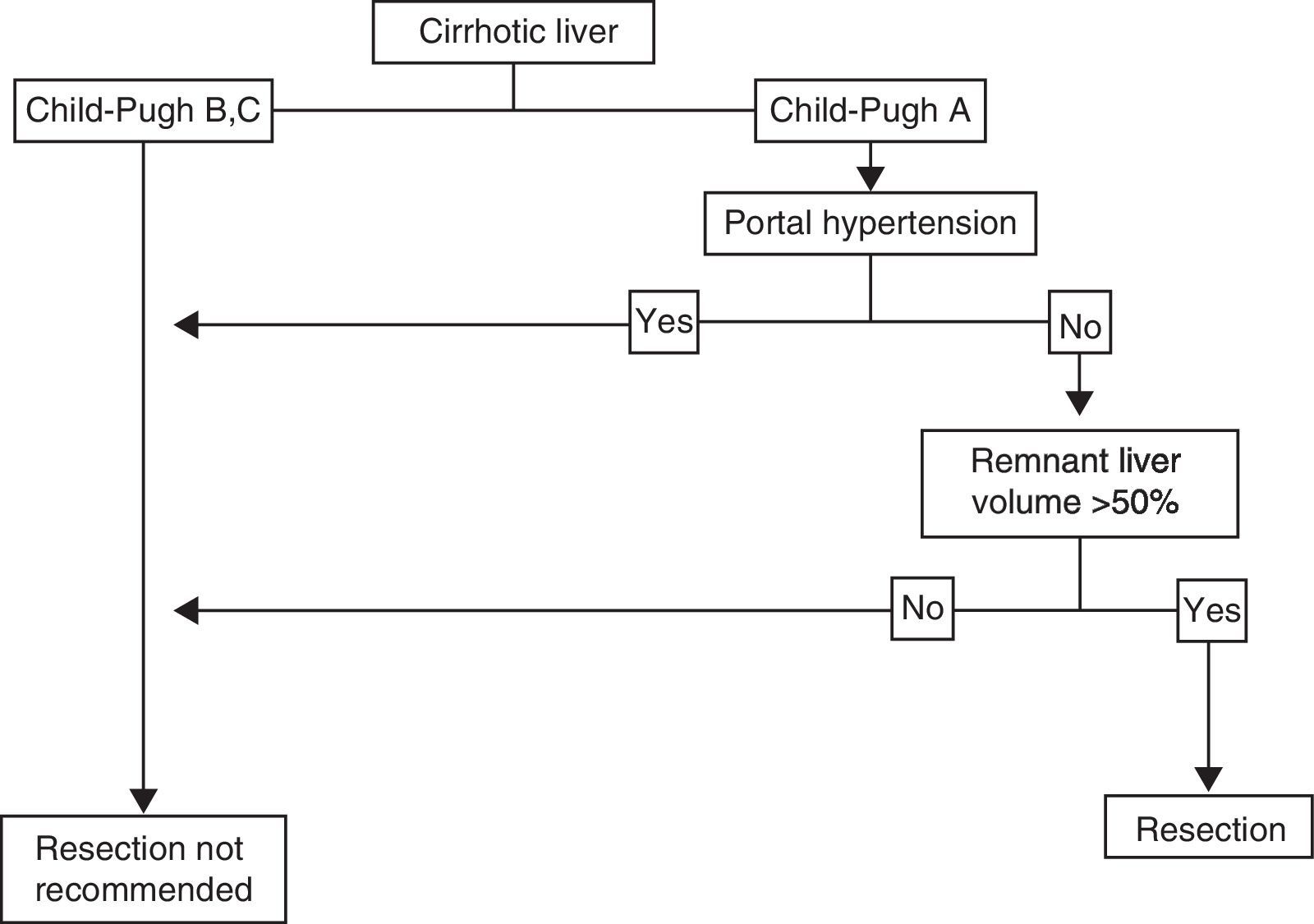
Surveillance is indicated in high-risk groups, specifically in patients with cirrhosis of the liver of any etiology, with sufficient liver reserve (Child-Pugh stage A and B) and adequate functional capacity. Contrastingly, screening for HCC is not recommended for patients with Child-Pugh C cirrhosis of the liver, except in patients considered for placement on a waiting list for liver transplantation.28
DiagnosisWhen a nodule smaller than one centimeter is identified through abdominal ultrasound In risk groups under surveillance, an ultrasound study every 3-4 months is recommended for a period of 18-24 months. If there is growth in the morphologic behavior of the image during this follow-up period, a dynamic study such as multiphase computed tomography or nuclear magnetic resonance should be carried out.16,29
To be considered technically adequate, dynamic studies should include the following phases: non-contrast phase, hepatic artery phase, portal vein phase, and late phase.16,30 It is very important to standardize the multiphase dynamic study to avoid interpretation variation and errors.
The choice between computed axial tomography (CAT) or magnetic resonance depends on experience and availability in relation to each hospital center. The typical dynamic study image of an HCC tumor is a hypo/isodense lesion in the noncontrast phase that is stronger in the early arterial phase and has an early venous washout.16
In a high-risk population, a dynamic study with an image typical of an HCC lesion is sufficient for making HCC diagnosis. If the image is atypical, another dynamic study should be carried out; if the studies are not concordant, then the recommendation is to perform a liver biopsy.16,17,31 Biopsy is not justified in high-risk groups with nodules regarded as typical lesions, and likewise, biopsy is not indicated in patients that are not candidates for any specific type of treatment.16,17
If a patient without cirrhosis has a lesion that is impossible to diagnose through imaging studies, biopsy of the lesion is always recommended.31 Ultrasound-guided or CT-guided Tru-Cut biopsy is suggested and should always be reviewed by a pathologist with training and/or experience in hepatic lesions. When the liver biopsy is difficult to interpret, histochemistry studies can be used, such as reticulum staining and immunohistochemistry with CD 34, glypican-3, heat shock protein 70, and glutamine synthetase. Unfortunately, the majority of these are not available for routine use; their foremost value is in distinguishing between regeneration nodule, dysplastic nodule, and HCC.31,32
According to current management recommendations,16,17,22 the determination of alpha-fetoprotein levels has no diagnostic usefulness, even though it could be useful in evaluating treatment response if levels were initially high.
There are 7 proposals in regard to staging and they include the TNM classification system created by the American Joint Committee on Cancer (AJCC), the Cancer of the Liver Italian Program (CLIP), the Japanese Integrated System (JIS), the Grupe d’Etude de Traitment du Carcinoma Hepatocellulaire (GRETCH), the Chinese University Prognostic Index (CUPI), the Okuda system, and the Barcelona Clinic Liver Cancer (BCLC) system. Traditionally, tumor prognosis is directly related to its size and extension, but in the case of HCC, liver function reserve also affects outcome and limits treatment. Therefore, the ideal classification should include variables related to the tumor, as well as liver function classification and functional capacity of the individual presenting with 2 diseases, cirrhosis and cancer, and finally, it should include the efficacy of the treatment that was administered. The Okuda, TNM, CLIP, and JIS classifications do not include variables that evaluate the functional status of the individual, limiting their prognostic capacity. Both the CUPI and the GRETCH consider indirect liver function reserve data. Of these 7 systems, the only scale that includes the abovementioned points is the BCLC, originally published in 1999.33 Marrero et al.34 compared the 7 different existing scales in a consecutive cohort of 239 individuals with HCC and found that the main independent survival predictors were tumor diameter, the presence of portal vein thrombosis, the Model of End Stage Liver Diseases (MELD) score, the functional capacity of the individual, and the administered treatment. Of the 7 scales, the BCLC had the greatest independent survival predictive power, because all these parameters are included in its variables, and therefore it is the staging classification recommended by the American and European guidelines, as well as by our group.16,17 Even though recent information suggests that stage B (intermediate) may be sufficiently heterogeneous to merit being a subclassification, this remains to be defined in the future.35
Recommendations:
- -
Surveillance is recommended for groups at high risk for developing HCC in Child-Pugh stages A or B, and also stage C, but only if the patient is on a waiting list or considered a candidate for liver transplantation (22/24 = 91.6%).
- -
Surveillance for risk groups should be carried out with abdominal ultrasound every six months (23/24 = 95.8%).
- -
All new lesions or those not previously identified in high-risk patients merit an initial evaluation with either a multiphase computed tomography or nuclear magnetic resonance dynamic study (23/24 = 95.8%).
- -
A dynamic study with a characteristic lesion > 1cm in a high-risk population is sufficient for making diagnosis (23/24 = 95.8%).
- -
A dynamic study with an undetermined lesion in this population makes another dynamic study or guided liver biopsy obligatory (23/24 = 95.8%).
- -
Any recently appearing lesion in a healthy liver that cannot be diagnosed through an imaging study merits a liver biopsy (20/24 = 83.3%).
F.J. Bosques Padilla, M. Guerrero Hernández, R.J. Mondragón Sánchez, J.F. Rivera Ramos, J.F. Sánchez Ávila, M. Vilatobá Chapa
Three curative treatment approaches, in accordance with BCLC classification, are available: liver resection, liver transplantation, and local ablation. In general terms, liver resection is the treatment of choice in individuals with HCC in a healthy liver and is an excellent alternative for individuals with cirrhosis, following strict criteria that will be discussed shortly. In the presence of cirrhosis and under the Milan criteria,36 liver transplantation is the treatment of choice, given that it treats not only the tumor, but also the underlying disease. The great disadvantage, particularly in Mexico, is in relation to the availability of this strategy. Finally, if liver transplantation is contraindicated and falls outside of the surgical criteria, the alternative curative treatment is locoregional therapy. Of these therapies, radiofrequency ablation (RFA) achieves the best results in relation to tumor control and survival. A description of each of the strategies follows.
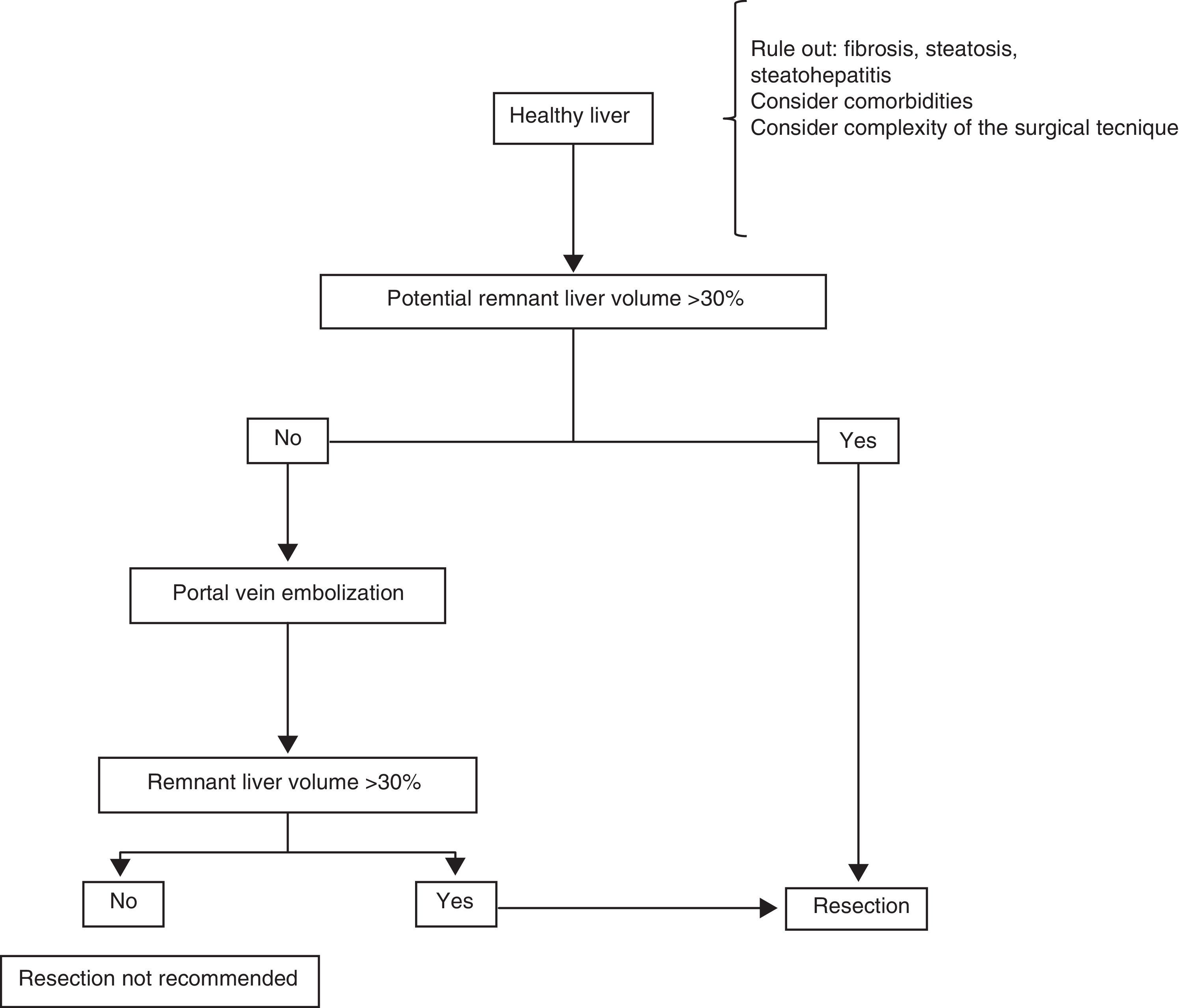
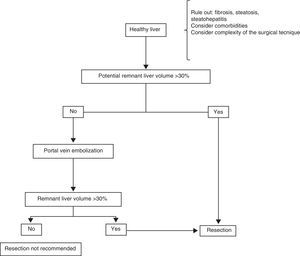
Surgical treatmentResectionHealthy liverPatients with HCC that arises in a healthy liver should be considered for surgical resection provided that extrahepatic disease is excluded through thoracic and abdominal CAT scans and bone scintigraphy, and a remnant liver volume (RLV) that is documented through tomography-assisted volumetry, is taken into consideration.37,38 If the estimated RLV is < 30%, portal embolization is suggested in order to increase it (figure 1).39–41 Some authors believe that RLV values of 20% are adequate, but nevertheless, it should be kept in mind that this method can overestimate the real hepatic volume. Other added risk factors associated with post-resection liver failure should also be taken into account individually, always in centers with experience in liver surgery (table 2).39,42
Risk factors for post-resection liver failure.
| Above 70 years of age |
| Cirrhosis |
| Hepatitis |
| Intraoperative blood loss |
| Ischemia |
| Obstructive Cholestasis |
| Chemotherapy |
| Steatosis |
Adapted from Clavien et al.42
In addition, it is very uncommon for HCC to present in a liver with no underlying pathology, and thus the importance of evaluating the presence of fibrosis, steatosis, or steatohepatitis in the patient being considered for resection. When there is doubt, non-neoplastic liver tissue biopsy should be performed before the procedure.43 Furthermore, each patient should be treated individually in relation to comorbidity. The surgical technique should be carefully evaluated, identifying the cases that are «high complexity» ones from the surgical perspective. Such cases can result in a period of prolonged hepatic ischemia (Pringle maneuver) or abundant intraoperative blood loss, both of which are important intraoperative predictive factors for the development of postoperative liver failure.44,45
Portal embolization can be carried out percutaneously or during a laparotomy, ligating the desired portal branch under fluoroscopic guidance.46,47 The aim is to favor the hypertrophy of the liver tissue that will be the future remnant by excluding the embolized section to be resected. At approximately 6 to 8 weeks after embolization, a new triphasic CAT scan with new volume measurements is carried out to calculate the new RLV. If the desired minimum is not achieved, the patient is not a good candidate for liver resection.46–48
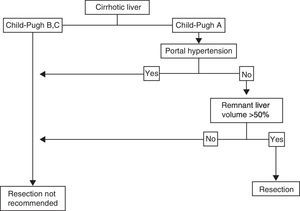
Cirrhotic LiverThe individual with HCC that has a localized lesion, cirrhosis of the liver with Child-Pugh stage A and no evidence of portal hypertension, normal bilirubin, and a RLV > 50%, is a candidate for liver resection.33,44,45 Unfortunately, a minority of cases are diagnosed in this phase and the surgery must also be performed in centers that specialize in liver surgery. Anatomic resection should be carried out, if possible, as it has shown greater survival and lower recurrence rates.49,50 If ample resection is considered (>50%), under ideal circumstances, in addition to the above-stated, the indocyanine green retention test should be carried out51,52 (figure 2); lamentably, this dynamic liver function test is not available in Mexico. Therefore, the decision to perform surgery and its extension depends on the previously mentioned parameters and must be carried out in centers that have experience in hepatic surgery management,33,53,54 given that current standards in centers that perform tumor resection in patients with cirrhosis include mortality < 3%, transfusions < 10%, and 5-year survival of at least 50%.55,56 According to different meta-analyses, 5-year survival in these patients is above 50%, and can reach 80% in super-selected patients (well-compensated liver disease with lesions < 2cm).57 Unfortunately, tumor recurrence, whether true or de novo, is frequent and fluctuates between 60 and 100% at 5 years, because cirrhosis, the main risk factor, remains and progresses.44 We did not find many published reports on the national statistics of liver resection due to HCC in Mexican cirrhotic patients.
Liver TransplantationAs mentioned above, liver transplantation is an excellent alternative, but its availability is scarce in Mexico. Therefore experience is limited and recommendations are based on international consensuses. In general terms, patients with advanced cirrhosis of the liver that meet the Milan or UCSF criteria are regarded as candidates for liver transplantation.58–60 Staging should be carried out through thoracic and abdominal CAT scan and bone scintigraphy.
Milan criteria:36
- -
One lesion < de 5cm
- -
Three lesions, none of which are > 3cm
UCSF criteria:59
One lesion < 6.5cm
Two to 3 lesions, none of which are > 4.5cm, with a total diameter < 8cm
Waiting list organ assignation is done according to patient disease severity and priority is given to those that have a greater probability of dying. The MELD classification is used for this because patients with HCC can present with stable disease. The MELD score does not reflect disease severity or the risk for death from tumor progression, and so the points are defined as follows:61
- •
HCC > 2cm: 22 MELD points at the time of enrollment (points are added every 3 months to the waiting list, with a 10% increase in risk for death)
- •
HCC < 2cm: no additional points are figured in
Because HCC patients are expected to be on the waiting list for more than 6 months, locoregional therapies (RFA or transarterial chemoembolization) are a good «treatment bridge» option if liver function allows it (Child-Pugh A or B).17,60,62 Stage can be lowered provided that the aim of tumor size reduction is met, staying within the Milan or UCSF criteria.63–65 Liver transplantation can be regarded as «salvage therapy» in patients that underwent previous liver resection and then presented with recurrence.66
Live donor liver transplantation between 2 adults can be a good option in relation to HCC. The same guidelines for cadaveric donor transplantation should be followed exactly and the donor should be clearly aware of the risks and possible complications.60,67
Even though it is difficult to compare liver resection vs liver transplantation, it has been determined that patients that undergo transplantation present with greater 5-year survival (70%) and lower disease recurrence frequency (20%), under the selection criteria mentioned above.68–70
Locoregional treatmentAblative therapiesAblative therapies are regarded as curative strategies in those patients with cirrhosis and early-stage HCC (Child-Pugh A-B, with a solitary nodule ≤ 3cm or 3 nodules ≤ 3cm) in whom surgical treatment, whether resection or liver transplantation, are contraindicated.17
These therapies are based on the injection of substances into the tumor (acetic acid, ethanol) or in changes of temperature (RFA, laser, cryotherapy) that lead to tumor necrosis/destruction.71,72
The 2 most frequent strategies used are percutaneous ethanol injection (PEI) and RFA, insofar as other techniques such as microwave ablation, cryoablation, and irreversible electroporation are still being evaluated. The percutaneous route is employed in the majority of cases, but in certain specific situations the procedures may be performed laparoscopically.
PEI can achieve complete necrosis in 90% of the tumors < 2cm in diameter. However, results are considerably lower with larger lesions.71,73 The main limitation of PEI is the high local recurrence rate, reaching 43% in lesions > 3cm.74
RFA has been more widely assessed than PEI as treatment in this group of patients. It is believed that the energy that is generated, producing coagulative tumor necrosis, could also eliminate small, undetected satellite lesions, achieving better tumor control with fewer sessions. Numerous studies in favor of this have compared the efficacy and safety of the two techniques; better local disease control has consistently been shown with RFA vs PEI (2-year local recurrence rates of 2-18% vs 11-45%).75–77 Furthermore, 3 independent meta-analyses that included all randomized studies confirmed that RFA benefits survival, compared with PEI, in tumors > 2cm.78–80 The best results with RFA are obtained in patients presenting with cirrhosis, Child-Pugh A, and small solitary tumors (usually < 2cm), with a 5-year survival rate of 40 to 70%.81,82
Some studies have reported a higher major complication rate with RFA (4%; 95% CI: 1.8-6.4%), when compared with PEI (2.7%; 95% CI: 0.4-5.1%).80 Nevertheless, both procedures are considered safe in general, with no statistically significant difference in major adverse events.83
Thus it can be concluded that RFA is the best ablative treatment alternative, but with the following limitations: it cannot be used on tumors that are located close to other organs (colon, gallbladder, etc.) or a large blood vessel, because of the possibilities of heat damage and incomplete ablation. Moreover, approximately 10-15% of the tumors that are difficult to treat with RFA, can be treated with PEI.84
Recommendations:
- -
In cases of liver resection due to HCC in a healthy liver, a meticulous tumor staging study and an abdominal tomography scan for remnant liver volume determination are essential (23/24 = 95.8%).
- -
Because HCC is frequently associated with underlying hepatopathy, in the case of resection, the underlying hepatic alteration should be documented through liver biopsy if it has not been previously recorded (22/24 = 91.6%).
- -
In situations of HCC in the patient with cirrhosis, liver resection is an option, but it is only recommended in well-selected patients with good functional status (23/24 = 95.8%).
- -
Liver transplantation is an excellent option in well-selected patients that has the advantage of restoring liver function and removing the neoplasia, when based on the Milan and UCSF criteria (23/24 = 95.8%).
- -
Percutaneous ethanol injection and radiofrequency ablation are two options for tumor destruction that are available in Mexico and they offer good therapeutic response when performed on well-selected patients in centers with adequate experience in liver surgery (23/24 = 95.8%).
L.E. Cisneros Garza, G. Elizondo Rojas, F.D. Huitzil Meléndez, O. Quiroz Castro, T. Rizo Robles, M. Serna Camacho
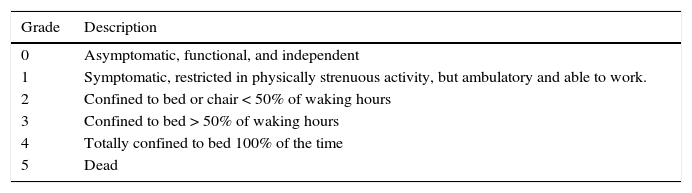
The treatment goal for advanced-stage HCC patients is to increase survival (stages B and C) and/or palliate the related symptoms (stage D). The patients that are susceptible to receiving non-curative treatment are those in categories B (intermediate), C (advanced), and D (terminal) of the BCLC classification. As commented beforehand, the different stages are defined according to tumor characteristics, liver reserve determined through the Child-Pugh classification, and functional classification by the ECOG scale (table 3).
Eastern Cooperative Oncology Group (ECOG) functional classification.
| Grade | Description |
|---|---|
| 0 | Asymptomatic, functional, and independent |
| 1 | Symptomatic, restricted in physically strenuous activity, but ambulatory and able to work. |
| 2 | Confined to bed or chair < 50% of waking hours |
| 3 | Confined to bed > 50% of waking hours |
| 4 | Totally confined to bed 100% of the time |
| 5 | Dead |
Adapted from Oken et al.101
Multidisciplinary evaluation is recommended for the category B patient to determine whether he or she is a candidate for liver transarterial chemoembolization.16,17
Early-stage HCC is not heavily vascularized and its blood supply comes from the portal vein. Once the tumor grows, the blood supply is arterialized, and thus obstruction of the hepatic artery in this disease phase is an effective therapeutic procedure for treating HCC. Acute arterial obstruction induces ischemic necrosis of the tumor. This obstruction of the hepatic artery is performed by an interventionist radiologist and is carried out through an angiographic procedure called arterial embolization. When this procedure is combined with the injection of chemotherapeutic agents through the same hepatic artery, and generally mixed with lipiodol, it is known as transarterial chemoembolization. Embolization can be carried out with a series of solid agents, the most widely used of which are polyvinyl alcohol particles; the mixture of doxorubicin with lipiodol is used as a chemotherapeutic agent.85
The patients with the following characteristics are considered candidates for this procedure:
- a.
In terms of liver functionality, patients with Child-Pugh category A are eligible.
- b.
In terms of tumor extension, the eligible patients are those that do not have the possibility of curative treatment because they present with a lesion > 5cm or multinodular disease (more than 3 nodules > 3cm) and no advanced disease data, meaning no portal invasion or extrahepatic metastasis.
- c.
In terms of physical performance status, patients with an ECOG score of 0, which implies no physical limitation, are eligible.
Chemoembolization is recommended as first-line non-curative treatment in patients with multifocal lesions > 5cm that do not present with vascular invasion or extrahepatic dissemination and that have good liver reserve. Because it has been demonstrated that chemoembolization benefits overall survival in this group of patients, it is recommended that all centers treating patients with HCC be equipped with the human and technical resources for performing this procedure.86 There is still no consensus in relation to the number of sessions for the patient to undergo or their frequency; this information requires further study.87 And finally, different techniques are being developed, such as radioembolization88,89 and drug-releasing solid particle chemoembolization;90,91 because they are currently being evaluated there is still not enough evidence available to be able to give a recommendation.
The category C patient should undergo a multidisciplinary evaluation to determine whether he or she is eligible for receiving specific molecular targeting treatment with the multikinase inhibitor, sorafenib. Sorafenib is currently considered the first-line treatment for patients with advanced HCC, and it is the only systemic therapy that has been approved for use in this disease,16,17 by demonstrating that it prolongs survival in advanced-stage patients, compared with placebo (7.9 vs 10.9 months; p < 0.001).92 There is evidence of certain signaling pathway alteration in these tumor cells, including the Raf/MEK/ERK and VEGF pathways.93 Likewise, sorafenib is a multikinase inhibitor that targets Raf-1, B-Raf, vascular endothelial growth factor receptor (VEGFR2), platelet-derived growth factor receptor (PDGFR), and c-Kit receptors, and inhibits tyrosine kinase and serine-threonine kinase receptors, acting as an anti-proliferative and anti-angiogenic agent. Its significant effect on overall survival and tumor progression time were documented in a phase II study94 and in the pivotal phase III SHARP study published in the New England Journal of Medicine in 2008.92Patients with the following characteristics are candidates for systemic sorafenib therapy:16,17
- a)
In terms of liver functionality the patients with Child-Pugh category A are eligible.
- b)
In terms of tumor extension the patients with data of advanced disease, including portal invasion and/or extrahepatic disease are eligible.
- c)
In terms of physical performance patients with an ECOG score of 0-2 are eligible.
Patients that do not have access to treatment with sorafenib could be evaluated to determine their eligibility for treatment with chemotherapy or with emerging protocols in clinical research analyzing molecules. Different specific molecular targets are currently being developed and studied, but there is not yet sufficient evidence for their recommendation.95–98
HCC has been shown to be chemo-resistant to the majority of chemotherapeutic agents; in general terms, the antitumor response is low (0-18%) and there is no evidence of survival improvement with either their isolated or combined use. The liver function disorder caused by cirrhosis may alter the metabolism of these agents and increase their toxicity, and so they are not recommended for routine use or to be used outside of research protocols.99,100
Finally, patients in category D, defined as terminal stage, with a life expectancy of about 3 months and characterized by an ECOG 3-4 physical performance grade and/or Child-Pugh stage C, should receive multidisciplinary management. It should be focused on improving quality of life through symptomatic palliative management that includes pain treatment, nutrition, and psychological management.16,17
Recommendations:
- -
HCC that is in the non-curative stage should be evaluated by a multidisciplinary team of experts in the field (23/24 = 95.8%).
- -
Treatment goals in stage B (intermediate) and stage C (advanced) patients include prolonging survival and disease progression time (23/24 = 95.8%).
- -
Stage B (intermediate) is characterized by being outside of curative treatment, but with no evidence of vascular or extrahepatic invasion, and with good liver reserve and physical performance status. The treatment of choice is transarterial chemoembolization (21/24 = 87.5%).
- -
Stage C (advanced) is defined by being outside of curative criteria, presenting with vascular invasion and/or extrahepatic disease, but with adequate liver reserve and physical performance status. The treatment of choice is systemic therapy with sorafenib (22/24 = 91.6%).
This article was funded in part by Bayer Healthcare.
Conflict of interestThe following participants declare having a conflict of interest: J.F. Sánchez Ávila: Merck, Gilead; J.M. Remes-Troche: BMS, Astra-Zeneca, Takeda, Janssen; L. Ladrón de Guevara: Bayer, MSD; R. Contreras Omaña: Bayer, MSD; R. Malé Velazquez: MSD; and M.S. González-Huezo: Roche, Bayer.
Aguirre García J. Hospital MIG, Mexico City.
Bosques Padilla FJ. School of Medicine and Hospital Universitario J. E. González UANL, Monterrey N.L.
Castillo Barradas M. Centro Médico Nacional “La Raza” Instituto Mexicano del Seguro Social, Mexico City.
Contreras Omaña R. Hospital General de Zona No. 1 Instituto Mexicano del Seguro Social, Pachuca, Hidalgo.
Cisneros Garza LE. UMAE # 25 Instituto Mexicano del Seguro Social, Monterrey. N.L. y Hospital San José TEC de Monterrey, Monterrey. N.L.
Elizondo Rojas G. Hospital Universitario, Monterrey, N.L.
González-Huezo MS. Centro Médico Issemym Metepec, Edo. Mex.
Guerrero Hernández M. Department of Radiology, INCMyNSZ, Mexico City.
Hernández Hernández C. Hospital Regional Presidente Juárez, Oaxaca Oax.
Huitzil Meléndez FD. Department of Oncology, INCMyNSZ, Mexico City.
Ladrón de Guevara L. Centro Médico Nacional “20 de Noviembre”, ISSSTE Mexico City.
Malé Velázquez M. Hospital del Carmen Guadalajara, Jal.
Méndez-Sánchez N. Fundación Clínica Médica Sur Mexico City.
Mondragón Sánchez RJ. Head of Transplantation Service, Centro Médico Issemym, Metepec Edo. Mex
Moreno Alcántar R. Centro Médico Nacional SXXI Instituto Mexicano del Seguro Social, Mexico City.
Quiroz Castro O. Hospital Ángeles del Pedregal, Mexico City.
Ramos Gómez M. Centro Médico Nacional “20 de Noviembre” ISSSTE, Mexico City.
Remes-Troche JM. Instituto de Investigaciones Médico-Biológicas Universidad Veracruzana, Veracruz
Rivera Ramos JF. Gastroenterology Service, Hospital Español de México. Mexico City.
Rizo Robles T. Centro Médico Nacional “La Raza”, Instituto Mexicano del Seguro Social, Mexico City.
Sánchez Ávila JF. Department of Gastroenterology, INCMyNSZ. Mexico City.
Serna Camacho M. Hospital Regional Lic. Adolfo López Mateos ISSSTE, Mexico City.
Vilatobá Chapa M. Head of Transplantation Department INCMyNSZ, Mexico City.
Zepeda-Florencio MC. Centro Médico Issemym, Metepec, Edo. Mex.
Collaborators of the Grupo Mexicano de Consenso de Carcinoma Hepatocelular can be found in Appendix A.
Please cite this article as: González Huezo MS, Sánchez Ávila JF, en representación de Asociación Mexicana de Hepatología, Asociación Mexicana de Gastroenterología, Sociedad Mexicana de Radiología e Imagen, Sociedad Mexicana de Oncología, World Gastroenterology Organisation, Grupo Mexicano de Consenso de Carcinoma Hepatocelular. Consenso mexicano de diagnóstico y manejo del carcinoma hepatocelular. Revista de Gastroenterología de México. 2014;79:250–262.