There is a wide heterogeneity in the reports of celiac disease prevalence in iron-deficiency anemia patients.
AimTo determine the prevalence of celiac disease in patients with iron-deficiency anemia.
Materials and methodsAdult patients with a diagnosis of iron-deficiency anemia were enrolled for upper endoscopy with duodenal biopsies. Volunteers that underwent upper endoscopy were enrolled as controls.
ResultsA total of 135 patients with iron-deficiency anemia and 133 controls were enrolled. Celiac disease prevalence was higher in the iron-deficiency anemia group [11.11 vs. 1.51%, OR: 8.18 (1.83-36.55), P=.001). Of the celiac disease patients in the iron-deficiency anemia group, 73.3% had at least one endoscopic sign suggesting villous atrophy, whereas 100% of the celiac disease patients in the control group presented with at least one endoscopic sign.
ConclusionsPatients with iron-deficiency anemia have an increased risk for celiac disease. Up to 25% of these patients may not present any endoscopic sign suggesting villous atrophy.
Existe una extensa heterogeneidad en los reportes de la prevalencia de enfermedad celíaca en el contexto de la anemia ferropénica.
ObjetivoDeterminar la prevalencia de enfermedad celíaca en pacientes con anemia ferropénica.
Materiales y métodosPacientes adultos con anemia ferropénica fueron reclutados para realizarse una endoscopia digestiva alta con biopsia duodenal. Se reclutaron asimismo voluntarios de la comunidad como controles.
ResultadosSe reclutó a 135 pacientes con anemia y 133 controles. La prevalencia de enfermedad celíaca fue mayor en el grupo de anemia (11.11% vs. 1.51%, OR 8.18 [1.83-36.55], p=0.001); el 73.3% de los celíacos en el grupo de anémicos presentaron algún signo endoscópico de atrofia vellositaria, mientras que el 100% de los celíacos en el grupo control presentaron por lo menos un signo endoscópico.
ConclusiónLos pacientes con anemia ferropénica presentan un riesgo incrementado de enfermedad celíaca. Hasta un 25% de estos pacientes pueden no presentar signos endoscópicos indicativos de atrofia vellositaria.
Celiac disease (CD) is an autoimmune disorder characterized by a chronic intestinal inflammatory response due to the ingestion of gluten, leading to some degree of villous atrophy and potential nutrient malabsorption.1 Its diagnosis is based on the presence of histologic signs of villous atrophy in duodenal biopsies and positive antibodies against specific targets, mainly tissue-transglutaminase, gliadin, or endomysium.2 Certain findings at upper endoscopy may suggest villous atrophy, such as scalloped folds or a mosaic pattern.3 However, CD may be diagnosed even in the absence of any endoscopic signs.4
Iron-deficiency anemia is one of the most common clinical features related to CD.5 It is the most common type of anemia among CD subjects,6 and may be the only feature present at diagnosis.
CD prevalence among patients with iron-deficiency anemia has been previously reported.6–9 However, the conspicuous methodological heterogeneity in the published studies has led to varied prevalence. Furthermore, there is little evidence of CD prevalence in patients with iron-deficiency anemia in the context of a Latin-American population.
Our aim was to determine the prevalence of CD in patients with iron-deficiency anemia. We also evaluated the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of endoscopic signs in the setting of iron-deficiency anemia.
Materials and methodsStudy populationA study with a case-control design was conducted. From January to December 2015, subjects over 18 years of age with a diagnosis of iron-deficiency anemia were enrolled and offered an upper endoscopy. Iron-deficiency anemia was defined as a hemoglobin value of less than 14g/dl in men and 12g/dl in women, with a ferritin value of less than 15 ug/l. Upper endoscopy was offered after performing the initial clinical evaluation and laboratory work-up, which was carried out to exclude other causes of anemia (e.g., folate or vitamin B12 deficiency, chronic disorder anemia). Tissue transglutaminase IgA antibodies (as well as total Ig A levels) were determined in every case. Colonoscopy was performed if necessary, according to our institution's protocol.
We also recruited healthy volunteers from the community, offering them an upper endoscopy with duodenal biopsy.
Subjects presenting with other symptoms, such as diarrhea, unexplained weight loss, recurrent abdominal pain, abdominal bloating, or melena were excluded.
Patients with a history of prior CD were also excluded.
Enrolled subjects were asked to sign a statement of informed consent. The study protocol was approved by the institutional Ethics Committee and financed by our institution's research funds.
Endoscopic and histopathologic proceduresUpper endoscopies were performed using High Definition Pentax® EPK-I 5010 endoscopes (Pentax Medical America, Montvale, NJ, USA). At least 4 to 6 duodenal biopsies were taken in each patient, including 2 duodenal bulb biopsies. These were sent to the pathology department where they were analyzed according to a previously published protocol.2 Histopathologic findings were classified according to the Marsh-Oberhuber classification. Biopsies were taken in every case, even in the absence of endoscopic signs suggesting villous atrophy. The following variables were recorded: age, sex, hemoglobin value, tissue transglutaminase antibody (anti-tTG) value, the presence of endoscopic signs suggesting villous atrophy (absence of duodenal folds, scalloping, or mosaic pattern in the duodenal mucosa), and the Marsh-Oberhuber classification.
CD was defined as the presence of any degree of villous atrophy and a significant increase in intraepithelial lymphocytes (IELs), with positive antibodies or histopathologic and serologic markers suggesting CD. These were corrected after the implementation of a gluten-free diet.
Statistical analysisStatistical analysis was performed using Stata software (v 11.1, Statacorp, College Station, TX). The categorical variables were described as percentages. The numerical variables were described as means with their standard deviations. For the comparison of the categorical variables, the chi square test was used. For the numerical variables, the Student's t test was used. Odds ratios (ORs) with their corresponding 95% confidence intervals (95%CI) were calculated. The prevalence of CD was compared between groups. A p value of less than 0.05 was considered statistically significant. The prevalence of endoscopic signs of villous atrophy was compared between CD patients in both groups. Sensitivity, specificity, PPV, and NPV of the endoscopic signs were calculated in the setting of patients with iron-deficiency anemia.
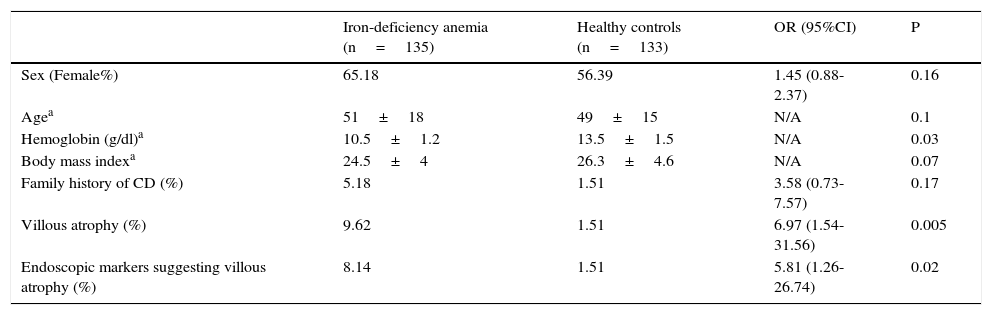
ResultsA total of 135 patients with iron-deficiency anemia and 133 controls were enrolled in the study. There were no significant differences in age and sex between groups as shown in Table 1. CD prevalence was significantly higher in the iron-deficiency anemia group vs. the control group (11.11 vs. 1.51%, OR: 8.18 [1.83-36.55], p=0.001). All CD patients had positive anti-t TG antibodies.
General characteristics of patients with iron-deficiency anemia and the healthy volunteers.
| Iron-deficiency anemia (n=135) | Healthy controls (n=133) | OR (95%CI) | P | |
|---|---|---|---|---|
| Sex (Female%) | 65.18 | 56.39 | 1.45 (0.88-2.37) | 0.16 |
| Agea | 51±18 | 49±15 | N/A | 0.1 |
| Hemoglobin (g/dl)a | 10.5±1.2 | 13.5±1.5 | N/A | 0.03 |
| Body mass indexa | 24.5±4 | 26.3±4.6 | N/A | 0.07 |
| Family history of CD (%) | 5.18 | 1.51 | 3.58 (0.73-7.57) | 0.17 |
| Villous atrophy (%) | 9.62 | 1.51 | 6.97 (1.54-31.56) | 0.005 |
| Endoscopic markers suggesting villous atrophy (%) | 8.14 | 1.51 | 5.81 (1.26-26.74) | 0.02 |
CD: Celiac disease.
In the iron-deficiency anemia group, 46.67% of the CD patients had subtotal villous atrophy (Marsh 3b), 40% had total villous atrophy (Marsh 3c), and 13.33% had only a significant increase in IEL number and crypt hyperplasia (Marsh II) upon histopathologic analysis. All CD patients in the control group had subtotal villous atrophy (Marsh 3b). None of the controls had anemia. All the CD patients in the control group had positive anti-tTG IgA antibodies (mean value: 40±8 U/ml).
When considering endoscopic markers, such as scalloped folds or a mosaic pattern in the duodenal mucosa, 73.3% of the CD patients in the iron-deficiency anemia group had at least one endoscopic marker. In contrast, 100% of the CD patients in the control group presented with at least one endoscopic marker. Sensitivity, specificity, PPV, and NPV of the endoscopic markers in the iron-deficiency anemia setting were as follows: 73.33, 95.83, 68.75, and 96.63%, respectively.
DiscussionOur results confirm the increased prevalence of CD among patients with iron-deficiency anemia. The main finding of our study was the high number of subjects with iron-deficiency anemia that had underlying CD and no endoscopic signs of villous atrophy. This finding highlights the necessity of carrying out duodenal biopsies when performing upper endoscopy in iron-deficiency anemia patients, even when there are no endoscopic signs.
Anemia due to malabsorption is one of the most common extraintestinal manifestations of subclinical CD.5 Among the different mechanisms responsible for anemia development in CD, iron-deficiency due to malabsorption is the most frequently encountered.
The prevalence of CD in subjects that present with iron-deficiency anemia has been previously described,6–9 with heterogeneous results. This is most likely due to the differences in study design: most of the analyses have used different definitions of CD, some have exclusively used antibody values as the only CD screening tool,9 and others have not taken subjects that do not present with villous atrophy (Marsh I and II) into account.
Our study was designed to avoid the abovementioned bias. We decided to initially evaluate all patients diagnosed with iron-deficiency anemia by performing upper endoscopy and duodenal biopsies (including bulb biopsies), and not only those that presented with positive antibodies or with iron-deficiency anemia of unknown origin (after an extensive work-up).
Regarding the endoscopic markers of villous atrophy, some authors, such as Mauriño et al.,3 showed that endoscopic markers had a high sensitivity and specificity. Interestingly, Oxentenko et al.10 demonstrated that, in subjects with iron-deficiency anemia, all endoscopic markers had a disappointingly low sensitivity with a relatively high specificity. Although our results showed that endoscopic markers had a high NPV, it is noteworthy that 25% of the iron-deficiency anemia patients that were diagnosed with CD had no endoscopic marker. This is a relevant finding, since it highlights the importance of taking duodenal biopsies in patients with iron-deficiency anemia, even in the absence of endoscopic signs of villous atrophy.
Our study has some limitations: we included a relatively small number of patients and we did not perform HLA testing on subjects with and without CD.
In conclusion, patients with iron-deficiency anemia have an increased risk of subclinical CD. Up to 25% of those patients may not present any endoscopic sign suggesting villous atrophy. This finding makes routine duodenal biopsy necessary, when performing upper endoscopy on iron-deficiency anemia patients.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Financial disclosureNo financial support was received in relation to this study.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Lasa JS, Olivera P, Soifer L, Moore R. La anemia ferropénica como presentación de enfermedad celíaca subclínica en una población argentina. Revista de Gastroenterología de México. 2017;82:270–273.