Stomach cancer (SC) and colorectal cancer (CRC) present with high rates of incidence and mortality in the worldwide population. These 2 tumors are characterized by great genetic heterogeneity. Up to now, there have been no molecular studies that analyze the mutations in the APC, KRAS, and TP53 genes in the Colombian/Latin American population.
ObjectivesTo analyze mutations in the APC, KRAS, and TP53 genes through direct sequencing in 59 patients with SC and CRC.
Patients and methodsTwenty-nine patients with SC and 30 with CRC were studied. An analysis of the mutations of the 3 genes was carried out using polymerase chain reaction and direct sequencing techniques.
ResultsA 30.5% total mutation frequency was found. The most frequently mutated gene was APC (15.3%), followed by KRAS (10.1%) and TP53 (5.1%). The CRC samples had a mutation frequency of 46.7% and it was 13.3% in the SC samples (P=.006). No mutations occurred simultaneously in the 3 genes. Mutations in 2 genes were found in only 6 tumor samples (10%). There was also a high frequency of polymorphisms in both types of cancer, the most common of which was the rs41115 polymorphism, located on the APC gene.
ConclusionThe APC, KRAS, and TP53 gene mutations were more common in CRC than in SC. Our results suggest the existence of different genetic pathways in the carcinogenesis of SC and CRC and they also reveal a particular mutation frequency in the Colombian patients studied; this could be influenced by factors related to the environment, ethnicity, and lifestyle of this population.
El cáncer de estómago (CE) y colorrectal (CCR) presentan altas tasas de incidencia y mortalidad en la población mundial. Estas 2 neoplasias se caracterizan por tener una gran heterogeneidad genética. Hasta el momento, no existen estudios moleculares que analicen las mutaciones en los genes APC, KRAS y TP53 en población colombiana/latinoamericana.
ObjetivoAnalizar mutaciones en los genes APC, KRAS y TP53 en 59 pacientes con CE y CCR mediante el secuenciamiento directo.
Pacientes y métodosSe estudió a 29 pacientes con CE y 30 con CCR. Se realizó un análisis de mutaciones en los 3 genes por las técnicas de reacción en cadena de la polimerasa y secuenciamiento directo.
ResultadosSe encontró una frecuencia total de mutaciones del 30.5%. El gen más frecuentemente mutado fue APC (15.3%), seguido de KRAS (10.1%) y TP53 (5.1%). Las muestras de CCR presentaron una frecuencia de mutaciones del 46,7% y las CE del 13.3% (p=0.006). No se encontraron mutaciones simultáneas en los 3 genes. En solo 6 muestras de tumores (10%) se detectaron mutaciones en 2 genes. Adicionalmente, se obtuvo una alta frecuencia de polimorfismos en ambos tipos de cáncer, el más común fue el rs41115 localizado en el gen APC.
ConclusiónLas mutaciones en los genes APC, KRAS y TP53 fueron más comunes en el CCR que en el CE; nuestros resultados indican la existencia de diferentes vías genéticas en la carcinogénesis del CE y del CCR, y revelan una frecuencia de mutaciones particular en los pacientes colombianos estudiados, que podría estar influida por factores ambientales y étnicos, y el estilo de vida de esta población.
Stomach cancer (SC) and colorectal cancer (CRC) have high incidence and mortality rates in the world population.1–3 The situation is more alarming in the developing countries because there is a tendency toward increase in the mortality rate; the majority of cases are diagnosed in the advanced stages and have a poor prognosis.4 Worldwide, SC is the fourth cause of death by cancer and is more frequent in men than in women.1 The incidence of SC varies geographically. There is a high incidence rate in the Asian countries of Korea, Japan, and China, as well as in some Latin American countries.1 In Colombia, SC holds second place in incidence in men and women and it is the first cause of death by cancer.1 More than 90% of the SC cases are adenocarcinomas and they are histologically classified as 2 types: diffuse and intestinal.5,6 There are multiple etiologic factors in the pathogenesis of SC and they include genetic, environmental, dietary, and lifestyle factors, as well as bacterial infection with Helicobacter pylori (H. pylori).7–9
On the other hand, CRC is the third most frequent tumor in men and women worldwide and the highest incidence rates are found in the developed countries.1 In Colombia, CRC holds sixth place in incidence and fourth in mortality, in both sexes.1 Similar to that observed in SC, CRC has a varied geographic distribution with genetic, environmental, ethnic, and lifestyle factors, among others, involved in CRC etiology.10 The majority of CRC cases are sporadic and a low percentage of them are related to a family history of the disease.11,12
In Colombia, SC and CRC have a varied geographic distribution. There are high incidences in the central, northeast, southern, southeastern, and southwestern regions of the country, whereas low incidences are observed in the Caribbean (northern) and Pacific regions. This disparity could be due to dietary habits that include the different types of home-cooked foods high in nitrites that are typical of each region and a high consumption of smoked red meats, as well as to alcohol ingestion and smoking.6
SC and CRC are very heterogeneous diseases that originate through different genetic pathways. Chromosomal alterations, oncogenic, tumor suppressing gene, and repair gene mutations, and methylation in the genes that control the cell cycle are common.13–15 Particularly in CRC, Fearon and Vogelstein proposed a molecular model that describes the sequence of colorectal carcinogenesis from adenoma to carcinoma, in which there are mutations in different oncogenes and tumor-suppressing genes.14
On the other hand, genetic studies in SC and CRC report that APC, KRAS, TP53, CDH1, MLH1, and ERBB2 gene alterations are among the most common molecular changes acquired in the carcinogenesis of these 2 malignancies.2,16–20 Thus it is proposed that these genes are essential for the transformation of normal cells into carcinoma. And so the molecular characterization of the genetic pathways of SC and CRC are of great importance for their later correlation with the clinical and pathologic characteristics and with patient outcome.21–23
In this study, we formulated the hypothesis that the mutation frequency in the APC, KRAS, and TP53 genes in the SC and CRC samples from Colombian patients is different from that of other populations.
The aim of our study was to analyze the somatic mutations in the APC, KRAS, and TP53 genes through direct sequencing in Colombian patients presenting with stomach cancer and colorectal cancer.
MethodsPopulation and sampleA cross-sectional descriptive study was conducted. The study population was made up of 59 patients, 29 presenting with SC and 30 with CRC, all of which were of the sporadic type. The study included 27 women and 32 men. None of the patients had a history of cancer nor had they received antineoplastic treatment prior to surgery. All the SC and CRC cases were confirmed through histopathologic study. The patients were asked to voluntarily participate in the study and they signed statements of informed consent. The study protocol was approved by the Bioethics Committee of the Universidad de Antioquia, Medellín, Colombia, for experimentation on humans.
The samples of the primary tumor tissues were obtained by the surgeons through surgical resection at 2 health institutions in Medellín, Colombia: the Hospital Universitario San Vicente Fundación and the Clínica León XIII within the time frame of 2010 to 2011. Each of the tumor samples was divided into 2 portions, one for histopathologic study performed by an expert pathologist and the other stored at –80°C for later genetic analysis.
DNA extractionDNA extraction from the primary tumor tissue was carried out using the commercial QIAamp DNA Mini Kit (Qiagen, Hilden, Germany), following the manufacturer's recommendations. The DNA was then quantified in a NanoDrop 2000c Spectrophotometer (Thermo Scientific, USA) determining its integrity through 1% agarose gel electrophoresis. The extracted DNA was stored at –20°C.
DNA amplification and the APC, KRAS, and TP53 gene mutation analysisThe isolated DNA was amplified through the polymerase chain reaction (PCR) method in a Gene Amp PCR System 9700 thermocycler (Applied Biosystems, USA), using specific primers for each gene. The mutation cluster region (MCR) located in exon 15 was amplified in the APC gene; exon 2 (codons 12 and 13) was amplified in the KRAS gene; and exons 5 to 8 were amplified in the TP53 gene. In short, the PCR was done at a volume of 35μl containing 300 ng of tumor DNA in a reagent mixture at the following concentrations: 1x for the 10x reaction buffer, 200μM of dNTPs, 0.4μM of each primer, and 1.4 U of Taq DNA polymerase (Invitrogen, USA). The MgCl2 concentrations were 1.05mM for APC and KRAS and 1.5mM for TP53. The PCR amplification products were examined through 2% agarose gel electrophoresis and stained with ethidium bromide; they were stored at –20°C until sequencing.
SequencingAll the amplified samples were purified and both chains were directly sequenced. Sequencing was performed with an automatic 3730xl DNA Analyzer from Applied Biosystems.
Result analysisThe chromatograms obtained were edited with the Chromas Pro program and were aligned with the reference sequences published in the GenBank (NCBI) whose access codes are: NT_034772.6 (APC), NT_009714.17 (KRAS), and NT_010718.16 (TP53).
Statistical analysisThe results were analyzed using the SPSS version 18 program. In the descriptive statistics, means and ranges were employed for the quantitative variables and the categorical variables were presented in frequencies and percentages. The Pearson chi-square test was used for the comparison of categorical variables such as sex and mutation frequency; when the expected values were lower than 5, the Yates correction was employed. The comparison between the continuous quantitative variables, like age, was carried out with the Student's t test for independent samples. All the calculated p values were bilateral and a value of p<0.05 was considered statistically significant.
ResultsA total of 59 patients with a mean age of 64.1 years (range 12-94) were studied. Patients with CRC and SC were stratified by age and sex. The mean age of the first group was 62.1 years (range 12-84) and the mean age of the second group was 66.1 years (range 36-94). No significant difference was observed between the 2 groups (p=0.332). In relation to sex, 63.3% of the patients presenting with CRC were women and 36.7% were men, whereas 27.6% of the patients with SC were women and 72.4% were men. There was a statistically significant difference between the 2 groups (p=0.005).
Among the most common signs and symptoms that patients with SC presented were: epigastric pain, emesis, asthenia, adynamia, anorexia, nausea, anemia, bleeding, weight loss, and bowel obstruction. The most frequent symptoms of the CRC patients were: rectal bleeding, weight loss, bowel obstruction, constipation, diffuse abdominal pain, melena, and abdominal bloating.
The total mutation frequency in the 59 samples analyzed was 30.5% (18/59) (Table 1). In addition, a high frequency of polymorphisms was found in the 3 genes, identifying a total of 7 different polymorphisms in the 59 samples; the most frequent was the rs41115 (c.4479G>A, p.Thr1493Thr) polymorphism, located in exon 15 of the APC gene. All the polymorphisms identified in this study are reported in the SNP database.24
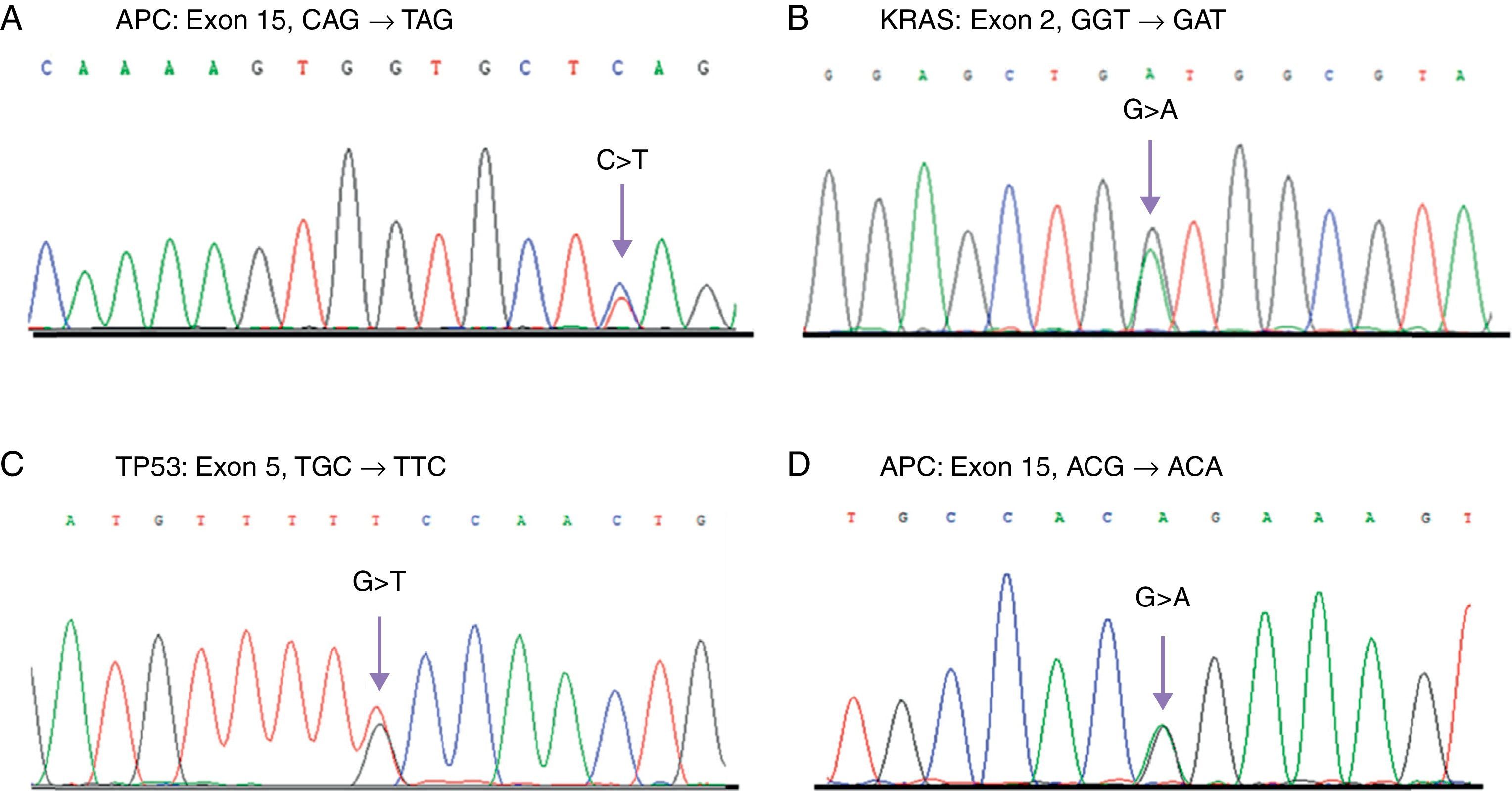
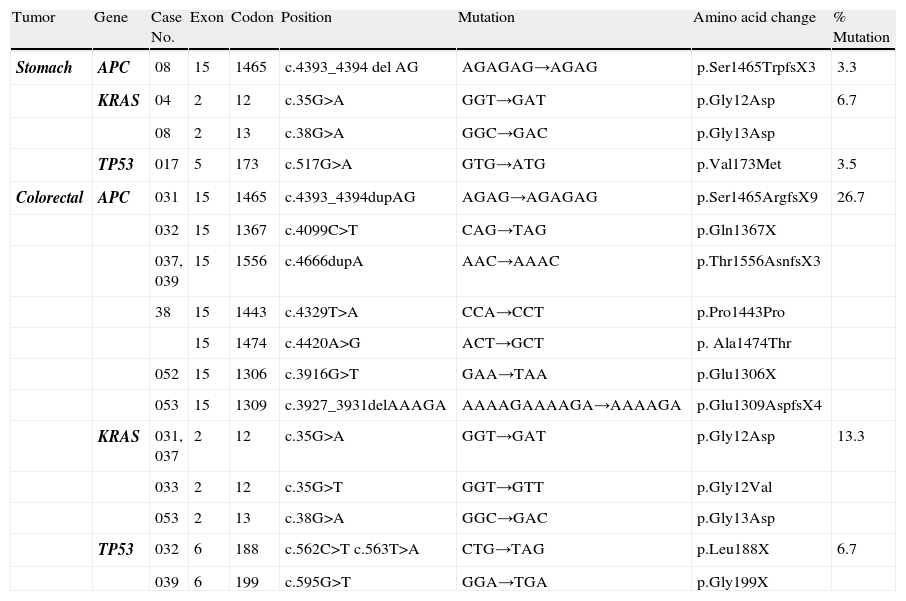
Mutations identified in the APC, KRAS, and TP53 genes in the SC and CRC samples analyzed.
| Tumor | Gene | Case No. | Exon | Codon | Position | Mutation | Amino acid change | % Mutation |
| Stomach | APC | 08 | 15 | 1465 | c.4393_4394 del AG | AGAGAG→AGAG | p.Ser1465TrpfsX3 | 3.3 |
| KRAS | 04 | 2 | 12 | c.35G>A | GGT→GAT | p.Gly12Asp | 6.7 | |
| 08 | 2 | 13 | c.38G>A | GGC→GAC | p.Gly13Asp | |||
| TP53 | 017 | 5 | 173 | c.517G>A | GTG→ATG | p.Val173Met | 3.5 | |
| Colorectal | APC | 031 | 15 | 1465 | c.4393_4394dupAG | AGAG→AGAGAG | p.Ser1465ArgfsX9 | 26.7 |
| 032 | 15 | 1367 | c.4099C>T | CAG→TAG | p.Gln1367X | |||
| 037, 039 | 15 | 1556 | c.4666dupA | AAC→AAAC | p.Thr1556AsnfsX3 | |||
| 38 | 15 | 1443 | c.4329T>A | CCA→CCT | p.Pro1443Pro | |||
| 15 | 1474 | c.4420A>G | ACT→GCT | p. Ala1474Thr | ||||
| 052 | 15 | 1306 | c.3916G>T | GAA→TAA | p.Glu1306X | |||
| 053 | 15 | 1309 | c.3927_3931delAAAGA | AAAAGAAAAGA→AAAAGA | p.Glu1309AspfsX4 | |||
| KRAS | 031, 037 | 2 | 12 | c.35G>A | GGT→GAT | p.Gly12Asp | 13.3 | |
| 033 | 2 | 12 | c.35G>T | GGT→GTT | p.Gly12Val | |||
| 053 | 2 | 13 | c.38G>A | GGC→GAC | p.Gly13Asp | |||
| TP53 | 032 | 6 | 188 | c.562C>T c.563T>A | CTG→TAG | p.Leu188X | 6.7 | |
| 039 | 6 | 199 | c.595G>T | GGA→TGA | p.Gly199X |
Upon comparing the APC mutation frequency between SC and CRC (p=0.006).
Upon comparing the KRAS mutation frequency between SC and CRC (p=0.06).
Upon comparing the TP53 mutation frequency between SC and CRC (p=0.98).
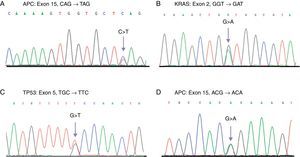
Only one mutation in the APC gene (3.5%) was found in the SC samples; this mutation was a deletion of 2 nucleotides (AG) in the c.4393_4394delAG position, which produces a premature stop codon in the protein (p.Ser1465ArgfsX9) (Table 1). In these same samples, the rs41115 polymorphism was also identified, with a high frequency of 86.2% (25/29) (Table 2) (Fig. 1). In contrast, the frequency of the mutations identified in the APC gene was 26.6% (8/30) in the CRC samples. Four of these mutations were frameshift mutations and the other 4 were point mutations; Table 1 describes the detected mutations in more detail (Fig. 1). Upon comparing the mutation frequency in the APC gene, a tendency toward a greater frequency in CRC than in SC was found (p=0.06). Furthermore, 2 polymorphisms were identified in these samples: the rs41115 polymorphism with a frequency of 56.7% (17/30) and the rs1801166 polymorphism (c.3949G>C, p.Glu1317Gln) with a frequency of 3.3% (1/30) (Table 2).
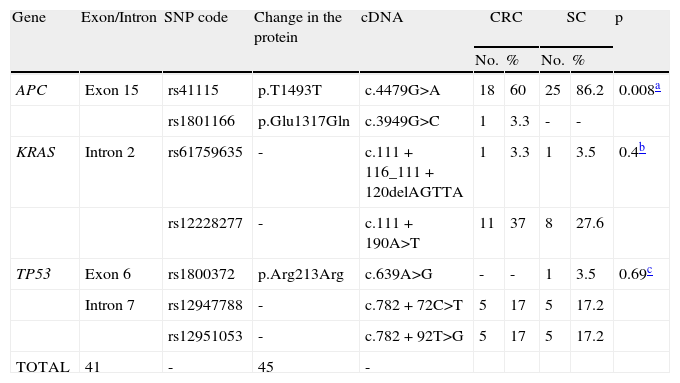
The polymorphisms identified in the APC, KRAS, and TP53 genes in the 59 samples of the SC and CRC patients evaluated.
| Gene | Exon/Intron | SNP code | Change in the protein | cDNA | CRC | SC | p | ||
| No. | % | No. | % | ||||||
| APC | Exon 15 | rs41115 | p.T1493T | c.4479G>A | 18 | 60 | 25 | 86.2 | 0.008a |
| rs1801166 | p.Glu1317Gln | c.3949G>C | 1 | 3.3 | - | - | |||
| KRAS | Intron 2 | rs61759635 | - | c.111+116_111+120delAGTTA | 1 | 3.3 | 1 | 3.5 | 0.4b |
| rs12228277 | - | c.111+190A>T | 11 | 37 | 8 | 27.6 | |||
| TP53 | Exon 6 | rs1800372 | p.Arg213Arg | c.639A>G | - | - | 1 | 3.5 | 0.69c |
| Intron 7 | rs12947788 | - | c.782+72C>T | 5 | 17 | 5 | 17.2 | ||
| rs12951053 | - | c.782+92T>G | 5 | 17 | 5 | 17.2 | |||
| TOTAL | 41 | - | 45 | - | |||||
SNP: Single nucleotide polymorphism; **CRC: Colorectal cancer; *** SC: Stomach cancer.
Direct sequencing chromatograms showing the different identified mutations. A) Mutation in APC found in a case of CRC. B) Mutation in KRAS identified in SC and CRC samples. C) Mutation in TP53 found in a case of SC. D) T1493 polymorphism in APC detected with a high frequency in SC and CRC patients. The arrows show the site of the base change.
On the other hand, the mutation frequency in the KRAS gene was 6.9% (2/29) in the SC samples (Table 1). The mutations were 2 base substitutions that occurred in codons 12 and 13; both mutations produced a change in the protein sequence: c.35G>A (p.Gly12Asp) and c.38G > A (p.Gly13Asp) (Table 1, Fig. 1). In addition, 2 polymorphisms in intron 2 were identified; the most frequent was the rs12228277 polymorphism (c.111+190 A > T) in 27.6% of the samples (8/29) (Table 2). The other was the rs61759635 polymorphism (c.111+116_111+120delTAACT); it was identified in only one sample (3.5%) and consisted of a deletion of 5 nucleotides. The mutation frequency of the KRAS gene in the CRC samples was 13.3% (4/30) (Table 1); all the detected mutations were base substitutions: 3 in codon 12 and one in codon 13 (Table 1). No differences were found upon comparing the mutation frequency in the KRAS gene between the SC and CRC samples (p=0.69). Just as in the genetic analysis of the KRAS gene in the SC samples, 2 polymorphisms with a high frequency of 40% (12/30) were identified in the CRC samples; the most frequent was the rs12228277 polymorphism with 36.7% (11/30), whereas the rs61759635 polymorphism presented in 3.3% (1/30) (Table 2).
Finally, a mutation frequency in the TP53 gene of 3.5% (1/29) was found in the SC samples (Fig. 1). The c.517G > A (p.Val173Met) mutation was detected in exon 5 (Table 1). Moreover, 3 polymorphisms were identified: the rs1800372 polymorphism (c.639A > G, p.Arg213Arg) in exon 6 with a frequency of 3.5% and 2 polymorphisms, rs12947788 (c.782+72C > T) and rs12951053 (c.782+92T > G), in intron 7, co-segregating together, with a frequency of 17.2% (5/29) (Table 2). Similarly to that found in the SC samples, 2 (6.6%) mutations in this gene were identified in the CRC samples (p=0.98); both mutations were base substitutions in exon 6 and they produced a premature stop codon (Table 1). Once again, 2 polymorphisms in intron 7 were identified, with a frequency of 16.7% (5/30) of the cases (Table 2).
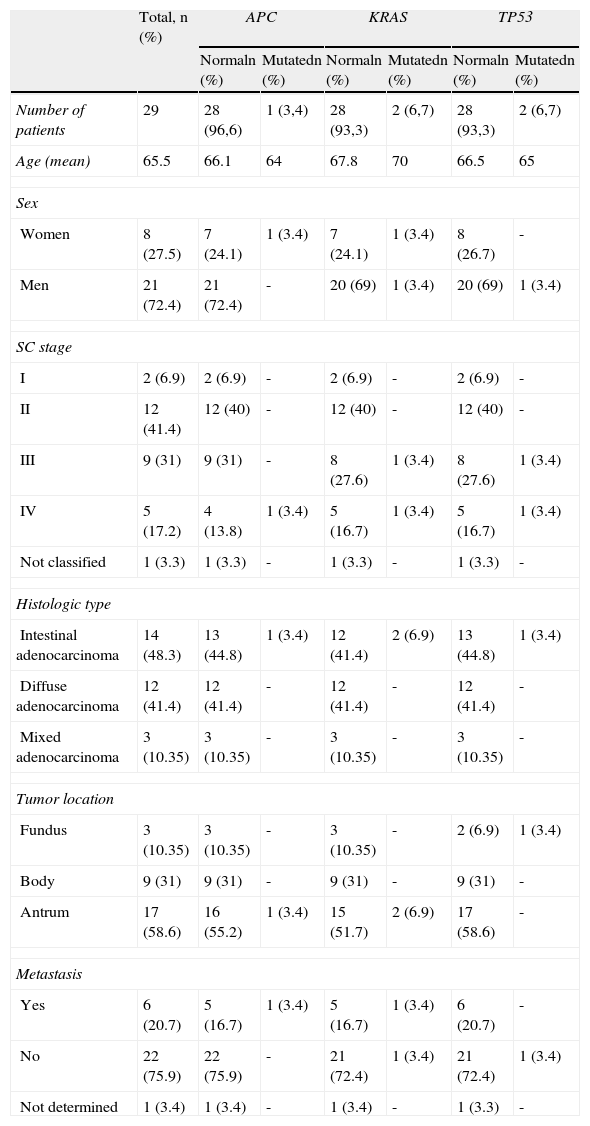
In relation to the histology of the SC cases, the intestinal type was the most common, with 48.3% (14/29), followed by the diffuse type, with 41.4% (12/29); half of the cases were found to present with advanced stages of the cancer (III and IV), the majority in stage III. Of this group of patients, 20.7% had metastases. The 4 mutations identified in the SC samples presented in the intestinal adenocarcinomas, which had an advanced stage of malignancy (stage IV). The location of the SC cases was mainly in the antrum, with 58.6% (17/29) (Table 3).
General description of the clinical and pathologic characteristics and the mutation frequency in the 29 SC patients analyzed.
| Total, n (%) | APC | KRAS | TP53 | ||||
| Normaln (%) | Mutatedn (%) | Normaln (%) | Mutatedn (%) | Normaln (%) | Mutatedn (%) | ||
| Number of patients | 29 | 28 (96,6) | 1 (3,4) | 28 (93,3) | 2 (6,7) | 28 (93,3) | 2 (6,7) |
| Age (mean) | 65.5 | 66.1 | 64 | 67.8 | 70 | 66.5 | 65 |
| Sex | |||||||
| Women | 8 (27.5) | 7 (24.1) | 1 (3.4) | 7 (24.1) | 1 (3.4) | 8 (26.7) | - |
| Men | 21 (72.4) | 21 (72.4) | - | 20 (69) | 1 (3.4) | 20 (69) | 1 (3.4) |
| SC stage | |||||||
| I | 2 (6.9) | 2 (6.9) | - | 2 (6.9) | - | 2 (6.9) | - |
| II | 12 (41.4) | 12 (40) | - | 12 (40) | - | 12 (40) | - |
| III | 9 (31) | 9 (31) | - | 8 (27.6) | 1 (3.4) | 8 (27.6) | 1 (3.4) |
| IV | 5 (17.2) | 4 (13.8) | 1 (3.4) | 5 (16.7) | 1 (3.4) | 5 (16.7) | 1 (3.4) |
| Not classified | 1 (3.3) | 1 (3.3) | - | 1 (3.3) | - | 1 (3.3) | - |
| Histologic type | |||||||
| Intestinal adenocarcinoma | 14 (48.3) | 13 (44.8) | 1 (3.4) | 12 (41.4) | 2 (6.9) | 13 (44.8) | 1 (3.4) |
| Diffuse adenocarcinoma | 12 (41.4) | 12 (41.4) | - | 12 (41.4) | - | 12 (41.4) | - |
| Mixed adenocarcinoma | 3 (10.35) | 3 (10.35) | - | 3 (10.35) | - | 3 (10.35) | - |
| Tumor location | |||||||
| Fundus | 3 (10.35) | 3 (10.35) | - | 3 (10.35) | - | 2 (6.9) | 1 (3.4) |
| Body | 9 (31) | 9 (31) | - | 9 (31) | - | 9 (31) | - |
| Antrum | 17 (58.6) | 16 (55.2) | 1 (3.4) | 15 (51.7) | 2 (6.9) | 17 (58.6) | - |
| Metastasis | |||||||
| Yes | 6 (20.7) | 5 (16.7) | 1 (3.4) | 5 (16.7) | 1 (3.4) | 6 (20.7) | - |
| No | 22 (75.9) | 22 (75.9) | - | 21 (72.4) | 1 (3.4) | 21 (72.4) | 1 (3.4) |
| Not determined | 1 (3.4) | 1 (3.4) | - | 1 (3.4) | - | 1 (3.3) | - |
SC: Stomach cancer.
Regarding the CRC cases, almost half the cases were in an advanced stage of the cancer, with the majority in stage IV. Of the 13 cases with mutations in the 3 genes, the high percentage of 77% (10/13) were identified in well differentiated adenocarcinomas. Stage IV was the most common in these cases (7/13). The mutations were more frequently located in the descending colon (9/13), followed by the ascending colon (3/13) (Table 4).
General description of the clinical and pathologic characteristics and the mutation frequency in the 30 sporadic CRC patients analyzed.
| APC | KRAS | TP53 | |||||
| n (%) | Normaln (%) | Mutatedn (%) | Normaln (%) | Mutatedn (%) | Normaln (%) | Mutatedn (%) | |
| Number of patients | 30 | 23 (76.7) | 7 (23.3) | 26 (86.7) | 4 (13.3) | 28 (93.3) | 2 (6.6) |
| Age (mean) | 62 | 56 | 56 | 63 | 59 | 62.5 | 53 |
| Sex | |||||||
| Women | 19 (63.3) | 12 (40) | 7 (23.3) | 15 (50) | 4 (13.3) | 17 (56.7) | 2 (6.6) |
| Men | 11 (36.7) | 11 (36.7) | - | 11 (36.7) | - | 11 (36.7) | - |
| CRC stage | |||||||
| I | 3 (10) | 2 (6.6) | 1 (3.3) | 3 (10) | - | 3 (10) | - |
| II | 10 (33.3) | 8 (26.7) | 2 (6.6) | 8 (26.7) | 2 (6.6) | 10 (33.3) | - |
| III | 2 (6,6) | 2 (6,6) | - | 2 (6,6) | - | 2 (6,6) | - |
| IV | 10 (33.3) | 6 (20) | 4 (13.3) | 9 (30) | 1 (3.3) | 8 (26.7) | 2 (6.6) |
| Not classified | 5 (16.7) | 5 (16.7) | - | 4 (13.3) | 1 (3.3) | 5 (16.7) | - |
| Histologic type | |||||||
| Well differentiated adenocarcinoma | 17 (56.7) | 12 (40) | 5 (16.7) | 13 (43.3) | 4 (13.3) | 16 (53.3) | 1 (3.3) |
| Moderately differentiated adenocarcinoma | 7 (23.3) | 6 (20) | 1 (3.3) | 7 (23.3) | - | 6 (20) | 1 (3.3) |
| Mucinous adenocarcinoma | 2 (6.6) | 2 (6.6) | - | 2 (6,6) | - | 2 (6.6) | - |
| Neuroendocrine carcinoma | 2 (6.6) | 1 (3.3) | 1 (3.3) | 2 (6.6) | - | 2 (6.6) | - |
| Other | 2 (6.6) | 2 (6.6) | - | 2 (6.6) | - | 2 (6.6) | - |
| Tumor location | |||||||
| Ascending | 6 (20) | 4 (13.3) | 2 (6.6) | 5 (16.7) | 1 (3.3) | 6 (20) | - |
| Transverse | 3 (10) | 3 (10) | - | 2 (6.6) | 1 (3.3) | 3 (10) | - |
| Descending | 16 (53.3) | 11 (36.7) | (16.7) | 14 (46.7) | 2 (6.6) | 14 (46.7) | 2 (6.6) |
| Rectum | 4 (13.3) | 4 (13.3) | - | 4 (13.3) | - | 4 (13.3) | - |
| Not classified | 1 (3.3) | 1 (3.3) | - | 1 (3.3) | - | 1 (3.3) | - |
| Metastasis | |||||||
| Yes | 12 (40) | 8 (26.7) | 4 (13.3) | 11 (36.7) | 1 (3.3) | 10 (33.3) | 2 (6.6) |
| No | 15 (50) | 12 (40) | 3 (10) | 12 (40) | 3 (10) | 15 (50) | - |
| Not determined | 2 (6.6) | - | 2 (6.6) | - | 2 (6.6) | 2 (6.6) | - |
*CRC: Colorectal cancer.
In summary, the results showed a greater total mutation frequency in the APC, KRAS, and TP53 genes in the CRC samples (46.7%) than in the SC samples (13.3%) (p=0.006). In the 59 tumor samples analyzed, no simultaneous mutations were identified in the 3 genes; only 6 tumors (10%) had mutations in 2 genes, distributed as follows: in the CRC samples, 3 tumors had simultaneous mutations in the APC-KRAS genes and 2 tumors had them in the APC-TP53 genes, whereas only one case in the SC samples had mutations in the APC-KRAS genes.
Lastly, in this study a high frequency of polymorphisms was found with no differences between the patients with SC and CRC (p=0.108). The total polymorphism frequency for the APC, KRAS, and TP53 genes in the 59 tumor samples was 74.6%, 35.6%, and 35.6%, respectively (Table 2).
DiscussionThis is the first study in Latin America analyzing the mutations in the APC, KRAS, and TP53 genes and conducted on 59 patients with stomach cancer (SC) and colorectal cancer (CRC). A total of 18 (30.5%) of the 59 samples had mutations.
The mutation frequency in the 3 genes analyzed was found to be greater in the CRC samples than in the SC samples. These results are consistent with those of other authors11,25 that reported a higher frequency of APC, KRAS, and TP53 gene mutations in CRC, according to the model proposed by Fearon and Volgestein in the adenoma-carcinoma sequence.13,21 On the other hand, our results in the SC samples showed that mutations in these 3 genes were unusual, similar to that reported in other studies.3,5,26,27 It could thus be suggested that SC and CRC develop by means of different genetic pathways, such as chromosomal alterations, microsatellite instability, different gene promoter methylation, and alterations in other genes directly related to greater SC risk.
In this study, of the 3 genes analyzed, the APC gene was the most frequently mutated in the CRC samples, whereas it had a low mutation frequency in the SC samples; however, this difference was not statistically significant. This finding is similar to the results reported by Lee et al., who found a frequency of 2.5% in the analysis of 237 SC samples in patients in Korea28 and to those of Horii et al. and Yamashita et al. who reported frequencies of 6.8% and 3.4%, respectively.3,29 These collective results indicate that mutations in the APC gene are not common in SC; likewise, these studies suggest a negative relation between mutations in the APC gene and the development of SC, which concurs with other studies reporting that APC gene mutations are rare in extracolonic neoplasias. Different genetic pathways have been described in relation to the carcinogenesis of SC, such as chromosomal instability (CIN), microsatellite instability (MSI that occurs between 30 and 40%), β-catenin mutations, and APC, MLH1, and RPRM gene methylation.3,6,14,15,30 On the other hand, in regard to SC carcinogenesis, other non-genetic factors should be taken into account that include diet, especially high red meat consumption, smoked foods, and those preserved with salt, the exposure to certain environmental agents, and H. pylori infection, given that this infection has a prevalence of up to 85% in the Colombian population.10
Contrastingly, in the CRC samples, there was a greater mutation frequency in the APC gene. However, the frequency reported in our study (26.7%) was lower than that found by other authors who report a mutation frequency of up to 60% when the entire coding region of the gene was analyzed.11,19,31,32 We only analyzed exon 15 of the APC gene, where the MCR region is found, and where the greater mutation frequency occurred. It must also be kept in mind that population differences have been reported in relation to APC mutation frequency, possibly due to ethnic composition, sample size, mutations outside the MCR region, and other APC genetic alterations such as large deletions and the loss of heterozygosity (LOH) in the 5q21 region.2,11,33,34
Furthermore, a low mutation frequency in the KRAS gene was found in the SC and CRC samples analyzed. The frequency reported for SC concurred with those reported in the literature, which are in a range from 0 to 10%.35–37 In the Asian population, which has a high incidence of SC, low mutation frequencies in the KRAS gene were also reported.35 Thus, mutations in the KRAS gene are considered to be very rare in SC. In the CRC samples, the mutation frequency found was lower than had been expected (13.3%); a mutation frequency from 26 to 50% is reported in the literature.31,38,39 Nevertheless, the mutation frequency reported in our study was similar to that found by Akkiprik et al. in 43 samples of sporadic CRC, which was 12%.18 A high mutation frequency of the KRAS gene has been observed in colorectal adenomas; in addition, it has been found that the mutations could be lost through selection in the cells that progress from adenoma to carcinoma.31,40
With respect to the TP53 gene mutations, a low mutation frequency was found in CRC and SC that was within the range reported in the literature.41 However, in the CRC samples, the frequency reported in this study was surprisingly low, compared with reports in other populations, which were up to 50%42 when the entire coding region of the gene was analyzed. We only evaluated codons 5 through 8, which is the region in which the greatest percentage of mutations has been identified.43 Nevertheless, the presence of mutations in the exons that were not evaluated cannot be excluded. The differences in the reported frequencies could also be due to exposure to given carcinogenic agents that induce specific mutations in the TP53 gene and to other genetic alterations common to this gene, such as microdeletions and LOH in the 17p.13.1 locus.3,20,42,44–46
In the genetic analysis of the CRC samples (25 from the colon and 4 from the rectum), our study revealed that all the detected mutations occurred in the colon, and most frequently in the descending colon. In contrast, no mutations were found in the cancer samples from the rectum, possibly due to the low number of tumors analyzed.
In addition, no simultaneous mutations in the 3 genes analyzed were found in the SC or CRC samples; this result concurs with those of other studies.11,12,47 This simultaneous mutation is regarded as an infrequent event, especially in cases of sporadic CRC in which these genes have been widely studied, reporting a mutation frequency for them under 7%.11,12,47 Concomitant mutations in 2 genes were found in only 6 cases (10.2%) and the APC and KRAS gene combination was the most frequent; these results are similar to those reported in other studies.12 It could therefore be suggested that the mutations in the APC and KRAS genes occur in the early stages of CRC, favoring cancer progression.12 In relation to the sporadic CRC carcinogenesis model, it is posited that the accumulation of mutations in these 3 genes is not a prerequisite for the development of many sporadic CRCs, as shown in our study and in those of other authors, and that at least one third of the CRCs do not follow this sequential model.11,12,47 Therefore, it is proposed that the APC, KRAS, and TP53 gene mutations occur through independent genetic pathways.12,18,19
Finally, contrary to the low mutation frequencies found in the APC, KRAS, and TP53 genes in the SC and CRC samples, we found a high frequency of polymorphisms in the 3 genes analyzed in our study. Of the 7 polymorphisms identified in the 59 study samples, the most frequent was the rs41115 polymorphism in the APC gene. The high frequencies of this polymorphism found in the SC (86.2%) and the CRC (60%) samples may be the highest reported up to now in these types of cancer. All the polymorphisms identified in our study are reported in the literature and in the SNP database of the NCBI.24,48–52 The association of these polymorphisms with the risk for developing cancer is not yet well defined.53
The rs41115 polymorphism has been identified in different tumors,48,49,54–57 particularly in families presenting with familial adenomatous polyposis (FAP), and it has been observed to cosegregate with germline mutations in the APC gene; therefore it is suggested that the individuals in families with FAP could be at greater risk for developing CRC.50 On the other hand, this first time identification of the polymorphisms reported in our study is very important in relation to the genetic component of the Colombian population. This population has historically been regarded as a genetic isolate composed of an ethnic mixture of Europeans in its majority, followed by Africans, and then to a lesser degree, Amerindians;58 thus, these genetic variants would make up part of the genetic composition characteristic of this population. Future studies with a greater number of samples are needed to validate these results, as well as to clarify the association of the identified polymorphisms with the risk for developing SC and CRC.
In summary, this is the first study analyzing the APC, KRAS, and TP53 gene mutations in patients with SC and CRC conducted in Latin America. Our results provide important information in relation to the genetic component of the Colombian population from the pattern of mutations and polymorphisms in these 3 genes. The results of this study confirm previous reported data on the mutation frequencies in SC, but they differ considerably with those found in CRC, especially those in the TP53 gene. And finally, different genetic pathways such as the MSI, CIN, and the epigenetic pathway are described in the carcinogenesis of SC and CRC.3,6,14,15,30
ConclusionsThe APC, KRAS, and TP53 gene mutation frequencies were greater in the CRC samples than in the SC samples; our results suggest that there are different genetic pathways in the carcinogenesis of SC and CRC and they reveal a pattern of mutations and polymorphisms that is particular to the Colombian patients studied.
Financial disclosureThis study was financed by the Universidad de Antioquia, Medellín, Colombia, through the 2009-2011 Genética Médica group sustainability program, CPT-0917.
Conflict of interestThe authors declare that there is no conflict of interest.
The authors wish to thank the patients for their willing participation in the study, the Hospital Universitario San Vicente Fundación, and the Clínica León XIII in Medellín, Colombia, where the study samples were collected, and Dr. Jorge Botero Garcés for his assessment of the statistical analyses.
Please cite this article as: Palacio-Rúa KA, Isaza-Jiménez LF, Ahumada-Rodríguez E, Muñetón-Peña CM. Análisis genético en APC, KRAS y TP53 en pacientes con cáncer de estómago y colon. Revista de Gastroenterología de México. 2014;79:79–89.