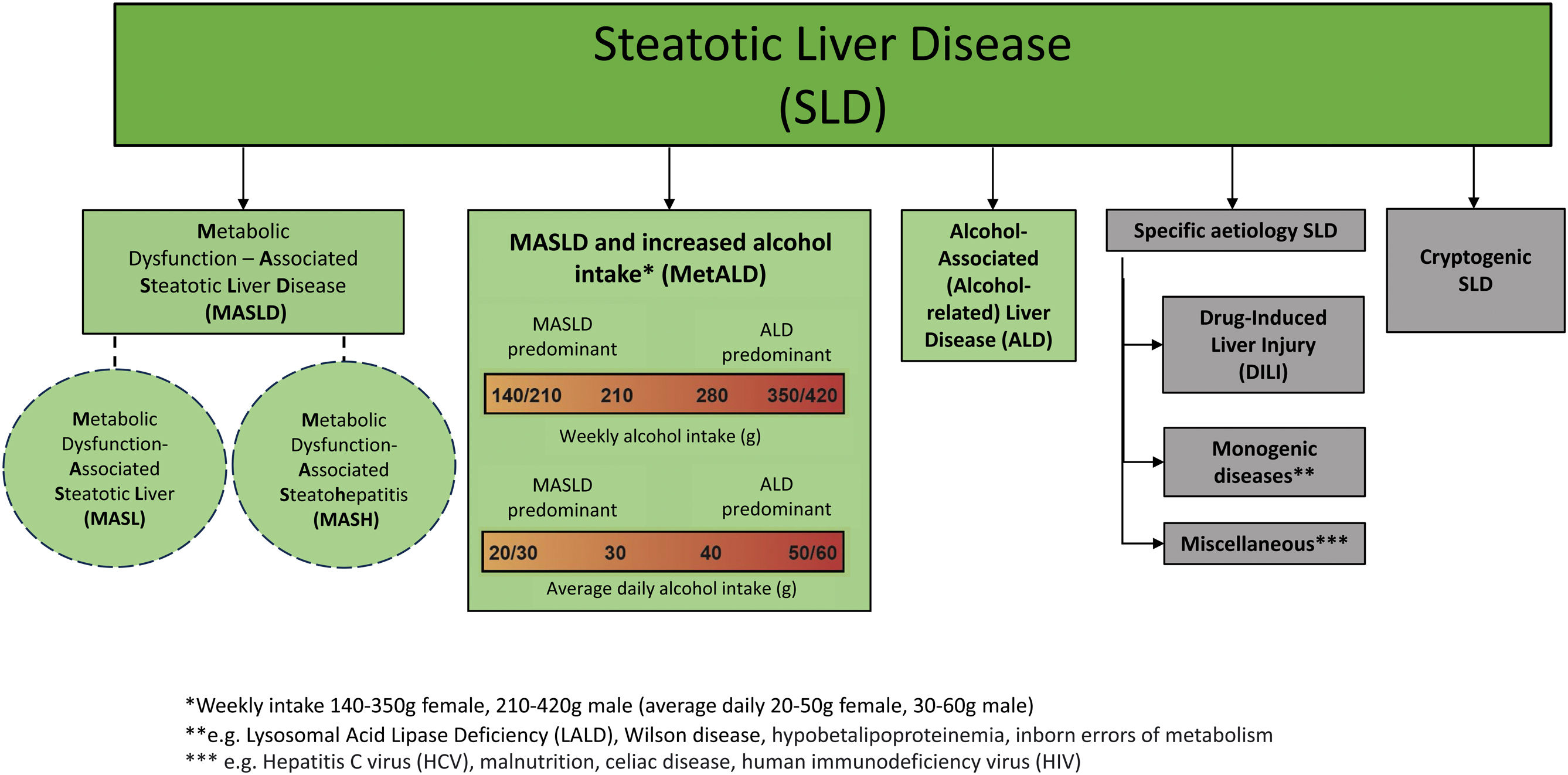
After nearly 3 years of joint work by the American Association for the Study of Liver Diseases, the European Association for the Study of the Liver, the Asociación Latinoamericana para el Estudio del Hígado, and other national associations and patient advocacy organizations, we present herein the new hepatic steatosis nomenclature (Fig. 1).
No more NAFLD!“Steatotic liver disease” (SLD) is the new general term, resulting in the consequent nomenclature, “metabolic dysfunction-associated steatotic liver disease” (MASLD).
Let’s dig deeper!
What you need to know about the new nomenclature:
- •
“Steatotic liver disease” (SLD) was chosen as the general term to encompass the different etiologies of steatosis.
- •
Because “steatohepatitis” was felt to describe an important pathophysiologic concept, the term should be retained, with nonalcoholic steatohepatitis (NASH) being replaced by metabolic dysfunction-associated steatohepatitis (MASH).
- •
Nonalcoholic fatty liver disease (NAFLD) is now to be called “metabolic dysfunction-associated steatotic liver disease” (MASLD) and includes patients that have simple metabolic dysfunction-associated steatotic liver (MASL) or metabolic dysfunction-associated steatohepatitis (MASH) and at least one of 5 cardiometabolic risk factors.
- •
A new category, outside of pure MASLD, was established, called metabolic and alcohol related/associated liver disease (MetALD). It describes patients with MASLD that consume quantities of alcohol per day or per week that are higher than the upper limits of intake established in MASLD. The following are the ranges of alcohol intake considered for presenting with MetALD: 20-50 g/day and 30-60 g/day or 140-350 g/week and 210-420 g/week, for women and men, respectively.
- •
Alcohol-associated (alcohol-related) liver disease (ALD) is the next category.
- •
Then follows steatotic liver disease due to known causes (medications, hepatitis C virus infection, genetic causes, and others).
- •
When there are no metabolic parameters or a known cause of disease, the new denomination is cryptogenic steatotic liver disease.
MASLD is the most common chronic liver disease worldwide, affecting more than 30% of the global population. Thus, it was vital for the world liver community to come together to provide an affirmative, non-stigmatizing nomenclature and diagnosis. Ultimately, the global members of the Nomenclature Development Initiative concentrated on ensuring that the medical community find a standardized nomenclature, increasing awareness, better directing research and resources, and increasing survival.
Consensus was defined as a supermajority vote (67%) and after 6 stages, which included 4 online surveys and 2 face-to-face meetings, the average response rate was above 75% in the 4 data collection rounds; the final response rate was 89% and the final recommendation approval rate was 85%. Numerous societies and organizations also supported the recommendations. Through the transparent and collaborative Delphi process, the determination that we would no longer use the excluding, negative, and confusing terms containing potentially stigmatizing language, such as “nonalcoholic fatty liver disease” and “nonalcoholic steatohepatitis” was identified and agreed upon.
The Delphi panel understands that agreement on this topic is not unanimous. No one can determine the tolerable threshold for the number of persons that feel stigmatized but said stigmatization should simply be avoided whenever possible. The new nomenclature provides an affirmative name and diagnosis, without using stigmatizing language.
To be clear: the term SLD does not alter the natural history of the disease, clinical trials, or biomarkers, nor does it impede the development of future research in this field. The classification and severity levels we use today will remain the same and the findings from previous studies on NAFLD are still valid under the new MASLD definition, given that the discrepancy between MASLD and NAFLD is minimal.2
The Delphi panel has defined and delineated MetALD, a category that has not been studied. Patients in that group will benefit from being included in clinical trials and integrated into care and attention pathways that have been proposed for SLD.
The Spanish-speaking directive committee agreed upon always using the same English-language acronyms, even in papers written in other languages, to facilitate and standardize the use of this new nomenclature (MASLD, MASL, MASH, MetALD, ALD).
We invite you to utilize this new nomenclature in your presentations and publications, as well as using it with your colleagues and patients in your clinical practice.
The moment is now here for us to raise awareness of the disease, reduce stigma, and accelerate the development of drugs and biomarkers, to benefit the patients with SLD.
There is much more to know about the new nomenclature for SLD. Read everything about it in the joint publications available in the Annals of Hepatology, Hepatology, and the Journal of Hepatology.3–5
Ethical considerationsThe authors of this work declare that no experiments were conducted on humans or animals for this article and there are no confidentiality conflicts, given that no patient data appear in this scientific letter. In addition, because no personal or patient data were used, obtaining informed consent from participants was not necessary.
Financial disclosureNo financial support was received in relation to this article.
Conflict of interestThe authors declare that there is no conflict of interest.