Circulating anti-CdtB/anti-vinculin antibodies have been validated as biomarkers to distinguish IBS-D from IBD, but there is no experience with them in Latin America.
Materials and methodsThe analysis was carried out on patients seen at a FGIDs/motility clinic over the last 7 months for diarrhea with abdominal pain and/or bloating who were tested for these antibodies. The patients were diagnosed according to the Rome III criteria or with organic disorders, and those presenting with IBS were further classified as post-infectious (PI) or non-PI IBS.
ResultsThirty patients were studied. Positive biomarkers were found in IBS-D y IBS-D Overlap (58.8%) and IBS-M (33.3%), with no differences between PI-IBS (71.4%) vs. non PI-IBS (41.7%) subjects (P=.21). There was no positivity in patients with other FGIDs or organic diarrhea, except for one with small intestinal bacterial overgrowth (SIBO).
ConclusionsOur findings support the use of this test as a first-line diagnostic tool to confirm the presence of IBS-D/IBS-M according to the Rome III criteria.
Se ha validado el uso de los anticuerpos anti-CdtB/anti-vinculina como biomarcadores para discriminar el SII-D de la EII, más no hay experiencia con ellos en Latinoamérica.
Materiales y métodosSe analizaron pacientes que consultaron a una clínica de TGIF/motilidad en los últimos 7 meses por diarrea con dolor/distensión abdominal, y a quienes se les solicitó estos anticuerpos. Los pacientes fueron diagnosticados mediante los criterios de Roma III o con trastornos orgánicos. Se clasificaron a los pacientes con SII en SII-post infeccioso (PI) o SII-no PI.
ResultadosTreinta pacientes fueron estudiados. Se encontraron biomarcadores positivos en sujetos con SII-D y SII-D con sobreposición (58.8%) y SII-M (33.3%), sin diferencias entre aquellos con SII-PI (71.4%) vs. SII-no PI (41.7%) (p=0.21). No hubo positividad en pacientes con otros TGIF o diarrea orgánica, excepto en uno con sobrecrecimiento bacteriano en el intestino delgado (SBID).
ConclusiónNuestros datos apoyan el uso de esta prueba como una herramienta de diagnóstico de primera línea para confirmar la presencia de SII-D/SII-M según los criterios de Roma III.
The multidimensional clinical profile (MDCP) has recently been published by The Rome Foundation to capture the full dimensionality of patients with functional gastrointestinal disorders (FGIDs) and plan an individualized treatment.1 The MDCP comprises five categories: 1. Categorical diagnosis (Rome III criteria); 2. Clinical modifiers (e.g. bowel habit subtype: irritable bowel syndrome with diarrhea [IBS-D], with constipation [IBS-C], mixed-IBS [IBS-M], post-infectious [PI-IBS], gluten sensitivity); 3. Self-perceived impact (i.e. mild, moderate, and severe); 4. Psychosocial influences (e.g. DSM-5 categories, history of abuse); and 5. Physiologic modifiers and biomarkers (which may enhance the understanding of the diagnosis or have treatment implications).1
Circulating antibodies to cytolethal distending toxin B (CdtB) and vinculin have been validated in the United States as biomarkers to distinguish IBS-D from inflammatory bowel disease (IBD).2 CdtB is produced by bacteria that cause acute gastroenteritis including Campylobacter, Salmonella typhi, Escherichia coli, and Shigella dysenteriae species species.3 A post-C. jejuni animal model demonstrated that host antibodies to CdtB cross-react with vinculin in the gut, producing a PI-IBS-like phenotype.4,5 Therefore, anti-CdtB/anti-vinculin antibodies were investigated in Rome III IBS-D patients and compared with patients with IBD, celiac disease (CD), and healthy controls. An optical density (OD) ≥ 2.80 for the anti-CdtB antibody and ≥ 1.68 for the anti-vinculin antibody provided a specificity of 91.6% and 83.8%, respectively, for discriminating IBS-D.2 If either one were present, the test was considered positive.
There is no clinical experience with these biomarkers in Latin America. However, a cost-minimization model showed that using them as first-line diagnostic investigation in patients suspected of having IBS-D in Mexico lowered the costs by 16.3%, with the possibility of reaching a 25% savings.6 Furthermore, the recently published Mexican Consensus on IBS acknowledged that the anti-CdtB/anti-vinculin antibodies were well studied and validated, although no recommendations were provided regarding their use as diagnostic tools.7 Therefore, we sought to describe herein the clinical experience in the use of these biomarkers in patients seen at a private FGIDs/Motility Clinic in Mexico City.
Materials and methodsWe retrospectively reviewed the clinical charts of patients with chronic diarrhea tested for the anti-CdtB/anti-vinculin antibodies as part of their diagnostic work-up between 1/09/2015 (when the biomarkers became available) and 31/03/2016. Patients were seen by one gastroenterologist (MS) and were diagnosed with IBS-D, IBS-M, functional diarrhea, or unspecified functional bowel disorder (UFBD) if they met the respective Rome III criteria, and were further classified as PI-IBS if they had a previous history of infectious gastroenteritis as a triggering factor. Patients that did not have any of the above disorders received organic diagnoses according to their clinical presentation and other tests (e.g. CD serology, upper endoscopy, colonoscopy, duodenal and/or colonic biopsies, small bowel transit, etc). We described the prevalence of positive anti-CdtB/anti-vinculin antibodies and OD values according to diagnoses, especially in IBS, and between PI-IBS and non-PI-IBS subjects. Categorical variables were reported in percentages and compared using the X2 test. Continuous variables were expressed in median and range and mean ± SD when appropriate, and were compared using the Student's t test. A p < 0.05 was considered statistically significant.
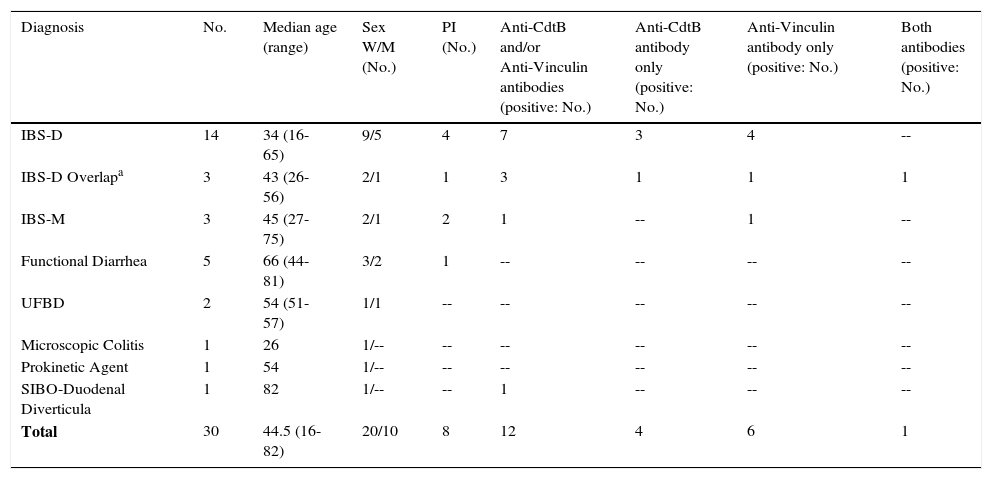
ResultsA total of 30 patients were studied. Their mean age was 49.1 ± 17.5 years and 63.3% were women. The majority of the initial diagnoses were IBS-D (N): 14; functional diarrhea: 5; IBS-M: 3; UFBD: 2; non-functional chronic diarrhea: 2; microscopic colitis (MC): 2; tropical sprue (TS): 1; and CD: 1. Those patients with TS, CD, and one with MC, previously responded to the specific treatments for those conditions and then developed relapsing diarrhea with abdominal pain/bloating consistent with IBS-D (herein labeled as IBS-D Overlap). Overall, 20 patients were diagnosed with IBS (Table 1).
Positive anti-CdtB and/or anti-vinculin antibodies in patients with diarrhea-related IBS and others.
| Diagnosis | No. | Median age (range) | Sex W/M (No.) | PI (No.) | Anti-CdtB and/or Anti-Vinculin antibodies (positive: No.) | Anti-CdtB antibody only (positive: No.) | Anti-Vinculin antibody only (positive: No.) | Both antibodies (positive: No.) |
|---|---|---|---|---|---|---|---|---|
| IBS-D | 14 | 34 (16-65) | 9/5 | 4 | 7 | 3 | 4 | -- |
| IBS-D Overlapa | 3 | 43 (26-56) | 2/1 | 1 | 3 | 1 | 1 | 1 |
| IBS-M | 3 | 45 (27-75) | 2/1 | 2 | 1 | -- | 1 | -- |
| Functional Diarrhea | 5 | 66 (44-81) | 3/2 | 1 | -- | -- | -- | -- |
| UFBD | 2 | 54 (51-57) | 1/1 | -- | -- | -- | -- | -- |
| Microscopic Colitis | 1 | 26 | 1/-- | -- | -- | -- | -- | -- |
| Prokinetic Agent | 1 | 54 | 1/-- | -- | -- | -- | -- | -- |
| SIBO-Duodenal Diverticula | 1 | 82 | 1/-- | -- | 1 | -- | -- | -- |
| Total | 30 | 44.5 (16-82) | 20/10 | 8 | 12 | 4 | 6 | 1 |
IBS: irritable bowel syndrome; IBS-D: irritable bowel syndrome with diarrhea; IBS-M: mixed irritable bowel syndrome; M: men; PI: post-infectious; SIBO: small intestinal bacterial overgrowth; UFBD: unspecified functional bowel disorder; W: women.
A positive biomarker was present in 47.1% of the IBS-D patients, in all of the patients with IBS-D Overlap, and in 33.3% of those presenting with IBS-M (Table 1). There were no positive results in patients with functional diarrhea, UFBD, MC, or in a patient with diarrhea that was later found to be a side effect of prucalopride. One exception was an 82-year-old woman with diverticula in the second and third segments of the duodenum, who tested positive for the anti-CdtB antibody. She was diagnosed with secondary small intestinal bacterial overgrowth (SIBO).
Thirty-five percent of the IBS patients had PI-IBS. However, positive biomarkers were present in the PI-IBS subjects: 5/7 (71.4%), as well as in the non-PI-IBS ones: 5/12 (41.7%), p = 0.21. In addition, there were no differences in the OD values between the PI-IBS patients vs. the non-PI-IBS patients for the anti-CdtB (3.019 ± 0.189 vs. 2.98.6 ± 0.232, p = 0.69) and the anti-vinculin (2.224 ± 0.616 vs. 2.151 ± 0.503, p = 0.14) antibodies.
ConclusionsIn this clinical experience in Mexico with anti-CdtB/anti-vinculin antibody testing on a small number of patients, only those subjects with a diarrhea-related IBS diagnosis according to Rome III had a positive result. In contrast, no patients with other diarrhea-related FGIDs or organic diarrhea without IBS-D Overlap were positive. Anti-CdtB/anti-vinculin antibodies were present in 8 patients (47.1%) with “pure” IBS. However, if we add the positivity among those with IBS-D Overlap, 55% of all patients with a diarrhea-related IBS diagnosis were positive. These results are similar to the 58.6% found by Pimentel et al. in their validation study.2
There was only one patient with organic diarrhea, presumably having SIBO secondary to duodenal diverticula, that tested positive for the anti-CdtB antibody. This can be explained by the hypothetical pathophysiologic mechanism underlying the presence of these antibodies. In a PI-IBS rat model,4 a significant reduction of interstitial cells of Cajal (ICCs) was observed and SIBO was developed.8 There was also a cross-reaction of anti-CdtB antibodies with vinculin,5 a cytosolic protein present in ICCs and myenteric ganglia.9 Vinculin is also related to actin that binds to cadherins, which are necessary for intestinal smooth muscle contraction.9 The loss of vinculin, ICCs, and myenteric ganglia probably decreased small bowel motility, leading to the development of bacterial overgrowth.5,8 This may explain the positive anti-CdtB antibody in our patient.
Not only did we find positive anti-CdtB or anti-vinculin antibodies in the PI-IBS subgroup, but also in the non-PI-IBS patients. This suggests the possible relation of other mechanisms not associated with a previous enteric infection. If anti-CdtB antibodies cross-react with vinculin, the presence of anti-vinculin antibodies needs to be explained. One may speculate that the anti-CdtB antibody alters the properties of vinculin and therefore anti-vinculin antibodies are produced. If that is the scenario, both antibodies should be present in every patient, which was not the case in the validation study or in our case series. Thus, an autoimmune mechanism could explain the generation of anti-vinculin antibodies. In fact, a previous study found that anti-enteric neuronal antibodies were more frequent in IBS patients than in controls, resulting in the proposal of autoimmune degenerative neuropathy.10
The present results have implications in clinical practice. The inclusion of this blood panel in the diagnostic process of patients with IBS according to Rome III has the potential for significant cost savings, because unnecessary testing to rule out organic causes of chronic diarrhea would be avoided.6 Moreover, an objective result would most likely be reassuring to patients. Nevertheless, our study has some limitations. First, it is a small retrospective series and not all of the patients had the same diagnostic tests and procedures to rule out other possible comorbidities. Second, we only had two cases of MC, and the behavior of the anti-CdtB/anti-vinculin antibody in a disease characterized by low-grade inflammation that could trigger autoimmunity is unknown. However, our patient with IBS-D that overlapped with MC was positive for both antibodies, whereas the patient with MC, alone, was not. Third, no patient with IBD was studied, given that it is unethical to perform this test in patients previously diagnosed through the use of other validated biomarkers, as was the case with our IBD patients.
In conclusion, more than half of this small group of patients with diarrhea-related IBS according to the Rome III criteria was positive for the anti-CdtB and/or anti-vinculin antibody, regardless of PI-IBS. These findings support the use of this test as a first-line diagnostic tool to confirm the IBS-D/IBS-M Rome III diagnosis in the patients that test positive. Future studies are warranted to determine if the antibody levels can be used to monitor and predict treatment response and/or IBS recurrence.
Ethical responsibilitiesProtection of persons and animalsThe authors declare that the procedures followed conformed to the ethical standards of the responsible committee on human experimentation and were in accordance with the World Medical Association and the Declaration of Helsinki.
Data confidentialityThe authors declare that they have followed the protocols of their work center in relation to the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Financial disclosureNo financial support was received in relation to this study.
Conflict of interestMax Schmulson has received grant supports from Alfa Wassermann and Nestle Ltd. He has served on the Advisory Board of Alfa Wassermann and has been a consultant for Almirall, Commonwealth Laboratories Inc, Commonwealth Diagnostics International Inc, Janssen, Nestle Ltd, Novartis, Procter and Gamble, Senosiain, and Takeda Mexico. He has also been a speaker for Alfa Wassermann, Janssen, Mayoli-Spindler, and Takeda Mexico.
Please cite this article as: Schmulson M, Balbuena R, Corona de Law C. Experiencia clínica con el uso de los anticuerpos anti-CdtB y anti-vinculina en pacientes con diarrea en México. Revista de Gastroenterología de México. 2016;81:236–239.