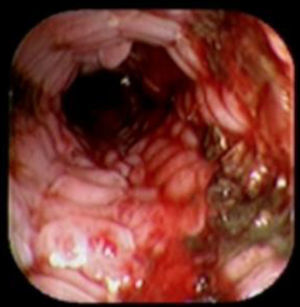
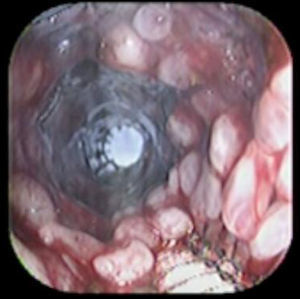
A 41-year-old man underwent a Heller myotomy due to achalasia. An unexpected puncture in the esophageal wall was identified during the procedure. A partially covered 15cm stent (PSEMS) (UltraFlex) was placed at the mucosal defect. It was removed 26 days later and the endoscopic image showed esophageal tissue embedded in the covered portion of the stent. PSEMS covering loss is a rare complication that can have serious consequences.
Se presenta el caso de un hombre de 41 años de edad al cual se le realizó una miotomía de Heller por acalasia. Durante el procedimiento se identificó una punción inadvertida de la pared del esófago. Se decide la aplicación de un stent parcialmente cubierto (PSEMS) de 15cm (UltraFlex) sobre el defecto de la mucosa. El stent fue removido 26 días después y la imagen endoscópica mostró la presencia de crecimiento de tejido esofágico sobre la sección cubierta del stent. La pérdida de la cubierta de las PSEMS es una complicación infrecuente que, sin embargo, puede llevar a serias complicaciones.
Covered self-expandable metallic stents, initially developed to avoid esophageal malignant tissue in- and/or overgrowth, are now being used successfully to treat other disorders such as anastomotic leaks, benign strictures, caustic injury stenoses, aorto-esophageal fistula, and spontaneous perforations.1,2 The development of covered stents has increased the posibility to make them removable and potentially useful in benign GI disease.3–5 We present the case of a patient with an esophageal leak treated with a partially covered self expandable metallic stent (PSEMS).
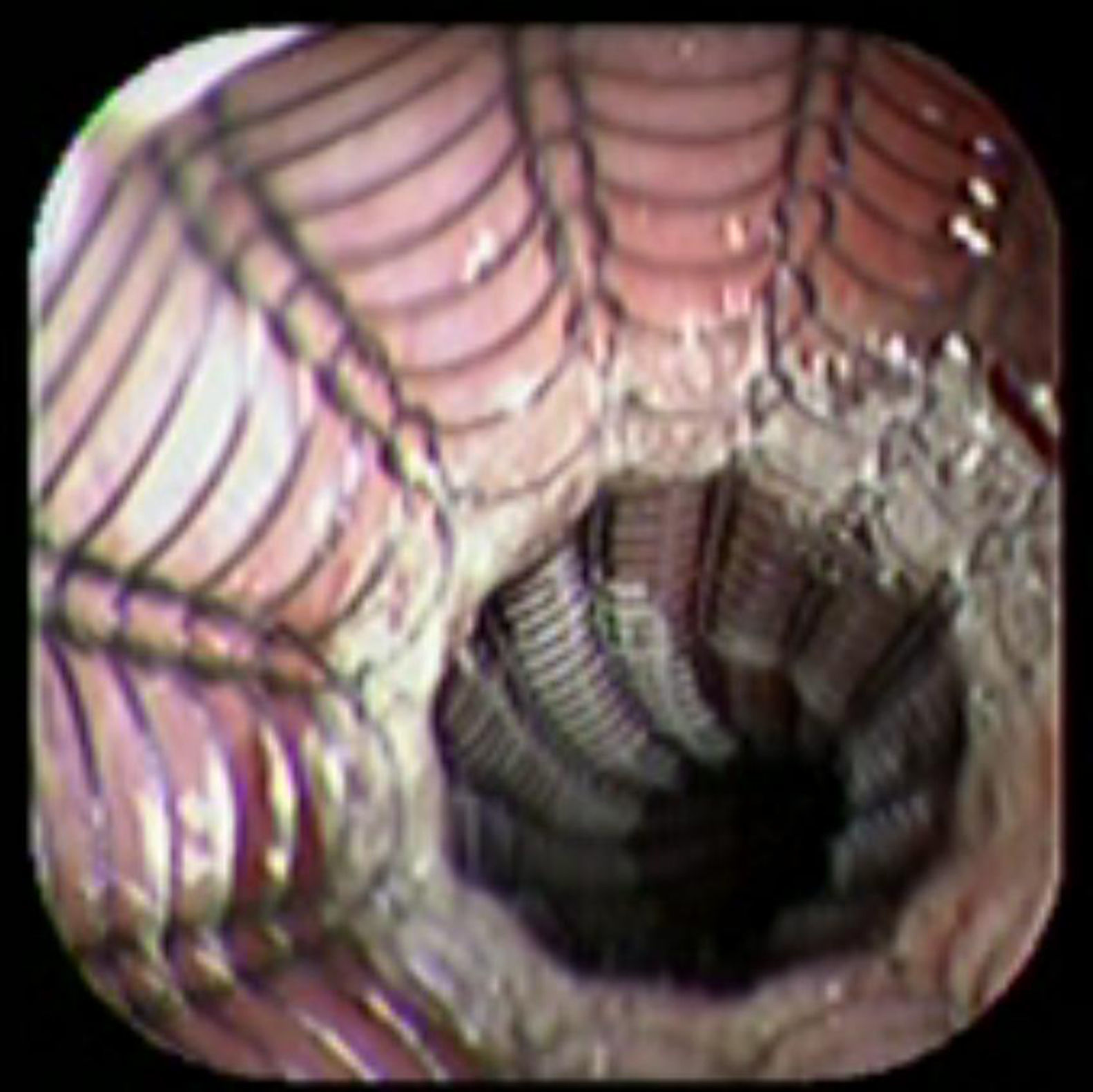
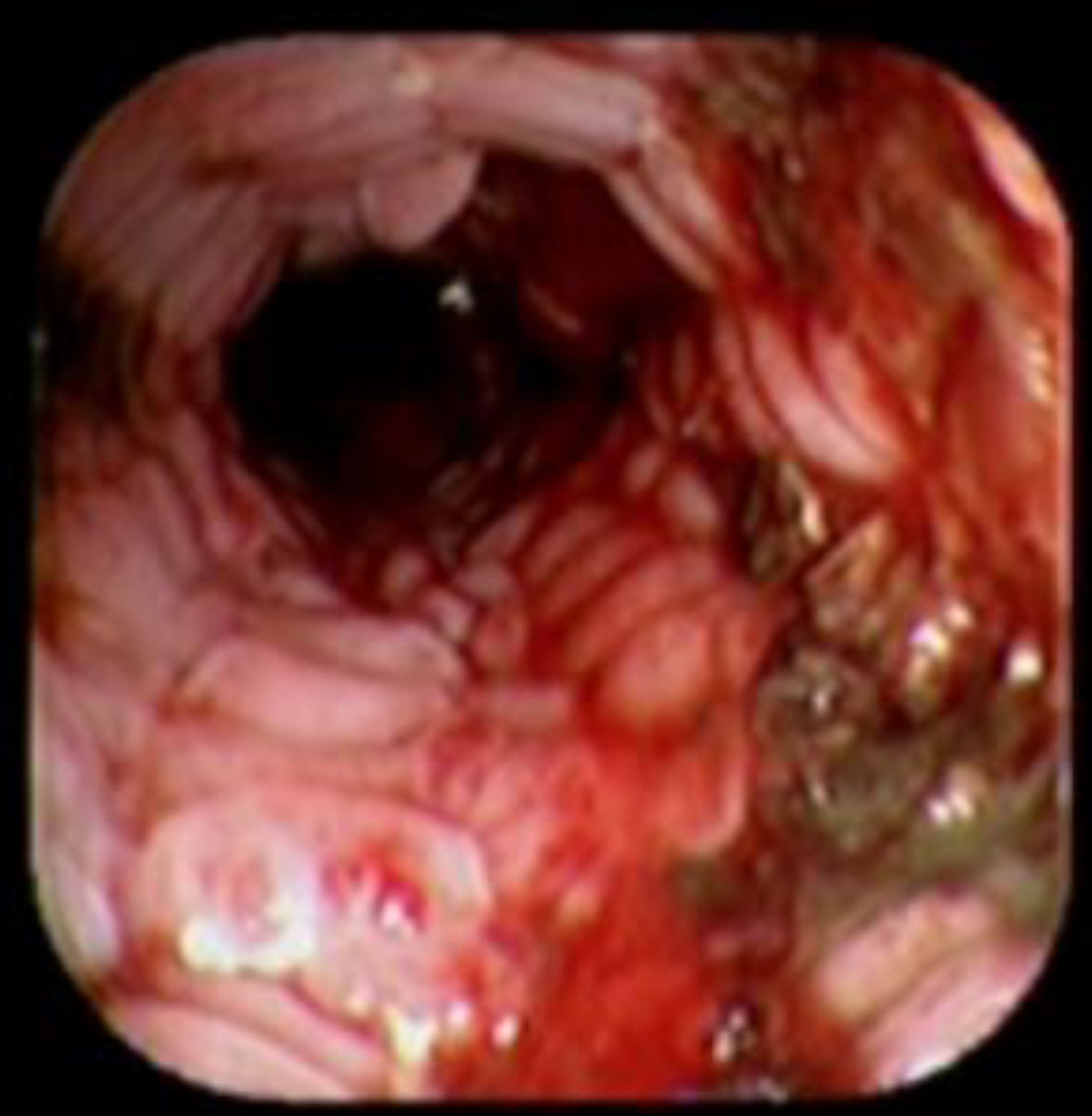
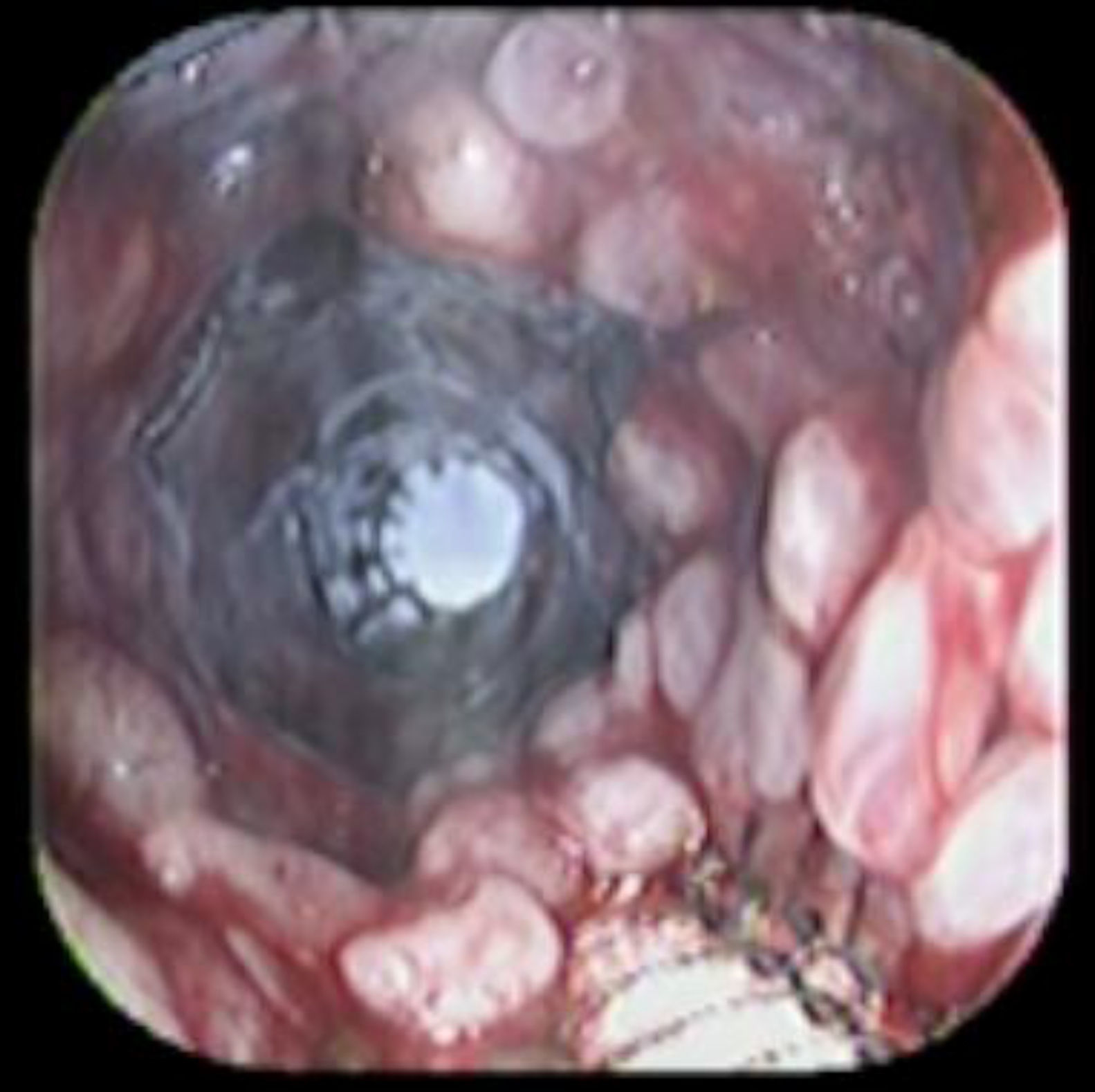
Case reportA 41-year-old man underwent elective laparoscopic Heller myotomy for achalasia; during the procedure a perforation of the esophageal wall was observed, and primary closure of the defect was attempted. The patient started with PO on day three, but he complained of chest pain and fever. Immediately CT scan with oral contrast was performed. A free leakage at esophago-gastric junction was observed. A 15cm PSEMS (Ultraflex esophageal NG covered (120mm) stent, Boston Scientific, Natick, MA) was placed across the defect, leading to the distal 1.5cm non cover into the stomach and the proximal 1.5cm non cover at 30cm from incisor (Fig. 1). A control CT scan with oral contrast showed good position of the stent, without evidence of leakage. The patient's clinical status improved and began to swallow normally, but showed numerous episodes of reflux despite PPI use. We leave the PSEMS for 26 days beside the reflux symptoms. During the endoscopic procedure to remove the stent the presence of tissue embedding along the covered portion of the PSEMS was noted (Fig. 2). The stent was pulled out with an alligator forceps with out major complications and the cover of the PSEMS was seen to be totally destroyed (Fig. 3).
With the possible new applications of PSEMS in benign GI disease, one of the main concerns is about the duration and characteristics of the cover. The currently available esophageal stents are easy to insert and can usually be removed safely within a few weeks of the placement.6 Actually, the PSEMS are not FDA approved in benign GI diseases, but are used as alternative treatment in some particular cases.7
The Ultraflex covered stent is made of silicone a material with a good resistance to heat and acid exposure. The PSEMS complications are severe and expensive (stent in stent method or surgery).8 The used of covered self-expanding metallic stents for intra-thoracic leakage treatment after esophageal perforations had reported effectiveness of 77%, with a complications rate of 5.6–16%.2,9
In a PubMed search, we found a few reports about the safety and complications (damage of the cover) of PSEMS in benign GI disease. Recently, the stent migration and tissue embedding became one of the most frequent complications of the PSEMS.10 One study about the use of PSEMS in benign GI diseases showed cover rupture (Ultraflex) in seven of twenty-three cases.11 Another study, including 71 stents placement, reports a clinical effectiveness and mortality of 76% and 2% respectively. But, 19/71 stents need to be removed early because stent complications and 8/19 stents had tissue in- and/or overgrowth. However, the article does not specify whether tissue in- and/or overgrowth was on the cover or in the bare area of the stent.10
In our report, even when the PSEMS was early removed (26 days), the cover was totally destroyed. As we mentioned early, the patient suffered severe reflux episodes despite use of double doses of PPI and head bed elevation. We think that the use of PPI may totally suppress the gastric acid content and alkaline reflux was present. The alkaline content could be one important factor in damaging the stent cover. We have the theory that the tissue contact with the biliar content promotes an inflammatory and pro-thrombotic response that could be the potential explanation of early in- and/or tissue overgrowth in the bare area of the stent.12
More information is requiered about the use of PSEMS in benign esophageal disease. Several questions about the covered need to be answered: 1) the potential role of alkaline-acid reflux, 2) durability of the cover, 3) time of stent removal. In our experience we may recommend endoscopic surveillance of the stent and to removed it in less than 3 weeks.
Due to likely increase in the number indications of covered and PSEMS in benign GI diseases, we believe that a complete reevaluation of the stent composition is needed to avoid potentially fatal complications.
FundingThere was no funding for the implementation of this article.
Conflicts of interestsThe authors have no conflicts of interest to declare.