¿ Introduction
Although the prevalence of Helicobacter pylori infection varies considerably throughout the world, it remains the most common bacterial infection in man. Estimates suggest that a majority of the world's population is infected, which would make attempts at widespread eradication quite challenging.1 Therefore, before addressing the question "Should we have a stomach without H. pylori?" we must first determine whether we could have a stomach without H. pylori. This requires understanding several challenging issues. For example, the epidemiology of infection closely relates to socioeconomic, public health, and hygienic issues that promote bacterial transmission during early childhood.1 These same issues likely relate to adult acquired infection and reinfection after treatment. Some type of universal testing would be required to eliminate infection entirely, since most infected individuals are asymptomatic which would make targeted testing ineffective. To be practical such widespread testing for H. pylori should be inexpensive, accurate, and non-invasive.2,3 The same thing may be said about follow-up testing to confirm cure following treatment. Once infection is identified, a number of obstacles to effective therapy need to be dealt with. There is no single medication that can effectively cure H. pylori infections. Current treatment regimens are complex, expensive, difficult to take, and associated with occasional adverse events.4,5 Treatment efficacy is hampered by antimicrobial resistant organisms which appear to be increasing in prevalence.4,6 Administering an effective H. pylori vaccine could be the means to make the organism extinct. This strategy has been successful with other infectious diseases such as polio and smallpox. However, despite several decades of research and development, a candidate H. pylori vaccine has not emerged.7
If having a stomach without H. pylori were possible, should we have it? Keep in mind the principle, "first, do no harm." There are clear benefits to eliminating infection and these include reducing peptic ulcer disease, gastric cancer, mucosa-associated lymphoid tissue (MALT) lymphoma, and possibly dyspepsia.1,8,9 However, there may be downside consequences of eliminating infection entirely, especially if individuals being treated are asymptomatic. Gastroesophageal reflux disease (GERD), Barrett's esophagus, and esophageal adenocarcinoma are more common when H. pylori is absent.8 Treatment-emergent dyspepsia and reflux symptoms as well as adverse events related to antibiotic therapy can cause morbidity in otherwise asymptomatic bacterial carriers. Obesity might emerge as ghrelin levels increase following eradication.8 A "naïve immune system" not stimulated during childhood by H. pylori may increase the risk for subsequent autoimmune conditions in adulthood.8
While the benefits of eliminating H. pylori likely outweigh the risks, it is unrealistic at the present time to strive for a bacteria-free gastric environment in everyone. Until a better treatment becomes available or an effective vaccine is developed, emphasis should be on targeting bacterial identification and eradication resources to those at highest risk for a serious clinical infection-related outcome.
¿ Epidemiology of Helicobacter pylori infection
Before attempting large scale elimination of H. pylori, the facts of its epidemiology should be addressed. Mankind has likely harbored the organism for more than 58 000 years when humans first migrated from Africa.10 It remains one of the most common chronic infections in humans with an estimated 50 percent of the world's population affected.1 Infection is more common and acquired at an earlier age in developing countries where socioeconomic circumstances, living conditions, community sanitation, and hygienic resources are less than ideal and can foster bacterial transmission.1 Even in developed nations, infection tends to be more common in immigrant populations and certain ethnic and racial minority groups.11 Such resilience and widespread prevalence of infection and lack of financial and medical resources to address H. pylori in less developed areas of the world or in disadvantaged groups where it is most common do pose challenges for its successful elimination. However, a positive aspect of eradication is that reinfection, especially in adults, is unlikely to occur although the rate of recurrence or recrudescence of infection varies throughout the world.12 Global social and fiscal growth and awareness may be the major means to control H. pylori since a rapidly declining prevalence of infection parallels economic progress and improvement in hygiene and sanitation. For example, in countries such as the United States and Japan, the organism could eventually become extinct13 and a similar situation could also accompany improved socioeconomic and public health conditions in many other parts of the world.
Selecting whom to test and treat for H. pylori would also pose a challenge since most infected individuals are asymptomatic. While gastric inflammation is a universal sequel of infection, clinical symptoms and significant disease i.e, ulcers, gastric cancer and lymphoma develop only 10-15% of the time.1 Certain inherited cytokine polymorphisms can generally predict risk for untoward clinical outcomes14 but host genetic profiling is expensive, not readily available, and impractical at present time. In addition, while serologic identification of "more virulent" bacterial strains is possible, even "less virulent" stains can cause disease. Such obstacles would make a targeted eradication program difficult to implement.
¿ Testing for and Treating Helicobacter pylori Infection
Currently, recommendations for testing and treating H. pylori are specifically directed to patients with symptoms or clinical manifestations of the infection.2,15 To eliminate infection entirely, efforts would need to be expanded beyond patients to the population at large. With this scenario, testing would be limited to non-invasive methods such as serology, urea breath test (UBT), and fecal antigen test (FAT).2,3,15
Serology is inexpensive, noninvasive, and samples can be analyzed off-site in batches or at the point of testing. In the later situation results are immediately available; this precludes later follow-up of positive results and permits starting treatment at once. Unlike other non-invasive methods, serologic results are not influenced by concomitant medication use (e.g., antibiotics, proton pump inhibitors [PPIs]). However, the accuracy of serology is impacted by the prevalence of H. pylori in the population. Its sensitivity is quite good (90 to 100 percent) but its specificity varies (76 to 96 percent), especially when prevalence of H. pylori is low.2,3 In populations where infection is less common, as is the case in many developed countries, the negative predictive value of serology remains reliable and can effectively exclude infection. On the other hand, its positive predictive value is poor so positive serology results will require confirmation with another method such FAT or UBT to avoid unnecessary treatment. Serology is neither useful to follow up cure of infection since a "serologic scar" may remain for many years after successful treatment. Therefore, in any large scale detection program, serology would be limited to primary diagnosis of H. pylori in high prevalence populations.2
The UBT only detects active H. pylori infection so it is useful for primary diagnosis, for confirming the accuracy of serology, and for documenting successful treatment.2,3,15) While the radiation dose with the 14C UBT is very low, the nonradioactive 13C UBT is more practical for population testing.
The specificity of UBT is excellent so false positive results are uncommon but false negative results can occur in individuals taking PPIs, bismuth or antibiotics. Iterations of the UBT require fasting and "test meals", while breath analysis equipment is expensive and generally not portable. Test results may not be immediately available. Such limitations seem to limit UBT's potential as a large scale detection tool.
A FAT appears to be the most ideally suited non-invasive method for population H. pylori detection. Since it only detects actively infected individuals, FAT is suitable for primary diagnosis, for confirming the accuracy of serology, and for confirming eradication following treatment .2,3
Both FAT sensitivity and specificity are comparable to those of UBT and a rapid version of the test provides immediate results at the time of testing.16 While accuracy of stool testing is negatively impacted by PPIs, bismuth and antibiotics, this should not be an issue in most individuals and if necessary, testing could be postponed to a later date. Although not specifically verified, compliance with stool collection may be less than with blood or breath sampling.
Currently there is no "H. pylori-specific" or single antibiotic available to cure infection. Treatment involves several medications, usually two antibiotics dosed several times daily for 7-14 days along with acid suppressive medication.2,4,5,15,17 Such regimens can be expensive and their complexity impacts compliance and leads to side effects. Unfortunately, efforts to simplify or shorten the duration of therapy generally reduce effectiveness. Also, treatment success varies around the world and even within countries due to antibiotic resistant organisms, which are more common than previously appreciated.6,18 For these reasons practical, effective, and geographically appropriate treatment selection is based on factors such as cost, side effects, ease of administration, and impact of antibiotic resistance. To completely eliminate H. pylori, retreatment of those who fail initial treatment becomes important. This requires follow-up testing to confirm cure and selection of second line or "rescue" regimes that account for both primary and emergent antibiotic resistance. Costs and logistics involved in such an effort at a population level seem to make such an undertaking out of the question.
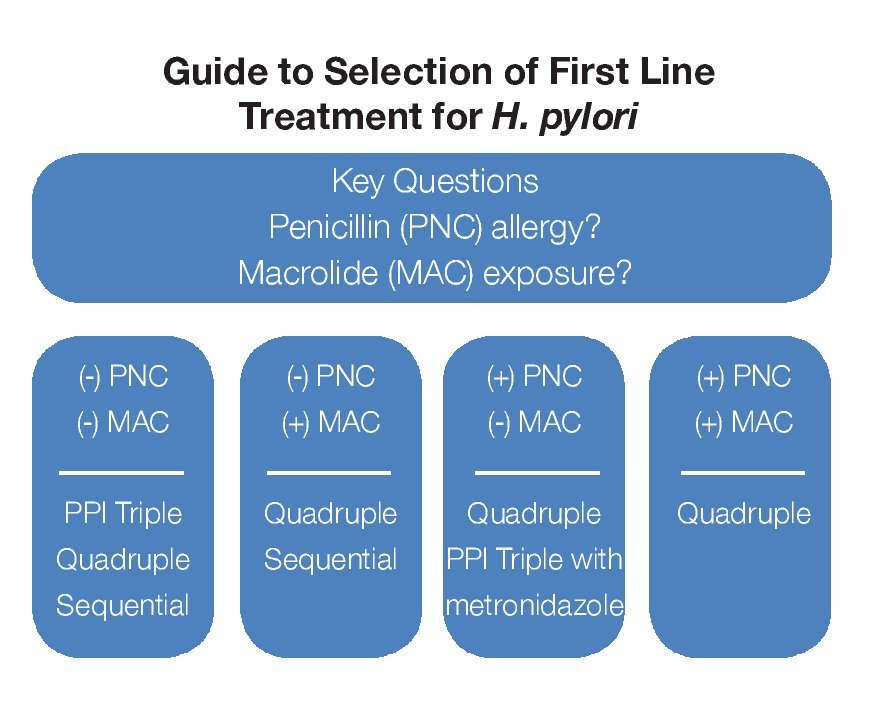
There are three commonly prescribed first-line treatment regimens that provide acceptable eradication rates (> 75-80%) but use of each may be limited by an individual's drug allergy/sensitivity or suspected antibiotic resistance.2,4,5,15,17 A guide to primary treatment selection is shown in Figure 1. A regimen comprised of two antibiotics, clarithromycin (500 mg BID) and amoxicillin (1 g BID) along with a PPI (BID) for 7 to 14 days is currently the most popular initial treatment for H. pylori but in some areas of the world clarithromycin is quite expensive and antibiotic resistance is quite high. Metronidazole (500 mg BID) can substitute either amoxicillin or clarithromycin in penicillin-allergic or macrolide-intolerant individuals or be used to reduce cost; however, metronidazole resistance is common and can reduce treatment success.
¿ Figure 1.Guide to selection of First Line Treatment for H. pylori.
A 10-day sequential regimen (PPI and amoxicillin 1 g each BID for the first 5 days, followed by a PPI, clarithromycin 500 mg and tinidazole 500 mg, each BID for the remaining 5 days) provides comparable efficacy to standard PPI triple therapy but seems better for clarithromycin-resistant bacteria.19 Experience with sequential therapy is largely limited to Mediterranean countries. There are reports of treatment success when metronidazole 500 mg is substituted for tinidazole.20 This regimen cannot be used in penicillin-allergic individuals.
One of the original treatments for H. pylori was bismuth-based therapy. This quadruple regimen combines a bismuth salt, metronidazole 500 mg and tetracycline 500 mg each given QID along with daily acid suppression for 14 days.2,4,15,17 It remains quite effective (> 80% eradication) but the number of daily pills and associated frequent minor side effects impact tolerability and compliance. Efficacy may be reduced in the setting of metronidazole resistance although this is generally not a problem when a higher metronidazole dose (500 mg) and longer duration of treatment (14 days) are used.
Failure of primary treatment generally suggests poor compliance and/or primary antibiotic resistance and portends emergence of secondary resistance, all of which can complicate retreatment efforts. Principles for subsequent treatments include emphasis on compliance, selecting regimens with new antibiotics, and extending treatment duration.5,15,17 Such principles are often difficult and costly to implement on a population level.
Based on the above discussion of diagnoses and treatment of H. pylori, the enormous challenges facing a global eradication effort become clear.
Vaccination to prevent infection or to augment host immunity to "self treat" infection appears to be the means to eliminate the bacteria entirely.7 Efforts to eliminate other global infections (e.g.. polio, smallpox) through widespread vaccination have been successful. Unfortunately, despite ongoing efforts and encouraging preclinical results, no candidate human vaccine is yet to emerge. Furthermore, vaccine development may be hampered by parochial research priorities since countries with the economic and biomedical infrastructure to develop a H. pylori vaccine are those with the least need of a vaccination program.
¿ Potential Benefits of Eliminating Helicobacter pylori
Chronic H. pylori infection is a major cause of altered gastric acid secretory physiology causing hypochlorhydria in infected individuals with certain host genetic profiles.14,21 Those individuals are at an increased risk for developing gastric cancer. Several lines of evidence suggest that eradication of infection can reduce subsequent cancer risk especially if organisms are eliminated early in the course of disease before irreversible histo-logic changes such as generalized metaplasia or dysplasia occur.22,23 After curing the infection, gastric secretory function also improves in most individuals and normal acid levels are recovered. It is less likely for more advanced precancerous gastric histology and associated acid secretory abnormalities to regress after antibiotic treatment so some risk of consequent cancer will remain despite elimination of infection.24 Therefore, to be most successful, chemoprevention of gastric cancer should be initiated during the earliest possible phase of H. pylori infection.
Gastric marginal B cell lymphoma or MALT B cell lymphoma is an uncommon malignancy linked to H. pylori infection; in fact, the great majority of those with gastric MALT lymphoma are infected. Bacterial colonization triggers mucosal lymphoid proliferation, largely B-cells, and sustained proliferation can promote monoclonal malignant transformation through acquisition of genetic abnormalities. In the past, treatment for MALT lymphoma involved chemotherapy, radiotherapy, and surgical resection but now H. pylori eradication has emerged as first-line therapy with complete remission achieved in the majority of early stage low-grade tumors.25,26 Long-term endoscopic surveillance remains important in anyone treated for MALT lymphoma since recurrence can occasionally occur even years after bacterial cure.
The Nobel Prize winning contribution of Marshall and Warren was linking H. pylori to duodenal ulcer disease and demonstrating that cure of infection reduced ulcer recurrence.27 As is the case with gastric cancer, host genetics plays a key role in ulcer disease. Active gastritis is more intense and confined primarily to the antrum in duodenal ulcer patients. This antral inflammation results in the down-regulation of somatostatin and the upregulation of gastrin with the net effect being enhanced acid secretion especially following meals.21 In some individuals this enhanced acid is sufficient to overwhelm mucosal defense and cause an ulcer. Following H. pylori eradication acid secretory physiology returns to normal, which explains why antibiotic treatment prevents ulcer recurrence. In less developed areas of the world, H. pylori remains the cause of nearly all ulcers and ulcer complications,28 while in more developed countries where prevalence of infection low, less than 50% of ulcers are infectious and curing the infection does not prevent all ulcer recurrences or complications: 20% of ulcers still return despite adequate antibiotic treatment.29
Dyspepsia is a poorly defined yet common clinical condition that probably has multiple causes. When evaluated most patients have functional dyspepsia, and no obvious structural cause of symptoms is found. Furthermore, evidence for H. pylori causing symptoms in the absence of an ulcer has been difficult to obtain because no specific symptoms differentiate H. pylori-related dyspepsia from other types of dyspepsia. Also the effect of H. pylori treatment on dyspeptic symptoms has been inconsistent.2,30 Nevertheless, some dyspeptic individuals certainly have symptoms related to infection and do respond to antibiotic treatment. Whether elimination of H. pylori could significantly reduce the population prevalence of dyspepsia is not known.
Associations of H. pylori infection and nongastroduodenal diseases including Raynaud's syndrome, scleroderma, idiopathic urticaria, acne rosacea, migraine headache, and thyroiditis have been reported but supporting evidence is weak.31 Associations with somewhat better levels of evidence include coronary artery disease, idiopathic thrombocytopenic purpura, and resistant iron deficiency anemia.15,32-35 Proposed mechanisms leading to these various conditions range from systemic immune reactions, cross-reactivity of bacterial and host proteins, and events secondary to gastric mucosal injury. How H. pylori elimination would affect the prevalence of such conditions is unknown.
¿ Potential Adverse Consequences of Eliminating Helicobacter pylori
The possible negative association between H. pylori and GERD remains an area of considerable controversy.8,9,36,37 GERD and its complications, esophagitis, Barrett's esophagus, and esophageal adenocarcinoma appear to be less common in those infected with H. pylori especially in Asian countries.36,38 This might be due to differences in bacterial strains and/or host genetics, since in Asia, CagA bacterial strains are more common and host genetics promote more intense corpus-predominant gastritis and hypochlorhydria as a consequence of infection. These differences could mitigate acid reflux and associated complications.8,9 However, in other parts of the world, a robust acid secre-tory response resulting from infection could actually promote GERD making clinical differences between infected and uninfected individuals less important.37 Thus, whether or not elimination of infection will have a negative impact on GERD depends on the pattern of H. pylori-related gastritis and acid secretion in the infected population.36 Normalization of acid secretion following antibiotic treatment in the setting of hypochlorhydria could cause de novo reflux symptoms in some Asian individuals or make treatment of present symptoms more difficult. Alternatively, if infection-related acid hypersecretion predominates, as is the case in most Western countries, eradication of infection could actually improve GERD.37
Could elimination of H. pylori further promote global obesity?8,9 Colonization of the stomach by the organism does affect the obesity-associated hormones leptin and ghrelin. While leptin inhibits appetite and controls body fat, ghrelin stimulates hunger and appetite. H. pylori-infected individuals produce less gastric ghrelin and have lower levels of circulating hormone. This effect is most profound in individuals with corpus predominant gastritis compared to antral prominent gastritis. Ghrelin levels increase after H. pylori eradication.39 While gastric leptin production is altered by infection, circulating hormone levels are not affected. In Asia, infected persons have a lower body mass index (BMI) than those who are not infected and H. pylori eradication can lead to weight gain.40 The obesity epidemic in Western countries has accompanied the disappearance of H. pylori from the population. Although there is no direct evidence that H. pylori-related changes in leptin or ghrelin protect against obesity, the data are intriguing and the hypothesis is biologically plausible. Only time will tell if a H. pylori-free population will become an "oversized" population.
It has been suggested that exposure to infections and external antigens during early childhood is essential for the immune system to appropriately develop and protect against autoimmune and allergic/atopic conditions later in life. One such protective infectious agent could be H. pylori. Epidemiologic studies have shown a negative association between the infection and several allergic/ atopic conditions such as asthma, allergic rhinitis and eczema.8,9,41,42 Whether elimination of a possibly important stimulus for early life immune development could pose problems for "immune naïve" individuals later in life remains to be seen.
Finally, any large scale H. pylori eradication program would undoubtedly generate a number of treatment-related side effects. While most would be self limited, some such as Clostridium difficile and allergic reactions to medications can be serious and contribute to morbidity and even occasional fatal cases. Emergence of antibiotic resistance among other organisms in the community that are incidentally exposed to antimicrobials would be a concern. Also some asymptomatic individuals who are treated would develop new dyspeptic or GERD symptoms that would generate unexpected healthcare-related costs.
¿ Conclusions
Overall, it appears that the benefits of elimination of H. pylori would outweigh the risks. Infection-related conditions such as peptic ulcer and gastric cancer impose a huge burden on the global healthcare system. The potential negative impact of bacterial elimination such as an increase in GERD, or rise in obesity and immune related disorders is speculative at best. However, it is currently highly unlikely that the logistic obstacles associated with a population-wide eradication effort could be overcome or the costs of such an effort be justified. In the meantime, it remains appropriate to identify and target treatment to those individuals at risk for serious infection-related outcomes. Total elimination of H. pylori infection could become a reality if an effective vaccine ever becomes available. In the long term, as global economies progress and public health and sanitation practices improve, the organism could ultimately become extinct on its own. Time will tell whether the net effect of extinction is good or bad.
Correspondence: David A Peura MD.
Division of Gastroenterology and Hepatology University of Virginia Health Systems. PO Box 800708 HSC UVA.
Charlottesville Virginia 22 908 0708
E-mail:dap8v@virginia.edu