Since the publication in 2009 of the Guidelines on the Diagnosis and Treatment of Irritable Bowel Syndrome of the Asociación Mexicana de Gastroenterología (2009 Guidelines), there have been significant advances in our knowledge of the epidemiology, pathophysiology, diagnosis, and treatment of this disease.
AimsTo present a consensus review of the most current knowledge of IBS, updating the 2009 Guidelines by incorporating new internationally published scientific evidence, with a special interest in Mexican studies.
MethodsThe PubMed literature from January 2009 to March 2015 was reviewed and complemented through a manual search. Articles in English and Spanish were included and preference was given to consensuses, guidelines, systematic reviews, and meta-analyses. Statements referring to the different aspects of the disease were formulated and voted upon by 24 gastroenterologists employing the Delphi method. Once a consensus on each statement was reached, the quality of evidence and strength of recommendation were determined through the GRADE system.
ResultsForty-eight statements were formulated, updating the information on IBS and adding the complementary data that did not appear in the 2009 Guidelines regarding the importance of exercise and diet, diagnostic strategies, and current therapy alternatives that were analyzed with more stringent scientific vigor or that emerged within the last 5 years.
ConclusionsWe present herein a consensus review of the most relevant advances in the study of IBS, updating and complementing the 2009 Guidelines. Several studies conducted in Mexico were included.
Desde la publicación de las guías de diagnóstico y tratamiento del síndrome del intestino irritable (SII) de la Asociación Mexicana de Gastroenterología en el 2009 (Guías 2009) se han producido avances significativos en el conocimiento de la epidemiología, fisiopatogenia, diagnóstico y tratamiento de esta enfermedad.
ObjetivosPresentar una revisión consensuada del estado actual de los conocimientos sobre el SII que actualicen las Guías 2009, integrando las nuevas evidencias científicas publicadas a nivel mundial con énfasis en estudios realizados en México.
MétodosSe realizó una revisión de la bibliografía en PubMed de enero del 2009 a marzo del 2015, que se complementó en forma manual. Se incluyeron todas las publicaciones en inglés y español, con preferencia por los consensos, guías, revisiones sistemáticas y metaanálisis. Se generaron enunciados en los diferentes aspectos de la enfermedad que fueron votados por 24 gastroenterólogos con el método Delphi. Una vez consensuado cada enunciado, se calificó el nivel de la evidencia y se otorgó la fuerza de la recomendación utilizando el sistema GRADE.
ResultadosSe generaron 48 enunciados que actualizaron la información sobre el SII y complementaron la información que no había sido incluida en las Guías 2009 con referencia al papel del ejercicio y la dieta, las estrategias diagnósticas, así como alternativas de tratamiento existentes que fueron evaluadas con mayor rigor o que surgieron en los 5 últimos años.
ConclusionesPresentamos una revisión consensuada de los progresos más relevantes en el SII, que actualizan y complementan las Guías 2009. Se incluyen diversos estudios realizados en México.
Irritable bowel syndrome (IBS) is the most frequently diagnosed gastrointestinal disorder in clinical practice.1 It is a functional disorder characterized by abdominal pain or discomfort that is associated with bowel habit alterations and other gastrointestinal symptoms, such as bloating and a sensation of abdominal inflammation, incomplete bowel movement, urgency, straining, and tenesmus.2–4 It is a clinical condition whose symptoms cannot be explained by organic, metabolic, or underlying infectious causes.
In 2009 the Asociación Mexicana de Gastroenterología brought together a group of gastroenterologists that formulated the Guidelines on the Diagnosis and Treatment of IBS.2–4 One year later, under the auspices of the same Association, a broad review of the pharmacologic treatment of IBS was published.5 Since then, there have been significant advances in different aspects of the disease, including epidemiology, pathophysiology, the role of the microbiota and diet, the use of probiotics, novelties in the use of diagnostic biomarkers, quality studies on the effectiveness of combined drugs and medications, as well as new drugs, some of which have recently arrived in Mexico. Innovative themes have also emerged in the international literature, such as fecal microbiota transplantation. All these advances justify the elaboration of a document complementing the 2009 Diagnosis and Treatment Guidelines. In January of 2015, the Asociación Mexicana de Gastroenterología summoned a group of experts to carry out a review of the advances made in relation to different aspects of IBS, evaluate the evidence, reach a quality consensus, and formulate statements for understanding the current status of IBS.
The aim of the 2015 Mexican Consensus on IBS is to present a consensus review of the current status of IBS to bring the 2009 Guidelines on the Diagnosis and Treatment of IBS up-to-date by incorporating the new internationally published scientific evidence, with a special interest in studies conducted in Mexico.
MethodsThe Delphi method was used to prepare this consensus.6 The consensus coordinators found the published articles to be reviewed through the search words “irritable bowel syndrome” and “IBS” combined with the following terms: “diagnosis” “diet”, “epidemiology”, “fecal transplant”, “FODMAP”, “gluten”, “guidelines”, “hypnotherapy”, “hypnosis”, “incidence”, “meta-analysis”, “microbiota”, “prevalence”, “probiotic”, “psychological”, “review, “symptoms”, “therapy”, “management” and “treatment”, as well as the equivalent terms in Spanish. The search was conducted using the PubMed database and included articles in both English and Spanish that were published within the time frame of January 2009 to March 2015. Preference was given to consensuses, guidelines, systematic reviews, and meta-analyses, but was not limited to these types of articles. Complementary online and manual searches were also carried out using the archives of the Revista de Gastroenterología de México and any of the publications that the coordinators felt were relevant up to March 2015.
After the review of each theme, a series of statements were formulated that covered the main aspects of the disease. These were then sent to all the 2015 IBS Consensus panel members for the first anonymous voting process carried out electronically, voting “in agreement” or “in disagreement” for each statement. When agreement equal to or greater than 75% was reached, it was determined that the statement could remain unchanged, passing to the next round of voting. Statements with disagreement of 75% or higher were eliminated from the consensus. The statements with less than 75% agreement or disagreement were restated by the coordinator of each working group, taking into account the comments of the participants. Three rounds of voting were carried out by email and an in-person vote was held at Boca del Río (Veracruz), in March 2015. In the final voting process, the votes were cast using a 6-point scale: A) in complete agreement; B) in agreement, with minor reservations; C) in agreement, with major reservations; D) in disagreement, with major reservations; E) in disagreement, with minor reservations; or F) in complete disagreement. In the present review, consensus was considered when 67% of the participants or more were in agreement (A, B, or C).
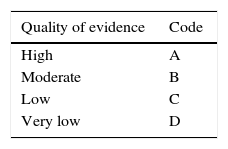
Once the final consensus statements were determined, the coordinators established the level of evidence that supported each statement and a recommendation grade when appropriate, using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) system.7 This system came about through an international initiative to optimize the evaluation of quality of evidence and the grading of strength of recommendation, in an effort to overcome the limitations of previous systems. In the GRADE system, the quality of evidence is not rated solely on study design or methodology, but in relation to a clearly posed question about a clearly formulated outcome measure.8 Even though, in general, the best evidence comes from controlled clinical trials and their meta-analyses, as well as from high quality cohort studies, whereas lower quality evidence comes from case-control studies, case series, cross-sectional surveys, and expert opinions, the GRADE system classifies the quality of evidence based on the design used to answer the specific question that has been posed a priori.8,9 In such a manner, the study methodology required is different, depending on the question, and its quality is judged according to the response to that question. Thus the quality of evidence can be high, moderate, low, or very low. It is defined as “high”, when more research will not modify our confidence in the estimated effect, “moderate”, when more research may modify our confidence in the estimated effect, “low” when more research will most likely importantly affect our confidence in the estimated effect, and “very low” when any estimated effect is highly uncertain. In addition, the GRADE system establishes strength of recommendation as strong or weak, for or against the intervention or statement. It employs a code for the quality of evidence, using capital letters followed by a number to indicate the strength of recommendation for or against the intervention or statement.8,9Table 1 shows the GRADE system codes.
GRADE system: Classification of the quality of evidence and the strength of recommendation.
| Quality of evidence | Code |
|---|---|
| High | A |
| Moderate | B |
| Low | C |
| Very low | D |
| Strength of recommendation | Code |
|---|---|
| Strong, in favor of the intervention | 1 |
| Weak, in favor of the intervention | 2 |
| Weak, against the intervention | 2 |
| Strong, against the intervention | 1 |
The consensus statements by section are listed below:
Definition, pathophysiology, and epidemiology of irritable bowel syndrome in adultsIrritable bowel syndrome is a functional disorder characterized by abdominal pain or discomfort that is associated with changes in bowel movement frequency or appearanceIBS is defined as a chronic and recurring functional disorder that is characterized by abdominal pain or discomfort, associated with changes in bowel movement frequency or appearance.2–4,10 It is considered one of the most common functional disorders, is presently incurable, and can affect patient quality of life to varying degrees.11
GRADE level of evidence and strength of recommendation: C1, strong, in favor of the statement
Level of agreement: In complete agreement 92%.
In agreement with minor reservations: 8%.
IBS incidence in Mexico is unknown and there are no reports on this subjectEven though several good quality studies have been conducted in Mexico on IBS etiology, none of them establishes the number of new cases of the disease having emerged at a given period of time, and therefore the incidence of this functional disorder in our country is presently unknown.12–15GRADE level of evidence and strength of recommendation: D1, strong, in favor of the statement.
Level of agreement: In complete agreement 100%
The reported prevalence of IBS in Mexico varies from 4.4 to 35%This wide variation in the epidemiologic results in Mexico is largely explained by the criteria used to define the presence of IBS. Some studies have pointed out that the Rome III questionnaires appear to have low sensitivity in the community for identifying IBS cases.15–17 In accordance with this, Amieva-Balmori et al.15 reported a prevalence of 4.4% using the Rome III criteria, whereas prevalences of up to 35% have been obtained in studies using the Rome II criteria.12–14GRADE level of evidence and strength of recommendation: C1, strong, in favor of the statement.
Level of agreement: In complete agreement 96%.
In agreement with minor reservations: 4%.
There is a higher IBS prevalence in women, regardless of the diagnostic criteria usedAs in the rest of the world, IBS in Mexico is more prevalent in women, regardless of the subtype. This fact has been consistently reported in epidemiologic studies conducted in our country.12–15GRADE level of evidence and strength of recommendation: B1, strong, in favor of the statement.
Level of agreement: In complete agreement 96%.
In agreement with minor reservations: 4%.
IBS has a more negative impact on quality of life in young adults than in older adultsDifferent studies conducted on Mexican patients coincide with the fact that IBS negatively affects quality of life, when measured through different instruments.18–22 At least one study conducted in the United States showed that young adults with this functional disorder have worse quality of life than older adults.23 This datum has not been reported in national studies.
GRADE level of evidence and strength of recommendation: C1, strong, in favor of the statement.
Level of agreement: In complete agreement 75%.
In agreement with minor reservations 24%.
In agreement with major reservations 1%.
The most frequent IBS subtypes in Mexico are those with a predominance of constipation and the mixed subtypeStudies in Mexico have found that the most frequent subtype of this disorder is the one in which there is a predominance of constipation (IBS-C), followed by the alternate or mixed subtype (IBS-M).12,13,15 Only one study has reported a higher frequency in the diarrhea-prominent subtype (IBS-D) than in the IBS-M subtype, but it still found the greatest frequency in IBS-C.14
GRADE level of evidence and strength of recommendation: B1, strong, in favor of the statement.
Level of agreement: In complete agreement 96%.
In agreement with minor reservations 4%.
IBS pathophysiology is multifactorial and varies among the affected individuals. At present, no universal factor has been establishedNumerous and different mechanisms intervene in IBS pathophysiology, among which are motor disorders, visceral hypersensitivity, gut microbiota alterations or dysbiosis, post-infectious intestinal dysfunction, small intestinal bacterial overgrowth, low-grade inflammation, immune regulation alterations, food intolerance and hypersensitivity, bile acid malabsorption, and psychosocial factors, but up to the present, no common factor for all cases has been established. 24–27
GRADE level of evidence and strength of recommendation: C1, strong, in favor of the statement.
Level of agreement: In complete agreement 100%.
The ingestion of lactose and other fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) may be associated with greater production of gas, visceral hypersensitivity, and perception of bloating, especially in the subgroup of patients with diarrhea-predominant IBSSome foods have been described that possibly aggravate IBS symptoms. A Mexican study showed that intolerance to fructose may be responsible for gastrointestinal symptoms in at least half of the patients with IBS, especially in those with IBS-D.28 Other authors report that lactose intolerance is more prevalent in patients with IBS-D than in healthy subjects, 29 and that they also have greater mucosal immunity activation and greater visceral hypersensitivity after lactose ingestion.29,30 One Mexican study found statistically significant improvement in 3 evaluated symptoms (abdominal pain, bloating, and flatulence) in the comparison of pre-low-FODMAP and post-low-FODMAP diet values.31 However, that study had the limitation of not having a control group that included the average or regular diet of the study population; another limitation was that both diets did not have the same nutritional content.32 Those findings concurred with other international studies reporting that FODMAPs appear to induce some symptoms in patients with IBS through increased luminal distension and if there is visceral hypersensitivity.33
GRADE level of evidence and strength of recommendation: C1, strong, in favor of the statement.
Level of agreement: In complete agreement 96%.
In agreement with minor reservations 4%.
The prevalence of small intestinal bacterial overgrowth in patients with IBS is quite variable, depending on the test and methodology employedThe presence of small intestinal bacterial growth has been pointed out, based on breath tests measuring the hydrogen in the exhaled breath of IBS patients. The reported prevalence is varied and depends on the type of test and methodology used: 28 to 84% with a lactulose breath test, 2 to 31% with a glucose breath test, and a lower prevalence (2 to 6%) when intestinal fluid cultures are used.27
GRADE level of evidence and strength of recommendation: C1, strong, in favor of the statement.
Level of agreement: In complete agreement 88%.
In agreement with minor reservations 8%.
In disagreement with major reservations 4%.
The incidence of post-infectious IBS is from 9 to 10%. The reported prevalence of post-infectious IBS varies from 3 to 17% and decreases in the years following the gastrointestinal infectionPost-infectious IBS (PI-IBS) incidence has been reported at 10% (range 4-36%) and prevalence varies from 3 to 17% and decreases in the years following the gastrointestinal infection.24,34,35 A recently published systematic review and meta-analysis that included 6 studies determined that the overall incidence of PI-IBS was 5.4% in patients that had presented with traveler's diarrhea compared with 1.4% of the control subjects, and the overall relative risk was 3.35 (95% CI: 2.22-5.05).36
GRADE level of evidence and strength of recommendation: C1, strong, in favor of the statement.
Level of agreement: In complete agreement 75%.
In agreement with minor reservations 21%.
In agreement with major reservations 4%.
In relation to PI-IBS, bacterial etiology is the best documented, but viral and parasitic causes also appear to be risk factors for developing PI-IBSPI-IBS has been studied in numerous cohort studies that conducted follow-up analyses of epidemic outbreaks of bacterial gastroenteritis and therefore this etiology is the best documented. Studies on acute gastroenteritis due to viruses and parasites have also been published, but these studies are much fewer and include a small number of patients.27
GRADE level of evidence and strength of recommendation: C1, strong, in favor of the statement.
Level of agreement: In complete agreement 92%.
In agreement with minor reservations 8%.
IBS has been associated with different intestinal and extraintestinal symptoms and syndromesSome community-based surveys have confirmed that IBS frequently overlaps with functional dyspepsia and with gastroesophageal reflux symptoms, especially in nonerosive reflux disease. IBS has also been associated with a variety of psychological disorders, but the evidence of an actual association is less solid. It has been suggested that psychopathology be considered a cofactor that, if present, will modify the response to the different IBS symptoms in each individual.37 In a study conducted in Mexico City utilizing the Rome II questionnaire, the researchers found that heartburn and other reflux symptoms were more frequent in patients with IBS than in controls, regardless of their body mass index.38 Another study also conducted in our country on patients with IBS showed differences in symptom association depending on the IBS subtype, using the Rome III questionnaire. Thus, the IBS-M subtype had a higher association with symptoms such as halitosis, vomiting, and greater intensity early satiety, and IBS-C was associated with straining and tenesmus, whereas IBS-D was associated with urgent bowel movements and daytime or nighttime fecal incontinence.39 Patients with inflammatory bowel disease (IBD) have also been reported to have a greater frequency of symptoms consistent with IBS compared with controls (non-IBD subjects), even among patients that appeared to be in remission. In addition, IBS symptoms were more frequent in patients with Crohn's disease than in those with ulcerative colitis (UC) and in those with active disease.40 These findings are very similar to those that have been reported in patients with celiac disease (CD).41
GRADE level of evidence and strength of recommendation: C1, strong, in favor of the statement.
Level of agreement: In complete agreement 88%.
In agreement with minor reservations 8%.
In agreement with major reservations 4%.
Irritable bowel syndrome diagnosis in adultsSymptom-based diagnostic criteria enable positive IBS diagnosis to be made in those patients with no alarm symptoms or risk factors. Nevertheless, their sensitivity and specificity is variable and some patients with organic disease have diagnostic IBS criteria, demonstrating the need for their modification in order to have greater diagnostic accuracyThere is clear evidence of the limitations of the Rome clinical criteria in diagnosing IBS. 42–45 Another systematic review that included studies published over a broad period of time that evaluated the 3 versions of the Rome criteria, as well as the Manning criteria, found that the latter had been more accurate and validated more often, whereas the Rome III criteria had not been validated or widely adopted in clinical research, up to the present.42 Among patients evaluated with Rome III criteria, 8.3% were observed to have Crohn's disease, 6.1% ulcerative colitis, and 2.3% cancer of the colon.45 Fifty-nine clinicians and researchers participating in an international survey were asked to review the diagnostic criteria and 77% stated that the Rome criteria did not adequately reflect IBS in their community or medical practice and 80% stated that new multinational diagnostic criteria were needed.46
GRADE level of evidence and strength of recommendation: B1, strong, in favor of the statement.
Level of agreement: In complete agreement 96%.
In agreement with minor reservations: 4%.
There is insufficient evidence for recommending a standard group of diagnostic tests in all patients meeting the symptom-based IBS criteriaDue to the fact that IBS is a frequent illness in the general population, its diagnostic evaluation is costly. Some studies systematically carrying out tests on subjects with IBS clinical criteria have shown an increased frequency of abnormal results, but these findings do not modify the diagnosis or clinical behavior of the disease.47–49 A systematic review of the theme determined that the existing evidence is insufficient for recommending the routine use of a battery of diagnostic tests in patients meeting the IBS clinical criteria.50
GRADE level of evidence and strength of recommendation: C1, strong, against the intervention.
Level of agreement: In complete agreement 100%.
It is recommendable to carry out complementary diagnostic tests in all patients that meet the symptom-based IBS clinical criteria and that present with alarm symptoms, refractory symptoms, or risk factors. Diagnostic test selection should be carried out individually, considering the clinical characteristics of IBS and the pre-test likelihood of organic diseases (e.g. celiac disease, inflammatory bowel disease, neoplasias, etc.)When patients presenting with IBS clinical criteria are first seen, a systematic search must be performed to rule out red flags, such as nocturnal symptoms, visible blood in stool, anemia, and significant weight loss; physical examination abnormalities, such as palpable masses; and risk factors, such as a family history of colorectal cancer, the recent appearance of symptoms, onset after 50 years of age, male sex, and recent antibiotic use.51 The positive predictive value of alarm symptoms is known to be low and 11-15 patients with these “red flags” need to be studied in order to detect one patient with colorectal cancer, inflammatory bowel disease, or malabsorption.52 A systematic review and meta-analysis that included 15 studies and over 19,000 patients showed that alarm symptoms had low sensitivity and specificity for diagnosing colorectal cancer.53 Therefore, it is indispensible that test selection be done individually, taking into account the characteristics and risk factors of each patient, as well as the local prevalence of the organic diseases that are being looked for.10,50,54 The evidence supporting this statement comes mainly from case series, case-control studies, and clinical guidelines.
GRADE level of evidence and strength of recommendation: C1, strong, in favor of the intervention.
Level of agreement: In complete agreement 96%.
In agreement with minor reservations: 4%.
It is recommendable to study celiac disease in patients with clinical criteria for IBS with diarrhea and in those with IBS with refractory symptomsIn some cases, IBS and CD can have a similar clinical presentation. IBS symptom prevalence in patients with CD is 38% and the risk for presenting with such symptoms is three times as great in those patients that do not adhere to a gluten-free diet.41 CD prevalence in patients meeting the IBS clinical criteria has been estimated to be 10 times higher than in the general public.50 However, a detailed analysis of the bowel habit of these patients is important, because the prevalence of antibodies associated with CD in IBS patients without constipation has been found to be similar to that of asymptomatic controls.55 For a long time, CD was considered a rare condition in Mexico, but several studies analyzing the prevalence of antibodies associated with CD in different settings (university population, type 1 diabetes mellitus patients, blood donors) suggest that the prevalence of this disease in our country is comparable to that found in other populations.56,57 A study conducted on Mexican patients with IBS (Rome III) found they had a prevalence of positive serology for CD that was twice as high as that of healthy controls and that the risk for presenting with CD confirmed by duodenal biopsy was 1.5 times higher.58 They also found that the prevalence of positive antibodies related to CD was even higher in the patients with IBS-D. Obviously, more and better studies are required in order to know the real CD prevalence in the general Mexican population and to distinguish our risk groups, but given the evidence we have at the present time, we recommend CD screening in patients with IBS clinical criteria, especially in those presenting with a predominance of diarrhea that are treatment-refractory.
GRADE level of evidence and strength of recommendation: C1, strong, in favor of the intervention.
Level of agreement: In complete agreement 92%.
In agreement with minor reservations: 4%.
In complete disagreement: 4%.
Colonoscopy should be performed in patients with IBS criteria that have risk factors for colon cancer, symptom onset at late stages of life, changes in bowel habit pattern, presence of blood in stool, and in all patients that do not respond to treatment. Colon biopsy should be performed in patients with refractory IBS-D, even in the absence of lesions, to intentionally search for microscopic colitisA systematic search should be carried out in all patients with IBS clinical criteria that present with alarm symptoms, such as nocturnal symptoms, visible blood in stool, anemia, and significant weight loss; physical examination abnormalities, such as palpable masses; and risk factors, such as a family history of colorectal cancer, recently appearing symptoms, onset after 50 years of age, male sex, and recent antibiotic use. 51 There is evidence of greater detection of organic disease in patients presenting with IBS clinical criteria with alarm symptoms and in patients with IBS-D or IBS-M criteria.59 The American College of Gastroenterology proposes the performance of screening tests for the detection of colorectal cancer in patients above 50 years of age.60 Some authors have suggested the intentional search for microscopic colitis in subjects with IBS-D criteria.61,62 At least 2 studies conducted in Mexico have found an increased prevalence of microscopic colitis in patients fitting the clinical criteria of IBS and IBS-D, in whom colon biopsies were systematically taken.63,64 It is important that the search for microscopic colitis in these patients be carried out through biopsies of all the segments of the colon, even in the presence of normal mucosa and when there is no evidence of lesions.
GRADE level of evidence and strength of recommendation: B1, strong, in favor of the intervention.
Level of agreement: In complete agreement 96%.
In agreement with minor reservations: 4%.
Imaging studies (barium enema, ultrasound, computerized tomography, magnetic resonance, etc.) are of little use in patients with IBS symptoms in the absence of alarm symptoms or comorbiditiesRadiologic studies are not necessary in patients that do not present with alarm symptoms, but they should be considered when these symptoms are present. The choice of study should be individualized and determined by the predominant symptoms. Routine abdominal ultrasound in IBS patients is unnecessary.65 A recent review determined that there is a surprising lack of information on the usefulness of imaging studies in IBS.66
GRADE level of evidence and strength of recommendation: C1, strong, against the intervention.
Level of agreement: In complete agreement 100%.
There is insufficient evidence for recommending the routine use of tests for detecting small intestinal bacterial overgrowth in patients with IBSAs mentioned beforehand, a recent systematic review found that the presence of small intestinal bacterial overgrowth in IBS patients varies widely and the methodology for making this diagnosis has not been standardized.27 In addition, it is not possible to establish with certainty the cause-and-effect relation between symptoms and the presence of small intestinal bacterial overgrowth.67
GRADE level of evidence and strength of recommendation: C1, strong, against the intervention.
Level of agreement: In complete agreement 96%.
In agreement with minor reservations: 4%.
There is insufficient evidence for recommending the routine use of tests for detecting carbohydrate intolerance in all IBS patientsThe same as with small intestinal bacterial overgrowth, the prevalence of intolerance to different carbohydrates in patients with IBS varies widely and the methodology for making this diagnosis has not been standardized,28,68 nor is it possible to establish with certainty the cause-and-effect relation between symptoms and the presence of food intolerance.29,67 However, some experts state that these tests could be useful in patients with refractory symptoms for the purpose of carrying out a potentially beneficial dietary intervention.67
GRADE level of evidence and strength of recommendation: C1, strong, against the intervention.
Level of agreement: In complete agreement 96%.
In agreement with major reservations: 4%.
The questionnaires for evaluating quality of life provide a profile of state of health and can detect aspects of the disease that require special attention (e.g. physical function, emotional role, mental health). Symptom intensity is correlated with a negative impact on quality of lifeSymptom severity and intensity has a consistently negative effect on the quality of life of these patients. Quality of life is an important measure in the integrated management of the patient with IBS. Because it is a functional disorder with no organic marker, clinical decisions are dependent on asking the patient to evaluate and communicate, through different instruments, how he or she perceives his or her state of health.69,70GRADE level of evidence and strength of recommendation: B1, strong, against the intervention.
Level of agreement: In complete agreement 96%.
In agreement with minor reservations: 4%.
At present, there are no biomarkers for establishing IBS diagnosisA biomarker is an objective biologic indicator of normal function, pathogenic processes, or pharmacologic responses to a therapeutic intervention. The potential usefulness of biomarkers in IBS has been studied in 3 respects: in the differential diagnosis through the detection of organic disease (inflammatory, infectious, or neoplastic) manifested as nonspecific clinical data that substitute “limited study”; in the diagnosis made a priori through the detection of genetic patterns, molecular dysfunction markers, and histologic data of intestinal permeability or low-grade infection that are seen in IBS; and as response predicters.71 Fecal biomarkers of inflammation, such as calprotectin, lactoferrin, protein S100A12, polymorphonuclear elastase, myeloperoxidase, M2 pyruvate kinase, granins, defensins, and matrix metalloproteinases, among others, have been analyzed for establishing the differential diagnosis of IBS with inflammatory bowel disease, but not ruling out other diagnostic possibilities.71–73 Recently, the detection of anti-CdtB and anti-vinculin serum antibodies has been evaluated and validated for the diagnosis made a priori of patients with IBS-D with apparently good results.74 Unfortunately, these antibodies are not detectable in all patients with IBS-D and their prevalence in IBS patients is unknown. Antibody expression depends on the immunologic condition of the host and they have only been validated in healthy controls and in patients with CD and inflammatory bowel disease, without taking into account other possibilities, such as microscopic colitis, parasitosis, or neoplasias. Only one determined age range was included and the results cannot be extrapolated to all populations. Even though there have been great advances in the development of biomarkers for the diagnosis of IBS in a subgroup of patients (e.g. IBS-D) and it is clear that in the future costs could be reduced in the care of these patients, contributing to the development of drugs, some experts believe that they are not yet ready for practical application.75,76GRADE level of evidence and strength of recommendation: B1, strong, against the intervention.
Level of agreement: In complete agreement 92%.
In agreement with minor reservations: 8%.
Irritable bowel syndrome treatment in adultsIBS treatment should be directed at the most bothersome symptom or at the pathophysiologic mechanisms of the diseaseGiven that there is no single medication for treating all the patients with IBS, two types of strategies have been recommended: directing treatment at the symptom that is the most bothersome for the patient (pain, bloating, constipation, diarrhea) or at the pathophysiologic mechanisms involved in the production of symptoms, such as visceral hypersensitivity, motor alterations, dysbiosis, small intestinal bacterial overgrowth, fluid homeostasis, and neuroplasticity.4,77–79GRADE level of evidence and strength of recommendation: C2, weak, in favor of the intervention.
Level of agreement: In complete agreement 96%.
In agreement with minor reservations: 4%.
An adequate doctor-patient relationship has positive effects on overall improvement, symptom improvement, symptom severity grade, and quality of life of the patients with IBS. In addition, it reduces the number of medical consultations and increases patient satisfaction. The doctor-patient relationship is the most robust component of the placebo effectThe approach to IBS centered on the patient and the effective communication between the physician and patient is associated with therapeutic benefit. Asking open questions that allow the patient to express his or her needs, to be actively listened to, and to be shown empathy to strengthen the doctor-patient relationship are useful strategies.80 Calming the patient's fears regarding his or her disease during the initial medical visit has been demonstrated to significantly reduce the self-perception of disability.81 An attentive, warm, and confidence-inspiring doctor-patient relationship has been shown to have a more intense positive effect on the symptoms of the patients.82
GRADE level of evidence and strength of recommendation: C2, weak, in favor of the intervention.
Level of agreement: In complete agreement 100%.
Two controlled studies showed that exercise (20-60min, 3-5 times per week) produces improvement in IBS symptom grade, in IBS-associated quality of life, and that it reduces the risk for symptom worseningEven though the controlled studies are few, they are good quality and have demonstrated improvement in IBS symptom severity compared with controls. Exercise was capable of preventing symptom progression in the patients.83 This improvement persisted over time, given that the patients with an exercise plan maintained their symptom grade and quality of life improvement after 5.2 years.84
GRADE level of evidence and strength of recommendation: C2, weak, in favor of the intervention.
Level of agreement: In complete agreement 88%.
In agreement with minor reservations: 8%.
In agreement with major reservations: 4%.
Soluble fiber ingestion is beneficial in IBS. Bran ingestion does not improve IBS symptomsDietary fiber supplementation has a long history in the treatment of gastrointestinal disorders. However, caution has been expressed in regard to its use, due to the possibility that fiber can exacerbate some symptoms in certain patients.85,86 The recent meta-analysis of randomized and controlled studies by Moayyedi et al.87 showed the benefit of fiber in IBS symptoms, but only in the case of soluble fiber and not bran. It should be noted that no significant adverse effects were demonstrated with the use of bran. On the other hand, no beneficial effects of linseed in relation to IBS have been detected, but there is only one quality study on this topic.87
GRADE level of evidence and strength of recommendation:
For soluble fiber A2, strong, in favor of the intervention.
For bran B2, weak, against the intervention.
Level of agreement: In complete agreement 96%.
In agreement with minor reservations: 4%.
A low-FODMAP diet can improve overall symptoms, the perception of bloating, abdominal pain, and bowel habit in some patients with IBSThe fermentable oligosaccharides, disaccharides, monosaccharides, and polyols are the so-called FODMAPs and they include fructose, lactose, fructans, and fructooligosaccharides present in common foods such as fruits, legumes, and wheat. After several non-controlled studies on dietary FODMAP content and its effect on IBS symptoms,88 Halmos et al., 89 in a randomized and blind study, demonstrated improvement in IBS symptom grade, bloating, pain, and flatulence with the use of a low-FODMAP diet. A recent study conducted in Mexico showed significant beneficial results in symptoms of patients on a low-FODMAP diet. 31
GRADE level of evidence and strength of recommendation: B1, weak, in favor of the intervention.
Level of agreement: In complete agreement 92%.
In agreement with minor reservations: 4%.
In agreement with major reservations: 4%.
A low-FODMAP diet reduces symptoms in IBS patients with a self-reported sensitivity to gluten and no celiac disease, regardless of its gluten contentMany patients today associate IBS symptoms with the ingestion of products that contain gluten, suspending their consumption and reporting symptom improvement. This has been named non-celiac gluten sensitivity. Some studies have shown that the reintroduction of gluten in patients with non-celiac gluten sensitivity that are well controlled with a gluten-free diet causes a reappearance of symptoms that includes abdominal pain and fatigue.90 Biesiekierski et al.91 put patients with IBS and this sensitivity on a low-FODMAP diet and in a blind manner gave them different doses of gluten or placebo. The effect of gluten on symptoms or fatigue could not be demonstrated.
GRADE level of evidence and strength of recommendation: C1, weak, in favor of the intervention.
Level of agreement: In complete agreement 92%.
In agreement with minor reservations: 8%.
There is indirect evidence that the use of bile acid sequestrants, such as cholestyramine, available in Mexico, produces symptom improvement in IBS with diarrheaIt has been documented that some patients with IBS-D can have bile acid malabsorption.92–95 A systematic review that included 1,223 patients with IBS-D that had a TauroH-23-(Se) selena-25-homocholic acid (SeHCAT) test for diagnosing bile acid diarrhea found that 26, 32, and 10% had mild, moderate, and severe bile acid malabsorption, respectively.92 This group of patients may benefit from bile acid sequestrants, such as cholestyramine (available in Mexico), colestipol, colesevelam, aluminum hydroxide, or obeticholic acid.94,95 However, the evidence is indirect,96 given that there are no studies that specifically evaluate the use of cholestyramine in IBS-D.
GRADE level of evidence and strength of recommendation: B2, weak, in favor of the intervention.
Level of agreement: In complete agreement 92%.
In agreement with minor reservations: 8%.
Antispasmodic drugs are more efficacious than placebo for abdominal pain improvement, overall improvement, and symptom scores in IBSAntispasmodics are a group of medications that compete with acetylcholine in the parasympathetic postganglionic nerve terminals or block the calcium channels, inhibiting smooth muscle contraction.97 Several subgroups of antispasmodics have been described5: 1) direct relaxing agents (mebeverine, trimebutine); 2) scopolamine derivatives (butylhyoscine, levsin, hyoscyamine, cimetropium); 3) ammonium derivatives (that also block calcium channels, such as otilonium bromide and pinaverium bromide); and 4) calcium antagonists (alverine citrate, fenoverine, rociverine, pirenzepine, peppermint). A meta-analysis that included 29 studies and a total of 2,333 patients compared antispasmodics with placebo and reported that antispasmodics as a group were superior in abdominal pain improvement (58% of the patients treated with antispasmodics improved, compared with 46% of the control group, p<0.001), overall improvement (57% of the patients treated with antispasmodics improved, compared with 39% that received placebo, p<0.001), and in symptom score (37% of those treated with antispasmodics improved, compared with 22% with placebo, p<0.01), with a number necessary to treat (NNT) of 7, 5, and 3, respectively.98 Another systematic review and meta-analysis99 with 23 studies and 2,585 patients showed similar findings. Some sub-analyses have demonstrated improvement in particular outcomes with specific antispasmodics: otilonium bromide (reduced defecation alterations and overall improvement) and pinaverium bromide (reduced defecation discomfort).100 Other studies have shown improvement with an antispasmodic (mebeverine) only in non-controlled studies with placebo.99,100 Peppermint oil is a drug that has been considered alternative therapy. However, it has calcium antagonist properties101 and has been shown to be superior to placebo in a recent systematic review and meta-analysis in pain improvement and overall symptom improvement.102 Nevertheless, the majority of the studies have observed short-term improvement (6-8 weeks) and the presence of side effects increases with use. There is limited evidence in relation to long-term benefit in the main outcome measures, at least in one study that used otilonium bromide for 15 weeks.103
GRADE level of evidence and strength of recommendation: A1, strong, in favor of the intervention.
Level of agreement: In complete agreement 100%.
The combination of simethicone/dimethicone with antispasmodics appears to improve abdominal pain and distensionDimethicone/simethicone reduces the surface tension of gas bubbles, causing their coalescence. The combination of dimethicone with certain antispasmodics has been shown to be effective, particularly in the improvement of abdominal pain and distension. In the meta-analysis by Martínez-Vázquez, this same combination was superior to placebo in overall symptom improvement.99 The combination of pinaverium bromide with dimethicone and alverine with simethicone has also been superior to placebo in the improvement of abdominal distension.99,104,105 The combination of trimebutine/simethicone has not been specifically evaluated and so there is no evidence for recommending its use.
GRADE level of evidence and strength of recommendation: B1, strong, in favor of the intervention.
Level of agreement: In complete agreement 92%.
In agreement with minor reservations: 8%.
5-HT3 receptor antagonists, such as alosetron and ondansetron, improve the consistency, frequency, and urgency of bowel movements in IBS with diarrhea. Alosetron is not available in Mexico and its use is restricted due to serious side effectsMedications that act on the serotonin or 5-hydroxytriptamine (5-HT) receptors owe their effect to stimulation or antagonsim. The 5-HT3 receptor antagonists attenuate bowel transit and increase fluid absorption, thus improving IBS-D symptoms. A systematic review and meta-analysis that included 11 studies and 7,216 patients evaluated the efficacy of this group of medications.106 Alosetron proved to be superior to placebo (8 studies, n=4,987), with a NNT of 7 (overall improvement) and 8 (symptom persistence), but its use is restricted due to serious side effects (number needed to harm=10) that include severe constipation and ischemic colitis, and it is not available in Mexico.101 In that review, cilansetron also showed improvement over placebo in overall symptom reduction (3 studies, n=2,229), with a NNT of 6 and practically no side effects, but it is not available in Mexico either.106 Ondansetron, which is available in Mexico, is another 5-HT3 antagonist that has been used mainly as an antiemetic, but there is evidence of its usefulness in IBS-D.107 A crossover and placebo-controlled study that was conducted for 5 weeks in 120 patients with IBS-D concluded that ondansetron, titrated to response, improved the consistency (p<0.001), frequency (p=0.02), and urgency of bowel movements (p<0.001), in addition to improving bloating (p<0.001) in those patients with IBS-D.106
GRADE level of evidence and strength of recommendation: B1, strong, in favor of the intervention.
Level of agreement: In complete agreement 92%.
In agreement with minor reservations: 4%.
In agreement with major reservations: 4%.
Certain 5-HT4 receptor agonists can improve symptoms in IBS-C. Tegaserod has been shown to be effective, but it should not be used in subjects above 55 years of age or with other cardiovascular risksThe 5-HT4 receptor agonists increase colon motility, as well as the secretion of fluids and electrolytes, and thus can be useful in IBS-C. Tegaserod has shown benefit in overall improvement (NNT=14), abdominal pain, and improvement in bowel habit (NNT=20) in patients with IBS-C.108 In the systematic review by Ford, tegaserod was associated with less symptom persistence compared with placebo, with a NNT of 10.106 A sub-analysis showed a greater effect in men (p=0.003). The frequency of side effects, such as diarrhea, had a number necessary to harm of 20. However, tegaserod was taken off most of the international markets in 2007, due to a statistically higher frequency of adverse cardiovascular effects, such as acute myocardial infarction, unstable angina pectoris, cerebrovascular disease, and sudden death (0.11 vs 0.01%). The hypothetical interaction mechanism is at the 5-HT1B/D receptor level in the coronary arterioles, although it was later demonstrated that tegaserod did not have that type of agonism. It was reintroduced in the United States in July 2007 under a treatment investigational new drug protocol for IBS-C and chronic idiopathic constipation in women under the age of 55 years that had no risk for certain cardiovascular events. However, tegaserod was not approved for later use due to the opinion of the Committee for Medicinal Products for Human Use that the benefit was not superior to placebo and did not outweigh its risks.109 In Mexico, based on the recommendations of a group of experts from the Asociación Mexicana de Gastroenterología, the Federal Commission Against Health Risks restricted its use to patients under 55 years of age and with no cardiovascular risk (e.g. hypercholesterolemia, arrhythmias, high blood pressure, or the use of other medications that can have an effect on the QT segment of the electrocardiogram). Two other 5-HT4 agonists available in Mexico are prucalopride and mosapride. Prucalopride has shown benefit in chronic idiopathic constipation, but has not been evaluated in IBS-C.110 There is little evidence of the usefulness of mosapride in IBS, but a placebo-controlled pilot study with 37 IBS-C patients showed a reduction in the pain threshold and rectal perception in response to the barostat test after the administration of mosapride.111 Some drugs can have a mixed 5-HT4 agonist and 5-HT3 antagonist effect, such as cisapride and renzapride. The former, similar to tegaserod, was taken off the majority of markets due to a risk for arrhythmias associated with QT segment prolongation, but it is still available in Mexico. The latter is not available in Mexico and there is little evidence of benefit in IBS.106
GRADE level of evidence and strength of recommendation: B1, strong, in favor of the intervention.
Level of agreement: In complete agreement 96%.
In agreement with minor reservations: 4%.
Polyethylene glycol can be useful for managing constipation in IBS, although it is not superior to placebo in pain or distension managementPolyethylene glycol (PEG) 3350 (or macrogol) is an osmotic laxative that has been used for the treatment of chronic constipation in children and adults, including chronic idiopathic constipation. Numerous studies have confirmed its efficacy and safety. There is less evidence in relation to its use in IBS-C, but a recent study compared PEG 2250 plus electrolytes vs placebo in a group of patients with IBS-C (n=68 and n=71, respectively). An increase in the number of spontaneous bowel movements (SBMs) was observed in the two groups from the beginning of treatment, but from week 4 the PEG group had a statistically significant increase in the number of SBMs, complete spontaneous bowel movements, stool consistency, and straining severity. Nevertheless, no improvement in the severity of pain or abdominal distension was observed with respect to placebo.112
GRADE level of evidence and strength of recommendation: B1, strong, in favor of the intervention.
Level of agreement: In complete agreement 92%.
In agreement with minor reservations: 8%.
Linaclotide improves IBS-C symptoms, including the frequency of spontaneous bowel movements, complete spontaneous bowel movements, stool consistency, straining severity, bloating, gas, and abdominal discomfortLinaclotide, available in Mexico, is a guanylate cyclase C agonist that acts by inducing an increase in cGMP levels, causing accelerated gastrointestinal transit, augmented intestinal secretion, and a decrease in visceral hypersensitivity. Two pivotal studies evaluated linaclotide usefulness in the main symptoms of IBS-C. The so-called Study 31 was a double-blind, placebo-controlled analysis with crossover at 12 weeks, in which linaclotide significantly improved IBS symptoms, including SBM and complete SBM frequency (p<0.0001), consistency, strain severity, and abdominal symptoms (subjective bloating, gas, and discomfort) (p=0.0003).113 The second study, called Study 302, had the same design and evaluated the same outcomes at 26 weeks.114 The therapeutic gain over placebo was 17% and significant improvement was observed in all the endpoints and visual symptom scale and quality of life scale scores, with a NNT of 5.1 (overall response), 7 (pain), and 4 (complete SBMs).114 Later systematic reviews have confirmed these findings.115
GRADE level of evidence and strength of recommendation: A1, strong, in favor of the intervention.
Level of agreement: In complete agreement 96%.
In agreement with minor reservations: 4%.
Lubiprostone is beneficial in overall improvement, bloating, pain, stool form, and frequency of bowel movements in patients with IBS-C, but it is currently unavailable in MexicoLubiprostone is a drug that activates the type 2 chlorine channels, increasing gastrointestinal secretion and motility. Even though there is greater experience in chronic idiopathic constipation, its efficacy in IBS-C is supported by 3 studies. The first 2 show that the drug was superior to placebo in overall symptom improvement, pain, bloating, stool form, and frequency of bowel movements after follow-up at 1 and 2 months.116 In the third study, the same cohort was treated for 36 weeks and followed for 52 weeks and, again, lubiprostone was associated with a greater frequency of spontaneous bowel movements and lower scores for pain and abdominal distension, compared with placebo.117 This drug is not currently available in Mexico.
GRADE level of evidence and strength of recommendation: B1, strong, in favor of the intervention.
Level of agreement: In complete agreement 96%.
In agreement with minor reservations: 4%.
There is insufficient evidence for recommending the use of mesalazine in the treatment of IBSMesalazine (or mesalamine) is a topical salicylate with an unknown action mechanism. It has been shown to modulate proinflammatory cytokine production, reduce NF-kappa-b transcriptional activity and tumor necrosis factor activation, and inhibit prostaglandin and leukotriene synthesis.118 The use of mesalazine may be associated with improvement in low-grade inflammation of the colonic mucosa and changes in the gut microbiota profile.119 However, these anti-inflammatory changes have not resulted in clinical improvement in patients with IBS. A recent pilot study showed no significant changes compared with placebo in symptoms that included pain, bloating, or bowel habit, nor in overall improvement or quality of life in patients with PI-IBS.120
GRADE level of evidence and strength of recommendation: C2, weak, against the intervention.
Level of agreement: In complete agreement 96%.
In agreement with minor reservations: 4%.
In general, antidepressants, including the tricyclic antidepressants and selective serotonin reuptake inhibitors, have been shown to be effective in overall improvement of IBS symptomsAntidepressants have been evaluated for IBS treatment due to their visceral analgesic properties and have been shown to be useful mainly in the treatment of abdominal pain and overall symptom imporvement.121 In a recent meta-analysis,98 antidepressants in general were superior to placebo for abdominal pain improvement (p=0.03, NNT=5), overall improvement (p<0.001, NNT=4), and symptom score (p=0.001, NNT=4). Two analyses by Ford, published in 2009 and 2014, confirmed the same findings.122,123 The tricyclic antidepressants (e.g. amitriptyline [available in Mexico], imipramine, desipramine), as well as the selective serotonin reuptake inhibitors (e.g. sertraline, citalopram, paroxetine, fluoxetine [all available in Mexico]), have shown this benefit: both groups are superior to placebo in overall symptom improvement, but the tricyclic antidepressants are superior to the selective serotonin reuptake inhibitors in pain improvement.98 Their benefit is obtained 4-6 weeks after treatment and can be limited by side effects.121 The tricyclic antidepressants are associated with constipation, somnolence, and dry mouth, whereas the selective serotonin reuptake inhibitors are associated with nausea and diarrhea, making antidepressant selection dependent on IBS subgroup, side effects, and patient tolerance. The selective norepinephrine reuptake inhibitors, such as duloxetine and venlafaxine, have been used for pain in neuropathy and fibromyalgia, but there are no studies in IBS.124
GRADE level of evidence and strength of recommendation: A1, strong, in favor of the intervention.
Level of agreement: In complete agreement 96%.
In agreement with minor reservations: 4%.
Rifaximin produces overall improvement in non-constipation IBS, including abdominal distension and perception of bloating, as well as loose/watery stool consistency, with no significant adverse effectsDue to the possible abnormalities in the gut microbiota in patients with IBS, treatment with poorly absorbable antibiotics and luminal antibiotics has the potential to modulate the bacterial composition of the gastrointestinal tract and alter the natural history of the disease in the short term. Rifaximin is a broad-spectrum, synthetic, non-absorbable antibiotic that has been shown to be useful in small intestinal bacterial overgrowth and has recently been evaluated in the management of IBS without constipation. Two randomized, double-blind, and placebo-controlled studies, called TARGET 1 and TARGET 2, analyzed a total of 1,260 patients that were given 550mg of rifaximin three times a day for 2 weeks, with follow-up at 10 weeks.125,126 In both studies, rifaximin was significantly superior to placebo, in overall symptom improvement (p<0.001), in the perception of bloating (p<0.001), and in stool consistency improvement, with a NNT of 10.2 and no significant adverse effects.125–127 In addition, approximately half of the patients with IBS had a negative lactulose breath test after rifaximin treatment, which was associated with a decrease in IBS symptom intensity.27 Effectiveness in patients that require retreatment with rifaximin has been shown to be similar to that of the first treatment, even in evaluations of two retreatments, and with a mean effect duration of 4 months.128
GRADE level of evidence and strength of recommendation: A1, strong, for intervention.
Level of agreement: In complete agreement 84%.
In agreement with minor reservations: 16%.
Some probiotics or their combinations have been efficacious as IBS treatment in overall symptom improvement, as well as in relief from abdominal pain and bloating. However, it is not known which species or strains are the effective onesChanges in the gut microbiota of patients with IBS have been described. The differences in the composition of the microbiota are significant in patients with IBS compared with controls.129 From 23 randomized and controlled studies with different probiotics compared with placebo, it is concluded that probiotics significantly reduce the risk for symptom persistence. Even though the meta-analyses suggest that probiotics have beneficial effects on the grading of overall symptoms, as well as on abdominal pain, bloating, and flatulence, we still do not know which species or individual strains are the most beneficial.130
GRADE level of evidence and strength of recommendation: B2, weak, in favor of the intervention.
Level of agreement: In complete agreement 92%.
In agreement with minor reservations: 4%.
In agreement with major reservations: 4%.
There is insufficient evidence for recommending the use of prebiotics and synbiotics in IBSThe randomized and placebo-controlled studies for evaluating the effectiveness of prebiotics and the studies on synbiotics have heterogeneous results and therefore their efficacy cannot be affirmed.130
GRADE level of evidence and strength of recommendation: D2, strong, against the intervention.
Level of agreement: In complete agreement 100%.
Fecal microbiota transplantation in IBS has been investigated in non-controlled studies with results showing symptom improvement. The use of fecal microbiota transplantation in IBS should be restricted to research protocolsFecal microbiota transplantation has progressed dramatically in the last few years, together with the developing knowledge of the gastrointestinal microbiota. Differences have been shown in the microbiota of individuals with IBS compared with healthy subjects. However, we do not know if the administration of the fecal microbiota from healthy individuals can revert symptoms in the long term.131 Non-controlled open studies on the use of fecal microbiota transplantation for IBS have shown cure or symptom improvement in 52 to 69% of cases and thus have been reviewed.132 Fecal microbiota transplantation has potential risks, such as communicable disease transmission, and there are reports of the appearance of autoimmune diseases in the long-term follow-up after transplantation.133 Therefore, fecal microbiota transplantation for the treatment of IBS should only be carried out under strict research protocol.
GRADE level of evidence and strength of recommendation: D2, weak, for intervention.
Level of agreement: In complete agreement 96%.
In agreement with minor reservations: 4%.
In patients with IBS, the application of true acupuncture has shown no significant differences compared with the application of sham acupuncture, in relation to symptom severity or quality of lifeComplementary and alternative medicine is used by an important number of patients with functional gastrointestinal disorders, corresponding to 51% in patients with IBS.134 Due to the safety of acupuncture and the fact that we do not have highly effective treatments for IBS improvement, its evaluation is relevant.135 There is not a clear definition as to the meaning of placebo in acupuncture. It usually involves placing the needles in zones that are not considered the correct ones for acupuncture or not penetrating the skin with them (sham acupuncture). Studies controlled with sham acupuncture have shown no benefits in relation to IBS symptoms.135
GRADE level of evidence and strength of recommendation: D2, weak, against the intervention.
Level of agreement: In complete agreement 87%.
In agreement with minor reservations: 4%.
In disagreement with minor reservations: 4%.
In disagreement with major reservations: 4%.
There is not enough evidence to recommend moxibustion for the treatment of IBSMoxibustion is a technique associated with acupuncture that uses the burning of herbal preparations on acupuncture points. The systematic review and meta-analysis of randomized and placebo-controlled studies show inconsistent results and a high risk for bias, thus its usefulness cannot be affirmed.136
GRADE level of evidence and strength of recommendation: D2, weak, against the intervention.
Level of agreement: In complete agreement 92%.
In agreement with minor reservations: 4%.
In complete disagreement: 4%.
There is not enough evidence to conclude whether homeopathy has any beneficial effects in IBS treatmentThree randomized and controlled studies conducted more than 25 years ago showed very poor evidence due to the low quality of the reports, the high or unknown risk for bias, a short-term follow-up, and sparse data. Therefore there is no evidence for affirming or ruling out the usefulness of homeopathy in the treatment of IBS.137
GRADE level of evidence and strength of recommendation: D2, weak, against the intervention.
Level of agreement: In complete agreement 92%.
In agreement with minor reservations: 4%.
In complete disagreement: 4%.
Cognitive behavioral therapy, multicomponent psychological therapy, and dynamic psychotherapy administered by qualified personnel have been shown to improve IBS symptomsPatients with IBS present with higher levels of psychological comorbidity compared with healthy controls. Therefore, psychological therapies have been proposed as alternatives for IBS treatment.138,139 Even though there are randomized and controlled studies, the large majority are biased due to the impossibility of conducting blind studies.122
GRADE level of evidence and strength of recommendation: C2, weak, in favor of the intervention.
Level of agreement: In complete agreement 100%.
Relaxation therapy, self-administered or minimum contact behavioral therapy, cognitive behavioral therapy administered online, stress management therapy, multicomponent psychological therapy by telephone, and mindfulness therapy have not been shown to be effective in improving IBS symptomsThe application of numerous psychological treatments in IBS has been reported. Nevertheless, the meta-analyses of randomized and placebo-controlled studies have shown important heterogeneity in the results, the number of patients included in the studies is small, and it is impossible to conduct blind studies due to the nature of the treatment. In addition, these studies have the disadvantage that they do not report adverse effects, which potentially exist in any treatment.122,140
GRADE level of evidence and strength of recommendation: D2, weak, against the intervention.
Level of agreement: In complete agreement 92%.
In agreement with minor reservations: 4%.
In complete disagreement: 4%.
Hypnotherapy performed by qualified personnel is efficacious in IBS treatmentThe meta-analysis of 5 randomized studies showed the effectiveness of hypnotherapy in the improvement of IBS symptoms compared with controls.140
GRADE level of evidence and strength of recommendation: B2, weak, in favor of the intervention.
Level of agreement: In complete agreement 92%.
In agreement with minor reservations: 4%.
In agreement with major reservations: 4%.
ConclusionIBS is the most frequently diagnosed gastrointestinal disorder in daily practice and therefore it is of the utmost importance for the physician to be up-to-date in regard to all the changes and advances that have been made in the knowledge of this disease in the last few years. We present herein a consensus review of the most relevant progress in the understanding of this disorder, updating and complementing the 2009 Clinical Guidelines on the Diagnosis and Treatment of Irritable Bowel Syndrome of the Asociación Mexicana de Gastroenterología.
Financial disclosureFinancial support was received from Laboratorios Alfa Wassermann to partially cover the costs of the in-person vote meeting (transportation and accommodations). The authors received no remuneration for their participation.
Conflict of interestRamón Carmona-Sánchez is a Member of the Advisory Board of Mayoly-Spindler and a Speaker for Mayoly-Spindler and Asofarma.
María Eugenia Icaza-Chávez a Member of the Advisory Board of Mayoly-Spindler and a Speaker for Mayoly-Spindler and Asofarma.
María Victoria Bielsa-Fernández is a Speaker for Alfa Wassermann and Almirall and a Member of the Advisory Board of Alfa Wassermann.
Octavio Gómez-Escudero is a Member of the Advisory Boeard of Laboratorios Almirall and a Speaker for Laboratorios Takeda, Astra-Zéneca, Almirall, Asofarma, and Alfa Wassermann.
Francisco Bosques-Padilla is a member of the Advisory Board of Laboratorios Takeda and a Speaker for Laboratorios Abvie, Janssen, and Bristol-Myers Squibb México.
Enrique Coss-Adame is a Speaker for Laboratorios Takeda de México and has been a Consultant for and collaborates Laboratorios Asofarma de México.
Francisco Huerta-Iga is a Speaker for Takeda and Asofarma.
Aurelio López-Colombo is a Speaker for Laboratorios Takeda de México.
Alejandra Noble-Lugo is a Speaker for Laboratorios Takeda de México.
José Ramón Nogueira-de Rojas is a Speaker for AstraZeneca and HealthPro and participates in a clinical research project with Laboratorios Senosiain.
José María Remes-Troche is a Member of the Advisory Board of Takeda Pharmaceuticals, Alfa Wassermann and Almirall and a Speaker for Takeda, Asofarma, Alfa Wassermann, Almirall, and Astra-Zeneca.
Max J. Schmulson has been a Speaker for Alfa Wassermann and Takeda Mexico. He has received financial support and carried out research projects for Alfa Wassermann and Takeda México. He has been a Consultant and Member of the Advisory Boards of Alfa Wassermann, Commonwealth Laboratories Inc., and Senosiain.
José Luis Tamayo-de la Cuesta is a Speaker and External Consultant for Alfa Wassermann, Asofarma, Malloly Spindler and Takeda de México.
Miguel A. Valdovinos is a speaker for Laboratorios Takeda de México and Laboratorios Ferrer. He is a Member of the Advisory Board of Mayoly-Spindler. He is receiving financial support for a research project from Laboratorios Ferrer.
Francisco Esquivel-Ayanegui, Ángel Ricardo Flores-Rendón, Marina Alejandra González-Martínez, Tomás Héctor Méndez-Gutiérrez, Ricardo Huberto Raña-Garibay, Federico Roesch-Dietlen, Julio César Soto-Pérez. Luis F. Uscanga, Joaquín Valerio-Ureña, and Mónica R. Zavala-Solares declare that they have no conflict of interest.
The authors wish to thank Dr. Francisco Javier Bosques-Padilla, President of the Asociación Mexicana de Gastroenterología and the administrative personnel of the Association for the favorable conditions provided us during the preparation of this consensus.
Please cite this article as: Carmona-Sánchez R, Icaza-Chávez ME, Bielsa-Fernández MV, Gómez-Escudero O, Bosques-Padilla F, Coss-Adame E, et al. Consenso mexicano sobre el síndrome de intestino irritable. Revista de Gastroenterología de México. 2016;82:149–167.