Significant advances have been made in the knowledge and understanding of the epidemiology, pathophysiology, diagnosis, and treatment of chronic constipation, since the publication of the 2011 guidelines on chronic constipation diagnosis and treatment in Mexico from the Asociación Mexicana de Gastroenterología.
AimsTo present a consensus review of the current state of knowledge about chronic constipation, providing updated information and integrating the new scientific evidence.
MethodsThree general coordinators reviewed the literature published within the time frame of January 2011 and January 2017. From that information, 62 initial statements were formulated and then sent to 12 national experts for their revision. The statements were voted upon, using the Delphi system in 3 voting rounds (2 electronic and one face-to-face). The statements were classified through the GRADE system and those that reached agreement >75% were included in the consensus.
Results and conclusionsThe present consensus is made up of 42 final statements that provide updated knowledge, supplementing the information that had not been included in the previous guidelines. The strength of recommendation and quality (level) of evidence were established for each statement. The current definitions of chronic constipation, functional constipation, and opioid-induced constipation are given, and diagnostic strategies based on the available diagnostic methods are described. The consensus treatment recommendations were established from evidence on the roles of diet and exercise, fiber, laxatives, new drugs (such as prucalopride, lubiprostone, linaclotide, plecanatide), biofeedback therapy, and surgery.
Desde la publicación de las guías de diagnóstico y tratamiento del estreñimiento crónico (EC) en México de la Asociación Mexicana de Gastroenterología en el 2011 se han producido avances significativos en el conocimiento de la epidemiología, fisiopatología, diagnóstico y tratamiento del EC.
ObjetivosPresentar una revisión consensuada del estado actual de los conocimientos sobre el EC que actualice e integre las nuevas evidencias científicas.
MétodosTres coordinadores generales realizaron una revisión de la bibliografía de enero del 2011 a enero del 2017. Con base en esta, se elaboraron 62 enunciados los cuales fueron enviados para su revisión a 12 expertos nacionales. Los enunciados fueron votados utilizando el sistema Delphi en 3 rondas de votaciones (2 electrónicas y una presencial) y calificados de acuerdo con el sistema GRADE. Aquellos que alcanzaron un acuerdo >75% fueron considerados en este consenso.
Resultados y conclusionesEl presente consenso consta de un total de 42 enunciados que actualizan la información sobre el EC y complementan la información que no había sido incluida en las guías previas. Para cada enunciado se presenta la fuerza de la recomendación y el grado de la evidencia. Se provee de una definición actualizada del EC y del EC funcional (EF), y del estreñimiento inducido por opioides (EIO). Se mencionan las estrategias diagnósticas con base en los métodos diagnósticos disponibles, y se emiten recomendaciones con respecto al tratamiento que incluye la evidencia del papel de la dieta y el ejercicio, la fibra, los laxantes, los nuevos fármacos (como prucaloprida, lubiprostona, linaclotida, plecanitida), la terapia de biorretroalimentación y la cirugía.
Chronic constipation (CC) is a very frequent condition that affects the general population and impacts the quality of life of those that suffer from it. In 2010, the Asociación Mexicana de Gastroenterología (AMG) brought together a group of experts that formulated the Guidelines for the Diagnosis and Treatment of Chronic Constipation in Mexico”, whose results were published in the Revista de Gastroenterología de México in 2011.1 Since then, national and international groups have produced relevant information on epidemiology, pathophysiology, diagnostic tests, quality of life, and on the efficacy of new drugs, some of which are now available in Mexico, or will be in the near future. Therefore, in January 2017, the Asociación Mexicana de Gastroenterología summoned together a group of experts to carry out a review of the advances made in relation to different aspects of CC, evaluating the quality of evidence and establishing useful recommendations for the medical community.
The aim of the Mexican consensus on CC was to provide a document on the epidemiology, diagnosis, and treatment of CC in adults. The recommendations are based on a thorough review of the literature and the consensus opinion of specialists.
MethodsThe consensus was developed using the Delphi process.2 The main steps of that process were: a) selecting the consensus group; b) identifying the areas of clinical importance; c) the systematic review of the literature to identify the evidence supporting the statements; d) the formulation of the statements; e) the anonymous electronic voting rounds with the discussion and analysis of the results and the correction and modification of the statements.
Three general coordinators of the consensus were designated (ALC, ECA, and JMRT) and 12 experts on the subject were invited to participate in the formulation of the document. The general coordinators performed an extensive search of the following databases: CENTRAL (The Cochrane Central Register of Controlled Trials), MEDLINE (Puede), EMBASE (Ovad), LILACS, CINAHL, Biomed Central, and the World Health Organization International Clinical Trials Registry Platform (ICTRP). The search covered the period of January 1, 2010 to December 31, 2016. The search criteria included the terms “constipation”, “functional constipation”, combined with the following terms: “epidemiology”, “incidence”, “prevalence”, “pathophysiology”, “inflammation”, “microbiota”, “diagnosis”, “differential diagnosis”, “treatment”, “dyssynergia”, “therapy”, “management”, “review”, “guidelines”, and “meta-analysis”, together with the equivalent terms in Spanish. The entire bibliography was available to the members of the consensus to be reviewed at any moment throughout the process.
The general coordinators then formulated 62 statements that were put to a first electronic anonymous vote (January 2-11, 2017) to evaluate the writing and content. The consensus participants cast their votes with the following responses: a) in complete agreement, b) in partial agreement, c) uncertain, d) in partial disagreement; and e) in complete disagreement.
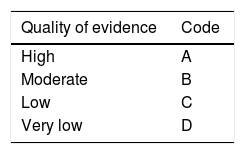
After the first vote, the coordinators carried out the modifications corresponding to each statement according to the results and comments of the participants. The statements with > 75% complete agreement remained and those with > 75% complete disagreement were eliminated. The statements with < 75% complete agreement and < 75% complete disagreement were reviewed and rewritten. In addition, a grade of recommendation was established for each new statement, with its corresponding quality of evidence to support the recommendation. This was done using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system.3 In the GRADE system, the quality of evidence is not rated solely on study design or methodology, but in relation to a clearly posed question about a clearly formulated outcome measure.4 Thus, evidence can be high, moderate, low, or very low. In addition, the GRADE system establishes the strength of the recommendations as strong or weak, in favor of or against an intervention or statement. It employs a code for the quality of evidence, using capital letters followed by a number to indicate the strength of recommendation for or against the intervention or statement. Table 1 shows the GRADE system codes.
Classification of the quality of evidence and strength of recommendation in accordance with the GRADE system.
| Quality of evidence | Code |
|---|---|
| High | A |
| Moderate | B |
| Low | C |
| Very low | D |
| Strength of recommendation | Code |
|---|---|
| Strong, in favor of the intervention | 1 |
| Weak, in favor of the intervention | 2 |
| Weak, against the intervention | 2 |
| Strong, against the intervention | 1 |
Source: version modified by Oñate-Ocaña and Ochoa-Carrillo.4
The statements reviewed and evaluated through the GRADE system underwent a second electronic anonymous vote (from January 30 to February 6, 2017) and the results were presented on March 9, 2017, at the face-to-face meeting in Boca del Río, Veracruz. At that meeting, the statements that had > 75% agreement were ratified. The statements that did not reach 75% agreement in the previous votes were discussed in an effort to either reach agreement or be eliminated, after which the third voting round was carried out.
Once all the statements making up the consensus were decided upon, the coordinators formulated the final manuscript, which was reviewed and approved by all the members of the consensus.
ResultsThe coordinators initially formulated 62 statements. In the first electronic voting round, 12 statements (27%) were eliminated because they did not reach consensus. Forty-five statements were included in the second voting round. According to the results of the second voting round, to be presented at the face-to-face meeting, one statement (2%) was to be eliminated, 24 (54%) were presented for ratification, and 20 (44%) to be voted on again. Fourteen of the 15 members of the consensus (93%) attended the final face-to-face vote. Of the 45 statements included in that third vote, 2 were eliminated, 2 were fused with other statements, and one new statement was added, for a total of 42 statements. The final statements resulting from that vote are presented below.
Definition1. Chronic constipation (CC) is defined as a decrease in the frequency of defecations, an increase in stool consistency, and difficulty in the passage of stools. The progression time of those characteristics should be at least 3 months.
Quality of evidence and strength of recommendation: B1 strong, in favor of the statement (in complete agreement: 100%).
The term “constipation” can have different meanings and vary among individuals, given that it mainly depends on how the individual perceives his or her bowel habit. However, the majority of patients with constipation refer to one or more of the following symptoms: infrequent defecations (< 3 per week) with hard or lumpy stools that are difficult to expulse and/or symptoms that include the sensation of incomplete evacuation, the sensation of anal blockage at the time of evacuation, or the aid of manual digital maneuvers to achieve evacuation.5 In a Mexican study conducted on 1,041 open population subjects, the symptom that best defined constipation was the sensation of pushing and/or straining to defecate (47%), followed by hard or lumpy stools (27%), a lower frequency of defecations than desired (8%), scant quantity of stools (8%), and the sensation of incomplete evacuation (5%).6
With respect to progression time, different epidemiologic studies, consensuses, and international guidelines (including the Latin American Consensus on CC)7–9 all state that those symptoms must be present for at least 3 months for the condition to be considered chronic.
Epidemiology2. The prevalence of CC varies from 2.4 to 22.3%. A meta-analysis of Mexican studies showed a prevalence of 14.4%. There are no data on incidence in Mexico.
Quality of evidence and strength of recommendation: C1 strong, in favor of the statement (in complete agreement:100%).
If patient perception of CC prevalence is taken into account, it is estimated to affect between 1.9 and 27.2% of the general population.10,11 In Mexico, several studies have shown that CC prevalence varies from 2.4 to 22.3%.6,12–14 In the previous guidelines for the treatment of CC in Mexico, that information was evaluated through a meta-analysis and CC prevalence in the Mexican population was estimated at 14.4% (95%CI: 12.6 to 16.6).1
Few studies have evaluated accumulated incidence, but it is calculated to be approximately 17% over a 12-year period.15 There are no studies on incidence in Mexico.
3. From the clinical perspective, CC is considered secondary when it is the consequence of metabolic or neurologic alterations, structural lesions, or medications. When other causes have been ruled out, constipation is considered primary, idiopathic, or functional (FC).
Quality and strength of evidence: B1 strong, in favor of the statement (in complete agreement: 93%; in partial agreement: 7%).
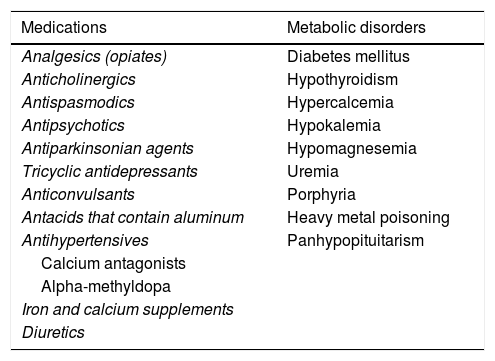
Symptoms of CC can often be manifestations of other diseases or be associated with medication use.1 That is known as secondary CC.1,6,9Table 2 lists a series of diseases and medications that can cause secondary CC.
Secondary causes of constipation.
| Medications | Metabolic disorders |
|---|---|
| Analgesics (opiates) | Diabetes mellitus |
| Anticholinergics | Hypothyroidism |
| Antispasmodics | Hypercalcemia |
| Antipsychotics | Hypokalemia |
| Antiparkinsonian agents | Hypomagnesemia |
| Tricyclic antidepressants | Uremia |
| Anticonvulsants | Porphyria |
| Antacids that contain aluminum | Heavy metal poisoning |
| Antihypertensives | Panhypopituitarism |
| Calcium antagonists | |
| Alpha-methyldopa | |
| Iron and calcium supplements | |
| Diuretics |
| Anorectal and colonic disorders | Psychiatric disorders |
|---|---|
| Hemorrhoidal disease | Eating disorders (bulimia-anorexia) |
| Anal fissure | Depression |
| Diverticulitis | Somatization disorders |
| Radiation proctitis | |
| Malignant neoplasias |
| Neurologic disorders | Others |
|---|---|
| Multiple sclerosis | Myopathies |
| Spinal cord injuries | Amyloidosis |
| Parkinson's disease | Scleroderma |
| Cerebrovascular disease | Cognitive disorders |
| Prolonged immobilization |
The chronic use of opioids has recently been established to cause, exacerbate, or be associated with CC.8 It is estimated that 5% of adults in the United States are currently under treatment with opiates, longer than 3 months, for causes unrelated to cancer, and between 40 and 90% of those patients experience symptoms of CC.16,17 The Rome IV group of experts believe opioid-induced effects on the gastrointestinal tract should not be regarded as “new” functional disorders, but rather as side effects, and they now include a new independent disorder within the intestinal disorders called opioid-induced constipation (OIC). That term describes patients whose constipation symptoms appear or worsen when opioid use is begun, changed, or increased. That entity has a growing epidemiologic behavior in countries such as the United States and is susceptible to treatment with specific medications (see the treatment section).
If a secondary cause is ruled out and there are no alarm signs, constipation is considered primary, idiopathic, or FC.
4. From the pathophysiologic perspective, FC can be classified as slow transit constipation, dyssynergic defecation (DD), and normal transit constipation.
Quality and strength of evidence: B1 strong, in favor of the statement (in complete agreement: 100%).
FC should essentially be considered a colorectal motility disorder, or a disorder of the mechanisms associated with defecation. Its pathophysiology can be multifactorial.8
Constipation can currently be classified into 3 subgroups: 1) constipation with slow colonic transit, 2) constipation associated with DD, and 3) constipation with normal colonic transit (FC).18,19 It is important to mention that there can also be overlap between the subgroups.
The pathophysiologic alterations described in slow transit constipation include: a reduced number of propagated high amplitude colonic contractions,20,21 an uncoordinated increase in the motor activity of the distal colon,22 alterations in the number of neurons in the myenteric plexus that express substance P (an excitatory neurotransmitter),23 a reduced production of inhibitory neurotransmitters, such as nitric oxide and vasoactive intestinal peptide,24 and a reduced number of interstitial cells of Cajal.25
DD, pelvic floor dysfunction or dyssynergia, or constipation due to outlet obstruction are the result of motor and sensory alterations in defecation-related mechanisms.26 The failure to evacuate is due to the incapacity to adequately coordinate the muscles of the abdomen and pelvic floor during defecation. Other contributing factors are the presence of anal pain secondary to fissures in the perianal region, intussusception, rectocele, excessive pelvic floor descent, and a history of physical and/or sexual abuse.27
Alterations of rectal sensitivity (especially hyposensitivity)28 or alterations in the perception of the frequency of defecations and stool consistency have been associated with normal transit in patients with FC. If the patient has abdominal pain related to defecation (improvement or worsening), he or she can be classified as having irritable bowel syndrome with constipation (IBS-C).8 Even though IBS-C and FC are commonly considered different disorders, it is important to underline that according to the new Rome IV criteria, those 2 entities can be same disease, manifesting on a continuum.
5. Female sex, aging, low liquid and fiber intake, and low physical activity are risk factors for FC.
Quality and strength of evidence: Female sex and aging: B1 strong, in favor of the statement. Low liquid and fiber intake and low physical activity: C2 weak, in favor of the statement (in complete agreement: 80%; in partial agreement: 20%).
In practically all the epidemiologic studies with respect to FC, female sex has been shown to be a predisposing factor for the condition. For example, in a systematic review, Queiroz and de Gouveia29 demonstrated that female sex conferred 2 to 3 times more risk of having FC, than male sex, in all 11 studies. Numerous factors can explain the greater prevalence of constipation in women and they include hormonal factors, a different pelvic floor anatomy, pregnancy, a greater prevalence of urogenital prolapse, and obstetric trauma, among others.30
The second most frequent factor associated with constipation is aging.31 According to a study conducted in the United States, 26% of women and 16% of men above 65 years of age present with constipation, but it can increase to 34 and 26%, respectively, after 84 years of age.32 Up to 50% of subjects that live in senior housing settings have constipation and 74% may utilize laxatives on a daily basis.33 The mechanisms associated with CC and aging include: a reduced number of myenteric plexus neurons, increased collagen deposit in the left colon that alters its distensibility and sensitivity, pelvic floor muscle atrophy, degenerative neuropathy, and greater use of medications that produce secondary constipation.31
Some epidemiologic studies have shown that low fiber consumption (< 10g per day) and low water intake (< 1,882ml per day) are factors associated with constipation.34 Even though evidence is more limited and there are controversial results, some studies have shown that constipation is associated with a sedentary lifestyle (OR 1.25, 95% CI: 1.17-1.34) and insufficient physical activity (OR 1.26, 95% CI: 1.16-1.36).35–37
6. FC has been shown to deteriorate the quality of life of those that present with it.
Quality and strength of evidence: B1 strong, in favor of the statement (in complete agreement: 93%; in partial agreement: 7%).
One of the aspects that most affects subjects that have FC is quality of life, in all its dimensions, when compared with that of healthy individuals.38,39 FC has been shown to affect the general health status of 80% of patients, it affects diet in 55%, interferes with appetite in 41%, 35% of patients have sleep alterations, and it affects work performance in 32% of cases.40
In Mexico, a study conducted by Ruiz-López and Coss-Adame39 on patients with IBS-C and FC, utilizing the PAC-QOL and SF-36 questionnaires, demonstrated a lower quality of life in Mexican patients with symptoms of constipation, especially in those suffering from IBS-C.
7. Healthcare costs related to FC are high. There are no data on said costs in Mexico.
Quality and strength of evidence: B1 strong, in favor of the statement (in complete agreement: 86%; in partial agreement: 14%).
Around 30% of the subjects that present with CC are estimated to seek out medical attention at some time.4 In the United States, for example, CC is responsible for more than 2.5 million medical visits and 92,000 hospitalizations per year.42 If a laxative was prescribed at some point to 85% of those patients, the estimated costs for 1994 were 840 million dollars.42 In a recent systematic review, direct costs in the United Sates were estimated to vary between $1,912 USD and $7,522 USD yearly per patient.4 In Europe, the medical care costs in the first year of treating CC vary from €310 to €845.44 Indirect costs are difficult to estimate, with few studies conducted on them. No studies on the direct or indirect costs related to FC have been carried out in Mexico.
Diagnosis8. The diagnosis of FC is based on clinical criteria (Rome IV) and complementary tests, when necessary.
Quality of evidence and strength of recommendation: B1 strong, in favor of the statement (in complete agreement: 79%; in partial agreement: 21%).
FC is a clinical syndrome that presents different symptoms that vary among individuals, depending on factors such as geographic location, diet, and physical activity. Clinical criteria are very useful for diagnosing FC and they help standardize patient populations for their inclusion in clinical trials.45 The current version of the Rome IV criteria is the most widely used for diagnosing FC.8
There are questionnaires for evaluating FC, but it is important to underline that none of them can identify constipation subtypes. That identification requires adequate physical examination and additional physiologic studies (see further ahead).46,47
There is no minimum study panel for diagnosing FC. Thyroid function tests, calcium tests, and fecal occult blood tests should be performed on an individual basis, when the clinician deems them necessary, given that their diagnostic yield has not been prospectively evaluated.48
9. Pictograms of stool shapes (Bristol Stool Scale [BSS]) are useful instruments for classifying bowel habit type and they correlate well with colonic transit velocity.
Quality of evidence and strength of recommendation: A1 strong, in favor of the recommendation (in complete agreement: 86%; in partial agreement: 14%).
Pictograms of stool shapes or the BSS have been considered a reliable instrument for evaluating colonic transit, in comparison with symptoms.49 In the beginning, the main function of the BSS was to provide both clinician and patient with a dependable tool for clarifying the type of defecation pattern (e.g., normal vs diarrhea) and correlating it with colonic transit.50,51 It was then evaluated for characterizing stool patterns, and was shown to be a reproducible and reliable method.52 As a result, the BSS was proposed as a useful instrument for classifying IBS subtypes (diarrhea, mixed, and constipation).8 Subsequent studies have correlated the BSS with radio-opaque marker and wireless motility capsule measurements of colonic transit velocity and found the correlation to be moderate with respect to the former (r=-0.45) and the latter (r=-0.61).53
The BSS is currently an easy method to use and improves understanding between the physician and the patient. It is also used in clinical trials for evaluating the effectiveness of therapeutic interventions. The ideal use of the scale is when the patient is not taking any medication (laxative or antidiarrheal agent) that potentially alters bowel transit.
10. Anorectal testing and digital rectal examination are indispensable evaluations in FC.
Quality of evidence and strength of recommendation: B1 strong, in favor of the recommendation (in complete agreement: 100%).
Evaluation of the anorectal region is indispensable for the initial approach in diagnosing the patient with CC and should routinely be included in all cases. Surveys have shown that digital rectal examination is not routinely taught to undergraduate students, and even specialists feel they lack the experience to adequately perform the maneuver.54,55
Revision of the anorectal region should be systematic, beginning with adequate inspection in search of scarring, dermatologic lesions, and external hemorrhoidal bunches. The complete circumference of the anal folds should be adequately exposed to look for fissures. The maneuver should be dynamic, asking the patient to voluntarily contract the sphincter, which can reveal an inability to contract or the existence of a half-open anus. The patient is then asked to push (defecation maneuver), making it possible to observe the perineal descent (normal being 1-3cm), and thus rule out hemorrhoidal prolapse or rectal prolapse, among others.56 A sensory evaluation of the anal region is performed, utilizing a cotton Q-tip that is gently moved along all the quadrants of the anus, which triggers a contraction reflex. If that does not occur, significant radicular lesion can be suspected.
Digital rectal examination can evaluate resting pressure, and in addition can assess an increase in pressure, as well as anal sphincter and puborectal muscle relaxation, through the dynamic maneuvers of contraction and the defecation push. Studies have compared digital rectal examination performed by an expert with anorectal manometry and determined 86% correct detection for resting pressure, 88% for adequate contraction, and 82% for alteration of pelvic floor relaxation. [1] Digital rectal examination has 75% sensitivity and 87% specificity, as well as a 97% positive predictive value for diagnosing dyssynergic defecation.57,58 Attempts have been made to standardize digital rectal examination, designing scales for its evaluation. The digital rectal examination scoring system (DRESS) is based on a visual analogue scale of 0 to 5 points that adequately correlates with resting pressure and the contraction maneuver obtained through anorectal manometry.59
11. Colonoscopic evaluation in patients with symptoms of FC is recommended when there are alarm symptoms/signs, in patients above the age of 50 years, or in patients with a family history of cancer.
Quality of evidence and strength of recommendation: B1 strong, in favor of the recommendation (in complete agreement: 100%).
It has been previously reported that the cost of healthcare for patients with CC is high and diagnostic tests, such as endoscopic studies, are among the high-cost factors.60 Colonoscopy is a widely used diagnostic method in patients with FC that have special characteristics, particularly to rule out colon cancer. Epidemiologic studies have shown that constipation is a frequent symptom in patients with colon cancer, which gives rise to recommending that diagnostic strategy in selected groups of patients.61,62 Based on studies analyzing the diagnostic yield of colonoscopy in patients with CC, the neoplasia detection rate has been shown to be similar to that of asymptomatic subjects above 50 years of age that undergo colon cancer screening.63 When there are no alarm symptoms, the diagnostic yield of colonoscopy for colon cancer detection is low.64,65
Therefore, the consensus recommends that colonoscopy be performed based on the presence of red flags other than constipation, such as in at-risk populations of patients over 50 years of age at the time of constipation onset or those with a family history of colon cancer. Colonoscopy is not recommended in subjects with constipation that do not have other alarm symptoms.
12. Physiologic studies (e.g., anorectal manometry, balloon expulsion, defecography, colonic transit) are recommended in patients with FC that have persistent symptoms, despite receiving medical treatment.
Quality of evidence and strength of recommendation: B1 strong, in favor of the recommendation (in complete agreement: 100%).
There is no consensus on the definition of FC patients with treatment failure. Many patients have symptom persistence, most likely due to a lack of treatment adherence. For example, treatment adherence after one month has been reported at only 38% in the pediatric population, and at 30% after 6 months.66 Thus, before carrying out diagnostic tests, the first step is to evaluate the type of treatment and its level of adherence.
Once secondary causes have been ruled out and there has been no response to a fiber and laxative challenge, physiologic studies are proposed.67,68 There is no consensus as to which should be the initial study to evaluate patients with FC, and it may depend on test availability and the experience in performing them.26–69 The American Gastroenterological Association recommends that anorectal manometry be the first study performed in patients with FC.68
13. The balloon expulsion test is useful in the initial approach to patients suspected of having dyssynergic defecation.
Quality of evidence and strength of recommendation: B1 strong, in favor of the recommendation (in complete agreement: 93%; in partial agreement: 7%).
The balloon expulsion test is a rapid, reproducible, and economic test that aids in the diagnosis of constipation and DD. Depending on the technique utilized, abnormality is determined if the balloon is not expulsed within 1-5min.70 That test has been described to have 87.5% sensitivity and 89% specificity for DD, with a negative predictive value of 97%.71 In the largest validation study conducted at present (286 patients and 40 controls), Chiarioni et al.72 showed that the balloon expulsion test has a high correlation with anorectal manometry and electromyography, and that the upper limit of normal should be 2min. Those authors recommend using a 16-Fr Foley catheter and filling it with 50ml of water at room temperature and having the patient defecate in a seated position. However, variations in the technique have been described, in relation to position (left lateral decubitus or seated), with or without traction, and different quantities of air or insufflated water. At present, there is no consensus on how to perform that test.73
In addition, it should be understood that asymptomatic subjects may not expel the balloon, whereas some patients with DD may do so.46,74
Given that the balloon expulsion test is widely available, low-cost, and easy to perform, the present consensus recommends its performance, together with other tests, for evaluating anorectal function when DD is suspected.
14. Anorectal manometry is the best diagnostic method for confirming the suspicion of DD, because pelvic floor relaxation alterations or a lack of rectal propulsion can be determined.
Quality of evidence and strength of recommendation: B1 strong, in favor of the recommendation (in complete agreement: 100%).
At present, anorectal manometry is the best method for confirming DD diagnosis, because it provides a thorough evaluation regarding pressure, sensitivity, and distensibility.75,76 Even though there are other methods for evaluating anorectal function, they have moderate-to-low evidence of determining DD and are used as complementary instruments in the assessment of those patients (see further ahead).77
Therefore, the consensus is of the opinion that anorectal manometry is the best method for evaluating patients with FC and suspected of having DD.
15. DD types can be classified through anorectal manometry. Diagnostic yield is influenced by the manometric techniques and types of probes (perfusion, solid state, high-resolution or high-definition) utilized.
Quality of evidence and strength of recommendation: B1 strong, in favor of the recommendation (in complete agreement: 93%; in partial agreement: 7%).
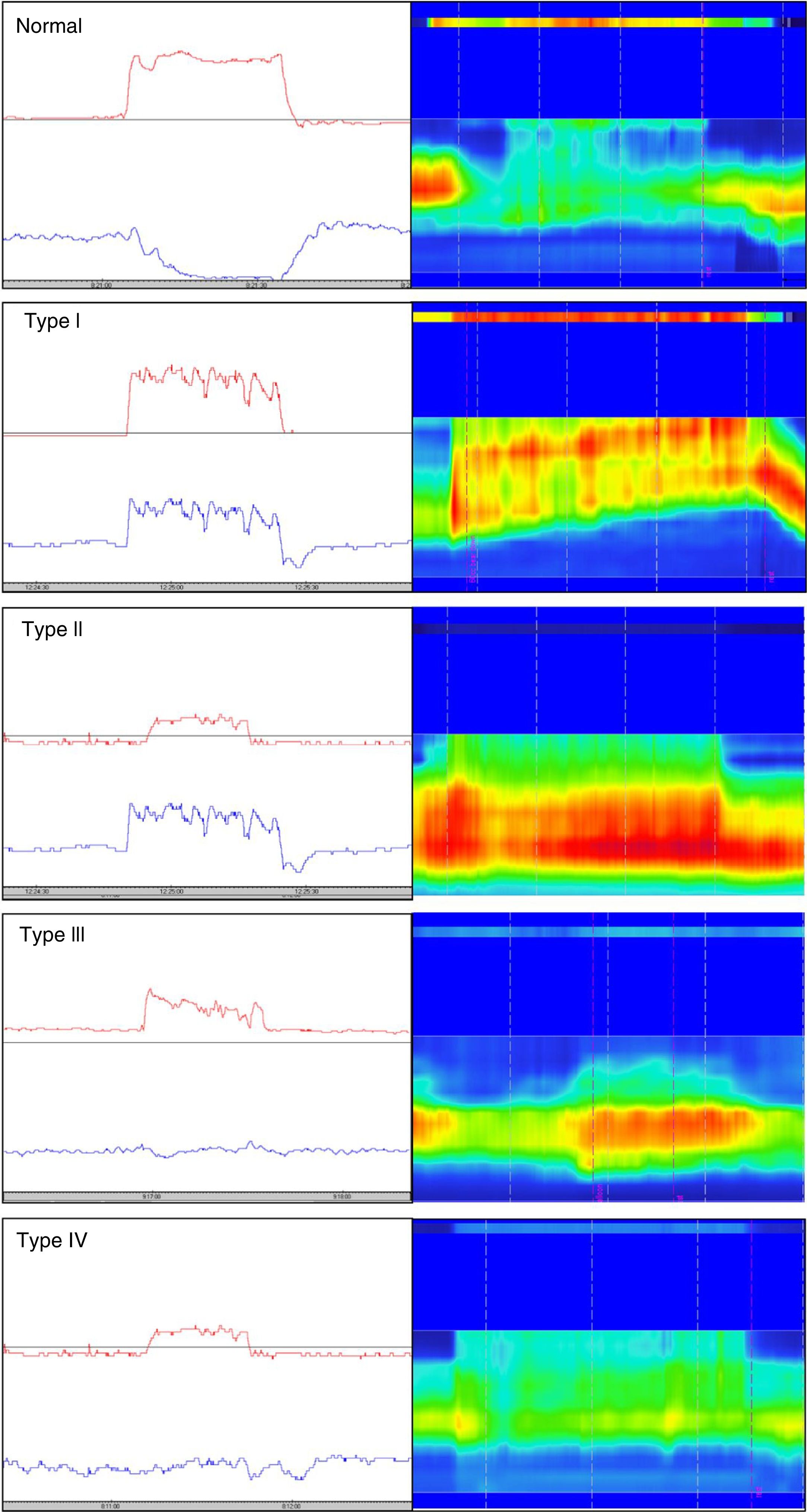
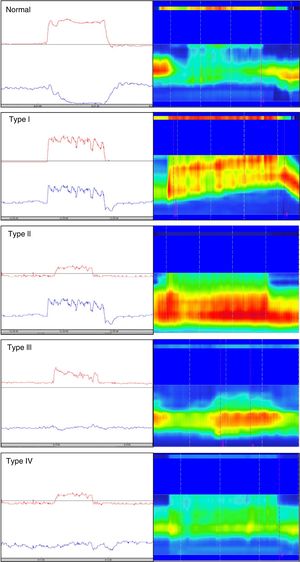
Different types of dyssynergia have been described that depend on the existence of propulsive force alterations or relaxation alterations of the anal sphincter structures (relaxation under 20%). The current Rome IV criteria recognize that said classification is useful and may have therapeutic implications.78 Traditionally, through the use of conventional anorectal manometry, 4 DD subtypes have been defined (fig. 1) that include the possible combinations of alterations of both propulsion and relaxation.79 Those 4 subtypes have been characterized with high-resolution anorectal manometry, making the pattern type more apparent (fig. 1).
Classification of dyssynergic defecation according to manometric pattern. Conventional manometric traces are on the left and high-resolution manometric traces are on the right. The red line in the conventional manometry represents the intrarectal pressure and the blue line the anal pressure during the defecation push maneuver. Types I and III are characterized by paradoxical contraction or absence of anal sphincter relaxation, whereas types II and IV are characterized by weakness or absence of rectal propulsion. The high-resolution manometry shows two color bands, the thin upper band (rectal) and the thick lower band (anal). The more intense colors represent greater pressure and the less intense colors represent lower pressure.
Conventional manometry can be performed with solid state or perfusion probes. The latter have the disadvantage of requiring the technique of extraction in stages, or pull-through technique, which consists of complete insertion of the probe and then its gradual removal to identify the zones that have an increase in pressure for each of the sensors. High-resolution technology has recently been incorporated, in both solid state and perfusion probes, and has done away with the need for pull-through extraction, thanks to more sensors and the fact that it provides a better topographic characterization of the anorectal region.80 Nevertheless, a higher number of false positives for DD has been described with that technique, most likely due to its more numerous sensors and the lack of specific parameters designed for that new technology. Finally, high-definition technology has been incorporated that can carry out a circumferential evaluation of the topography of anal sphincter pressure and reconstruct a 3-dimensional map through a specialized computer program.81,82
Regardless of the type of system employed, it is of the utmost importance that there are normality values, that the test is standardized, and that experts perform the data retrieval and interpretation.
16. The diagnosis of DD requires confirmation through anorectal manometry and an additional study, such as the balloon expulsion test, defecography, or radio-opaque marker testing.
Quality of evidence and strength of recommendation: B1 strong, in favor of the recommendation (in complete agreement: 93%; in partial agreement: 7%).
The presence per se of a manometric pattern of dyssynergia does not necessarily diagnose DD. As mentioned above, the presence of a manometric pattern consistent with dyssynergia may depend on the position of the patient when the test is being carried out and the type of equipment employed. Manometric patterns have also been described as a consequence of structural alterations of the pelvic floor, such as rectocele.83 Thus, to adequately establish the diagnosis of DD, the patient must have FC that does not respond to conventional treatment measures and a dyssynergic pattern associated with one of the following characteristics: 1) incapacity to expel the balloon (under 2min), 2) retention of 5 or more radio-opaque markers observed in an abdominopelvic x-ray at 120h after ingestion of a capsule with 24 markers, and 3) retention of more than 50% of the barium in defecography.8 It is important to stress that all of those tests are complementary, and not mutually exclusive.
17. Conventional defecography and magnetic resonance defecography identify anatomic alterations and pelvic dynamic variations in selected patients with FC. They are not standardized and lack availability.
Quality of evidence and strength of recommendation: B2 weak, in favor of the recommendation (in complete agreement: 86%; in partial agreement: 14%).
Defecography is useful for identifying anatomic alterations and the dynamics of the pelvic floor (e.g., rectocele, intussusception) that are present in some patients with FC.84 The identification of those abnormalities is relevant, given that they can lead to a change in treatment strategy. However, it is important to emphasize that the technique is not standardized, and it has limited availability. In addition, defecography can be normal in 10 to 75% of patients with DD, and there can be findings that do not correlate with symptoms in up to 77% of patients.85
Magnetic resonance defecography has recently been used to study functional and anatomic problems of the pelvic floor. The main advantage of magnetic resonance is that it offers a more adequate view of the adjacent anatomic structures and the joint identification of problems related to pelvic floor dynamics is more accurate.86,87 Nevertheless, the technique is not standardized, and its availability is still limited.
Therefore, the consensus considers that defecography is a complementary study for evaluating anorectal function that should not be used alone, but rather in conjunction with other tests, to diagnose patients with constipation and DD.
18. Colonic transit measurement through validated methods aids in the diagnosis of slow transit constipation.
Quality of evidence and strength of recommendation: B1 strong, in favor of the recommendation (in complete agreement: 86%; in partial agreement: 14%).
There are different methods for evaluating colonic transit in patients with FC. The most accessible and economic method is through radio-opaque markers. There are methodological variations for determining transit through markers. In the Hinton technique, a capsule with 24 markers is administered and an x-ray of the abdomen and pelvis is taken 120h later. Slow colonic transit is diagnosed when there are ≥ 5 markers after that time lapse.88 Another method is the Metcalf method, which includes the administration of a capsule with 24 markers every 24h for 3 days and the performance of an abdominopelvic x-ray on days 4 and 7. If the markers observed in the two x-rays add up to ≥ 68, slow colonic transit is diagnosed.89 The wireless motility capsule is a technology that incorporates a pH sensor, a temperature sensor, and a pressure sensor. With that technology, colonic transit is established as slow if it is ≥ 59h.90 The motility capsule is a method that enables the evaluation of gastric emptying and small bowel transit. It is limited by cost and availability.
Transit with radioisotopes through scintigraphy is only available at one center worldwide and has been used exclusively for research purposes.91
A high number of patients (2 out of every 3) with DD present with slow colonic transit. Therefore, it is not recommended to perform colonic transit study as a first step in subjects with a poor response to medical treatment, since initial treatment is not affected if those patients present with DD. Consequently, slow colonic transit evaluation is a complementary study in the evaluation of patients with FC.92
19. Colonic manometry and barostat study are methods used in highly selected patients and for research purposes.
Quality of evidence and strength of recommendation: C2 weak, in favor of the recommendation (in complete agreement: 100%).
Colonic manometry and barostat study are techniques that are employed in the evaluation of patients with slow transit FC, especially in selected cases in whom colon resection is proposed for the treatment of colonic inertia. Colonic manometry assesses the contractility of the resting colon after a meal with a standardized caloric content, as well as after treatment challenge, such as neostigmine or prucalopride.93 It can be performed for a few hours (stationary) or in an ambulatory manner (24h).
Barostat study is utilized to evaluate sensitivity, capacitance, and distensibility of the rectum or segments of the colon, with or without the association of colonic manometry.94 Whereas manometry assesses phasic contractility, the barostat study evaluates the tonic contractility of the colon. Those technologies are available in very few centers, limiting their applicability, and they are not considered part of the standard diagnostic approach to the patient with FC.
TreatmentHygienic-dietary measures20. Physical exercise is recommended in the treatment of FC because it can accelerate bowel transit.
Quality of evidence and strength of recommendation: C2 weak, in favor of the intervention (in complete agreement: 100%).
As described in statement 5, a sedentary lifestyle and lack of exercise predispose to FC. There is evidence that exercise improves bowel transit and symptoms of constipation.95,96 In a study in which 43 subjects were randomized to maintain regular physical exercise or to walk intensely for 30min for 12 weeks showed that the number of Rome II criteria decreased from 2.7 to 1.7 (p < 0.05) and total colonic transit decreased from 17.5 to 9.6 h (p < 0.05).97 The majority of studies are small and have methodological bias, making more and better studies necessary for determining the true effect and mechanisms of action of exercise on constipation. Nevertheless, there is no doubt that exercise has a positive effect on health.
21. Adopting a regular schedule, having adequate posture, and taking the sufficient time needed are all recommendations that should be made to patients in relation to defecation.
Quality of evidence and strength of recommendation: D2 weak, in favor of the intervention (in complete agreement: 100%).
Appropriate defecation requires adequate rectoanal coordination. To achieve that, it is important to adopt the correct posture (favoring the opening of the rectoanal angle) and perform a push maneuver without excessive force. In addition, the moments of greatest colonic motor activity (e.g., upon waking up or after food intake) should be taken advantage of.97 A squatting posture has been associated with faster defecation and less effort than the sitting posture.98 In a Mexican study on 343 healthy adults and 347 patients with FC, it was shown through pictograms that 98% of the healthy subjects utilized favorable postures during defecation, compared with only 71% of the patients with FC (p < 0.05).99 Leaning forward while sitting (“The Thinker” position) has recently been shown through cinedefecography to favor the opening of the rectoanal angle.100
With respect to the amount of time for defecation, a study on 518 adults demonstrated that 53% of that population read or played electronic games on the toilet. Fifty-five percent of them spent more than 5min on the toilet and 24% stated that they had hemorrhoidal disease. A statistically significant association was demonstrated between a longer time on the toilet and hemorrhoidal disease (p < 0.000006).101
Despite the evidence described, more studies are needed to adequately evaluate postures and schedules in patients with FC.
22. Patients with low fiber intake should eat foods with a high fiber content or take supplements, because they can increase the frequency of bowel movements. Liquid intake (1.5 to 2 l per day) can improve constipation and potentiate the effects of fiber in the diet and in the supplements.
Quality of evidence and strength of recommendation: Foods rich in fiber: C2 weak, in favor of the intervention. Fiber supplements: B1 strong, in favor of the intervention. Liquid intake: C2 weak, in favor of the intervention (in complete agreement: 100%).
The consumption of fiber through fiber-rich foods or fiber supplements can accelerate bowel transit because increased bolus formation is known to stimulate colonic peristalsis.102 Moreover, the fluid retention that fiber causes is useful in softening consistency and favoring stool passage.103
Very few studies have evaluated the consumption of fiber-rich foods for the treatment of constipation. In a study on 40 patients with FC that were randomized to receive 50g of prunes twice a day or 11g of psyllium twice a day for 3 weeks, prune intake was shown to be better for increasing the number of complete spontaneous bowel movements (CSBMs) (3.5 ± 0.2 vs 2.8 ± 0.2, p = 0.006) and improving stool consistency (3.2 vs 2.8, p = 0.02).104 In another study on 33 patients and 20 controls, eating 2 kiwis daily for 4 weeks improved the number of CSBMs, compared with the baseline (2.2 ± 2.6 vs 4.4 ± 4.6, p = 0.013), improved constipation symptoms (p = 0.02), and accelerated bowel transit (p = 0.003).105
With respect to fiber supplements, several meta-analyses have evaluated their beneficial effects.106,107 The most recent meta-analysis, in which 7 controlled clinical trials (CCTs) were evaluated, showed a response in 77% of the patients that received fiber supplements, compared with 44% that received placebo (relative risk [RR] for success of 1.71, 95% CI: 1.20-2.42; p = 0.003) and the estimated number necessary to treat (NNT) was 3 (95% CI: 2.6-3.4).108 Fiber increased the frequency of bowel movements (p = 0.03) and softened stool consistency (p = 0.02). It is important to point out that the CCTs included in that meta-analysis were very heterogeneous, given that they evaluated different types of fiber (e.g., psyllium, inulin, wheat bran) at different doses (10-22.5g/day), and the time periods varied from 2 to 8 weeks. Six of the 7 studies included soluble fiber and one only included insoluble fiber (wheat bran). The most widely studied fiber is Psyllium plantago, a fermentable fiber with intermediate solubility.109 There is limited evidence for recommending guar gum, pectin, methylcellulose, and polycarbophil and more studies are needed to evaluate their efficacy in FC.110
The evidence suggests that the therapeutic effect of fiber is greater when more than 15g are consumed daily.34 However, it is very important to mention that dietary fiber or supplements can aggravate symptoms associated with constipation, such as abdominal distention and flatulence in some patients. As a result, the recommended dose will be dependent on the tolerance of each patient.34
The daily consumption of 1.5 to 2 l of water is recommended because it can potentiate the effect of fiber and reduce side effects.111,112
Pharmacologic treatment23. Pharmacologic treatments are recommended in patients with FC that do not respond to initial measures of exercise, a diet high in fiber or supplements, and/or abundant liquid intake.
Quality of evidence and strength of recommendation: A1 strong, in favor of the intervention (in complete agreement: 86%; in partial agreement: 14%).
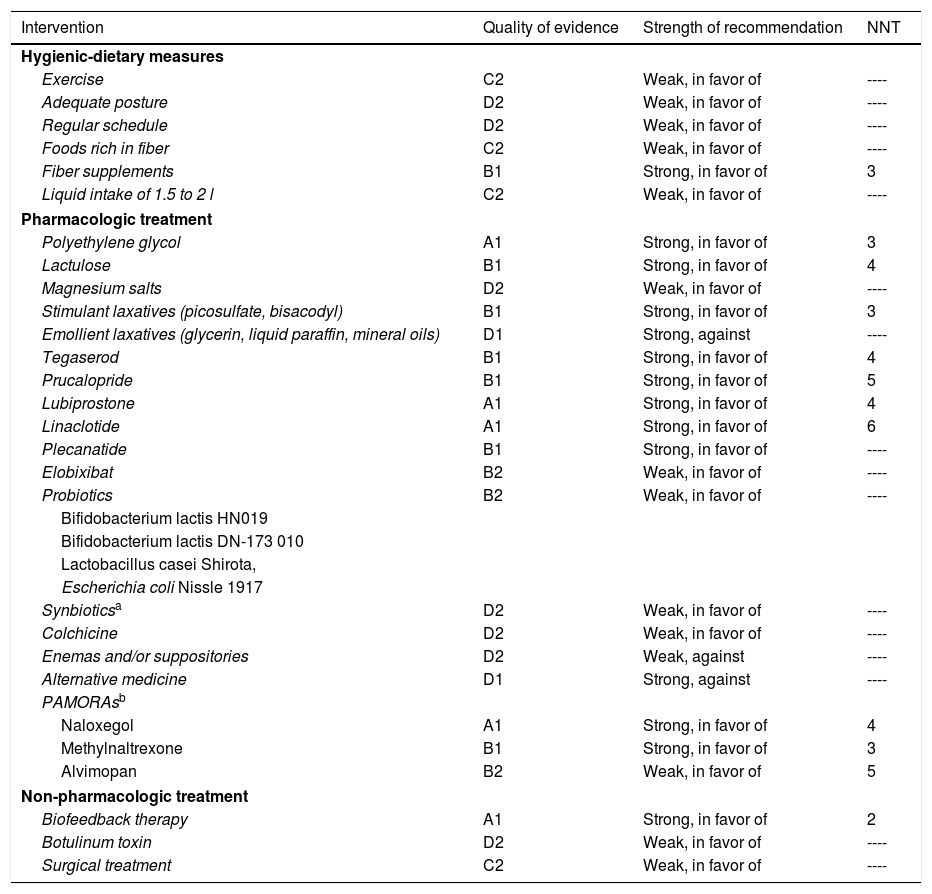
Due to the effects of FC on the quality of life of the individuals that suffer from it, pharmacologic treatment is recommended in those patients in whom initial measures have failed. There are numerous drugs that have been shown to be more efficacious than placebo and their choice should be individualized (Table 3).
Level of recommendation of pharmacologic therapy in chronic constipation.
| Intervention | Quality of evidence | Strength of recommendation | NNT |
|---|---|---|---|
| Hygienic-dietary measures | |||
| Exercise | C2 | Weak, in favor of | ---- |
| Adequate posture | D2 | Weak, in favor of | ---- |
| Regular schedule | D2 | Weak, in favor of | ---- |
| Foods rich in fiber | C2 | Weak, in favor of | ---- |
| Fiber supplements | B1 | Strong, in favor of | 3 |
| Liquid intake of 1.5 to 2 l | C2 | Weak, in favor of | ---- |
| Pharmacologic treatment | |||
| Polyethylene glycol | A1 | Strong, in favor of | 3 |
| Lactulose | B1 | Strong, in favor of | 4 |
| Magnesium salts | D2 | Weak, in favor of | ---- |
| Stimulant laxatives (picosulfate, bisacodyl) | B1 | Strong, in favor of | 3 |
| Emollient laxatives (glycerin, liquid paraffin, mineral oils) | D1 | Strong, against | ---- |
| Tegaserod | B1 | Strong, in favor of | 4 |
| Prucalopride | B1 | Strong, in favor of | 5 |
| Lubiprostone | A1 | Strong, in favor of | 4 |
| Linaclotide | A1 | Strong, in favor of | 6 |
| Plecanatide | B1 | Strong, in favor of | ---- |
| Elobixibat | B2 | Weak, in favor of | ---- |
| Probiotics | B2 | Weak, in favor of | ---- |
| Bifidobacterium lactis HN019 | |||
| Bifidobacterium lactis DN-173 010 | |||
| Lactobacillus casei Shirota, | |||
| Escherichia coli Nissle 1917 | |||
| Synbioticsa | D2 | Weak, in favor of | ---- |
| Colchicine | D2 | Weak, in favor of | ---- |
| Enemas and/or suppositories | D2 | Weak, against | ---- |
| Alternative medicine | D1 | Strong, against | ---- |
| PAMORAsb | |||
| Naloxegol | A1 | Strong, in favor of | 4 |
| Methylnaltrexone | B1 | Strong, in favor of | 3 |
| Alvimopan | B2 | Weak, in favor of | 5 |
| Non-pharmacologic treatment | |||
| Biofeedback therapy | A1 | Strong, in favor of | 2 |
| Botulinum toxin | D2 | Weak, in favor of | ---- |
| Surgical treatment | C2 | Weak, in favor of | ---- |
NNT: number needed to treat; PAMORAs: peripherally acting μ-opioid receptor antagonists.
Two combinations have been evaluated: one with fructooligosaccharides (FOS) and B. longum NCIMB 30182, B. breve NCIMB 30180, L. casei NCIMB1 30185, L. rhamnosus NCIMB 30188, L. acidophilus NCIMB 30184, L. bulgaricus NCIMB 30186, and S. thermophilus NCIMB 30189; and the other with FOS and B. lactis HN019, L. paracasei Lpc-37, L. rhamnosus HN001, and L. acidophilus NCFM.
24. Polyethylene glycol is the most widely studied laxative in FC and has been shown to increase defecation frequency and improve stool consistency.
Quality of evidence and strength of recommendation: A1 strong, in favor of the intervention (in complete agreement: 86%; in partial agreement: 14%).
Polyethylene glycol (PEG 3350) is an organic polymer whose osmotic activity is proportionate to the number of monomers that form it. It is metabolically inert, not metabolized or degraded by colonic bacteria, and interacts with water in a solution to increase osmotic pressure. There are multiple studies that demonstrate the effectiveness of PEG over placebo, lactulose, and other laxatives in the treatment of FC.113–118 In a recent meta-analysis,119 19 studies were evaluated (9 with PEG alone, 8 with PEG plus electrolytes, and 2 that compared PEG vs PEG plus electrolytes), demonstrating that the administration of PEG (with and without electrolytes) increased the number of bowel movements per week and softened stool consistency. According to the 2010 Cochrane review,120 PEG is superior to lactulose in increasing defecation frequency, softening stool consistency, and reducing the need for rescue laxatives. The NNT has been estimated at 3 (95% CI: 2-4) and the majority of the studies had less bias and heterogeneity than the studies on other drugs. The side effects reported were infrequent and the most common were abdominal pain and headache. Even though most of the studies had a follow-up under 6 months, PEG effectiveness did not appear to decrease after that period of time. The recommended dose is 17g of PEG diluted in at least 250ml of water.
25. Lactulose is an osmotic laxative that increases the frequency of bowel movements and improves stool consistency in FC.
Quality of evidence and strength of recommendation: B1 strong, in favor of the intervention (in complete agreement: 100%).
Lactulose is a synthetic disaccharide that can be hydrolyzed by intestinal lactase. Once in the colon, it is hydrolyzed by the bacteria, fermenting it in lactic acid, formic acid, and acetic acid. Those organic acids acidify the stools, forcing defecation.121 Its effect appears within the first 24 to 72h. In randomized clinical trials against placebo, superiority in the improvement of stool consistency and defecation frequency has been demonstrated, as well as a lower number of fecal impactions.122,123
The estimated NNT for lactulose is (95% CI: 2-7), but PEG is superior to lactulose, as mentioned above in the Cochrane review. Because lactulose is not systemically absorbed, it is considered a very safe drug in children and pregnant women and is regarded as first-line treatment in those groups of patients.124 It is important to point out that the capacity of lactulose to ferment in the colonic lumen is responsible for adverse effects, such as colic, flatulence, pain, and abdominal distension that some patients do not tolerate well, limiting its use.
26. Magnesium salts are useful in patients with acute constipation associated with immobilization and should not be used chronically because they produce hypermagnesemia, especially in patients with kidney failure.
Quality of evidence and strength of recommendation: D2 weak, in favor of the intervention (in complete agreement: 93%; in partial agreement: 7%).
Magnesium is found in various preparations of laxatives, including magnesium hydroxide (milk of magnesia), magnesium sulfate, and magnesium citrate. Because those salts are minimally absorbed, they have a very rapid-acting osmotic effect (6h). Their efficacy has been demonstrated in patients with periods of immobilization, related to short periods of admission to intensive care units.125 There is only one study on the chronic use of milk of magnesia for more than 8 weeks in patients above 65 years of age and the results are controversial.126 Due to its potential renal toxicity, magnesium is not recommended for prolonged periods or in patients with abnormal renal clearance. Cardiovascular effects associated with hypermagnesemia, such as hypotension, bradyarrhythmia, and conduction disorders have been described.127
27. Stimulant laxatives, such as bisacodyl or sodium picosulfate, can be used for short periods in patients with FC that do not respond to treatment with bulk-forming agents or osmotic laxatives.
Quality of evidence and strength of recommendation: B1 strong, in favor of the intervention (in complete agreement: 100%).
Stimulant laxatives are compounds that directly cause an increase in colonic peristalsis and favor the secretion of water and electrolytes. Sodium picosulfate and bisacodyl are diphenylmethanes that belong to that class of laxatives.
Bisacodyl is a diacetic acid ester that is hydrolyzed to its free form in the small bowel by endogenous esterases. Although its beneficial effect in CC was not shown in previous non-placebo controlled studies, in a double-blind, randomized, parallel, and placebo-controlled study on 55 patients, 10mg of bisacodyl significantly increased the number of bowel movements (1.8 vs 0.95 daily, p = 0.0061), improved consistency (p < 0.0001), and increased overall improvement, compared with placebo.128 In another study, Kamm et al. demonstrated that bisacodyl taken for 4 weeks not only improved defecation frequency and stool consistency in patients with FC, but also improved their quality of life.129,130
Sodium picosulfate is a prodrug that is hydrolyzed to its free form by bacterial enzymes, making it active in the colon after the bacterial gut microbiota transform it into the same molecule as bisacodyl. In a double-blind, parallel, randomized, placebo-controlled, multicenter German study, the administration of sodium picosulfate was associated with a higher percentage of patients with > 3 bowel movements per week (51.1% vs 18%, p < 0.0001), an increase of > 1 additional spontaneous bowel movement per week (65.6% vs 32.3%, p < 0.0001), a higher number of patients having their first bowel movement within the first 24h (69% vs 53%), and improved quality of life.131 The NNT for bisacodyl and sodium picosulfate was estimated at 3 (95% CI: 2-3.5).
The most common side effects of the diphenylmethanes are colicky pain, bloating, and fluid and electrolyte imbalance, but cutaneous eruptions, Stevens-Johnson syndrome, lupus-like reactions, and enteropathy with protein loss have also been reported.
There is no evidence for recommending the use of other stimulant laxatives, such as the anthraquinones (e.g., senna, cascara sagrada bark, senna leaves), in patients with FC. That group of medications can be useful for occasional constipation and for short periods. Their established side effects are allergic reactions, fluid and electrolyte imbalance, and melanosis coli. Melanosis coli, or pseudo-melanosis coli, is due to the deposit of lipofuscin and other pigments in the macrophages. Most patients develop tolerance, requiring a progressively increasing dose. There is no evidence that its chronic use causes megacolon or colorectal cancer.48,132
28. There is limited evidence for recommending the use of emollient or lubricating laxatives (sodium docusate, paraffin, glycerin, mineral oils) in FC.
Quality of evidence and strength of recommendation: D1 strong, against the intervention (in complete agreement: 86%; in partial agreement: 14%).
That group of laxatives has a detergent effect and softens stools. There is a limited number of studies on docusate (only 2) and they have conflicting results, and so its use is not recommended for the treatment of FC.133,134
Liquid paraffin appears to be more effective than placebo in increasing the frequency of bowel movements, although experience is limited to the pediatric population. Because there are no studies on the use of liquid paraffin in patients with FC, it cannot be recommended. There is a combination of lactulose with paraffin in gel, and based on the one available study, no recommendation for its use can be made.135,136 There are no studies on the use of mineral oil, glycerin, and ricinoleic oil in the treatment of FC, and considering their long-term effects, such as liposoluble vitamin malabsorption, risk for bronchoaspiration in infants and the elderly, foreign body reactions that include lipoid pneumonia, and anal incontinence, we do not recommend their use.137
29. Tegaserod is a nonselective 5HT4 receptor agonist with demonstrated efficacy in patients with FC that improves defecation frequency and stool consistency. Its use has been associated with severe cardiovascular events, and therefore in Mexico its recommendation in women is only for those under 55 years of age that have no cardiovascular risk factors.
Quality of evidence and strength of recommendation: B1 strong, in favor of the intervention (in complete agreement: 86%; in partial agreement: 14%)
Tegaserod is a selective 5-HT4 receptor agonist with no activity on 5-HT3 receptors. Its efficacy in relation to FC and IBS-C has been demonstrated in numerous clinical trials. According to the Cochrane review on FC,138 the RR for being a responder, considering the number of bowel movements per week with 12mg of tegaserod, is 1.54 (95% CI: 1.35-1.75), compared with 0.6 (95% CI: 0.42-0.78) with placebo. The improvement of symptoms, such as abdominal distension, stool consistency, and pushing is not consistent in the studies evaluated, and so its clinical efficacy must be regarded as moderate. In March of 2007, The U.S. Food and Drug Administration (FDA) restricted the commercialization of tegaserod due to the increased incidence of cardiovascular events. That link was not clear, and in Mexico, the Federal Commission for the Protection against Health Risks (COFEPRIS, the Spanish acronym) decided not to suspend its commercialization in the country and its use is restricted to patients with no cardiovascular risks (women under 55 years of age with no high blood pressure and no hypercholesterolemia).139
30. Prucalopride is a selective 5-HT4 receptor agonist that improves the number of bowel movements and stool consistency, as well as quality of life, and reduces the need for laxatives.
Quality of evidence and strength of recommendation: B1 strong, in favor of the intervention (in complete agreement: 100%).
Prucalopride is a highly selective intestinal 5-HT4 serotoninergic receptor agonist. It has less affinity for the hERG protein that is related to the adverse cardiovascular effects of the other 5HT4 receptors, like tegaserod. Numerous studies have demonstrated its efficacy over placebo. A meta-analysis of 16 studies140 that included 3,943 patients showed that it is efficacious, increasing the frequency and consistency of complete spontaneous bowel movements at all its doses (1mg, 2mg, and 4mg). In another meta-analysis of 6 studies, its efficacy was evaluated by sex, and it was shown to be efficacious in both men and women for producing at least 3 complete spontaneous bowel movements with greater frequency than placebo (27.8% vs 13.2%, OR 2.68, 95% CI: 2.16-3.33, p = 0.001).141 In addition, studies have shown that patients are satisfied with treatment with prucalopride and have significantly improved quality of life (measured through the PAC-QOL questionnaire).142–144 In a follow-up analysis of 3 pivot studies,142–144 in which patients that had responded for 12 weeks were left with continuous treatment (up to 18 months), 40-50% of patients did not need to take laxatives.145 The estimated NNT for prucalopride is 5 (95% CI: 4-8).
Nevertheless, in a recent study, the use of 2mg of prucalopride for 24 weeks was not superior to placebo for producing at least 3 complete spontaneous bowel movements per week (25.1% vs 20.7%, p = 0.36).146 According to the analysis by Ford et al.,147 in the American College of Gastroenterology guidelines for the management of IBS and CC, and taking into account the heterogeneity of the published studies, prucalopride is considered to have a strong recommendation in favor of its use, but with moderate evidence. A similar recommendation was recently emitted in the clinical practice guidelines on the management of CC in Spain.148
The most common adverse effects reported are headache and diarrhea and it is important to stress that its use in patients above 65 years of age has been shown to be safe, producing no electrocardiographic alterations or vital sign disturbances.
31. Lubiprostone is an activator of intestinal epithelium chloride channels that improves defecation frequency, stool consistency, and the quality of life in patients with FC.
Quality of evidence and strength of recommendation: A1 strong, in favor of the intervention (in complete agreement: 93%; in partial agreement: 7%).
Lubiprostone is a bicyclic fatty acid that activates the chloride channels (ClC2) of the apical intestinal membrane, thus promoting the intestinal secretion of water. In the multicenter, double-blind, randomized, and placebo-controlled pivot study by Johanson et al.,149 242 patients were assigned to 24μg of lubiprostone or placebo twice a day for 4 weeks. The 120 patients treated with lubiprostone had a higher mean of spontaneous bowel movements at week 1, compared with placebo (5.69 vs 3.46, p = 0.0001) and the effect lasted into weeks 2, 3, and 4. The most frequent side effects were nausea (31.7%) and headache (11.7%). In all the later studies and according to a meta-analysis of 9 trials with a total of 1,468 patients that received lubiprostone and 841 that received placebo, lubiprostone has been shown to significantly improve constipation symptom intensity, stool consistency, and quality of life.150 The estimated NNT for lubiprostone was 4 (95% CI: 3-6).151
The reported adverse effects of nausea, vomiting, and diarrhea are common (incidence varies from 2 to 75%), but the presence of serious adverse effects for which the drug must be suspended is less than 5%. The mechanism by which the drug produces nausea and vomiting is not known. At present, lubiprostone is not available in Mexico.
32. Linaclotide is a guanylate cyclase receptor agonist that increases defecation frequency, improves stool consistency, reduces the pushing force needed to defecate, and improves quality of life.
Quality of evidence and strength of recommendation: A1 strong, in favor of the intervention (in complete agreement: 93%; in partial agreement: 7%).
Linaclotide is a synthetic peptide made up of 14 amino acids that binds to the guanylate cyclase-C receptor (GC-C) on the surface of the enterocytes, increasing the intestinal secretion of water and electrolytes, in addition to having an antinociceptive effect.152 At a daily dose of 290μg, linaclotide has shown its efficacy in the treatment of IBS-C and it has recently been approved by the FDA for the treatment of FC at a daily dose of 145μg. That dose is now available in Mexico. In a meta-analysis of 3 pivot studies on linaclotide in FC that included 1,662 patients, the RR for reaching the primary success variable (more than 3 complete spontaneous bowel movements per week for more than 75% of treatment time) was 3.80 (95% CI: 2.20-6.55) with linaclotide, compared with placebo.153–156 In addition, linaclotide was shown to improve stool consistency, the pushing force needed to defecate, and other symptoms associated with constipation, as well as quality of life. The mean time for achieving the first spontaneous bowel movement after a dose of 145μg of linaclotide was 12.5h, compared with 28.1h with placebo (p < 0.05).157 The estimated NNT was 6 (95% CI: 5-8).
The most common adverse event was diarrhea and it presented in 14-16% patients, compared with 5% with placebo. However, severe diarrhea resulting in treatment suspension presented in 4.8% of the cases.153–157 The RR for developing diarrhea was estimated at 3.08 (95% CI: 1.27-7.48), with a number needed to harm (NNH) of 12 (95% CI: 7-38.5).
33. Plecanatide is a uroguanylin analog that increases defecation frequency and improves stool consistency, as well as the symptoms associated with constipation.
Quality of evidence and strength of recommendation: B1 strong, in favor of the intervention (in complete agreement: 100%).
Plecanatide is a peptide composed of 16 amino acids that acts as a uroguanylin analog and once it binds to the Gc-C receptor, it stimulates the secretion of water into the intestinal lumen, softening stool consistency and favoring passage. Like linaclotide, plecanatide has been described to have antinociceptive effects.158 A multicenter phase III trial has evaluated the effect of 3 or 6mg of plecanatide for 12 weeks, compared with placebo, on 1,394 patients with FC.159 In that study, the patients receiving plecanatide (3 or 6mg) had a better response (> 3 complete spontaneous bowel movements for 12 weeks of treatment), compared with placebo (21, 19.5, and 10.2%, p = 0.001). Plecanatide (3 or 6mg) also significantly increased the number of spontaneous bowel movements per week, compared with placebo (3.2, 3.1, and 1.3; p = 0.001). The most common adverse effect was diarrhea in 5.9% of the patients that received 3mg, 5.7% in those that received 6mg, and 1.3% in the placebo group.
Plecanatide is not available in Mexico.
34. Some specific probiotic and synbiotic strains appear to be beneficial in FC, increasing defecation frequency and improving stool consistency. There is no evidence for recommending the isolated use of prebiotics.
Quality of evidence and strength of recommendation: Probiotics: B2 weak, in favor of the intervention. Synbiotics: D2 weak, in favor of the intervention (in complete agreement: 100%).
The administration of specific probiotics in patients with FC has been shown to accelerate bowel transit and increase the frequency of bowel movements. There are 3 meta-analyses: the first included 14 controlled and randomized trials with a total of 1,182 patients,160 the second with 11 studies and a total of 464 patients,161 and the third that analyzed IBS-C and FC with a total of 245 patients,162 as well as a systematic review that included 5 clinical trials on 266 adults.163
The first meta-analysis included 10 studies that evaluated defecation frequency as the primary aim of the intervention with probiotics. The results showed that the administration of specific strains of probiotics, compared with control groups, increased the frequency of bowel movements per week by 1.3 (95% CI: 0.7-1.9, p < 0.0001, I2 = 90%) (p= 0.00001). The Bifidobacterium lactis strain favored a higher increase of 1.5 (95% CI 0.7-2.3, p = 0.0003).160 Stool consistency was evaluated in 11 studies, based on the BSS, and there was a change in stool consistency from hard to soft (RR 0.55; 95% CI: 0.27-0.82; p = 0.0001; I2 = 80%). In the subgroup analysis, B. lactis improved stool consistency the most. Colonic transit time has also been shown to decrease by a mean of 12.4h (95% CI: -2.5 to -22.3; p = 0.01; I2 = 23%) after the administration of B. lactis HN019 (2 trials) and B. lactis DN-173 010 (3 trials),160–163 especially at the rectosigmoid level. The meta-analysis by Ford et al.162 included a limited number of clinical trials on subjects with FC and the authors concluded that the value of probiotics, prebiotics, and synbiotics was uncertain in FC. In their systematic review, Chmielewska and Szajewska163 showed a favorable effect of treatment with B. lactis DN-173 010, Lactobacillus casei Shirota, and Escherichia coli Nissle 1917 on adults with constipation, in relation to the frequency of bowel movements and stool consistency.
There are only 2 studies on synbiotics in FC,164,165 and together they included 166 patients. In one of them, a combination of fructooligosaccharides (FOS) and 7 probiotics (Bifidobacterium longum NCIMB 30182, Bifidobacterium breve NCIMB 30180, L. casei NCIMB1 30185, Lactobacillus rhamnosus NCIMB 30188, Lactobacillus acidophilus NCIMB 30184, Lactobacillus bulgaricus NCIMB 30186, and Streptococcus thermophilus NCIMB 30189) was administered and in the other, a combination of FOS and 4 probiotics (B. lactis HN019, Lactobacillus paracasei Lpc-37, L. rhamnosus HN001, and L. acidophilus NCFM) was administered for 4 weeks. According to the meta-analysis by Ford et al.,162 synbiotics can have a beneficial effect on FC, given that the RR for response failure was 0.78 (95% CI: 0.67-0.92).
Although the meta-analyses showed the benefits of probiotics and synbiotics in FC, they all emphasized the fact of high risk for bias and study heterogeneity, and therefore were cautious in their recommendations.
35. There is scant evidence showing that colchicine improves constipation symptoms in patients with slow colonic transit.
Quality of evidence and strength of recommendation: D2 weak, in favor of the intervention (in complete agreement: 100%).
There are medications whose side effects cause diarrhea, such as colchicine, which is indicated for treating acute attacks of gout. At least 2 studies have evaluated the use of colchicine in FC with favorable results.166,167 In a double-blind, randomized, cross-over study on 16 patients with a 4-week follow-up, 0.6mg of colchicine administered TID increased defecation frequency, accelerated colonic transit, and reduced pain and bloating in patients with constipation.166 In the other study, 60 patients were randomized to receive 1mg of colchicine daily or placebo for 8 weeks and symptom improvement was evaluated through the Knowles-Eccersley-Scott Symptom (KESS) questionnaire. At the end of 2 months, the KESS score in the patients that received colchicine was 11.67 ± 3.91 vs 18.66 ± 3.72 in the patients that received placebo (p = 0.0001).168 Even though the studies showed colchicine to be efficacious, they had small samples and the results should be interpreted with care.
36. There is no evidence for recommending the chronic use of enemas or suppositories in patients with FC.
Quality of evidence and strength of recommendation: D2 weak, against the intervention (in complete agreement: 100%).
Patients with refractory FC and symptoms of anorectal blockage frequently utilize rectal laxatives that include suppositories and enemas containing monosubstances or combinations of agents that act by increasing the secretion of water into the rectum. Their effects depend on the quantity of liquid applied, intraluminal pressure, temperature of the enema, and on additional substances. The most widely utilized agent in enemas and suppositories is glycerin, but they can also contain phosphates, sorbitol, bisacodyl, or saline solution.168 They can be used for short periods, but there are no studies that support their use and therefore we consider that they should not be employed in FC.
37. There is no evidence for recommending the use of alternative or complementary medicine, such as acupuncture, herbal medicine, moxibustion, or colonic lavages in the treatment of CC.
Quality of evidence and strength of recommendation: D1 strong, against the intervention (in complete agreement: 93%; in partial agreement: 7%).
Complementary medicine has frequently been used in patients with FC and the evidence supporting that measure is null or scarce. In a 2015 review article,169 acupuncture was found to be the most widely used method, followed by herbal medicine. There are 11 studies on acupuncture, but the results are extremely heterogeneous and biased, and therefore, even though it is a method that could be useful, it is difficult to make a recommendation.169 With respect to herbal medicine, 21 studies had adequate quality, but like those on acupuncture, there was great heterogeneity.170 There is no evidence regarding the use of moxibustion, massages, or colonic lavage. Those types of therapies can be associated with severe complications that include ulceration and perforation of the colon.171
38. Elobixibat, a bile acid transport inhibitor at the ileal level, increases defecation frequency in patients with FC.
Quality of evidence and strength of recommendation: B2 weak, in favor of the intervention (in complete agreement: 93%; in partial agreement: 7%).
Elobixibat is an inhibitor of the ileal bile acid transporter that has secretory and motor effects at the colonic level.172 There are 3 studies that have evaluated elobixibat in FC, showing that it is capable of accelerating bowel transit, increasing the frequency of bowel movements, and softening stool consistency.173–175 In the largest study, conducted by Chey et al.,174 190 patients were randomized to receive 5mg,10mg, or 15mg of elobixibat or placebo once a day for 8 weeks. At the end of the study, the number of bowel movements increased to 1.7 (95% CI: 0.7-2.8) with the placebo, compared with 2.5 (95% CI: 1.5-3.5), 4.0 (95% CI: 2.9-5.0), and 5.4 (95% CI: 4.4-6.4) with 5mg, 10mg (p < 0.002), and 15mg (p < 0.001) of elobixibat, respectively. Likewise, abdominal distension and pushing decreased with elobixibat, compared with placebo. The most common adverse effect was diarrhea (2-13%).
39. The peripherally acting μ-opioid receptor antagonists (PAMORAs), such as naloxegol, methylnaltrexone, and alvimopan, have been shown to be efficacious for treating opioid-induced constipation (OIC). However, those medications are not yet available in Mexico.
Quality of evidence and strength of recommendation: Naloxegol: A1 strong, in favor of the intervention. Methylnaltrexone: B1 strong, in favor of the intervention. Alvimopan: B2 weak, in favor of the intervention (in complete agreement: 100%).
PAMORAs have become the treatment of choice for OIC. Naloxegol has been the most widely studied. It is a conjugated polymer of naloxone with polyethylene glycol that does not cross the blood-brain barrier.176–180 In the largest studies (KODIAC 04 and KODIAC 05) that included 1,352 non-cancer patients with OIC, response with 25mg of naloxegol after 12 weeks was shown in 44% of the patients, compared with 29% that received placebo (p = 0.001).179 The drug shortened the time for achieving the first bowel movement and increased the number of days with complete spontaneous bowel movements, compared with placebo. The most frequent side effects were abdominal pain (10-19%) and diarrhea (7-9%).177–180
Methylnaltrexone is another PAMORA which, like naloxegol, crosses the blood-brain barrier very little and has few secondary effects on the digestive tract, such as delayed gastric emptying.181 In their meta-analysis of 6 trials with 1,610 patients, Ford et al.180 reported that methylnaltrexone was more effective in 1,095 patients, compared with placebo, for the treatment of OIC, with a RR for treatment failure of 0.67 (95% CI: 0.54-0 .84). The estimated NNT for preventing treatment failure in a patient with OIC was 3 (95% CI: 2-10). However, the doses administered (12-450mg), the administration routes (subcutaneous or oral), and treatment duration (4-8 weeks) were heterogeneous in the studies evaluated. The most common side effects were abdominal pain (27%), flatulence (13%), and nausea (9%).182
Alvimopan is the most recently evaluated PAMORA. It is administered orally and has the advantage of not reversing the analgesic effect of opioids or causing withdrawal symptoms.183 Three clinical trials184–186 have shown that alvimopan, at a dose of 0.5-1mg daily, improved the number of spontaneous bowel movements related to constipation in patients with OIC. Even though the results were not significantly greater than those with placebo in all of the studies, the estimated therapeutic gain varied from 13-24%, compared with placebo. The most common side effects reported were nausea, dizziness, and diarrhea.
Non-pharmacologic treatment40. Biofeedback therapy (BFT) is the short-term and long-term treatment of choice for patients with constipation and dyssynergic defecation.
Quality of evidence and strength of recommendation: A1 strong, in favor of the intervention (in complete agreement: 93%; in partial agreement: 7%).
The goal of BFT in patients with DD is to restore the coordination between the rectum and the anus during defecation.187 That is achieved through a process of teaching and learning, utilizing auditory and visual techniques that make it possible to correct the lack of abdominopelvic coordination those patients have.188 Even though there are different methodologies, at least 7 studies have shown that short-term BFT (4 weeks) and long-term BFT (12 months) are superior to exercise, PEG, and benzodiazepines for correcting DD.101 The estimated OR for successful BFT was 8.86 (95% CI: 2.2-15.8) and harder stools, disposition of the patient, anal sphincter hypertonia, and an abnormal balloon expulsion test were predictive factors for good response.189
Biofeedback therapy is not associated with side effects or complications, but its limitations include: restricted availability and variability of equipment, techniques, and protocols. In Mexico, there are referral centers with experience in performing BFT.
41. The application of botulinum toxin can be effective in some cases of pelvic floor dyssynergia (hypertonia or lack of anal sphincter relaxation), but its effect is transitory, and evidence is limited.
Quality of evidence and strength of recommendation: D2 weak, in favor of the intervention (in complete agreement: 93%; in partial agreement: 7%).
Botulinum toxin type A produces a strong neuromuscular inhibitory effect that induces relaxation of smooth muscle and striated muscle. Based on that principle, studies with small samples have used the toxin to correct anal sphincter hypertonia (anismus), chronic anal pain, and dyssynergic defecation.190 In a Chinese study, 191 31 patients diagnosed with anismus had ultrasound-guided injection of 100 units of botulinum toxin into the puborectal muscle and the external anal sphincter and also underwent BFT. That combination produced symptom improvement in 77% of the cases and was sustained for up to 8.4 months. Nevertheless, in the study by Rao et al.192 on patients diagnosed with levator ani syndrome, there were no significant differences between botulinum toxin and placebo.
Treatment with botulinum toxin type A is costly, and its side effects include pain and fecal incontinence. More studies of better quality need to be conducted on botulinum toxin application, before a recommendation can be made.
42. Surgical treatment through colectomy should be reserved for exceptional cases in patients with treatment-refractory slow colonic transit and with no dyssynergic defecation and/or other motility alteration of the digestive tract.
Quality of evidence and strength of recommendation: C2 weak, in favor of the intervention (in complete agreement: 79%; in partial agreement: 21%).
The surgical indications in cases of FC are not well-defined. In general terms, patients that are candidates for surgery are those that do not respond to thorough conventional medical treatment. It is essential that constipation due to pelvic floor dysfunction be ruled out. To do so requires a complete diagnostic approach that includes studies for excluding metabolic diseases and structural alterations of the colon and rectum. The diagnosis of colonic inertia should be confirmed through colonic transit study. Anorectal manometry, defecography, and the balloon expulsion test are necessary for ruling out pelvic floor dysfunction. A thorough assessment of psychiatric comorbidity is recommended.
The decision to perform a surgical intervention is based on the severity of symptoms and the deterioration of quality of life of the patient.193–195 Currently, colectomy with ileorectal or ileosigmoidal anastomosis is considered the most effective type of resection.194–196 Around 80% of the patients have favorable or satisfactory results.
ConclusionsThere is a high prevalence of CC in Mexico, affecting the quality of life of the individuals that present with it. Female sex, advanced age, low fiber intake, and low physical activity are risk factors for CC. The first step in the approach to patients with CC is adequate semiology and physical examination, emphasizing the importance of performing digital rectal examination. Diagnostic studies, such as colonoscopy, anorectal manometry, colonic transit, and defecography, when available, should be recommended individually for each case.
Pharmacologic treatment is recommended in the patients that do not respond to the initial measures of exercise, a diet high in fiber or the taking of supplements, and/or abundant liquid intake. Currently there are useful and efficacious medications for CC management. BFT is efficacious for the treatment of patients with CC and dyssynergic defecation. Surgical treatment is reserved for exceptional cases.
Financial disclosureThe present consensus was sponsored by Laboratorio Allergan for transportation and lodging during the face-to-face voting. The participants received fees from the Laboratory that did not affect the design, collection, conduction, analysis, interpretation, preparation, or writing of the document for its publication.
Conflict of interestJosé María Remes-Troche is a member of the advisory board of Takeda Pharmaceuticals, Alfa-Wassermann, and Almirall. He received research funds from Sanfer. He is a speaker for Takeda, Asofarma, Alfa-Wassermann, Carnot, Menarini, Almirall, and Astra-Zeneca.
Enrique Coss-Adame is a member of the advisory board of Takeda Pharmaceuticals, Allergan, Carnot Laboratorios. He is a speaker for Takeda, Asofarma, Alfa-Wassermann, Carnot, Allergan.
Aurelio López-Colombo is a speaker for Takeda and Sanfer.
Mercedes Amieva-Balmori is a speaker for Takeda and Sanfer.
Ramón Carmona-Sánchez is a member of the advisory board of Mayoly-Spindler, a speaker for Mayoly-Spindler and Grünenthal, and participates in research protocols sponsored by Laboratorios Senosian and Asofarma.
Luis Charúa Gunidic has no conflict of interest.
Ricardo Flores Rendón received research funds from Asofarma and Takeda Pharmaceuticals. He is a speaker for Janssen.
Octavio Gómez Escudero is a member of the advisory board of Allergan and Sanofi. He is a speaker for Asofarma, Alfa-Wassermann, Allergan, Carnot, Mayoli-Spindler, Sanofi, and Takeda Pharmaceuticals.
Marina González-Martínez is a speaker for Grünenthal.
María Eugenia Icaza Chávez is a speaker for Takeda and Asofarma.
Miguel Morales Arambúla is a speaker for Takeda and Asofarma.
Max Schmulson has been a consultant for Alfa Wassermann, Allergan, Commonwealth Diagnostics International Inc, and Takeda. He has been a speaker for Alfa Wassermann, Commowealth Diagnostics Inc, Mayoly-Spindler, and Takeda and has received research grants from Alfa Wassermann, Nycomed/Takeda, and Genova Diagnostics Inc.
José Luis Tamayo is a speaker and external advisor for the laboratories of Alfa-Wassermann, Asofarma, Mayoly-Spindler, and Takeda de México, and participates in a research protocol sponsored by Laboratorios Asofarma.
Genaro Vázquez Elizondo is a speaker and external advisor of the laboratories of Alfa-Wassermann and Asofarma.
Miguel A. Valdovinos is a member of the advisory board and a lecturer for Takeda, Biocodex, Mayoly-Spindler, and Sanofi. He is a speaker for Carnot.
Please cite this article as: Remes-Troche JM, Coss-Adame E, Lopéz-Colombo A, Amieva-Balmori M, Carmona Sánchez R, Charúa Guindic L, et al. Consenso mexicano sobre estreñimiento crónico. Revista de Gastroenterología de México. 2018;83:168–189.