Hepatocellular carcinoma (HCC) staging provides a basis for calculating disease prognosis and therapeutic guidance. Liver resection and transplantation are curative options, and ablation therapies are applied to patients that are not candidates for curative treatment. Survival after liver resection or ablation therapies varies.
AimsTo describe the presentation, staging, management, and outcome in patients with HCC in our center.
Patients and methodsForty-two patients had a 7-year prospective follow-up. Survival was calculated with the Kaplan-Meier analysis and the log-rank test was used for its comparison between the staging systems (Okuda, BCLC, and CLIP) and types of treatment (liver resection, radiofrequency ablation, and no surgical treatment).
ResultsThe mean age of the patients was 68.9 ± 9.5 years; 57% were women. A total of 54% of the patients presented with cirrhosis and 31% were infected with hepatitis C virus (HCV). The mean tumor size was 6.48 ± 2.52cm. The CLIP 0, Okuda I, and BCLC A stages had better survival rates than the other stages (P<0.05). Survival with resection was superior (median of 32 months and survival at 1, 3, and 5 years of 83, 39, and 19.7%, respectively) to that of both radiofrequency ablation (median of 25 months and survival at 1 and 3 years of 90 and 17.2%, respectively) and no surgical treatment (1 year < 5%) (P<0.05).
ConclusionThe patients at our center were diagnosed at late stages of HCC, as is the case in other Mexican populations. Outcome in relation to CLIP and BCLC was similar to the prognoses reported in the literature. The best results were observed in the patients with early stage disease and those that underwent HCC resection surgery.
La estadificación en el carcinoma hepatocelular (CHC) otorga pronóstico y orientación terapéutica. La resección y el trasplante hepático son opciones curativas y las terapias de ablación se aplican a pacientes que no reciben tratamiento curativo. La sobrevida tras la resección hepática o terapias de ablación es variada.
ObjetivoDescribir la presentación, la estadificación, el manejo y la evolución de los pacientes con CHC en nuestro centro.
Pacientes y métodosCuarenta y dos pacientes fueron seguidos prospectivamente durante 7 años. La sobrevida se calculó mediante Kaplan-Meier y log-rank entre los sistemas de estadificación (Okuda, BCLC y CLIP) y tipos de tratamiento (resección hepática, ablación por radiofrecuencia y ningún tratamiento quirúrgico).
ResultadosLa edad media ± desviación estándar de los pacientes fue 68,9 ± 9,5 años; el 57% fueron mujeres y el 54% cirróticos. El 31% tenía infección por VHC. El tamaño medio del tumor fue 6.48 ± 2.52cm. Los estadios CLIP 0, Okuda I y BCLC A tuvieron mejor sobrevida que otros estadios (p<0.05). La resección tuvo mejor sobrevida (mediana: 32 meses y sobrevida a 1, 3 y 5 años del 83, el 39 y 19.7%) que ablación por radiofrecuencia (25 meses, y el 90 y el 17.2% a 1 y 3 años) y que ningún tratamiento quirúrgico (1 año<5%) (p<0.05).
ConclusiónLos pacientes con CHC en nuestro centro al igual que otra población en México son diagnosticados tardíamente. El pronóstico usando CLIP y BCLC es similar a la literatura. Los mejores resultados se observaron en estadios tempranos y los que tuvieron resección quirúrgica del CHC.
Hepatocellular carcinoma (HCC) is a very important health problem worldwide. HCC is the fifth most common cancer in the world and the most frequent primary hepatic neoplasia.1,2 Its estimated incidence is 0.5 to one million new cases per year.1–4 Even though the recognized risk factors include hemochromatosis and certain environmental toxins, hepatitis B virus (HBV) and hepatitis C virus (HCV) are the most predominant causal factors in HCC development worldwide. Cirrhosis of the liver is present in 50-80% of the patients that develop HCC;5 this disease has a critical impact in Mexico, given that it is the third cause of death in our population. HCC represents > 90% of the primary hepatic tumors in Mexico, as in other countries, and the HCC mortality rate in Mexico showed a 14% increase from the year 2000 to 2006.6,7
HCC staging systems are important for predicting patient outcome and guiding the therapeutic approach. Conventional prognostic systems for HCC such as the Okuda classification have certain limitations. New systems have currently been proposed and validated, such as the Barcelona Clinic Liver Cancer (BCLC) system that links disease stage with treatment strategy and the Cancer of the Liver Italian Program (CLIP) that is used in patients with advanced disease.8
HCC outcome continues to be very poor, with a 5-year survival rate of < 5% when there is no type of treatment.1 Up to now, resection and liver transplantation are the primary curative options for HCC. Liver transplantation offers a potential cure for HCC and also attends to the underlying cirrhosis. However, less than 20% of the patients receive curative liver resection (LR) and an even lower number of them receive a liver transplantation.9–11 Ablation therapy potential allows locoregional therapies to be carried out in HCC patients that would otherwise not be candidates for curative surgical treatment. Ablation therapy options for HCC include percutaneous ethanol injection, cryotherapy, radiofrequency ablation (RFA), and intra-arterial chemoembolization alone or combined with RFA.9,11,12In some cases these locoregional therapies are used as a bridge to liver transplantation for the patient.11 Survival after resection or ablation therapies in HCC has a wide percentage range due to the differences in the HCC stages analyzed in all the studies.10,12–17 The options of systemic treatment for unresectable HCC are limited. Recently, sorafenib has been approved by the Food and Drug Administration for systemic treatment of HCC.18 Likewise, few studies have analyzed HCC presentation and its results in Mexico; the majority have been carried out in medical centers in Mexico City at different points in time.19–25
AimThe aim of our study was to describe HCC presentation, staging, management, and results in patients presenting with the disease at our center.
MethodsForty-two patients diagnosed with HCC were evaluated for surgical treatment at the Centro Médico Nacional «Adolfo Ruiz Cortines» of the Instituto Mexicano del Seguro Social in Veracruz, Mexico within the time frame of July 2005 and March 2012. The patients were followed prospectively from their initial evaluation. The study was approved by the local institutional ethics committee. The factors analyzed included demographics (age, sex, and body mass index), the presence of cirrhosis, viral hepatitis detection, and laboratory values. The patients were assessed using the Child-Pugh score26 and the model for end-stage liver disease (MELD) scoring system.27
HCC diagnosis was made during the evaluation or before referral to our center. The laboratory values included in the study were for coagulation (prothrombin time and international normalized ratio), liver function tests (total bilirubin and serum albumin), serum creatinine, viral hepatitis detection, and alpha-fetoprotein (AFP). Radiologic studies such as computed axial tomography (CAT) and nuclear magnetic resonance (NMR) were carried out to determine the number of tumors and their size. All the patients that underwent LR or RFA had platelet counts above 100 x 103cells/mm3. All the patients were evaluated through chest CAT scan and bone scintigraphy to rule out the presence of extrahepatic metastases. Diagnosis was based on the established criteria8,11for 2cm lesions in cirrhotic patients: arterially hypervascularized lesions in 2 studies (CAT scan and NMR) or only in one when AFP was > 400 ng/ml. Conventional histopathologic studies with fine needle aspiration biopsy were ordered in the non-cirrhotic patients.
The patients were classified according to the Okuda system and the BCLC and CLIP classifications. The patients were grouped into 3 categories for this manuscript: no surgical treatment (NT), LR, and RFA. All RFAs were carried out through exploratory laparotomy or by laparoscopy when this was available to the patient (n = 2). Two cycles of RFA were performed when the lesions were smaller than 5cm and 3 to 4 cycles when they were larger than 5cm. Liver function tests were carried out and serum levels of AFP were determined each month in the outpatient follow-up. Liver ultrasound was ordered every 2 months and thoracoabdominal CAT scan every 6 months or sooner when there was recurrence suspicion. Tumor recurrence was defined by the increase in AFP and the appearance of radiologic tumors in the patients that underwent LR. Sorafenib use, when available to our unit, was recorded. Overall survival was the primary aim of the study. Survival was calculated from the moment of evaluation up to death or loss of follow-up. The patients were evaluated by treatment modality and classification/staging system.
Statistical analysisThe data were analyzed using the SPSS 21 (SPSS, 2012, Chicago, IL, USA) software. Means ± standard deviation and range were used for describing the continuous variables and frequency and percentages for the categorical variables. The variance analysis (ANOVA) was used for the continuous variables with normal distribution between the treatment groups and the chi-square test was used for the categorical variables. Survival was calculated with the Kaplan-Meier test and the log-rank (Mantel-Cox) test was employed to compare survival between groups. Median survival was recorded in months. Statistical significance was set at a p < 0.05.
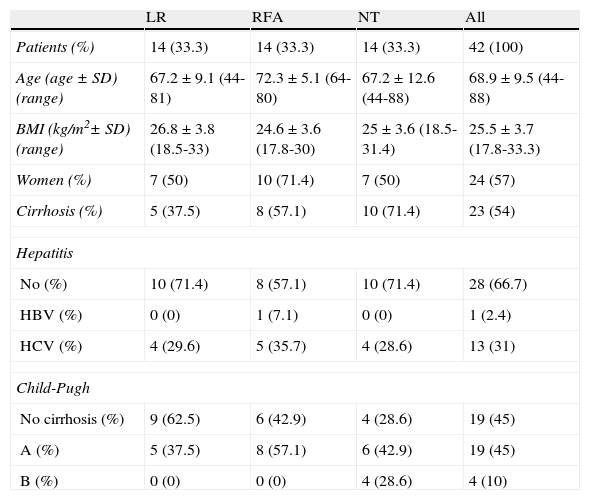
ResultsThe mean age of all the patients was 68.9 ± 9.5 years. The majority of patients were women (57%) and more than half of the patients presented with cirrhosis (54%). The most common viral hepatitis in our population was HCV (31%) and none of the patients were classified as Child-Pugh C. Patient demographic data, the presence of cirrhosis, viral hepatitis, and the Child-Pugh classification for all the patients and by treatment modality are shown in Table 1. Coincidentally, the same number of patients received surgical treatment (either LR or RFA) as those that did not (NT) (n = 14). Both RFA and LR were performed in the same procedure in one patient.
Clinical characteristics of the patients by groupa
| LR | RFA | NT | All | |
| Patients (%) | 14 (33.3) | 14 (33.3) | 14 (33.3) | 42 (100) |
| Age (age ± SD) (range) | 67.2 ± 9.1 (44-81) | 72.3 ± 5.1 (64-80) | 67.2 ± 12.6 (44-88) | 68.9 ± 9.5 (44-88) |
| BMI (kg/m2± SD) (range) | 26.8 ± 3.8 (18.5-33) | 24.6 ± 3.6 (17.8-30) | 25 ± 3.6 (18.5-31.4) | 25.5 ± 3.7 (17.8-33.3) |
| Women (%) | 7 (50) | 10 (71.4) | 7 (50) | 24 (57) |
| Cirrhosis (%) | 5 (37.5) | 8 (57.1) | 10 (71.4) | 23 (54) |
| Hepatitis | ||||
| No (%) | 10 (71.4) | 8 (57.1) | 10 (71.4) | 28 (66.7) |
| HBV (%) | 0 (0) | 1 (7.1) | 0 (0) | 1 (2.4) |
| HCV (%) | 4 (29.6) | 5 (35.7) | 4 (28.6) | 13 (31) |
| Child-Pugh | ||||
| No cirrhosis (%) | 9 (62.5) | 6 (42.9) | 4 (28.6) | 19 (45) |
| A (%) | 5 (37.5) | 8 (57.1) | 6 (42.9) | 19 (45) |
| B (%) | 0 (0) | 0 (0) | 4 (28.6) | 4 (10) |
RFA: radiofrequency ablation; BMI: body mass index; NT: no surgical treatment; LR: liver resection; HBV: hepatitis B virus; HCV: hepatitis C virus.
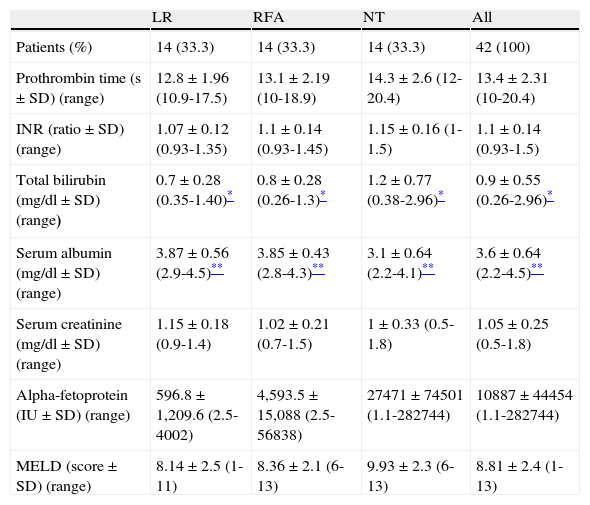
The liver function tests were worse in the NT patients than in those that underwent surgical treatment (either LR or RFA). There were statistically significant differences in total bilirubin and serum albumin values between the patients that underwent LR, RFA, and the NT group. Likewise, the NT patients had higher AFP values and MELD scores than the patients that underwent LR or RFA, but those differences were not statistically significant. Serum creatinine levels were similar in all the patients. All the laboratory values are shown in Table 2.
Patient laboratory values by treatment modality.
| LR | RFA | NT | All | |
| Patients (%) | 14 (33.3) | 14 (33.3) | 14 (33.3) | 42 (100) |
| Prothrombin time (s ± SD) (range) | 12.8 ± 1.96 (10.9-17.5) | 13.1 ± 2.19 (10-18.9) | 14.3 ± 2.6 (12-20.4) | 13.4 ± 2.31 (10-20.4) |
| INR (ratio ± SD) (range) | 1.07 ± 0.12 (0.93-1.35) | 1.1 ± 0.14 (0.93-1.45) | 1.15 ± 0.16 (1-1.5) | 1.1 ± 0.14 (0.93-1.5) |
| Total bilirubin (mg/dl ± SD) (range) | 0.7 ± 0.28 (0.35-1.40)* | 0.8 ± 0.28 (0.26-1.3)* | 1.2 ± 0.77 (0.38-2.96)* | 0.9 ± 0.55 (0.26-2.96)* |
| Serum albumin (mg/dl ± SD) (range) | 3.87 ± 0.56 (2.9-4.5)** | 3.85 ± 0.43 (2.8-4.3)** | 3.1 ± 0.64 (2.2-4.1)** | 3.6 ± 0.64 (2.2-4.5)** |
| Serum creatinine (mg/dl ± SD) (range) | 1.15 ± 0.18 (0.9-1.4) | 1.02 ± 0.21 (0.7-1.5) | 1 ± 0.33 (0.5-1.8) | 1.05 ± 0.25 (0.5-1.8) |
| Alpha-fetoprotein (IU ± SD) (range) | 596.8 ± 1,209.6 (2.5-4002) | 4,593.5 ± 15,088 (2.5-56838) | 27471 ± 74501 (1.1-282744) | 10887 ± 44454 (1.1-282744) |
| MELD (score ± SD) (range) | 8.14 ± 2.5 (1-11) | 8.36 ± 2.1 (6-13) | 9.93 ± 2.3 (6-13) | 8.81 ± 2.4 (1-13) |
RFA: radiofrequency ablation; INR: international normalized ratio; MELD: Model for End-Stage Liver Disease; NT: no surgical treatment; LR: liver resection.
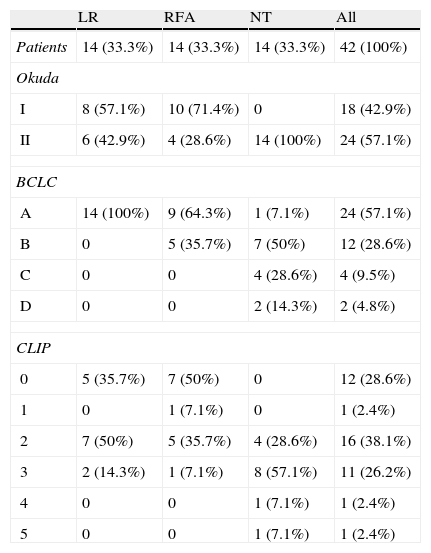
The mean tumor size was 6.48 ± 2.52cm (range: 2-13cm) and the median size was 6cm. Twenty-three patients (54.8%) had tumors larger than 5cm. The NT patients had larger tumors (7.36 ± 2.37cm, range: 5-13cm) than the patients that underwent RFA (6.07 ± 2.9cm, range: 3-13cm) and LR (6 ± 2.48cm, range: 2-9cm), but with no statistical significance. Thirteen LR patients (92.9%) had a solitary tumor and only one patient (7.1%) had 3 tumors. Likewise, in the RFA patients, 12 (85.7%) had one tumor and the rest of the patients in that group (n=2, 14.3%) had 2 tumors. Three NT patients (21.4%) had 3 tumors and 10 (71.4%) had only one tumor. The tumors were larger than 5cm in all the patients with more than one tumor. Fourteen patients (33.3%) had preoperative biopsy and a histopathologic diagnosis of HCC (5 patients that underwent LR, 5 that had RFA, and 4 NT patients).Table 3 shows patient staging in accordance with the Okuda, BCLC, and CLIP systems. The majority of the patients were classified as Okuda stage II (57.1%). All the patients that underwent LR were classified as initial BCLC stage (A). The majority of the NT patients (78.6%) had intermediate and advanced BCLC stages (B and C). The CLIP scores were also higher in the NT patients.
Patient staging by treatment type.
| LR | RFA | NT | All | |
| Patients | 14 (33.3%) | 14 (33.3%) | 14 (33.3%) | 42 (100%) |
| Okuda | ||||
| I | 8 (57.1%) | 10 (71.4%) | 0 | 18 (42.9%) |
| II | 6 (42.9%) | 4 (28.6%) | 14 (100%) | 24 (57.1%) |
| BCLC | ||||
| A | 14 (100%) | 9 (64.3%) | 1 (7.1%) | 24 (57.1%) |
| B | 0 | 5 (35.7%) | 7 (50%) | 12 (28.6%) |
| C | 0 | 0 | 4 (28.6%) | 4 (9.5%) |
| D | 0 | 0 | 2 (14.3%) | 2 (4.8%) |
| CLIP | ||||
| 0 | 5 (35.7%) | 7 (50%) | 0 | 12 (28.6%) |
| 1 | 0 | 1 (7.1%) | 0 | 1 (2.4%) |
| 2 | 7 (50%) | 5 (35.7%) | 4 (28.6%) | 16 (38.1%) |
| 3 | 2 (14.3%) | 1 (7.1%) | 8 (57.1%) | 11 (26.2%) |
| 4 | 0 | 0 | 1 (7.1%) | 1 (2.4%) |
| 5 | 0 | 0 | 1 (7.1%) | 1 (2.4%) |
RFA: radiofrequency ablation; BCLC: Barcelona Clinic Liver Cancer; CLIP: Cancer of the Liver Italian Program; NT: no surgical treatment; LR: liver resection.
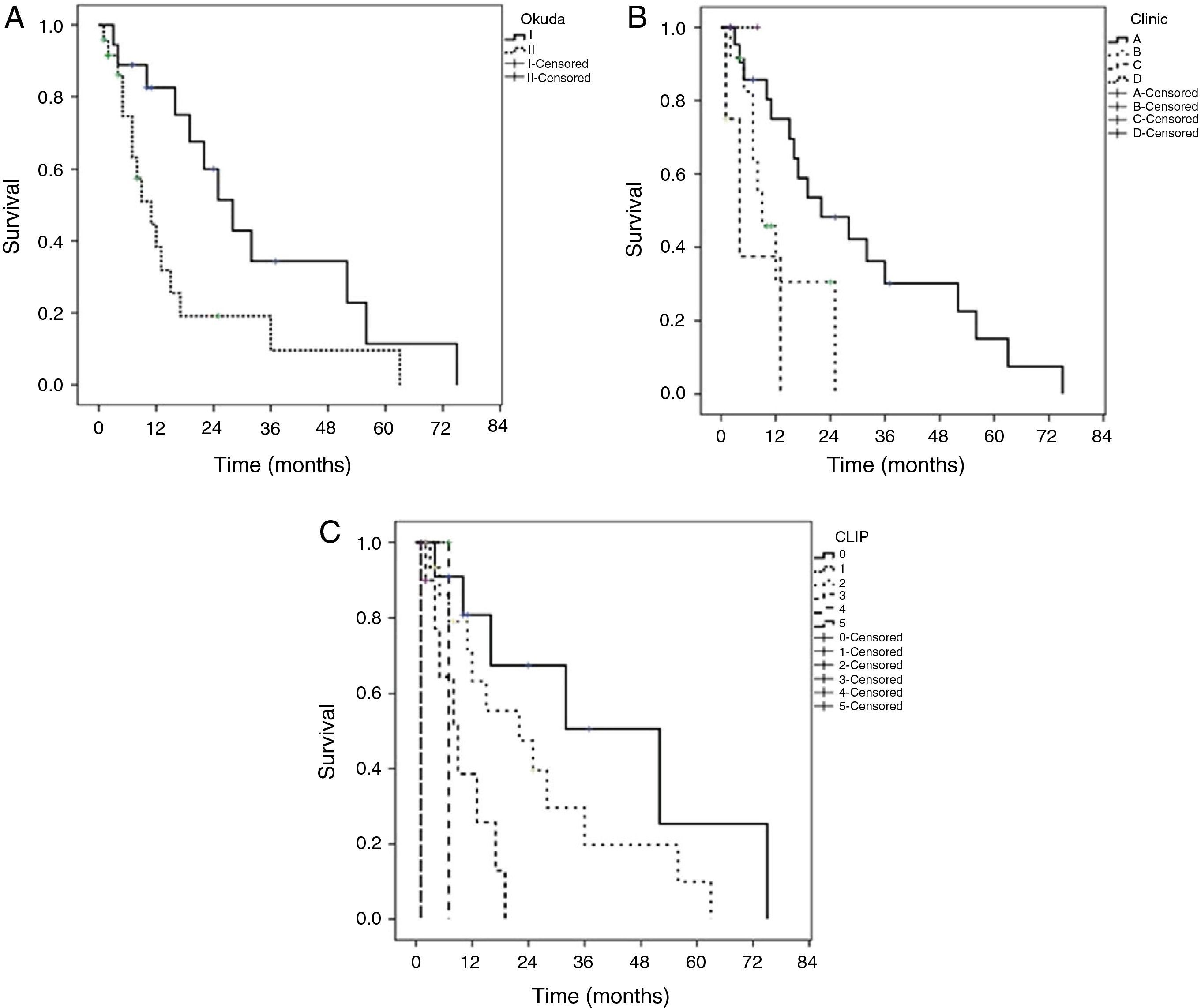
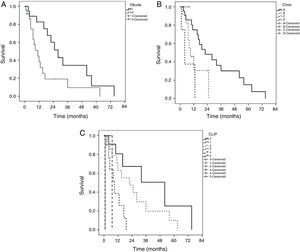
The median of survival was 16 ± 3.8 months (95% CI, 8.5-23.4) in all the patients, being higher in the patients that underwent LR with a low CLIP score and early stages according to the BCLC. Actuarial survival (Kaplan-Meier) was carried out in the Okuda, BCLC, and CLIP systems (fig. 1). The differences in the CLIP scores were statistically more significant (p = 0.0001). The patients with a CLIP score of 0 had a survival of 80.1, 50.6, and 25.1% at 1, 3, and 5 years, respectively. The patients with a CLIP score of 2 had a survival at 1, 3, and 5 years of 63.2, 19.7, and 10.1%, respectively. The patients with a CLIP score of 3 had a 1-year survival of 38.5% and a maximum survival of 19 months. Maximum survival for the patients with CLIP scores of 4 and 5 was 6.8 and 1.1 months, respectively. Only one patient with a CLIP score of 1 had a maximum survival of 6.8 months. The patients with an early phase BCLC classification (stage A) had the best actuarial survival rate (75.2, 30.1, and 15.3% for 1, 3, and 5 years, respectively.) The patients in the intermediate (B) and advanced (C) stages had a survival rate at 1 year of 30% and 37%, respectively. These differences were statistically significant between stages (p = 0.01). None of the patients classified as advanced stage (D) survived more than 12 months. Likewise, there were no patients in stages B and C that reached a 3-year period of survival. The patients in Okuda stage I had a better survival at 1, 3, and 5 years (88.9, 34.2, and 11.5%) than Okuda stage II (82.6, 19.1, and 9.6%). These differences were statistically significant (p = 0.023).
Kaplan-Meier curves by staging system. A) Okuda (p=0.023) classification between groups (log-rank test). B) BCLC (p=0.010) classification between groups (log-rank test). C) CLIP (p=0.0001) classification between groups (log-rank test).
BCLC: Barcelona Clinic Liver Cancer; CLIP: Cancer of the Liver Italian Program.
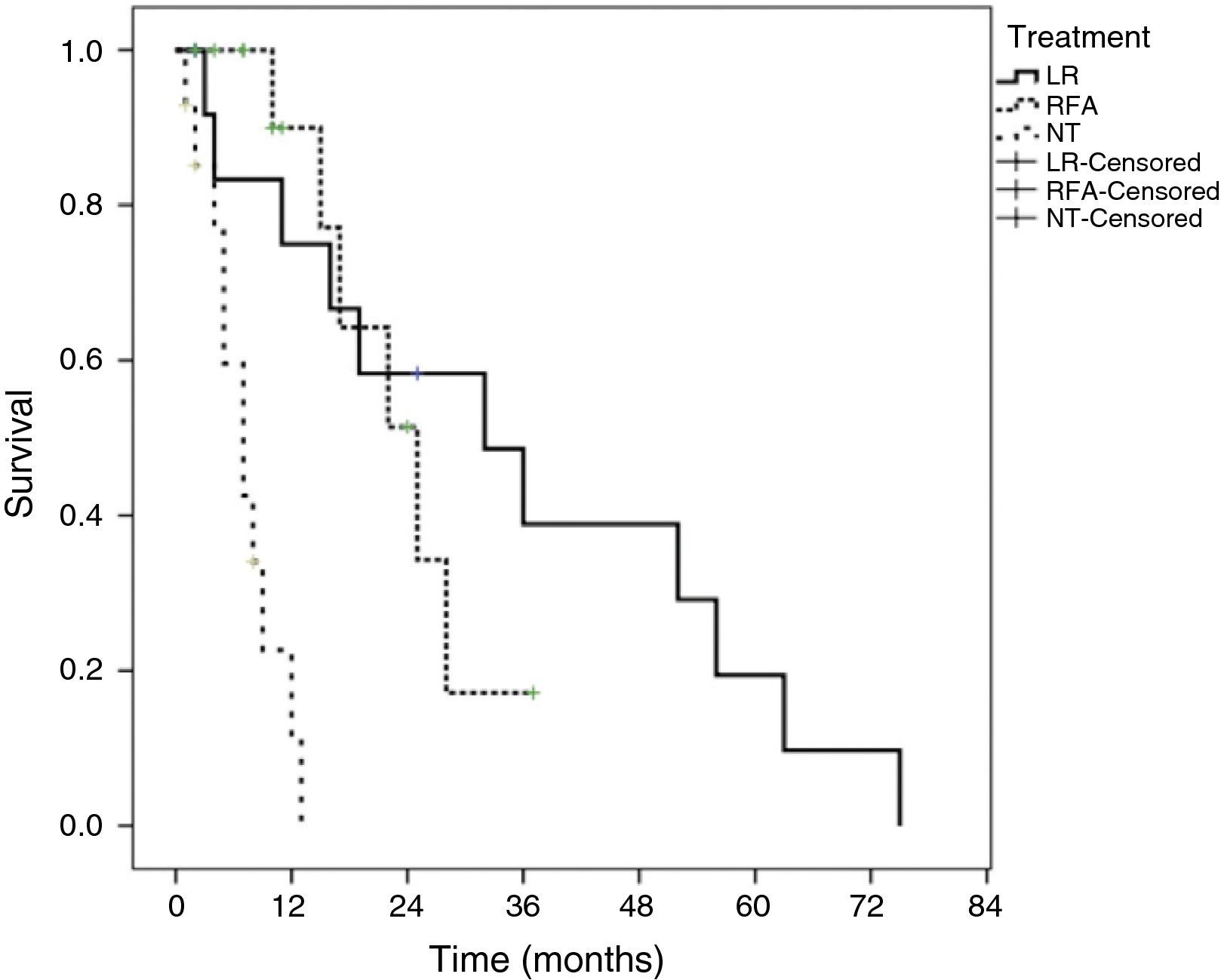
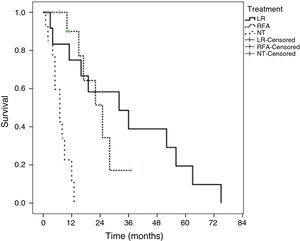
Survival according to treatment modality showed that patients that underwent LR had a better survival rate than the patients that underwent RFA or the NT patients (p = 0.0001). The median survival of the patients with LR was 32 ± 12.9 months (95% CI, 6.6-57.3). The median survival of the patients that underwent RFA was 25 ± 4.8 months (95% CI, 15.4 to 34.5) and it was 7 ± 1.6 months (95% CI, 8.5 to 23.4) for the NT patients. The actuarial survival rate at 1, 3, and 5 years of the patients with LR was 83, 39.1, and 19.7%, respectively. The patients that underwent RFA had a survival rate at 1 year of 90% and 17.2% at 3 years. Survival at one year in the patients that received no surgical treatment (NT) was under 5%. Figure 2 shows the Kaplan-Meier survival curves for all the treatment modalities.
And finally, tumor recurrence in the patients with LR was 21.4% (n = 3) at 18, 40, and 50 months, all at the surgical margin and in patients with anatomic resections. Two of these patients received sorafenib and the other had no treatment. Seven patients received sorafenib: 2 after LR, 2 NT patients, and 3 patients that underwent RFA.
DiscussionThere were similarities in the clinical characteristics of our patients with HCC in relation to comparisons with international and Mexican populations. HCC usually appears in patients above the age of 55 years, as was seen in our study, regardless of the geographic distribution (the United States and Asian countries). 13,16,19,24 Since 1999, Tsukuma, et al.5 demonstrated that cirrhosis was present in 50-80% of the patients that finally developed HCC. In 1999, Fong, et al.13 reported a 70% incidence of cirrhosis of the liver in HCC patients. The Mexican studies from that same year reported a 56% incidence of cirrhosis in the population19 and these high figures have been maintained with percentages close to or above 50%: thirty-eight percent in the study by Meza-Junco et al., 22 and 72% in Mexico City federal employees.24 Even though HBV is more common in the Asian countries in which HVC has reached up to 75%,5,12the association of HBV and HCV with HCC has also been described in the North American population.1,6,7 In Mexico, HCV is the second cause of cirrhosis of the liver, which is intimately related to HCC;6,7 studies conducted in Mexican institutions showed that HCV was present in up to 60% of the patients with HCC.21 Similar characteristics were found in our center, given that half of our patients had cirrhosis of the liver (54%) and HCV was present in almost one third of our population.
There is no current classification system for HCC that is accepted worldwide. The traditional classification systems, such as Okuda or Child-Pugh, do not include different outcome variables, such as portal hypertension grade, AFP, tumor size, and the functional status of the patient, and so they should be employed concomitantly with other systems.8 The BCLC classification and the CLIP score include the Child-Pugh classification and they are utilized with different outcome prediction capacities and patient treatment.8,9 The prognostic capacity of the Okuda classification is lower than the others8 and the prognostic strength of the CLIP score is better in the Western population.8 The BCLC classification also serves as a guide for the treatment of HCC in its first stages.9 The majority of the Mexican studies conducted on HCC use a single staging system, such as the Okuda19–22 or BCLC.24 Our study demonstrated predictive capacity in all the systems; the advanced stages had a statistically significant worse outcome than the early and intermediate stages. The CLIP score and the BCLC system had significantly better validity than the Okuda system.
Liver transplantation offers the best survival at 5 years in patients with HCC. However, less than 20% of the patients meet the Milan transplantation criteria (a single HCC smaller than 5cm or fewer than 3 nodules smaller than 3cm).9–11In Mexico, results of liver transplantation for HCC are limited28–32and there are no Mexican studies that describe complete experiences with all the HCC therapies. In the United States, experiences from individual institutions have been published on treatment results in patients with HCC in which 75% had tumors > 5cm, with a median survival of 39 months and an actuarial survival at 1, 3, and 5 years of 81, 54, and 37%, respectively.13 Ablation therapies (not RFA) had a median survival of 15 months.13 A sub-analysis of tumors at the same center that were >10cm described a median survival of 32 months and an actuarial survival at 5 years of 33% with no significant differences between the small (<10cm) and large (> 10cm) tumors.14 Another Western center10 found that only 30% of the evaluated patients with HCC were operated on (transplantation 13%, LR 12%, and RFA 5%). The best median survival corresponded to transplantation patients (100.3 months), followed by LR (44.5 months), and RFA (31.6 months) (p <0.05). Survival at one year for LR and RFA was 81 and 86%, respectively, survival at 3 years was close to 50% in both modalities (LR 57% and RFA 47%), and survival at 5 years was 47% for LR and 36% for RFA. The relation between HCC therapies and overall survival has recently been analyzed from 1973 to 2003 using the SEER database.17 The mean tumor size was 5cm with a solitary lesion in 52%. LR was performed in 16% of the patients with a 5-year survival rate of 35%. RFA was carried out in 25% of the patients with a 20% survival at 5 years.17 A SEER sub-analysis in patients with HCC under 5cm showed a mean survival of 45 months and a 39% 5-year survival rate after LR. Chinese studies have reported better mean and actuarial survival results with both LR16 and RFA,12 compared with Western studies. Our center's study had certain similarities to Western studies, such as mean tumor size, the number of solitary lesions, and a good actuarial 1-year survival rate with LR and RFA, but our mean survival rate was slightly lower for LR and RFA and the 5-year survival rate was lower when compared with the abovementioned centers. It is worth noting that even though our median survival was adequate, the 95% confidence interval was very wide, which could modify our estimated actuarial 5-year survival rate. Other factors could also have an influence on this same survival rate, such as age, patient comorbidities, and aspects related to LR itself, but these were not taken into consideration in our statistical analysis. The comparison of all treatment modalities was not feasible in our report because we do not perform liver transplantation. However, it should be mentioned that 42.8% (n = 18) of our patients would not be considered liver transplantation candidates according to the BCLC9 classification and 69% (n = 29) did not fit the Milan transplantation criteria. 9–11Among its services, our hospital functions as an institutional referral center for liver transplantation; as part of its referral criteria, patients must be under 65 years of age, and in relation to HCC, the Milan criteria is employed, both of which would limit the referral of our patients.
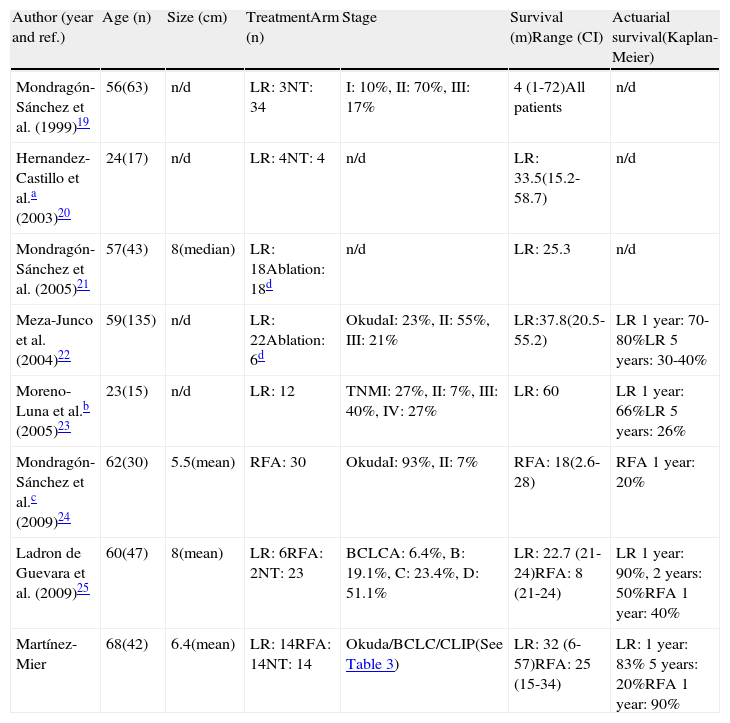
Few Mexican studies have been published that analyze the different HCC treatment modalities (Table 4). There were certain differences between those studies and our results: a) not taking into account the HCC studies on patients < 40 years of age 20 and the patients with fibrolamellar HCC,23 the patients at our center were older than those in the other studies, b) there was a similar number of LR and RFA procedures in our study, c) only 2 studies, besides ours, included RFA as therapy for HCC, and one of them included all types of hepatic tumors,24d) our study included various staging systems for HCC, and e) the median survival of patients that underwent RFA was higher in our study and the survival rate in LR was similar or superior. Despite the fact that these differences strengthen our study, we recognize that it has the following limitations, in addition to the lack of liver transplantation results: our center does not perform transhepatic arterial chemoembolization,23,25 as has been suggested for patients with BCLC stage B, which has resulted in a more abundant performance of RFA since our initial utilization of this procedure in 2005,33 thus possibly modifying our results; and the utilization of sorafenib in our center is irregular, as well, which prevented us from statistically analyzing its use.
Mexican studies on HCC presentation and results.
| Author (year and ref.) | Age (n) | Size (cm) | TreatmentArm (n) | Stage | Survival (m)Range (CI) | Actuarial survival(Kaplan-Meier) |
| Mondragón-Sánchez et al. (1999)19 | 56(63) | n/d | LR: 3NT: 34 | I: 10%, II: 70%, III: 17% | 4 (1-72)All patients | n/d |
| Hernandez-Castillo et al.a (2003)20 | 24(17) | n/d | LR: 4NT: 4 | n/d | LR: 33.5(15.2-58.7) | n/d |
| Mondragón-Sánchez et al. (2005)21 | 57(43) | 8(median) | LR: 18Ablation: 18d | n/d | LR: 25.3 | n/d |
| Meza-Junco et al. (2004)22 | 59(135) | n/d | LR: 22Ablation: 6d | OkudaI: 23%, II: 55%, III: 21% | LR:37.8(20.5-55.2) | LR 1year: 70-80%LR 5years: 30-40% |
| Moreno-Luna et al.b (2005)23 | 23(15) | n/d | LR: 12 | TNMI: 27%, II: 7%, III: 40%, IV: 27% | LR: 60 | LR 1year: 66%LR 5years: 26% |
| Mondragón-Sánchez et al.c (2009)24 | 62(30) | 5.5(mean) | RFA: 30 | OkudaI: 93%, II: 7% | RFA: 18(2.6-28) | RFA 1year: 20% |
| Ladron de Guevara et al. (2009)25 | 60(47) | 8(mean) | LR: 6RFA: 2NT: 23 | BCLCA: 6.4%, B: 19.1%, C: 23.4%, D: 51.1% | LR: 22.7 (21-24)RFA: 8 (21-24) | LR 1year: 90%, 2years: 50%RFA 1year: 40% |
| Martínez-Mier | 68(42) | 6.4(mean) | LR: 14RFA: 14NT: 14 | Okuda/BCLC/CLIP(See Table 3) | LR: 32 (6-57)RFA: 25 (15-34) | LR: 1year: 83% 5years: 20%RFA 1year: 90% |
RFA: radiofrequency ablation; BCLC: Barcelona Clinic Liver Cancer; CLIP: Cancer of the Liver Italian Program; n/d: not determined; NT: no surgical treatment; LR: liver resection; TNM: tumor node metastasis.
In conclusion, the patients with HCC in our center that were diagnosed in late stages of the disease have similar characteristics to those of other Mexican populations. Based on the CLIP and BCLC scoring systems, the outcome for our patients was similar to that reported in the literature. The best results were observed in patients with early stage disease and in those that underwent surgical resection of the HCC. Opportune diagnosis of these patients is essential for establishing their disease management and, in turn, referring them to the specialized centers that offer the treatment option corresponding to their disease stage.
Financial disclosureNo financial support was received in relation to this article.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Martinez-Mier G, Esquivel-Torres S, Medina Granados JP, Luna-Castillo M, Castillo-Chiquete R, Calzada-Grijalva JF, et al. Presentación, clasificación y evolución de los pacientes con carcinoma hepatocelular en un centro de Veracruz, México. Revista de Gastroenterología de México. 2014;79:171–179.