Management of the patient with cirrhosis of the liver that requires surgical treatment has been relatively unexplored. In Mexico, there is currently no formal stance or expert recommendations to guide clinical decision-making in this context.
AimsThe present position paper reviews the existing evidence on risks, prognoses, precautions, special care, and specific management or procedures for patients with cirrhosis that require surgical interventions or invasive procedures. Our aim is to provide recommendations by an expert panel, based on the best published evidence, and consequently ensure timely, quality, efficient, and low-risk care for this specific group of patients.
ResultsTwenty-seven recommendations were developed that address preoperative considerations, intraoperative settings, and postoperative follow-up and care.
ConclusionsThe assessment and care of patients with cirrhosis that require major surgical or invasive procedures should be overseen by a multidisciplinary team that includes the anesthesiologist, hepatologist, gastroenterologist, and clinical nutritionist. With respect to decompensated patients, a nephrology specialist may be required, given that kidney function is also a parameter involved in the prognosis of these patients.
El terreno del paciente con cirrosis que requiere de una intervención quirúrgica ha sido poco explorado. En México, a la fecha no contamos con un posicionamiento formal o recomendaciones de expertos que ayuden a la toma de decisiones clínicas en este contexto.
ObjetivosRevisar la evidencia existente sobre el riesgo, pronóstico, precauciones, cuidados especiales y manejo o proceder específico para los pacientes con cirrosis que requieren ser intervenidos quirúrgicamente o mediante procedimientos invasivos, para emitir recomendaciones por un panel experto, basadas en la mejor evidencia publicada para la atención oportuna, de calidad, eficiente y con el menor riesgo posible en este grupo específico de pacientes.
ResultadosSe obtuvieron 27 recomendaciones, en donde se abordan el terreno preoperatorio, el escenario transoperatorio y el seguimiento y cuidados postoperatorios.
ConclusionesLa valoración y cuidado del paciente con cirrosis que requiere un procedimiento quirúrgico o invasivo mayor, debe estar a cargo de un equipo multidisciplinario que brinde soporte al cirujano, durante todo el perioperatorio, este equipo debe incluir al anestesiólogo, hepatólogo, gastroenterólogo, nutriólogo clínico. En el paciente descompensado, puede ser necesario involucrar especialistas en nefrología ya que la función renal es un parámetro implicado también en el pronóstico de estos pacientes.
Liver fibrosis and cirrhosis of the liver are chronic conditions that increase patient morbidity and mortality.1–6 These patients are also more vulnerable to complications from invasive or surgical procedures, requiring special care to prevent decompensation of their underlying disease after a surgical event. Thus, they should undergo a detailed preoperative evaluation, monitored to prevent volume overload during the intraoperative period, especially if they also present with clinically significant portal hypertension (CSPH).7 Few studies have evaluated this context, and in Mexico, there are no formal recommendations for the optimum care of these patients. Thus, the present consensus panel reviewed the existing evidence on risks, prognoses, precautions, special care, and specific management or actions, regarding patients with cirrhosis that require surgical intervention or invasive procedures, to issue expert recommendations, based on the best published evidence, for the specific aim of providing timely, quality, efficient, and low-risk care to this group of patients.
MethodologyThe board of directors and scientific committee of the Asociación Mexicana de Gastroenterología A.C. (AMG) designated two general coordinators, José Antonio Velarde Ruiz Velasco (JAVRV) and Fátima Higuera de la Tijera (FHT), who equally contributed to the conceptualization of the present manuscript. JAVRV and FHT carried out a review of the bibliography, utilizing the words “surgery, cirrhosis” as search criteria in combination with the following terms: “care” or “evaluation”, “preoperative period”, “postoperative period”, “perioperative period”, “intraoperative period”, “prognosis”, “complications”, “special care”, “scales”; and the equivalent terms in Spanish. The search was conducted on PubMed, Google Scholar, Scopus, Medline, Embase, Science Direct, and the TRIP Database, considering articles published within the period of January 2010 to June 2023. All publications in English and Spanish were included. The search gave preference, but was not limited to, consensuses, guidelines, systematic reviews and meta-analyses, clinical trials, and cohort studies. Complementary electronic and manual searches of the archives of the Revista de Gastroenterología de México were also conducted, as well as of all publications up to June 2023 that the coordinators considered relevant. After this search and the analysis of the publications, it became apparent that there were very few high-quality original studies evaluating the theme at hand, and not enough systematic reviews and meta-analyses, clinical trials, and cohort studies with sufficient methodological quality for designing a clinical guideline. Therefore, the decision to carry out a consensus utilizing the modified Delphi method8 was made, to arrive at a general agreement among experts on the theme.
During the consensus process, the following sections were specifically reviewed: 1) Evaluation of the preoperative panorama in cirrhosis, 2) Intraoperative setting, and 3) Follow-up and postoperative care. After carrying out an exhaustive search of each theme, the Patient, Intervention, Comparison, Outcomes (PICO) system was employed for creating questions with four characteristics: P: referred/affected population, I: type of intervention to analyze, C: comparator, and O: results obtained after the data analysis or systematic review. From there, the representative statements were formulated and sent to all the members of the expert panel (except JAVRV, who acted as data monitor and analyst) for a first online, anonymous round of voting, utilizing the following scale: A) complete agreement, B) partial agreement, C) uncertain, D) partial disagreement, E) complete disagreement. In this first round of voting, the panelists could also make suggestions for improving the writing and quality of each statement. When agreement was above 75% (sum of A and B), the statement could be ratified in the next round of voting. Statements in which disagreement was 75% or higher (sum of D and E) were eliminated. The statements with agreement or disagreement below 75% were reformulated, taking into account the comments of the panelists. Two rounds of anonymous online voting and one round conducted on a live Zoom® call were needed to produce the statements. Twenty-seven final statements were obtained, the evidence sustaining each statement was evaluated and graded according to the Grading of Recommendations Assessment, Development and Evaluation” (GRADE) system9 (Table 1).
GRADE system.9
| Levels of evidence | |
|---|---|
| High | We are relatively confident that the true effect of the intervention is similar to the estimated effect |
| Moderate | The true effect is probably close to the estimated effect but there is the possibility that it is markedly different |
| Low | The true effect of the intervention might be markedly different from the estimated effect |
| Very low | The true effect is probably markedly different from the estimated effect |
| Grades of recommendation | ||
|---|---|---|
| Strong recommendationa | Weak recommendationa | |
| Meaning | The recommended alternative can be followed by all or almost all patients. A detailed conversation with the patient or a careful review of the evidence the recommendation is based on may not be necessary | Even though the recommended alternative is appropriate for the majority of patients, the decision should be individualized, ideally through a shared decision-making process |
| Quality of evidence | Generally high or moderate (low or very low in certain exceptional circumstances) | Low or very low |
| Risk-benefit balance | An alternative is clearly superior | There is a close risk-benefit balance |
| Patient values and preferences | All or almost all informed patients make the same decision | There is substantial variation or uncertainty in decisions made by informed patients |
| Resource considerations | The cost of the intervention is thoroughly justified | The cost of the intervention may not be justified in all circumstances |
GRADE: Grading of Recommendations Assessment, Development and Evaluation.
- 1
In patients that present with a chronic liver disease of any etiology and require an elective surgical procedure, evaluating the presence of advanced fibrosis through noninvasive methods is recommended.
Complete agreement 76.6%, partial agreement 21.4%
Level of evidence, moderate; strong, in favor of the recommendation
The condition that increases the risk of postoperative complications in patients with chronic liver disease is the impairment of hepatic synthesis, in conjunction with the presence of CSPH. Given this, chronic liver disease with no fibrosis should not substantially increase the risk for surgical complications. However, patients with compensated cirrhosis are asymptomatic, which is why the noninvasive evaluation of liver fibrosis prior to an elective surgical procedure is recommended in patients with chronic liver disease, regardless of etiology (viral hepatitis, autoimmunity, metabolic dysfunction-associated steatotic liver disease [MASLD], and alcohol use, among others) that do not have known diagnostic or clinical manifestations of cirrhosis and CSPH.1–2
The fibrosis-4 index (FIB-4) is one of the most validated noninvasive serologic methods, in the context of different etiologies that condition cirrhosis, and is considered adequate for the initial patient evaluation.3 A historic cohort study that included 19,861 subjects found that 10% presented with advanced fibrosis according to the FIB-4. In an adjusted multivariate model, a FIB-4 ≥ 2.67 (advanced fibrosis/cirrhosis) was independently associated with an increase in intraoperative mortality (odds ratio [OR] 3.63, 95% confidence interval [CI] 1.25–10.58), greater in-hospital mortality (OR 3.14, 95% CI 2.37–4.16), as well as within 30 postoperative days (OR 2.46, 95% CI 1.95–3.10). An increase is mortality was evident, the higher the predefined FIB-4 category: ≤ 1.3 (reference), > 1.3 and < 2.67, and ≥ 2.67, respectively; during hospitalization (OR 1.89, 95% CI 1.34–2.65 and OR 4.70, 95% CI 3.27–6.76), and within 30 postoperative days (OR 1.77, 95% CI 1.36–2.31 and OR 3.55, 95% CI 2.65–4.77). In a 1:1 propensity-matched sample (n = 1,994 per group), the differences in mortality persisted. Comparing the FIB-4 ≥ 2.67 vs the FIB-4 < 2.67 groups, respectively, mortality during hospitalization was 5.1% vs 2.2% (OR 2.70, 95% CI 1.81–4.02) and mortality at 30 days was 6.6% vs 3.4% (OR 2.26, 95% CI 1.62–3.14).4
Of the radiologic noninvasive methods, transient elastography (Fibroscan®) has also been shown to be useful for estimating prognosis in patients that will electively be programmed for surgery. In 105 patients that underwent heart surgery, preoperative liver stiffness measurement (LSM) ≥ 9.5 kPa was associated with significantly longer postoperative hospitalization, compared with patients with a LSM < 6 kPa. Said association appeared to be independent of the preoperative comorbidities commonly associated with coronary disease.5
Patients with advanced fibrosis and those with cirrhosis have a higher risk of postoperative complications. After colorectal surgery, the general anastomotic leak rate has been estimated at 2.7%. In patients with cirrhosis or advanced fibrosis, the anastomotic leak rate has been reported at 12.5%, whereas it has been reported at only 2.5% in patients without cirrhosis or advanced fibrosis (p = 0.024).6
- 2
The staging of liver disease severity in patients with cirrhosis should be evaluated through the MELD and Child-Pugh scales.
Complete agreement 76.6%, partial agreement 21.4%
Level of evidence, high; strong, in favor of the recommendation
The preoperative evaluation of patients with cirrhosis should focus on identifying the factors that can increase perioperative morbidity and mortality. In addition to evaluating cardiovascular risk, comorbidities, and functional status, as is performed on any patient that will undergo surgery, evaluating the grade of liver dysfunction by determining the presence of CSPH and current or previous clinical decompensation (ascites, variceal bleeding and/or hepatic encephalopathy [HE]) is indispensable.7 The Child-Turcotte-Pugh (CTP) and Model for End-stage Liver Disease (MELD) scores are the most widely used for evaluating liver disease severity.10 The CTP classification predicts long-term survival in patients with cirrhosis.11 Higher CTP scores are associated with an increase in perioperative morbidity and mortality. Patients with CTP class A have a 10% risk of in-hospital death after surgery, whereas those with class B have a 30% risk. Said risk increases to 76–82% in patients with CTP class C.12 Likewise, the MELD score predicts mortality at 3 months in patients with cirrhosis that are on the liver transplant waiting list.13,14 It has been shown to be a good preoperative predictor for surgical mortality; for every MELD point above 20, there is a 2% increase in the mortality rate.15 The performance of the two prognostic scales for predicting the surgical outcome in patients with cirrhosis is similar (area under the curve [AUROC] 0.755 ± 0.066 for MELD vs AUROC 0.696 ± 0.070 for CTP, p = 0.3).2 Some studies have also shown that the combination of the CTP and MELD scores is a better mortality predictor than each of the scales on their own.16
Serum sodium (Na) levels are an independent mortality predictor in cirrhosis.17 New models based on the MELD scale have been described. The MELD-Na incorporates the level of sodium, the integrated iMELD incorporates sodium and age, and the MESO is the MELD to sodium ratio, and they are superior to the MELD score for predicting short-term and intermediate-term prognoses in patients with decompensated cirrhosis.18 These indexes based on MELD have been shown to have an adequate prognostic performance in patients with cirrhosis that undergo elective surgery. The iMELD is the best predictive parameter of operative mortality (AUROC 0.80, 95% CI 0.63–0.97, p = 0.04), with a predictive potential superior to the CTP and MELD.19
Nevertheless, both MELD and MELD-Na utilize the serum creatinine level, which could overestimate kidney function in patients with sarcopenia and women with poor muscle mass. Due to those limitations, new prognostic scores, such as the MELD 3.0, have been developed. The MELD 3.0 includes female sex and the serum albumin level, the interactions between bilirubin and sodium, the interactions between albumin and creatinine, and has an upper limit for creatinine (3.0 mg/dl). It has been shown to predict mortality with greater accuracy, compared with the MELD-Na, in patients with cirrhosis that are on the transplant waiting list.20
Hospital mortality varies substantially regarding the type of surgical procedure.21 In general, the outcome is better in elective surgeries,10 reporting a postoperative mortality up to 6-times higher in emergency surgery.22 Abdominal wall surgeries, such as umbilical and inguinal hernioplasties, cholecystectomy, and minimally invasive surgeries have less morbidity and mortality, whereas pancreatic surgery, major abdominal surgery, cardiovascular surgery, and trauma surgery have the highest morbidity and mortality rates.21,23,24 The majority of procedures can be safely performed in patients with no CSPH, with CTP A, or with low MELD scores.13 In the elective repair of inguinal hernias, patients with a MELD score < 15 and that are CTP class A or B, can be operated on with no significant increase in mortality.25Table 2 summarizes the reported 30-day mortality rate for the different types of surgery, whether elective or emergency procedures.
- 3
All patients with cirrhosis that will undergo an elective surgical procedure should be evaluated for the presence of clinically significant portal hypertension and liver decompensation.
Mortality associated with the type of surgery in cirrhosis.22,23
| Type of surgery | 30-day mortality (%) | Reference | ||
|---|---|---|---|---|
| Elective | Emergency | |||
| Gastrointestinal surgery | Hernioplasty (inguinal/femoral or umbilical) | 0.6–1 | 8–12.7 | 20 |
| Cholecystectomy | 0.9 | 5.6 | ||
| Appendicectomy | 4.1 | 1.8 | ||
| Pancreatic surgery | 3.7 | 16.7 | ||
| Gastric surgery | 4.2 | 38.6 | ||
| Esophageal surgery | 5.5 | 33.3 | ||
| Colorectal resection | 7 | 35.4 | ||
| Cardiothoracic and vascular surgery | Cardiac surgery | 1.8–17 | 21 | |
| Lung cancer surgery | 2.7–6.5 | |||
| Trauma and orthopedic surgery | Trauma surgery | 11.5–45 | ||
| Hip and knee surgery | 60 | |||
Complete agreement 85.7%, partial agreement 14.3%
Level of evidence, moderate to high; strong, in favor of the recommendation
Portal hypertension is the main consequence of cirrhosis of the liver and the cause of the majority of its complications: ascites, variceal bleeding, and HE.26 Cirrhosis is classified into two distinct prognostic stages: compensated and decompensated.27 Compensated cirrhosis, or compensated advanced chronic liver disease (cACLD) is further subdivided, based on the presence or absence of CSPH, which is defined as a hepatic venous pressure gradient (HVPG) ≥ 10 mmHg or by the presence of clinical manifestations, such as ascites or esophagogastric varices,26 and is associated with an increased risk for developing events of clinical decompensation, esophageal variceal bleeding, and hepatocellular carcinoma.28–30
The presence of CSPH is associated with an increase in perioperative mortality.31 Therefore, in addition to complete questioning about previous clinical decompensation and the physical examination for evaluating the presence of clinical manifestations of CSPH, the performance of an esophagogastroduodenoscopy (EGD) for determining the presence of esophageal varices and/or portal hypertensive gastropathy and an imaging study, such as ultrasound or computed tomography, for evaluating the presence of ascites, splenomegaly, and portosystemic collaterals, should be carried out in the preoperative evaluation of patients with cirrhosis.32
Measuring the HVPG, the gold standard for evaluating portal pressure, could be necessary in some cases. It is an invasive procedure that involves catheterization of the right or middle suprahepatic vein and is the gradient between the pressure of the hepatic sinusoidal capillary network and the systemic pressure.26,27,33 HVPG values ≥ 16 mmHg, especially a value ≥ 20 mmHg, have been shown to be associated with an elevated risk of postoperative mortality, whereas there is less risk of decompensation in the postoperative period, with a HVPG value < 10 mmHg.34
The LSM correlates with the HVPG.35 A recent publication reports that a LSM ≥ 25 kPa in patients with cACLD is sufficient for diagnosing CSPH, and a measurement ≤ 15 kPa and platelet count > 150 × 109/l rule it out. In addition, patients with LSM between 20 and 25 kPa and platelet count < 150 × 109/l or LSM between 15 and 20 kPa and platelet count < 110 × 109/l, have close to a 60% risk of CSPH.26,27,35–37
Spleen stiffness measurement (SSM) significantly improves the capacity to diagnose CSPH. The study by Dajti et al.38 validated the Baveno VII (LSM ≤ 15 kPa + platelet count ≥150 × 109/l for ruling out CSPH and LSM > 25 kPa for confirming it) diagnostic algorithm. However, between 40 and 60% of patients remained in the gray zone. The addition of the SSM (40 kPa) to the model significantly reduced the gray zone to 7–15%, maintaining adequate negative predictive values and positive predictive values. All the decompensation events occurred in the “confirmation” zone of the model that included the SSM. Therefore, when said tool is available, it is recommendable to incorporate it into the patient evaluation.
- 4
Conducting a preoperative evaluation of nonhepatic comorbidities is recommended. It should include the habitual preoperative evaluation (electrocardiogram for estimating cardiovascular disease and the American Society of Anesthesiologists scale and Goldman index determinations).
Complete agreement 85.7%, partial agreement 14.3%
Level of evidence, high; strong, in favor of the recommendation
The surgical risk in patients with cirrhosis is based on multiple factors: the evaluation of liver function, the presence of CSPH, the urgency of the procedure, the type of procedure, and the presence of comorbidities. Patients with cirrhosis have a higher risk of presenting with coagulopathy, malnutrition, immune dysfunction, cirrhotic cardiomyopathy, pulmonary alterations, and kidney disorders.39,40
Regarding the estimation of cardiovascular function, the preoperative evaluation should include the American Society of Anesthesiologists (ASA) classification. Patients with cirrhosis in the compensated phase are classified as ASA III and in the decompensated phase as ASA IV, signifying that the surgical risk is considerable at the onset (Table 3).41
- 5
Prior to surgery, calculating the scores of the Mayo and VOCAL-Penn risk indexes is recommended for predicting the surgical risk and postoperative mortality of patients with cirrhosis.
Physical status classification of the American Society of Anesthesiologists (ASA).40
| Classification | Definition |
|---|---|
| ASA I | Healthy patient |
| ASA II | Patient with systemic disease with no functional limitations |
| ASA III | Patient with severe systemic disease with defined functional limitation |
| ASA IV | Patient with severe systemic disease that is life-threatening |
| ASA V | Moribund patient not expected to survive beyond the next 24 hours, with or without surgery |
Complete agreement 92.9%, partial agreement 7.1%
Level of evidence, moderate; strong, in favor of the recommendation
The preoperative evaluation of patients with cirrhosis of the liver requires a detailed clinical history, thorough physical examination, and the calculation of the Mayo and VOCAL-Penn scores. The Mayo score was the first model for predicting surgical risk in patients with cirrhosis of the liver. It evaluates the ASA classification (3 for compensated cirrhosis and 4 for decompensated cirrhosis), the international normalized ratio (INR), total bilirubin, creatinine, age, and liver disease etiology. It estimates mortality at 7 days, 30 days, 90 days, 1 year, and 5 years, with the limitations of risk overestimation and lack of stratification according to type of surgery, as well as not taking into account all etiologies. Access to this score is free and an online calculator is available at the website of the Mayo Clinic: https://www.mayoclinic.org/medical-professionals/transplant-medicine/calculators/post-operative-mortality-risk-in-patients-with-cirrhosis/itt-20434721.42 After its validation, a progressive increase in the overestimation of the risk of actual mortality, possibly due to improved surgical techniques, was observed, limiting its use to cardiac surgery, major abdominal surgery, and orthopedic surgery.43
The VOCAL-Penn score was recently developed for estimating the surgical risk in patients with cirrhosis. It includes the following parameters evaluated in the preoperative period: age, albumin, total bilirubin, platelet count, body mass index (BMI), etiology due to MASLD, type of surgical procedure (laparoscopic or open abdominal surgery, abdominal wall surgery, vascular surgery, orthopedic surgery, or cardiac surgery), indication (elective or emergency), and the ASA classification. Access to this score is free and available online at: https://www.vocalpennscore.com/. It estimates the risk of postoperative mortality at 30, 90, and 180 days, and its discrimination capacity is superior to the MELD, MELD-Na, CTP, and Mayo risk index. In addition, it can estimate the risk for liver decompensation at 90 days. This index includes variables dependent on the individual, type of surgery, type of hepatopathy, has been externally validated, predicts mortality and decompensation, is easy to use, and is free of charge, making it extremely useful. Perhaps its biggest drawback is the fact that the data have been obtained almost exclusively in men.44–46 It should be pointed out that these surgical risk predictors are helpful in the evaluation and clinical decision-making, but they do not take frailty or sarcopenia into account in the evaluation. Therefore, they do not substitute medical criteria when weighing the risk-benefit of the surgical interventions.22–24
- 6
In patients with cirrhosis that require an elective surgical procedure, a multidisciplinary evaluation is recommended that should include specialists in hepatology or gastroenterology, internal medicine, nutrition, anesthesiology, and the area of surgery involved.
Complete agreement 92.9%, partial agreement 7.1%
Level of evidence, low; weak, in favor of the recommendation
The postoperative morbidity and mortality rate is higher in patients with cirrhosis.22,23 This increase in mortality has been associated with different factors that characterize the patient with cirrhosis, such as clotting disorders, thrombocytopenia, platelet dysfunction, increased systemic inflammation, bacterial translocation, hyperdynamic circulation, cirrhotic myocardiopathy, sarcopenia, and micronutrient deficiency that lead to an increased risk of thrombosis, infections, bleeding, kidney injury, altered cicatrization, and liver decompensation. For these reasons, the preoperative evaluation should include the participation of a multidisciplinary team (hepatology, gastroenterology, internal medicine, nutrition, anesthesiology, cardiology, and surgery).32,39 Of course, in emergency surgery, the procedure should never be delayed if this multidisciplinary team is not available, and each case should be individualized, without putting the patient’s life at risk by deferring the procedure while waiting for said evaluations. Whenever viable or possible, patients with decompensated cirrhosis should be evaluated and treated at specialized centers.
Likewise, prehabilitation, a concept that is obviously only possible in elective surgery, is useful for achieving better conditions for the patient prior to being operated on. Considered complementary, it entails the prescription of a programmed physical exercise plan, aerobic or anaerobic, designed according to the functional situation and other comorbidities of the patient, supervised by a physical therapist, and aimed at results. In addition, the removal of toxic agents, especially alcohol and tobacco, and achieving glycemic control and the control of other comorbidities are relevant.39
- 7
Prior to a surgical intervention, having a complete nutritional evaluation that includes the detection of sarcopenia and frailty is recommended in patients with cirrhosis. In cases of elective surgery, strategies should be implemented that enable said conditions to be improved before the procedure, given that they both have a negative impact on postoperative prognosis and survival.
Complete agreement 85.7%, partial agreement 14.3%
Level of evidence, moderate to low; weak, in favor of the recommendation
Sarcopenia is associated with greater mortality in patients with cirrhosis, and with respect to surgery, is related to delayed wound healing and poor postoperative progression, a higher risk for liver decompensation, lower quality of life, higher risk of infection, and prolonged hospitalization. It increases the mortality rate in post-transplanted patients, as well.47,48
Ideally, all patients with cirrhosis should undergo a comprehensive nutritional evaluation. The prevalence of malnutrition and sarcopenia in patients with cirrhosis is reported at 80% and 25 to 70%, respectively.47 In Mexico, the prevalence of malnutrition has been reported at 59% in hospitalized patients with cirrhosis and at 88% for sarcopenia.49 In another Mexican study, a general prevalence of malnutrition was reported at 54%; compensated patients had a prevalence of malnutrition of 37–50%, whereas it reached 60–71% in decompensated patients. In the multivariate analysis, there was higher mortality in patients with malnutrition, with a hazard ratio (HR) of 2.15 and a 95% CI of 1.18–3.922 (p = 0.024).50 Likewise, malnutrition in patients with cirrhosis has been associated with a worse quality of life, greater generalized pain, dyspnea during daily activities, reduced appetite, generalized weakness, and a decreased level of energy.51
Nutritional status can be evaluated through several tools, and currently, the most highly recommended are the Royal Free Hospital-Nutritional Prioritizing Tool (RFH-NPT) and the Royal Free Hospital-Subjective Global Assessment (RFH-SGA). Importantly, the BMI is not an adequate tool, given that it underestimates malnutrition cases.47,49,52
Multiple instruments for detecting sarcopenia in the patient with cirrhosis have been proposed and the tomographic skeletal muscle index at L3 is considered the ideal method but it is not an easily accessible study. Other easily accessible and reproducible tools are anthropometric evaluation and handgrip strength, the latter of which can also be utilized as a tool for diagnosing frailty syndrome. Another accepted diagnostic tool for sarcopenia is bioelectric impedance, but results can be altered in patients with ascites.47,49
There is recent evidence on the use of ultrasound as a tool for diagnosing sarcopenia in cirrhotics, showing good correlation with tomography studies (r = 0.70), with an AUROC > 0.95. Advantages are its low cost and applicability at the bedside or office, but it is operator-dependent and requires a learning curve.53,54
- 8
The most efficient manner to evaluate coagulation, and when necessary, guide transfusion therapy in patients with cirrhosis that require a surgical procedure is through viscoelastic tests.
Complete agreement 92.9%, partial agreement 7.1%
Level of evidence, low; weak, in favor of the recommendation
Unlike hereditary coagulopathies, cirrhosis affects the entire spectrum of the coagulation cascade, i.e., it includes procoagulant and anticoagulant factors and antifibrinolytic and profibrinolytic proteins. In addition, it is associated with platelet hyperactivity and an increase in von Willebrand factor levels, giving rise to “rebalanced hemostasis”. This new balance is fragile and can be easily inclined toward a prohemorrhagic or prothrombotic phenotype. Therefore, there is no ideal test or study for evaluating clotting in cirrhotic patients, given that none of them include the variables of volume status, kidney function, or endothelial dysfunction.55 The INR is a mathematical manipulation of prothrombin time (PT) that measures the procoagulant factors I, II, V, VII, and X. It does not measure the deficit of anticoagulants, such as protein C. It is called “normalized” because it is standardized in patients anticoagulated with warfarin, based on the activity of a commercially available thromboplastin. The use of different commercial thromboplastins causes a significant variation in the INR in cirrhotics at different hospitals, depending on which commercial thromboplastin is used. Fibrinogen has recently emerged as a laboratory test potentially more specific than the INR, especially in critically ill cirrhotic patients. Levels of 120–150 mg/dl have been suggested as safe, together with platelet levels, for predicting bleeding risk in invasive procedures in cirrhotics.56,57 There are no clinical trials supporting a specific cutoff point for platelets. The cutoff point of 50,000/μl has been used in in vitro trials, in which this platelet level was associated with normal thrombin production. Expert recommendation currently suggests it should be individualized, according to the bleeding risk of the procedure and the clinical setting, proposing platelet levels of 30,000–75,000/μl as safe, albeit the more commonly used figure is 50,000/μl.56,58
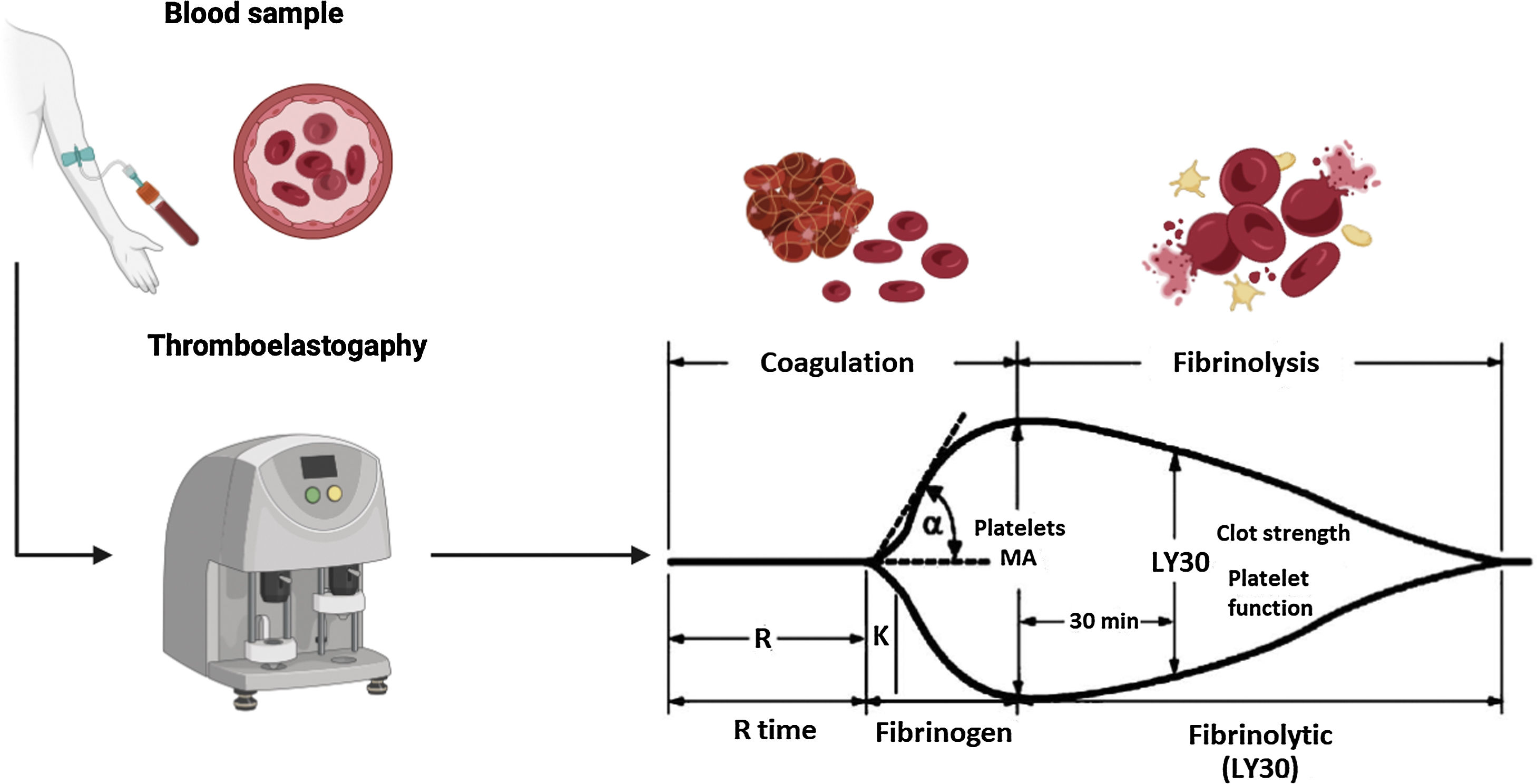
The viscoelastic tests of thromboelastography (TEG) and thromboelastometry (TEM) are considered the best strategy for guiding transfusion therapy in cirrhotic patients undergoing invasive procedures. These tests report clot formation in blood that is not centrifuged, as well as clot strength and the presence of hyperfibrinolysis or premature clot dissolution.59 In North America and Mexico, the most well-known and commercially available is TEG. This test measures the properties of the clot, using a small recipient that contains a blood sample. The sample is rotated slowly to form the clot. A pin is suspended in the center of the cup of blood and tension is exerted on it through a torsion wire, bringing the recipient and the pin together as the clot forms. Increased rotational forces are transferred from the cup through the forming of the clot to the pin and are converted into electrical signals, and the strength of the clot is directly proportional to them (Fig. 1). Maximum amplitude (MA) is one of the parameters determined by TEG. Its normal values are between 50–70 mm and it reflects the strength of the clot (general clot stability) and directly shows the interaction of platelet function and plasma coagulation factors.59,60 In a recent study, Zanetto and García-Tsao60 demonstrated that a MA < 30 mm can be converted into the limit for identifying patients with decompensated cirrhosis with a higher risk for procedure-related bleeding, in whom the use of blood products should be considered before the procedure.
Thromboelastography parameters.58 Thromboelastography (TEG) provides us with several parameters:
• R time. Defined as the time from the start of the TEG tracing until the trace reaches an amplitude of 2 mm (normal: 5–10 min), representing the initial clot formation rate (initiation phase) that is primarily related to the activity of the procoagulant and anticoagulant coagulation factors. Its prolongation is the result of deficiencies in the coagulation factors or severe hypofibrinogenemia.
• K time. Defined as the time from the end of the “R time” until the trace reaches an amplitude of 20 mm (normal: 1–3 min), representing dynamic clot formation (amplification phase) that is related to the activity of clotting factors, fibrinogen, and platelets.
• α. The angle formed by the slope of a tangent line, traced from the initiation of clotting (normal range: 53° to 72°); it measures the rate at which fibrin crosslinking occurs (thrombin propagation phase) and the function of platelets and plasma factors on the surface of the platelets.
• MA. Maximum amplitude of the TEG trace (normal: 50–70 mm), reflecting the maximum clot strength (general stability of the clot) and directly showing the interaction of platelet function and plasma coagulation factors.
• LY30. The percentage of the decrease in trace amplitude 30 minutes after the MA (normal 0–8%), reflecting initial clot dissolution (fibrinolysis).
Figure created using Biorender.com with data modified from Turco et al.59
A systematic review that included 8 studies (n = 118) concluded that patients, in whom transfusion therapy was guided through TEG, utilized fewer fresh frozen plasma (FFP), platelet, and cryoprecipitate transfusions, there was no increase in bleeding, and there were fewer adverse effects associated with transfusion (30.6% vs 74.5%, p = 0.01).61 Likewise, in a meta-analysis that included 7 studies (n = 421), the patients that had viscoelastic tests to guide transfusion therapy had fewer FFP transfusions (relative risk [RR] 0.52, 95% CI 0.35–0.77), fewer platelet transfusions (RR 0.34, 95% CI 0.16–0.73), and less risk for transfusion-associated adverse effects (RR 0.42, 95% CI 0.27–0.65). There were no changes in mortality in the patients that underwent invasive procedures nor increases in bleeding events.62 The American Gastroenterological Association guidelines on coagulation in cirrhotics propose three parameters that should be met for operating on a patient with cirrhosis: hematocrit > 25%, platelets > 50,000/μl, and fibrinogen > 120 mg/dl, given that these parameters have greater clinical usefulness for guiding transfusion therapy.56
Neither the prophylactic correction of coagulation parameters nor the use of platelet concentrates or thrombopoietin receptor (r-TPO) agonists, when platelet levels are > 50,000/μl or when bleeding can be controlled with local hemostasis, is recommended. In high-risk procedures in patients with a platelet count < 50,000/μl, considering platelet transfusion one hour before an emergency procedure is recommended. In elective procedures, the recommendation is to prepare the patient 7 days earlier, with the use of r-TPO agonists (avatrombopag or lusutrombopag). The routine use of tranexamic acid for reducing the bleeding rate after the procedure is not recommended.63,64
- 9
In all patients with cirrhosis, whenever possible, but especially in those with decompensated cirrhosis, ascites, and therapy with diuretics, evaluating and correcting kidney function, serum electrolytes, and the acid-base balance is recommended.
Complete agreement 92.9%, partial agreement 7.1%
Level of evidence, moderate; strong, in favor of the recommendation
The presence of ascites increases the risk of peritoneal infection, fistulas, and wound dehiscence. To prevent said complications patients are usually treated with diuretics, a low-sodium diet, and large-volume paracentesis, which can lead to complications due to hypovolemia, such as acute kidney injury (AKI), electrolyte imbalance, and acid-base disorders.48
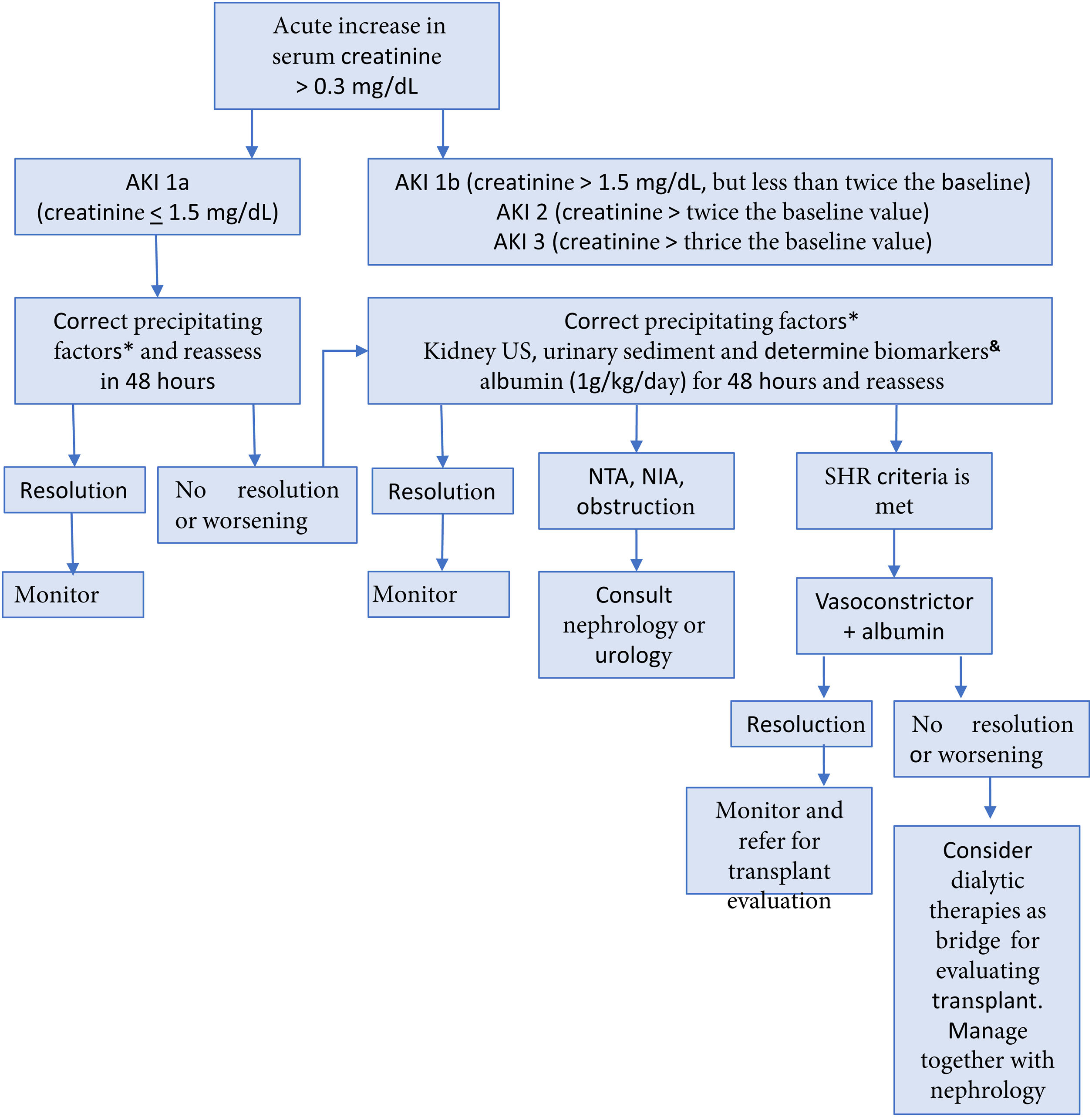
Up to 50% of hospitalized cirrhotic patients develop AKI, generally secondary to a combination of factors, such as the decrease of circulating volume, vasoconstriction, and altered kidney regulation. It is important to detect possible precipitants of AKI, such as infections, hyperbilirubinemia, gastrointestinal bleeding, and the use of diuretics and other nephrotoxic agents.65 In the patient with cirrhosis, AKI is defined when serum creatinine increases ≥ 0.3 mg/dl within 48 hours or increases ≥ 50%, with respect to the baseline value, or when urine production falls below 0.5 ml/kg/h for more than 6 hours.66,67 In the preoperative evaluation, the development of AKI in cirrhosis should be prevented by avoiding potentially nephrotoxic medications, such as nonsteroidal anti-inflammatory drugs (NSAIDs), excessive or unsupervised use of diuretics or nonselective beta blockers (NSBBs), large-volume paracentesis without replacing albumin, and alcohol use.66 In patients with cirrhosis that present with AKI, the recommendation is to follow the management algorithm shown in Fig. 2. Likewise, maintaining a mean arterial pressure (MAP) > 60 mmHg is advised, to ensure adequate renal perfusion pressure.68
Evaluation and management of acute kidney injury in patients with cirrhosis65,66
AIN: acute interstitial nephritis; AKI: acute kidney injury; ELISA: enzyme-linked immunosorbent assay; ATN: acute tubular necrosis; HRS: hepatorenal syndrome; US, ultrasound.
* Withdraw diuretics, nonselective beta-blockers, nonsteroidal anti-inflammatory drugs, and other nephrotoxic agents. Treat infections. Restore intravascular volume.
& The best cutoff values for neutrophilic gelatinase-associated lipocalin (NGAL) to differentiate between ATN and other types of AKI, including HRS-AKI, are 365 ng/ml and 220 μg/g of creatinine for the ELISA and particle-enhanced turbidimetry techniques, respectively. Fractional excretion of sodium (FENa) < 1% can suggest HRS.
Hyponatremia is defined as a serum sodium concentration < 135 mmol/l. It is present in 49% of patients with cirrhosis and ascites.67,68 Hypervolemic hyponatremia is most frequent in the cirrhotic patient with ascites and edema, due to the expansion of extracellular volume. It is secondary to the activation of the renin-angiotensin-aldosterone system due to the decrease in the circulating volume associated with splanchnic vasodilation. Hyponatremia should only be treated when serum sodium is < 125 mmol/l. Treatment consists of restricting fluids to < 1,000 ml/day and the suspension of diuretics, to achieve a negative balance. In cases with severe symptoms, such as convulsive crises (serum sodium < 120 mmol/l), hypertonic saline solutions can be administered. They rapidly improve the natremia but increase volume overload, in turn, worsening the ascites and edema. In the setting of a patient that will be operated on that has severe acute hyponatremia with severe symptoms, the administration of saline solution at 3% in boluses of 100 ml for 15–30 minutes is recommended. The dose can be repeated up to three times, with the goal of a 4–6 mmol/l increase of serum sodium in the first 6 hours. In cases of severe or symptomatic chronic hyponatremia, saline solution of 0.9% at a rate of 15–30 ml/h can be administered. The concentration of serum sodium should not be increased more than 8 mmol/l, to prevent pontine myelinolysis. However, recent evidence suggests that increasing serum sodium concentration >10 mmol/l is associated with a lower mortality rate and shorter hospital stay, without increasing the risk of pontine myelinolysis.65,69
Another current option for correcting hyponatremia is the administration of intravenous albumin, which produces the increase in serum sodium by augmenting the urinary free water clearance secondary to intravascular volume expansion. A higher response rate to hyponatremia, as well as improvement in 30-day survival, has been shown in hospitalized cirrhotic patients.70 The use of vasopressin receptor antagonists in cirrhotics with hypervolemic hyponatremia can increase the concentrations of serum sodium during treatment. Nevertheless, they should be used for short periods < 30 days due to the risk of hepatocellular damage.67 They are not available in Mexico.
Hypokalemia in cirrhotics is multifactorial and the main causes are loop diuretic use, gastrointestinal losses (diarrhea and vomiting), respiratory alkalosis, secondary hyperaldosteronism, renal tubular acidosis, and hypomagnesemia due to chronic malnutrition. Hypokalemia induces intracellular acidosis in the cells of the proximal tubule that produces an increase in the reabsorption and metabolism of glutamine, leading to ammoniagenesis and the risk of HE. Therefore, optimum serum potassium levels are ≥ 4 mEq/l. In patients that present with a deficit, correction should be slow. Oral potassium salts can be used. If oral administration cannot be used or rapid corrections are needed, parenteral administration can be utilized.65,71,72
On the other hand, cirrhotic patients can also develop hyperkalemia, generally secondary to the use of potassium-saving diuretics (spironolactone, amiloride, or eplerenone). Patients with AKI and patients with a dose of spironolactone > 100 mg/day have a higher risk for developing it. If a patient presents with serum potassium levels > 6 mEq/l, the suspension of spironolactone is recommended.65,67,72
Every decompensated cirrhotic patient with metabolic acidosis should be evaluated and its cause determined. The most common causes are hypovolemia and infections. The timely correction of hypovolemia and infections are crucial for prognosis.67,73
- 10
In patients that require elective surgical procedures, the preoperative control of the etiologic factor of the cirrhosis, whenever possible, is advisable. However, in the patient that needs emergency surgery, surgical management should never be delayed, regardless of the cause of cirrhosis or its control.
Complete agreement 92.9%, partial agreement 7.1%
Level of evidence, moderate to low; weak, in favor of the recommendation
Cirrhosis has various causes and it is well-known that controlling the etiologic factor is crucial for preventing decompensation in compensated patients, preventing decompensation progression, improving survival in patients that already have decompensated cirrhosis at initial presentation,74,75 and reducing the development of hepatocellular carcinoma.73 Likewise, we know that surgical procedures can be the cause of decompensation in the patient with cirrhosis,7,24,76 and so it is advisable, whenever possible, to identify the cause of the cirrhosis in the preoperative stage,77–82 treat and control it, in addition to establishing other patient optimization measures26,68,77,83 summarized in Table 4. However, in cases of emergency surgery, etiologic factor control takes a backseat, given that deferring the surgical procedure could result in greater morbidity and mortality for the patient. For example, in a general context, the mortality rate at 28 days was significantly higher in patients whose treatment for emergency conditions was delayed, compared with patients that were treated opportunely (10.4% vs 2.5%; OR 4.6, 95% CI 1.3–16.5; p = 0.038).84
- 11
Overt hepatic encephalopathy should be corrected whenever possible before an elective surgical procedure.
The most important preoperative management aspects for reducing surgical risk in patients with advanced chronic liver disease/cirrhosis.76
| Identify, treat, and control the etiologic factor | •Hepatitis B virus infection77Maneuver. All patients with compensated or decompensated cirrhosis ideally should be receiving treatment with nucleos(t)ide analogues with a high barrier to resistance (TDF, TAF, or ETV). Post-liver transplant patients should continue treatment with TDF, TAF, or ETV + HBIG to prevent recurrence Goal. Undetectable viral loada |
| •Hepatitis C virus infection78Maneuver. Treatment with DAAs (protease inhibitor-free regimens in the decompensated patient) ideally should be guaranteed in the period prior to elective surgery. In liver transplant surgery, some decompensated patients could require undergoing transplantation first (Child-Pugh C, MELD > 20) and then receive treatment with DAAs Goal. Sustained virologic response at post-treatment week 12 | |
| •Autoimmune hepatitis79Maneuver. Immunosuppressive therapyGoal. Induce remission defined as the normalization of aminotransferases and immune globulin G at six months, and then maintain remission | |
| •Alcohol-related liver disease80Maneuver. Family, psychosocial, and pharmacologic support Goal. Maintain abstinence | |
| •Metabolic dysfunction-associated steatotic liver disease81Maneuver. Changes in lifestyle, metabolic control, and strategies for reducing cardiovascular riskGoal. Control of cardiometabolic factors, improvement of steatosis, steatohepatitis, and liver fibroses | |
| Management and control of decompensation manifestations | •Ascites. Optimization of diuretics, restriction of excess sodium in the diet67 |
| •Hepatic encephalopathy. Control of the precipitating factor, nutritional optimization to prevent or correct sarcopenia, specific therapy or prophylaxis (lactulose, rifaximin, L-ornithine L-aspartate)82 | |
| •Prevention of variceal bleeding. NSBBs are considered the first-line strategy. Only in the case of intolerance to NSBBs, consider endoscopic band ligation (it reduces risk of bleeding but has no effect on preventing the development or progression of decompensation or on survival). In secondary prophylaxis, the combination strategy (NSBB + endoscopic band ligation) is considered the most efficacious. TIPS placement should be considered individually26 | |
| Nutritional status and physical conditioning | •Dietary optimization. Varied diet that includes between 25 and 35 kcal/kg/day and 1.2 to 1.5 g of protein/kg/day. Frequent meals, including nighttime snack76Exercise. Favor aerobic exercise76 |
Regarding the etiology of cirrhosis, this table describes the most common causes.
DAA: direct acting antiviral agents; ETV: entecavir; HBIG: hepatitis B immune globulin; MELD: Model for End-stage Liver Disease; NSBB: nonselective beta-blocker; TAF: tenofovir alafenamide; TDF: tenofovir disoproxil fumarate; TIPS: transjugular intrahepatic portosystemic shunt.
Complete agreement 100%
Level of evidence, low to very low; weak, in favor of the recommendation
Surgery is a factor related to the risk of decompensation in the patient with cirrhosis. Therefore, regarding elective procedures, it is crucial to correct all possible adverse factors in the preoperative period.77 For example, patients that underwent orthopedic surgery had more decompensation, within 90 days after the procedure, than paired controls (12.8% vs 4.9%).76 During the postoperative period, patients with cirrhosis have a higher risk of presenting with HE, ascites, sepsis, and bleeding, signifying that surgical procedures in patients with cirrhosis lead to greater mortality.85 After an elective surgical procedure, the factors that have been independently associated with a higher risk of mortality at one year after surgery are alkaline phosphatase levels, MELD score, and the presence of preoperative HE. This last factor has an HR of 4.4 (95% CI 1.3–15.4) as an independent risk factor associated with mortality at one year of follow-up after surgery.86
In addition, it is well-known that certain sedative or anesthetic agents can be related to a higher risk of HE.83 Therefore, it is important to correct or improve overt HE prior to an elective surgical procedure. Likewise, the anesthesiologist must take into account aspects of safety and efficacy characteristic of the sedative and anesthetic agents. Several studies have found that monotherapy with propofol is an equally effective sedation strategy, but safer in patients with compensated or decompensated cirrhosis, when compared with midazolam or the combination of propofol plus midazolam, given that it has been related to a lower risk for the development and progression of minimal and overt HE.87–91
- 12
In patients with cirrhosis that are indicated for emergency surgery, the procedure should never be delayed by prioritizing the evaluation of other parameters, knowing that the length of delay could risk the life of the patient.
Complete agreement 100%
Level of evidence, low; strong, in favor of the recommendation
As stated above, delay in emergency care of the patient confers greater mortality.84 There is little information on the specific context of the patient with cirrhosis, but it can be extrapolated from the general context. As such, limiting expeditious and timely access to patients that require emergency surgery has resulted in an increase in intraoperative complications, general morbidity, length of hospital stay, length of stay in the intensive care unit, and mortality.92
- 13
Despite the fact that immune dysfunction in the patient with cirrhosis increases the risk of infections, there is no evidence supporting the prophylactic administration of antibiotics in patients for having cirrhosis per se. The indication for antibiotic prophylaxis before a surgery will depend on the type of surgical procedure and be applied to patients with cirrhosis in the same way it is usually indicated in patients without cirrhosis.
Complete agreement 92.9%, partial agreement 7.1%
Level of evidence, moderate to low; weak, in favor of the recommendation
Patients with cirrhosis, especially those that are decompensated, have immune system dysfunction. Thus, bacterial infection is a relatively common event in those patients and it can also worsen portal hypertension, impair liver function, and affect extrahepatic organs.93,94 Among the pharmacologic strategies for preventing infection, antibiotic prophylaxis continues to be the first option in patients with acute variceal bleeding, low proteins in ascites, and previous episodes of spontaneous bacterial peritonitis (SBP) and should not be suspended if the patient is programmed for an elective or emergency surgical intervention.95,96
On the other hand, routine antibiotic prophylaxis is not recommended for elective interventional procedures in patients with cirrhosis. It should be carefully considered in high-risk patients, such as those with bilioenteric anastomosis, whereas it can be routinely adopted in patients with primary sclerosing cholangitis that undergo endoscopic retrograde cholangiopancreatography (ERCP).97
- 14
Patients with cirrhosis, who, in accordance with the Baveno VII consensus are receiving primary or secondary prophylaxis with nonselective beta-blockers, should continue to do so, as long as there are no specific contraindications for maintaining it before certain surgical or anesthetic procedures. If variceal ligation were indicated, it should be performed before the elective surgical procedure.
Complete agreement 85.7%, partial agreement 14.3%
Level of evidence, high; strong, in favor of the recommendation
Treatment with NSBBs is indicated for the prevention of decompensation in patients with CSPH, as well as in primary and secondary prophylaxis of variceal bleeding. Carvedilol has intrinsic anti-alpha 1-adrenergic vasodilating effects that contribute to a greater reduction of portal pressure, compared with conventional NSBBs.26 In addition to the hemodynamic effect, NSBBs reduce abnormal gastrointestinal permeability and bacterial translocation and regulate the phagocytic activity of monocytes and granulocytes after exposure to bacterial products.98 Contraindications for NSBB use are bronchial hyper-reactivity, chronic obstructive pulmonary disease, and second and third-degree atrioventricular block.99 The benefit NSBBs provide to patients with cirrhosis is based on treatment adherence. They should not be suspended unless there is a side effect (severe bradycardia or hypotension). They do not need to be suspended ahead of elective surgery. Once the patient tolerates oral diet, is hemodynamically stable, without AKI or infectious processes, NSBBs can be restarted upon discharge from the hospital. In patients programmed for elective surgery that will undergo variceal ligation, the procedure should be performed 2 to 3 weeks before the elective surgery.26
- 15
Transjugular intrahepatic portosystemic shunt (TIPS) placement can reduce the risk for presenting with gastrointestinal bleeding associated with severe portal hypertension.
Complete agreement 78.6%, partial agreement 7.1%, uncertain 14.3%
Level of evidence, moderate; strong, in favor of the recommendation
CSPH is a primary complication of cirrhosis and is characterized by the development of varices, variceal bleeding, and ascites.100 Said complications are associated with significant morbidity and mortality, requiring efficacious treatment and/or their prevention. Transjugular intrahepatic portosystemic shunt (TIPS) placement is an efficacious and safe interventional treatment of portal hypertension.101 Importantly, evidence-based allocation for TIPS placement, as well as optimum patient selection before the implantation, are essential for improving prognosis. In patients with variceal bleeding, TIPS placement can be carried out as salvage therapy (rescue TIPS), as preventive TIPS (early), or for the secondary prophylaxis of variceal bleeding. In certain patients, endoscopic treatment does not achieve stopping the bleeding, and in such situations, TIPS placement with coil embolization of the bleeding varices, as rescue therapy, is highly efficacious, with a success rate of approximately 90%.102 Several studies have demonstrated a significant reduction in bleeding recurrence rates. In addition, those studies have also shown significant improvement in one-year survival.103 In accordance with those positive studies, the Baveno consensus recommended preventive TIPS placement 72 hours after the variceal bleeding event in patients at high risk for bleeding recurrence.68 Even patients with acute-on-chronic liver failure (ACLF) can benefit from TIPS placement after presenting with variceal bleeding.104
There are several areas of opportunity, with respect to preoperative TIPS placement, such as:
- a)
There is no routine indication for TIPS placement before a surgical event.
- b)
There is scant bibliographic material on the theme.
- c)
In decompensated patients, TIPS placement can “recompensate” liver disease. A careful evaluation of the TIPS indication is very important in decompensated patients because perhaps it would be indicated, regardless of the surgical event.
- d)
In compensated patients, there are no clear selection criteria, but in high-risk surgeries, perhaps a gradient > 16 mmHg could be useful. Nevertheless, it is clear that TIPS placement should never postpone a necessary surgery, and especially not an emergency surgery.35
- e)
If there is a clear indication for TIPS placement, deferring the surgery until TIPS placement, whenever possible, is advisable. If TIPS placement is not possible at the center where the patient is being managed, it is advantageous to refer the patient to a specialized center where the procedure can be performed.
- 16
The presence of ascites increases the risk for peritoneal infection, fluid leakage, and wound dehiscence in abdominal surgery. Therefore, optimum control of ascites, before an elective surgery, is recommended.
Complete agreement 100%
Level of agreement, low; weak, in favor of the recommendation
The accumulation of fluid in the peritoneal cavity, not only increases the difficulty of the surgical technique, but also significantly elevates the risk of peritoneal infection, fluid leakage, and surgical wound dehiscence. The presence of ascites in patients with cirrhosis is associated with a mortality rate of 37 to 83%.105 It can cause respiratory involvement by reducing pulmonary expansion, abdominal wall eventration, and suture dehiscence. The efficacious approach to and control of ascites before elective surgery is essential for improving postoperative results and morbidity and mortality in high-risk patients. Fleming et al.106 demonstrated that the presence of ascites was a predictive factor for high risk in the occurrence of surgical complications and was also associated with higher mortality. Both situations were unrelated to the MELD score. In that same study, the authors suggest that the MELD score can underestimate the surgical risk in patients with ascites. To improve the accuracy of perioperative risk evaluations for these complex patients, predictive models should include ascites as a critical parameter. It is important for clinical decision-making and underlines the need for a nuanced approach to surgical interventions in the presence of ascites.
Certain procedures are contraindicated in patients with ascites. For example, percutaneous gastrostomy is contraindicated in patients with ascites due to the high risk of infection, and in general, should be avoided in patients with portal hypertension.107
Overall, ascites should be treated aggressively with diuretics and/or large-volume paracentesis and albumin administration, if needed. If drainage cannot be achieved before surgery, it should be done during laparotomy, quantifying the amount drained and replacing the necessary albumin, similar to large-volume paracentesis (6 to 8 g/l evacuated, from > 5 l drained).68 Ascites should be analyzed to rule out SBP, and treat it, if present.107
Patients that require emergency surgery have a significant risk for developing liver dysfunction due to less opportunity for correcting reversible factors, such as electrolytic alterations, coagulopathy, and the portal hypertension manifestations of ascites and HE. Emergency surgery is a poor prognosis factor, with a significant difference, compared with patients that undergo elective surgery (44% mortality in emergency surgery at 3 months vs 21% in elective surgery).108
- 17
In the patient with cirrhosis that will undergo an endoscopic or invasive procedure classified as high risk, no significant benefit in reducing the risk for bleeding upon correcting the prolonged INR by transfusing blood products has been demonstrated. Transfusing platelet concentrates or thrombopoietin agonists when the platelet count is > 50,000/μl is not indicated, either.
Complete agreement 100%
Level of evidence, moderate to low; weak, in favor of the recommendation
The prophylactic administration of blood products for the purpose of preventing bleeding during the procedure in patients with cirrhosis should not be carried out routinely, especially when the procedures to be performed are considered low risk. In that context, optimizing the general measures described in Table 5 is much more relevant for preventing and reducing the risk of bleeding.109
Periprocedural surgical, nonsurgical, and endoscopic management in the patient with cirrhosis.55,108,111
| General measures | • Optimize anemia by restoring potential deficiencies: iron, B-complex, folic acid |
| • Treat infections | |
| • Optimize kidney function | |
| • Correct acid-base imbalance (acidosis) | |
| • Patients with anticoagulant or antiplatelet therapy should be carefully evaluated and the therapy removed prior to the elective procedure; in emergency cases, explain the risk-benefit to the patient | |
| • Multidisciplinary assessment for better decision-making | |
| Tests of hemostasis | • Prothrombin time and INR do not predict the risk of bleeding |
| • Whether platelet count and fibrinogenic figures accurately predict the risk of bleeding is uncertain | |
| • Whether viscoelastic tests accurately predict the risk of bleeding is uncertain, but they do more accurately help guide the need for transfusion therapy in the patient with cirrhosis, compared with other conventional tests | |
| • Routine hemostasis testing to predict the risk of bleeding is not recommended, but said tests are useful prior to a procedure to have a baseline value in case bleeding occurs | |
| • They are disease severity indicators | |
| Prophylaxis in patients with altered hemostasis tests | • The prophylactic administration of fresh frozen plasma is not recommended, even in the presence of prolonged prothrombin time and INR |
| • The routine administration of platelet concentrates, fibrinogen concentrates, or cryoprecipitates for prophylaxis is not recommended | |
| • In patients that will undergo high-risk surgical procedures and that have a platelet count < 50,000/ml or fibrinogen < 120 mg/dl, a multidisciplinary group discussion and individualized decision is recommended, in addition to maintaining hematocrit > 25%. Some experts recommend INR < 2, although said parameter is still very controversial | |
| • In patients that will undergo high-risk nonsurgical invasive procedures and that have a platelet count < 50,000/ml or fibrinogen < 100 mg/dl, a multidisciplinary group discussion and individualized decision is recommended. It is advantageous to maintain hematocrit > 25%. Some experts recommend INR < 2, although said parameter is still very controversial | |
| • In patients that will undergo low-risk nonsurgical invasive procedures and that have a platelet count < 30,000/ml, a multidisciplinary group discussion and individualized decision is recommended. Other parameters need not be adjusted |
It should be kept in mind that patients with cirrhosis face an increased risk of bleeding that is multifactorial in origin. Not only is it due to hemostatic alterations associated with liver disease, including thrombocytopenia and a decrease in coagulation factors, but it is also associated with CSPH itself, and more importantly, related to the nature of the procedure to be performed.110–112
In a study that included 1,187 hospitalized patients with cirrhosis, in whom 3,006 nonsurgical procedures were performed at 20 international centers, bleeding, in general terms, was reported in 3% of the procedures. Major bleeding was reported in 2.3% of the admitted patients and in 0.9% of the procedures. The patients with bleeding had more probabilities of presenting with metabolic dysfunction-associated steatohepatitis (MASH) (43.9% vs 30%), higher BMI (31.2 vs 29.5), and a higher MELD score (24.5 vs 18.5). In the multivariate analysis, regarding the potential variability between the participating centers, high-risk procedure (OR 4.64, 95% CI 2.44–8.84), MELD score (OR 2.37, 95% CI 1.46–3.86), and a higher BMI (OR 1.40, 95% CI 1.10–1.80) were found to independently predict the risk of bleeding. In contrast, neither INR prior to the procedure, platelet level, nor the use of antithrombotic agents were predictive of bleeding.113
Procedures with an incidence of bleeding above 1.5% are classified as high risk110 (Table 6). One of the most frequently performed high-risk endoscopic procedures is polypectomy and its post-resection bleeding rates range from 0.3 to 6.%. Pure cutting current is more commonly used in the procedure. The most relevant risk factors for presenting with post-polypectomy bleeding are age above 65 years, a polyp larger than 1 cm, cardiovascular disease, and the use of antithrombotic therapy.114,115
Classification of the procedures according to risk in patients with cirrhosis.111
| Low-risk proceduresEndoscopicEndoscopic retrograde cholangiopancreatography (ERCP) with biliary or pancreatic stent placement without sphincterotomy Endoscopic hemostasis with argon plasma Endoscopic capsule Endoscopic ultrasound with no fine-needle aspiration Enteral stent removalPolypectomy < 1 cmDiagnostic panendoscopy with or without biopsies Diagnostic balloon-assisted enteroscopy Push enteroscopyFlexible rectosigmoidoscopy with or without biopsies Diagnostic colonoscopy with or without biopsiesVascularCentral venous catheter placement Central venous catheter placement via peripheral routeRemoval of central vascular accessCardiac catheterizationTransesophageal echocardiographyDiagnostic coronary angiographyInferior vena cava placement filter HepaticTransjugular liver biopsy Hepatic venous pressure gradient measurement Diagnostic and therapeutic paracentesis PulmonaryThoracocentesisBronchoscopy with no biopsy UrologicCystoscopyUreteroscopyGynecologicColposcopy with cervical biopsy Diagnostic hysteroscopy MiscellaneousDental cleaningIntra-articular injectionPercutaneous lymph node biopsy Skin biopsyDrainage catheter replacement |
| Procedures lacking consensus on riskEndoscopicERCP with balloon papillary dilation without sphincterotomy Stricture dilation Endoscopic ultrasound with fine-needle aspirationVariceal ligationInjection of gastric varicesTherapeutic balloon-assisted enteroscopy VascularArterial line placementTherapeutic coronary angiographyAngiography or venography with interventionRadiologicRadiofrequency ablationHepaticPercutaneous liver biopsyLaparoscopic liver biopsy Portal recanalizationTransjugular intrahepatic portosystemic shunt (TIPS)Percutaneous ablation of hepatocellular carcinomaAscites drainage catheter placementPulmonaryBronchoscopy with biopsiesTherapeutic bronchoscopy Intrapleural catheter placementUrologicLithotripsy (kidneys, bladder, ureter)NeurologicLumbar punctureGynecologicHysteroscopy with biopsyAmniocentesisMiscellaneousDental extractionIntra-articular puncture |
| High-risk proceduresEndoscopicERCP with biliary or pancreatic sphincterotomy Mucosal resection Submucosal dissectionCystogastrostomyPolypectomy > 1 cmPeroral endoscopic myotomyAmpullar resectionGastrostomy JejunostomyHepatic or biliaryPercutaneous biliary drainageCholecystostomyPulmonaryIntrathoracic biopsyUrologicProstate biopsyPercutaneous kidney biopsyNephrostomy catheter placementNeurologicEpidural catheter placementProcedures that involve the central nervous systemMiscellaneousNonhepatic intra-abdominal solid organ biopsy |
Modified from Riescher-Tuczkiewicz et al.112
As already mentioned herein, ideally, transfusion therapy in patients with cirrhosis should be guided by viscoelastic tests.59 When they are not available, the following conduct before endoscopic or other high-risk nonsurgical procedures, similar to that of surgical procedures, could be considered110,111 (Table 6), all the while utilizing clinical judgement and individualizing each case. This conduct includes optimizing clot formation through the general measures109 listed in Table 5 and maintaining the following parameters: hematocrit > 25%, platelet count > 50,000/μl, and fibrinogen > 120 mg/dl.56 This last parameter should preferably be corrected through fibrinogen concentrates, given that the very low volume, the standardization of the fibrinogen content, and the fact that cross-over tests are not needed favor its use in cirrhosis.111 It should be emphasized that correcting the INR in this context is not supported by evidence and excess transfusion of FFP could be deleterious, because it would increase the risk for bleeding associated with the increase in portal hypertension.109,116–118
- 18
In patients with cirrhosis that require a low-risk endoscopic or invasive procedure, the administration of blood products, for correcting alterations in conventional hemostatic tests, is not recommended.
Complete agreement 100%
Level of evidence, low to very low; weak, in favor of the recommendation
A recent study showed that patients with cirrhosis, especially decompensated ones, had significantly increased whole blood platelet aggregation. This particularly elevated platelet aggregation was associated with the risk for major complications and death.119 In the PROLIVER study that prospectively studied 280 patients with stable cirrhosis, the bleeding risk was 3.5% per year for major bleeding events and 1.8% per year for minor bleeding events. There were 3 complications due to bleeding that mainly occurred in the gastrointestinal tract and no relation between platelet count and bleeding was found.120 There is not sufficient or convincing evidence in favor of recommending platelet correction in patients that will undergo low-risk procedures. In patients with severe thrombocytopenia (< 30,000/μl) the decision should be made based on multidisciplinary team discussion. The correction of other parameters, such as INR, fibrinogen, and PT, before low-risk procedures, is not justified.112
Intraoperative setting- 19
The need for blood product transfusion during surgery should be guided by the magnitude of intraoperative bleeding, the hemodynamic status of the patient, serum fibrinogen and platelet values, and viscoelastic tests, when available.
Complete agreement 100%
Level of evidence, moderate; weak, in favor of the recommendation
The viscoelastic tests previously discussed herein appear to be the best alternative at present for guiding transfusion therapy during a surgical procedure. Two recent studies showed that the majority of patients with compensated advanced chronic liver disease had values in the normal range in TEG.121,122 When viscoelastic tests are not available, the need for transfusion can be guided according to the recommendations shown in Table 5.
Similar to the recommendations for endoscopic or invasive procedures described above, prophylactic FFP transfusions should not be performed, even in patients with abnormal PT/INR levels, because it can result in a significant increase in portal pressure, bleeding risk, and adverse pulmonary effects.56
Prothrombin complex, fibrinogen, or cryoprecipitate (that contain fibrinogen, von Willebrand actor, and factor VIII) concentrates are preferred because they balance hypofibrinogenemia, with less volume overload. The use of antifibrinolytic drugs, such as tranexamic acid, is not advised. Severe thrombocytopenia is related to periprocedural bleeding events but there is no evidence that prophylactic platelet transfusion improves the hemostatic potential.111 In patients with severe coagulopathy (platelet count < 50,000/μl or fibrinogen level < 100 mg/dl) undergoing high-risk procedures with no possibility of local hemostasis, the prophylactic administration of thrombopoietin analogues (avatrombopag or lusutrombopag), platelet concentrates, and fibrinogen concentrates can be considered.63,109
- 20
To limit liver injury in patients with cirrhosis, general intraoperative goals should be to maintain the hepatic blood flow and oxygen supply, as well as to minimize exposure to hepatotoxic agents.
Complete agreement 100%
Level of evidence, moderate; strong, in favor of the recommendation
Fluids and electrolytes should be strictly monitored and corrected, when necessary. The development or worsening of hyponatremia is considered an important finding because it involves the fluid overload resulting from reduced solute-free water clearance. It can cause severe ascites, HE, and AKI, and increase the length of hospital stay.123 The evaluation of volume status with invasive or noninvasive methods is recommended, depending on availability at the center where the patient is being treated and also understanding the limitation of said tools in patients with cirrhosis. Methods that can be employed include MAP, echocardiography with point-of-care ultrasound (PoCUS), and pulmonary artery catheter.124 Due to the scant evidence on hemodynamic goals in patients with cirrhosis, maintaining a MAP ≥ 60 mmHg is recommended.125 In cases of persistent MAP < 60 mmHg, early vasopressor use should be considered, when fluid resuscitation fails. Norepinephrine is the vasopressor of choice.126
Patients with cirrhosis have a higher risk of hypoxia secondary to restrictive pulmonary disease due to the presence of ascites or pleural effusion, diffusion anomalies due to arterial and venous vasodilation and vascular shunts, and the presence of pulmonary hypertension. Patients with large-volume ascites are also predisposed to bronchopulmonary aspiration. Therefore, sufficient oxygen supply during the surgical procedure, ensuring airway permeability at all times, is recommended.127
Different NSAIDs tend to be used for managing surgical pain, even during the intraoperative period to prevent postoperative pain. However, they are conducive to a high risk for developing gastrointestinal bleeding and kidney injury in patients with cirrhosis and should be avoided. Other drugs to avoid are opioids and long-acting benzodiazepines.128
- 21
Conventional anesthetic medications can be used in patients with cirrhosis, and in those that require major surgery, general anesthesia is preferred.
Complete agreement 100%
Level of evidence, low to very low; weak, in favor of the recommendation
Liver disease modifies the pharmacokinetics of anesthetics by altering the binding to proteins, metabolism, and volume distribution. However, in patients with compensated disease, conventional drugs, similar to those used in other patients, can be selected.129
Modifications should be considered (e.g., dose reduction) in patients with advanced or decompensated disease, especially when accompanied by portal hypertension (such as in patients with esophageal varices, ascites, or gastropathy/portal colopathy) or kidney injury.130
Regarding hypnotic sedatives, patients with liver disease have similar rates of elimination of the induction doses used in habitual clinical practice, such as propofol, etomidate, and methohexital, compared with patients with cirrhosis. Mean elimination time and drug-free levels of benzodiazepines increase in cirrhosis, with the exception of oxazepam and temazepam.129–131
Dexmedetomidine has reduced clearance, and the elimination half-life can be prolonged in patients with severe liver disease. Dosage can be limited due to bradycardia.132
For the procedures that require sedation, propofol is preferable to benzodiazepines because it provides more rapid sedation and recovery, in patients with cirrhosis.133 Propofol is the induction agent of choice for general anesthesia due to its rapid redistribution, but it can cause vasodilation and potentially reduce liver perfusion. Propofol can also be administered by target-controlled infusion, requiring a lower quantity.134
Thiopentone can have a prolonged duration of action due to a decrease in plasma proteins, resulting in an increase in the unbound fraction of the drug.135
Reduced doses of opioids should be used at longer intervals to avoid drug accumulation. Long-acting opioids, such as morphine and meperidine, should be avoided but short-acting opioids, such as fentanyl and hydromorphone, are well-tolerated when used at lower doses.129,130,135
The duration of action of the aminosteroid neuromuscular blockers, rocuronium and vecuronium, can be prolonged in cases of liver failure. The neuromuscular blocking agents, benzylisoquinoline, atracurium, and cisatracurium, are not affected in liver failure. Succinylcholine is metabolized by plasma cholinesterase and even though it is long-acting, it is not clinically significant.135,136
Modern halogenated volatile anesthetics are safe in cirrhotic patients. Isoflurane and desflurane are metabolized to trifluoroacetyl chloride (TFA), less than halothane. TFA has been shown to be involved in the liver toxicity of halothane (20% for halothane, 0.2% for isoflurane, and 0.02% for desflurane). Liver injury from isoflurane and desflurane is extremely rare if it exists at all. Sevoflurane is not metabolized to TFA and has not been associated with immune-mediated liver injury.137
Postoperative follow-up and carePostoperative liver dysfunction is characterized by the decline in synthetic, excretory, and detoxification properties. It usually appears within the first five days after surgery. The severity and nature of the liver dysfunction can vary according to different factors, such as the type of surgery, degree of prior liver function decline of the patient, and other individual comorbid factors. Some of the common causes of postoperative liver dysfunction include hepatic ischemia, lesions during surgery, reactions to medications, postoperative infections, and complications of anesthesia. Obviously, this condition has a negative impact on patient survival, and so strict and careful monitoring during the postoperative period is very important.138
- 22
In the postoperative period, nutrition, thromboprophylaxis indication, analgesia use, surgical wound condition (dehiscence, bleeding, infection), restarting of medications used for controlling cirrhosis, and the development of decompensations characteristic of cirrhosis must all be monitored.
Complete agreement 92.9%, partial agreement 7.1%
Level of evidence, moderate to low; strong, in favor of the recommendation
The main sections of this recommendation are broken down into the statements that follow below.
- 23
NUTRITION. Malnutrition and hypoalbuminemia are poor prognosis risk factors, and so the reestablishment of early feeding, preferably by the enteral route, should be prioritized.
Complete agreement 100%
Level of evidence, moderate; strong, in favor of the recommendation
Once the baseline nutritional status is established (ideally in the preoperative stage), action should be taken accordingly, i.e., if malnutrition, protein depletion, or trace element deficiency is identified, an attempt must be made to reestablish the best possible status before the procedure. In the postoperative period, it is assumed that the nutritional status has already been established and necessary strategies have been implemented to improve the deficiencies identified. However, patients should be reevaluated and reclassified in the postoperative period because surgery is considered an aggressive factor and potential cause of greater decompensation of the disease, and in turn, of malnutrition. There are associated factors, some of which are related to the disease, whereas others are related to specific medical management.139
Disease-related factors. Some patients can develop decompensations of cirrhosis, such as HE, tense ascites, or gastrointestinal bleeding, which can impede adequate feeding. Other factors can be gastrointestinal motility disorders, postoperative ileus, metabolic ileus, etc. Albumin levels decrease after episodes of trauma or surgery and can even be related to the level of severity. In such settings, there is vascular permeability and albumin leakage, leading to albumin redistribution.139 Serum albumin levels are included in the preoperative VOCAL-Penn score, which is why it has been studied as a prognostic marker of decompensations and mortality. Lower albumin levels result in a higher postoperative decompensation rate and risk for death.44 Nevertheless, the intravenous administration of albumin has shown no benefit, and the dietary supplementation of albumin has only been indicated as an expert recommendation.139
Specific medical management-related factors. During hospitalization for a surgical event, medications are usually used that can cause hyporexia, nausea, and abdominal discomfort, among others, and in turn, impede adequate feeding in the postoperative period. It is important to be aware of such alterations and manage them in a timely manner. In addition, when making decisions, the time of restarting diet, ideally by the enteral route, should be specified, and take place within the first 12–24 hours after surgery. This decision should not be delayed unjustifiably. If it is not possible to restart the enteral diet, parenteral nutrition is superior to fasting and the administration of intravenous fluids and electrolytes.140
- 24
THROMBOPROPHYLAXIS. Despite the potential coagulation dysfunction status inherent in cirrhosis, thromboprophylaxis should be considered and carried out in high-risk patients.
Complete agreement 92.9%, partial agreement 7.1%
Level of evidence, low; weak, in favor of the recommendation
The incidence of deep vein thrombosis (DVT) and pulmonary thromboembolism (PTE) in the patient with cirrhosis is 0.5–7%.64 The risk of developing DVT or PTE is similar to that of patients without cirrhosis but confers greater mortality.63
Thromboembolic risk. Risk is evaluated through the predictive Padua or IMPROVE scales. Even though there is little evidence in the setting of patients with cirrhosis of the liver, the current recommendation is to evaluate the risk with either of those two scales. Evaluation with laboratory tests (PT, INR, platelet count, fibrinogen levels) is not recommended, nor are viscoelastic tests, because they have not been widely validated.63,141
Mechanical prophylaxis. In low-risk patients, nonpharmacologic thromboprophylaxis measures are recommended. The mechanical devices for preventing venous thromboembolism act on venous stasis and include static systems: graduated compression measures (elastic stockings or anti-embolism stockings) and dynamic systems: intermittent pneumatic compression and venous foot pump.142
Pharmacologic tools. In high-risk patients, the use of low-molecular-weight heparin is preferred over standard dose unfractionated heparin. Enoxaparin has a reasonable safety profile in patients with Child-Pugh A, B, and C cirrhosis, but no clear efficacy has presently been shown. In cases of kidney failure, the treatment of choice is low-molecular-weight heparin, rather than unfractionated heparin. Direct-acting anticoagulants are considered safe for patients with Child-Pugh A and B cirrhosis. Despite having a reasonable safety profile, there is limited evidence on their efficacy in these patients. They are not recommended in patients with Child-Pugh C cirrhosis.142
- 25
ANALGESIA. Analgesia should be selected according to the best safety profile of the drugs.
Complete agreement 100%
Level of evidence, low; weak, in favor of the recommendation
Analgesia can be started with nonopioid/opioid analgesics, according to the values stated by the patient, using the Visual Analogue Scale as reference. The use of NSAIDs is contraindicated.143
NSAIDs. NSAIDs should be avoided in patients with cirrhosis and there is presently not enough evidence for defining their safety profile in patients with cirrhosis.
Paracetamol. Paracetamol does not inhibit the action of cyclooxygenase and therefore has no anti-inflammatory activity.144 Its use in patients with cirrhosis of the liver has been determined to be safe, as long as the dose does not exceed 2 g/day.145
Opioids. The use of opioids in these patients is safe but due to cirrhosis of the liver, the half-lives of these drugs can be longer, increasing the risk of HE. Immediate-release formulations are preferred in these patients, avoiding extended-release formulations. Likewise, extended intervals between prescriptions are preferred, in lapses of 6 to 12 hours.145,146
Tramadol. Tramadol can cause less sedation and respiratory depression, but lower tolerance has been reported, compared with other opioids. The initial dose is 50 mg every 12 hours in extended intervals, with special care in patients that take selective serotonin reuptake inhibitors or tricyclic antidepressants because there is a potential risk for developing convulsive crises.145
Morphine. Morphine has analgesic properties with potential neurotoxic effects, such as confusion, convulsions, and respiratory depression, mainly in settings of kidney failure. The recommendation is to start with 5 mg every 6 hours and avoid its use in patients with kidney failure.145
Fentanyl. In cases of prolonged pain, transdermal fentanyl is the first-choice drug because it does not produce active metabolites, but it can be accumulated in adipose tissue at the visceral level for several days, after which it is purified by the liver.145
- 26
Recommendations on restarting medications used for controlling cirrhosis:
Complete agreement 92.9%, partial agreement 7.1%
- a)
The early start or restart of the drugs (lactulose, rifaximin, L-ornithine/L-aspartate) for HE prophylaxis is recommended.77
- b)
There is no evidence for suspending diuretics in patients with a proven preoperative tolerance of these drugs. In the absence of hypovolemia or kidney injury, they can be used during postoperative hospitalization.77
- c)
There is no evidence for suspending beta-blockers in the perioperative period. If they were to be suspended, restarting them is adequate, upon ensuring hemodynamic stability during the in-hospital postoperative period.77
- d)
The use of intravenous albumin in the postoperative period is not indicated unless the patient presents with a decompensation justifying its administration.77
- a)
- 27
Monitoring the development of the decompensations characteristic of cirrhosis, such as ascites, variceal bleeding, hepatic encephalopathy, and acute-on-chronic liver failure, is recommended.
Complete agreement 100%
Level of evidence, moderate to low; strong, in favor of the recommendation
Mortality in patients with cirrhosis that undergo a surgical procedure is higher than in patients without cirrhosis, with an increased risk of decompensation within 90 days after the surgical procedure. Therefore, special care should be given to the most common decompensation factors of ascites, bleeding, and HE.77
- a)
Ascites. Restricting sodium in the diet and restarting diuretics if they had been suspended. Daily weight measuring is recommended to monitor ascites and its respective adjustment with diuretic therapy. Surgical drains should be removed as soon as possible. Likewise, monitor the development of SBP and establish specific management, if needed. TIPS therapy can be considered an option in patients that present with difficult-to-control postoperative ascites (untreatable ascites).147
- b)
Variceal bleeding. The most important added factor associated with surgery and the management of intraoperative volume is the inevitable increase in portal pressure. Management should be established according to the Baveno VII consensus.26
- c)
HE. Preventing constipation is crucial. Extubation, in case of mechanical ventilation, should be carried out as soon as possible, to evaluate the neurologic status of the patient. HE prophylaxis should be restarted with anti-ammonia measures, as soon as the patient tolerates the oral administration route. Lactulose is the first-line treatment if tolerated by the patient. Frequently associated with bloating, it can be difficult to tolerate after abdominal surgery. Rifaximin or L-ornithine-L-aspartate can be considered an option as concomitant drugs.83
- d)
ACLF. The determination of creatinine levels and urine output requires strict follow-up for the early identification of kidney injury. In the presence of liver decompensation, infection should be ruled out as a first possibility. The serum bilirubin level and INR should be monitored as more sensitive early indicators of liver failure, and the development of ACLF should be evaluated through the corresponding scoring scales. An intensive care unit should be available.147
The evaluation and care of the patient with cirrhosis that requires a major surgical or invasive procedure should be managed by a multidisciplinary team that offers support to the surgeon during the entire perioperative period. This team should include an anesthesiologist, hepatologist, gastroenterologist, and clinical nutritionist. In the decompensated patient, the participation of a nephrology specialist may be needed, given that kidney function is a parameter that is also involved in the prognosis of these patients.
Financial disclosureThis consensus was carried out exclusively with the support of the Asociación Mexicana de Gastroenterología A.C. and no type of external economic resource was received. None of the authors received honoraria for their participation.
Conflict of interestThe authors declare that they have no conflict of interest.