Coagulation management in the patient with cirrhosis has undergone a significant transformation since the beginning of this century, with the concept of a rebalancing between procoagulant and anticoagulant factors. The paradigm that patients with cirrhosis have a greater bleeding tendency has changed, as a result of this rebalancing. In addition, it has brought to light the presence of complications related to thrombotic events in this group of patients.
These guidelines detail aspects related to pathophysiologic mechanisms that intervene in the maintenance of hemostasis in the patient with cirrhosis, the relevance of portal hypertension, mechanical factors for the development of bleeding, modifications in the hepatic synthesis of coagulation factors, and the changes in the reticuloendothelial system in acute hepatic decompensation and acute-on-chronic liver failure. They address new aspects related to the hemorrhagic complications in patients with cirrhosis, considering the risk for bleeding during diagnostic or therapeutic procedures, as well as the usefulness of different tools for diagnosing coagulation and recommendations on the pharmacologic treatment and blood-product transfusion in the context of hemorrhage. These guidelines also update the knowledge regarding hypercoagulability in the patient with cirrhosis, as well as the efficacy and safety of treatment with the different anticoagulation regimens. Lastly, they provide recommendations on coagulation management in the context of acute-on-chronic liver failure, acute liver decompensation, and specific aspects related to the patient undergoing liver transplantation.
El manejo de la coagulación en el paciente con cirrosis ha sufrido una transformación significativa a partir de principios de este siglo, con el concepto de un rebalanceo entre factores procoagulantes y anticoagulantes. Esto ha cambiado el paradigma de que los pacientes con cirrosis tienen una mayor tendencia a la hemorragia, ya que existe un rebalanceo entre factores procoagulantes y anticoagulantes. Además, ha traído a la luz la presencia de complicaciones relacionadas a eventos trombóticos en este grupo de pacientes.
En estas guías se detallan aspectos relacionados con los mecanismos fisiopatológicos que intervienen en el mantenimiento de la hemostasia en el paciente con cirrosis, la relevancia de la hipertensión portal, factores mecánicos para el desarrollo de sangrado, modificaciones en la síntesis hepática de factores de coagulación, y los cambios en el sistema reticuloendotelial en la descompensación hepática aguda e insuficiencia hepática aguda sobre crónica (IHAC). Se abordan nuevos aspectos relacionados a las complicaciones hemorrágicas en pacientes con cirrosis, considerando el riesgo de hemorragia durante procedimientos diagnósticos o terapéuticos, así como la utilidad de diferentes herramientas diagnósticas de la coagulación y recomendaciones en el tratamiento farmacológico y transfusión de hemoderivados en el contexto de hemorragia. En estas guías se actualiza el conocimiento respecto al diagnóstico y abordaje de complicaciones relacionadas a hipercoagulabilidad en el paciente con cirrosis, así como la eficacia y seguridad del tratamiento con diferentes esquemas de anticoagulación. Finalmente, se mencionarán recomendaciones en el manejo de la coagulación en el contexto de IHAC, descompensación hepática aguda y aspectos específicos relacionados al paciente en protocolo de trasplante hepático.
Patients with cirrhosis frequently present with alterations in the hemostatic system, identified through conventional coagulation studies, such as the international normalized ratio (INR), prothrombin time (PT), activated partial thromboplastin time (aPTT), and platelet count. Historically, it was believed that there was a greater tendency toward bleeding in patients with cirrhosis, but the function of the hemostatic system may not be adequately represented through conventional hemostatic tests due to a rebalancing between procoagulant and anticoagulant factors.
In addition, even though patients with chronic liver disease can experience bleeding complications, the majority of cases are recognized as not being related to alterations in the hemostatic system. Portal hypertension and mechanical vascular lesions during invasive procedures play an important role in the cause of hemorrhage in these patients. Likewise, patients with cirrhosis can be susceptible to requiring preventive or therapeutic anticoagulant therapy, with respect to thrombotic episodes, which can be controversial in the context of hemostatic system alterations in those cases. Correspondingly, there is a lack of randomized controlled clinical trials, and the design and methodology of the existing ones are considerably heterogeneous. Nevertheless, in accordance with the experience of the present panel of experts in hepatology and the available clinical evidence, we consider it relevant to provide guidelines whose purpose is to modify previous paradigms, regarding the approach to these patients.
The executive board of the Asociación Mexicana de Hepatología (AMH) designated a panel of experts in the approach to patients with advanced chronic liver disease, to formulate the present document. Its aim was to provide clinical practice guidelines with clinical applicability in the management of patients with cirrhosis and hemostatic system alterations. Scientific evidence based on clinical and biochemical fundamentals regarding the prevention of and approach to bleeding or thrombotic complications in patients with cirrhosis was compiled. The panel of experts was organized to work on four themes considered relevant for the clinician that are a practical necessity in decision-making, which were: 1) bleeding and coagulation physiology and pathophysiology in the patient with cirrhosis, 2) complications related to bleeding in the hospitalized patient with cirrhosis undergoing different diagnostic and therapeutic procedures, 3) complications related to hypercoagulability in the patient with cirrhosis and its approach, and 4) coagulation management in patients with acute-on-chronic liver failure (ACLF) or undergoing liver transplantation (LT). Each theme was developed under the supervision of a coordinator who was an expert in the area of interest.
An extensive bibliographic search was carried out on the PubMed, Embase, Cochrane, and Scopus databases, utilizing the keywords: “coagulation”, “hemostasis”, “thrombocytopenia”, “hypofibrinogenemia”, “fibrinolysis”, “thrombolysis”, “hemorrhage” OR “bleeding secondary to procedures” OR “hemostasis” OR “conventional coagulation tests” OR “viscoelastic tests” OR “thrombosis” OR “vitamin K antagonist” OR “low molecular weight heparin” OR “direct-acting oral anticoagulants” AND “cirrhosis of the liver” OR “chronic liver disease”. The scientific information collected for formulating the recommendations was classified according to quality of evidence into 1) meta-analyses, 2) systematic reviews of randomized controlled studies, randomized controlled trials, or observational studies with adequate design, 3) systematic reviews of nonrandomized or retrospective studies, 4) nonrandomized cohort studies, follow-up studies with a control arm, 5) case series, case-control studies, and 6) expert opinion based on biochemical fundamentals. Given that information in the literature is insufficient for generating an expert consensus, these guidelines were created; their recommendations were discussed remotely, due to the contact restrictions in place at the time, related to the risk for COVID-19 infection.
Bleeding and coagulation physiology and pathophysiology in the patient with cirrhosisRecommendation 1. In patients with cirrhosis, there is a rebalancing of procoagulant and anticoagulant factors, increasing the risk for thrombotic events.
Patients with advanced chronic liver disease present with a greater frequency of upper gastrointestinal bleeding complications. These patients have a deficiency in the production of coagulation factors upon the deterioration of the synthetic functions of the liver. In addition, the identification of thrombocytopenia related to splenic sequestration due to an increase in portal and splenic vein pressures, is frequent. Thus, these patients have traditionally been categorized as patients with acquired coagulopathy, i.e., as “naturally autoanticoagulated” patients.1 However, there are other simultaneous factors in this population of patients, such as elevated levels of von Willebrand factor (vWF) and factor VIII, as well as reduced levels of anticoagulant factors that are also synthesized in the liver, such as protein C, protein S, and antithrombin III.2,3 Consequently, a rebalancing between procoagulant and anticoagulant factors is produced, creating a state of adequate hemostasis. Importantly, this balance is more fragile, compared with healthy subjects.
Recommendation 2. Hemorrhagic events in the patient with cirrhosis are primarily due to complications related to portal hypertension or mechanical injury to a blood vessel.
Hemorrhagic events are related to factors other than coagulation disorders, the most important of which are portal hypertension and vascular puncture or rupture. The most advanced stages of liver disease and clinically significant portal hypertension have been related to a greater risk for bleeding in this context.4 Prospective studies have shown that, despite a higher risk for bleeding in patients with a platelet count under 30,000/μL, fibrinogen levels below 60mg/dL, and aPTT above 100s, these were attributed to complications due to portal hypertension, vascular injury, or post-procedure lesion.5
In a study carried out on candidates for LT, Giannini et al. reported an incidence of bleeding complications of 20% in patients with a platelet count below 75,000/μL. Despite that observation, the patients that received prophylactic platelet transfusion prior to the procedure were more likely to present with hemorrhagic events, suggesting that severe thrombocytopenia (fewer than 50,000 platelets/μL) is a phenomenon related to complications of cirrhosis, and that the pathophysiologic mechanism responsible for bleeding is the increase in the portal venous pressure gradient.6 Therefore, the most important aspect of treatment in these patients are interventions directed specifically at controlling the source of the bleeding, such as the prevention and control of portal hypertension, the eradication protocols of esophageal varices, and the repair of vascular lesions.
Recommendation 3. There is a defect in the hepatic synthesis capacity of coagulation, natural anticoagulant, and fibrinolytic factors in patients with cirrhosis.
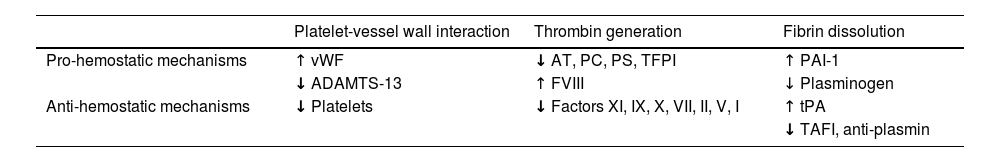
The patient with cirrhosis shows multiple hemostatic system disorders, the most relevant of which are: 1) defects in the hepatic synthesis capacity of coagulation factors (FII, FV, FVII, FIX, FX, and FXI), which in turn, is counterbalanced with a concomitant decrease in coagulation inhibitors (protein C, protein S, and antithrombin) and fibrinolytic factors (plasminogen).7 This results in a balance that reasonably impedes the expected bleeding that could result from the decrease in procoagulant factors; 2) increase in the synthesis of hemostatic proteins arising from the reticuloendothelial system (vWF, tissue plasminogen activator, plasminogen activator inhibitor-1) due to a chronic activation of endothelial cells (Table 1).
Rebalanced hemostasis in patients with cirrhosis.
| Platelet-vessel wall interaction | Thrombin generation | Fibrin dissolution | |
|---|---|---|---|
| Pro-hemostatic mechanisms | ↑ vWF | ↓ AT, PC, PS, TFPI | ↑ PAI-1 |
| ↓ ADAMTS-13 | ↑ FVIII | ↓ Plasminogen | |
| Anti-hemostatic mechanisms | ↓ Platelets | ↓ Factors XI, IX, X, VII, II, V, I | ↑ tPA |
| ↓ TAFI, anti-plasmin |
AT: antithrombin; FVIII: factor VIII; PAI-1: type 1 plasminogen activator inhibitor; PC: protein C; PS: protein S; TAFI: thrombin activatable fibrinolysis inhibitor; TFPI: tissue factor pathway inhibitor; tPA: tissue-type plasminogen activator; vWF: von Willebrand factor.
Recommendation 4. The decrease in platelet synthesis reduction, splenic sequestration, and accelerated platelet destruction is compensated by an increase in platelet adhesion protein synthesis, vWF, and a decrease in a disintegrin and metalloproteinase with thrombospondin type 1 motif, member 13 (ADAMTS-13).
Thrombocytopenia affects 70% of patients with cirrhosis and the decrease can be mild (100,000-150,000/μL) to moderate (50,000-100,000/μL), and lower values are considered severe.8 Multiple factors contribute to the development of thrombocytopenia and they can be divided into those that cause decreased production, splenic sequestration, and increased destruction. In patients with cirrhosis, there is a balance in platelet function, given that cirrhotics present with thrombocytopenia related to portal hypertension or underlying liver disease, reduced ADAMTS-13 metalloproteinase, and marginalization alterations caused by the decrease in hematocrit. In addition, there are compensatory factors, such as elevated vWF levels.
Patients with cirrhosis and thrombocytopenia have lower levels of circulating thrombopoietin than patients with normal platelet levels. There is also a decrease in the production of platelets in the bone marrow, derived from the decrease in thrombopoietin or from direct toxicity, especially in patients with alcohol consumption, certain chronic viral infections (hepatitis B virus [HBV], hepatitis C virus [HCV]), iron overload, and certain medications (azathioprine, interferon, antibiotics).9 One of the first and main causes of thrombocytopenia described in patients with cirrhosis is splenic sequestration. Another cause of thrombocytopenia to consider, especially in patients with chronic liver injury of autoimmune origin or due to HCV infection, is the destruction mediated by antiplatelet antibodies. Specific situations involving immune thrombocytopenia include immune thrombocytopenic purpura (ITP), chronic C hepatitis, infection, and certain medications.10–12 Patients with cirrhosis are considered immunodepressed and have a greater risk of contracting infections. In infectious processes, especially in sepsis, multiple inflammatory cytokines are released; among the most important are interleukin 6 (IL-6) and tumor necrosis factor-alpha (TNF-α) that accelerate platelet destruction and increase fibrinolysis.13,14 One of the reasons vWF is elevated is due to an increase in synthesis by endothelial cells and lower activity of serum ADAMTS-13, a metalloproteinase synthesized by the liver, that binds to and inactivates vWF.15–18
Recommendation 5. Conventional coagulation tests (CCTs) (PT, INR, aPTT, and platelet count) do not accurately reflect hemostatic changes and risk for bleeding in the patient with cirrhosis.
The liver has a key role in the coagulation process, given that it is the organ in which the majority of coagulation factors, as well as their inhibitors, are synthsized.19,20 Hemostasis is the result of a complex interaction between platelets, coagulation factors, and endothelium.21,22 PT and INR are tests designed for monitoring anticoagulation in patients treated with vitamin K antagonists. Vieira et al.4 showed that the conventional tests for hemostasis (INR and platelet determination) do not adequately predict the risk for hemorrhage in patients with cirrhosis and band ligation of esophageal varices and do not adequately reflect coagulation status in vivo in the patient with cirrhosis. The INR only evaluates the procoagulant pathway and does not reflect the defects in the anticoagulant pathway, and so is not adequate for evaluating patients with cirrhosis that are going to undergo a procedure.23,24
Complications related to hemorrhage in the hospitalized patient with cirrhosis undergoing different diagnostic and therapeutic proceduresRecommendation 6. Thrombocytopenia in patients with cirrhosis is correlated with the presence of clinically significant portal hypertension, but its utility as a predictor of risk for bleeding is not well established.
Results are contradictory, regarding the association of severe thrombocytopenia with the presence of hemorrhagic events after procedures, but the majority of evidence supports the idea that there is no direct relation between platelet count and risk for bleeding.25,26 Patients with advanced liver disease and severe thrombocytopenia (platelets ≤ 50×103/μL) undergoing an invasive procedure can have a higher risk for bleeding, compared with moderate thrombocytopenia. However, there is a discrepancy regarding the platelet cutoff point and risk for bleeding in different clinical practice guidelines.27 We believe the clinical utility of platelet count as a risk factor for bleeding in patients with cirrhosis should be analyzed in prospective studies.
The evaluation of risk for bleeding in diagnostic or therapeutic procedures should take into account the grade of portal hypertension, disease stage, comorbidities, systemic factors, and operator experience. The risk for bleeding is largely determined by the type of procedure and the experience of the surgeon. However, the grade of liver failure, systemic factors such as the presence of acute kidney injury (AKI), and infection, can contribute to increasing said risk.28 In a prospective study, Shah et al. reported that a group of patients with cirrhosis undergoing low-risk procedures (mainly paracentesis) that had alterations in conventional coagulation parameters (INR>1.5, platelets < 50,000) showed no hemorrhagic complications, when compared with a group of patients with normal, or slightly altered, coagulation tests.29
Recommendation 7. Viscoelastic tests (VETs) enable a more complete and detailed evaluation of coagulation homeostasis to be made in patients with cirrhosis, compared with conventional tests.
Thromboelastography (TEG) is a laboratory test used for evaluating blood clotting in real time. It provides comprehensive information on the formation, stabilization, and lysis of the clot, enabling a more complete evaluation of coagulation function to be made, compared with CCTs. TEG measures the viscoelasticity of blood during clot formation. It consists of a blood sample that is placed in a chamber and receives an activation stimulus, such as kaolin acid or calcium citrate. As the clot is forming, various parameters are measured, including coagulation starting time (R), clot formation time (K), alpha angle (α), maximum amplitude (MA), and clot lysis time (LY30).
TEG provides valuable information on platelet function and coagulation factors in the formation of a clot. The R reflects the velocity of clot formation and is primarily influenced by platelets and fibrinogen; K indicates the velocity at which the clot becomes sufficiently stable to support tension; α represents the velocity of fibrin formation and is related to thrombin activity; MA indicates the firmness of the clot and is influenced by platelet function and thrombin activity; and LY30 evaluates the capacity of the clot to dissolve and reflects fibrinolytic activity.
The clinical utility of TEG lies in its capacity to detect coagulation disorders, both hereditary and acquired, and to identify coagulation factor deficiencies, platelet dysfunction, and hyperfibrinolysis. In addition, TEG is useful in monitoring anticoagulation therapy, evaluating coagulopathy in critically ill patients, and predicting perioperative bleeding.30
Recommendation 8. In patients with cirrhosis that will undergo invasive or surgical procedures, determining the risk associated with the procedure is important, to establish intervention guidelines.
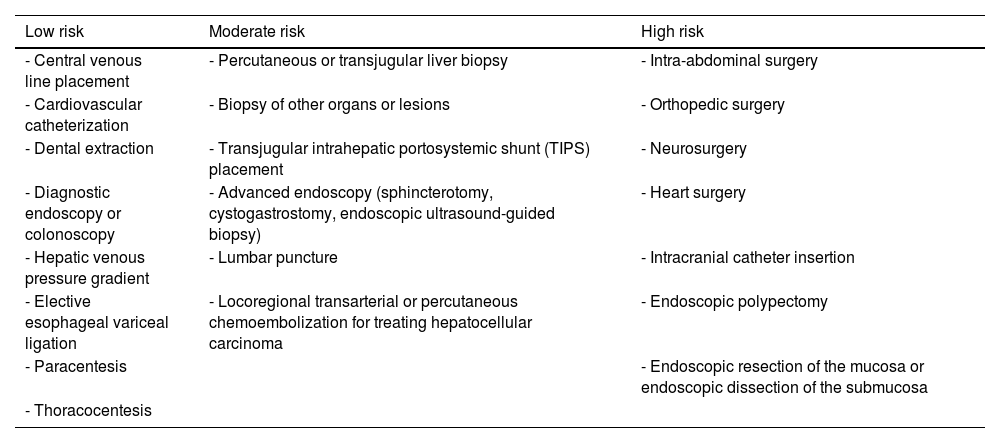
Patients with cirrhosis have a higher risk for bleeding, regarding certain invasive procedures, and the mechanisms related to a greater risk for bleeding are multifactorial. Outstanding factors are the type of procedure, grade of liver decompensation, and comorbidities, such as chronic kidney disease, medications, and vasculature alterations due to increased portal pressure.31,32 Procedures considered to have a low risk for bleeding are characterized by an estimated risk < 1.5% of significant bleeding, as well as adequate access for controlling active bleeding in case it occurs. In contrast, high-risk procedures confer a higher risk for bleeding (estimated risk > 1.5% of major bleeding) and/or difficult control of the bleeding, with catastrophic consequences, even in small quantities.33,34
The abovementioned classification is based on expert opinion and generally in the context of therapeutic anticoagulation prior to a procedure. Nevertheless, it is useful when making a multidisciplinary evaluation of bleeding risk. The technical skill and experience of the operator has a direct impact on the risk for bleeding. The use of ultrasound guidance in performing different procedures, such as thoracentesis in patients with coagulation disorders, or those that require portal vein access during transjugular intrahepatic shunt (TIPS) placement, reduces the risk for bleeding35 (Table 2).
Classification of the most frequent invasive and surgical procedures in the patient with cirrhosis, according to risk for bleeding.
| Low risk | Moderate risk | High risk |
|---|---|---|
| - Central venous line placement | - Percutaneous or transjugular liver biopsy | - Intra-abdominal surgery |
| - Cardiovascular catheterization | - Biopsy of other organs or lesions | - Orthopedic surgery |
| - Dental extraction | - Transjugular intrahepatic portosystemic shunt (TIPS) placement | - Neurosurgery |
| - Diagnostic endoscopy or colonoscopy | - Advanced endoscopy (sphincterotomy, cystogastrostomy, endoscopic ultrasound-guided biopsy) | - Heart surgery |
| - Hepatic venous pressure gradient | - Lumbar puncture | - Intracranial catheter insertion |
| - Elective esophageal variceal ligation | - Locoregional transarterial or percutaneous chemoembolization for treating hepatocellular carcinoma | - Endoscopic polypectomy |
| - Paracentesis | - Endoscopic resection of the mucosa or endoscopic dissection of the submucosa | |
| - Thoracocentesis |
In patients with cirrhosis that will undergo low-risk procedures, platelet transfusion is not recommended. The recommendation is to maintain a platelet level > 50,000/μL in patients that will undergo moderate-risk procedures and a platelet count > 100,000/μL prior to procedures with a high risk for bleeding, such as spinal surgery, heart surgery, or TIPS placement.36,37
The transfusion of five to six platelet concentrates or a platelet apheresis achieves an increase in the platelet count of 5,000 to 10,000/μL in the patient with a total infused volume of approximately 250mL of plasma, which can be conducive to a harmful effect in portal hypertension hemodynamics, as well as to a deleterious immunologic effect, conditioning little benefit.38
Recommendation 9. Thrombopoietin (TPO) agonist administration in patients with severe thrombocytopenia can be useful prior to invasive procedures.
Reduced platelet production by megakaryocytes due to a low thrombopoietin level is one of the factors that contributes to thrombocytopenia in patients with cirrhosis of the liver. TPO agonists are a good alternative to platelet transfusion, exclusively in the case of elective or programmed procedures, given that their effect is transitory and needs around 10 days to achieve the required elevation in the number of platelets. TPO agonists increase the platelet count by stimulating the TPO receptor. In a study that examined thrombin generation, platelet counts above 56,000/μL were associated with normal thrombin production.38 Two TPO agonists have shown efficacy and safety in patients with cirrhosis and severe thrombocytopenia that underwent moderate-to-high-risk invasive procedures. Avatrombopag is administered 10 to 13 days prior to the procedure at a daily dose of 40mg, if the patient’s platelet count is 40,000 to 50,000/μL, and at a daily dose of 60mg, if the patient’s platelet count is below 40,000/μL. It is administered orally, and the duration of this therapy is five days. The median increase in the platelet level reached is 26,500/μL and 34,500/μL, respectively, and the maximum time it takes to maintain a platelet level above 50,000/μL is about 20 days. However, the procedure should be programmed two to eight days after the final dose of avatrombopag, to ensure the required platelet count.
Lusutrombopag is administered orally at a daily dose of 3mg for seven days. It should be started eight to 14 days prior to the procedure, the median increase in the platelet level reached is 45,000/μL, and the maximum time it takes to maintain a platelet level above 50,000/μL is 20 days. Nevertheless, the procedure should be programmed five to eight days after the last dose of lusutrombopag, to ensure the required platelet count. Both TPO analogues have a risk for thrombosis similar to placebo and are contraindicated in pregnancy and in patients with a history of or presenting with portal vein thrombosis (PVT) or mesenteric thrombosis. Other TPO analogues, such as eltrombopag and romiplostim, have not been evaluated in this clinical context and so cannot yet be recommended.39–42
Recommendation 10. Routine use of fresh-frozen plasma (FFP) is not recommended due to the increased risk for volume overload and associated acute pulmonary injury, in patients with cirrhosis undergoing invasive procedures.
In patients with cirrhosis, every 100 mL of plasma increases portal pressure by 1.4 mmHg and transfusion of more than two units increases the circulating volume and portal pressure more than 7 mmHg, increasing the probability of hemorrhage due to portal hypertension. Studies on patients with cirrhosis have shown that even though FFP administration can correct the PT and the INR, it has no impact with respect to thrombin generation. A more recent study showed that although FFP transfusion improved the CCT results, it only improved thrombin generation in a very limited number of patients. Thirty-four percent of the cases analyzed even presented with a decrease in thrombin generation in response to FFP transfusion. Those authors suggested that FFP transfusion could reduce thrombin generation by replacing protein C, one more reason not to recommend its use for correcting the INR in patients with cirrhosis.43
Additional adverse events that have been related to FFP transfusion that should be taken into account are transfusion reactions, acute pulmonary injury, infection transmission, hypercoagulability, thrombotic event generation, systemic inflammatory response, greater mortality, and human leukocyte antigen (HLA) antibody formation.
Recommendation 11. Maintaining serum fibrinogen levels > 120 mg/dL, through the administration of synthetic fibrinogen or cryoprecipitates, is recommended in case of bleeding or prior to a high-risk invasive procedure.
Patients with compensated cirrhosis tend to have normal serum fibrinogen levels. Fibrinogen levels decrease as chronic liver disease progresses, occurring in up to 76% of the patients with cirrhosis. Dysfibrinogenemia has been reported in up to 76% of patients with cirrhosis. Hypofibrinogenemia is an independent risk factor related to an increase in bleeding in patients with cirrhosis. The frequency of severe hemorrhagic events, in particular, increases in patients with cirrhosis and a fibrinogen level under 60 mg/dL.43 In addition, fibrinogen levels are an independent predictor associated with greater mortality in patients with cirrhosis, showing an increase of 29% in mortality for each decrease of 100 mg/dL in serum fibrinogen. Currently, there are no studies that have evaluated fibrinogen and determined its optimum levels in patients with cirrhosis that will undergo moderate-to-high-risk invasive or surgical procedures. Most experts suggest that maintaining fibrinogen levels above 100-120 mg/dL can be appropriate, given that this strategy has been effective for hemostatic control in the context of patients with acute hemorrhage.44,45
For fibrinogen replacement, the clearest data have been in cases of severe trauma with cirrhosis, in which levels > 200 mg/dL are associated with more effective hemostasis; historically, in patients with cirrhosis, levels under 120 mg/dL have required correction.16,46 Its replacement has conventionally been carried out with cryoprecipitates, at a volume of 10 to 20mL/U. One unit of cryoprecipitate per 10 kg of body weight raises the plasma fibrinogen concentration by approximately 50 mg/dL. An average dose is 5 to 10 U (50 to 200 mL). Fibrinogen concentrates (50 mg/kg) have a lower volume, a more standardized fibrinogen content, and lack of cross-matching, favoring their use in cirrhosis, and recent data suggest a clear improvement in hemostasis, but more studies are needed to justify their use. There are isolated studies on prothrombin complex concentrates that have been used as anticoagulation therapy, given that the dose is based on factor IX content, at a dose of 25-30IU/kg. Recent data suggest that lower doses can be effective in cirrhosis, but more evidence is required, and the concern about an increased risk for thrombosis persists.31
FFP contains 200 to 450 mg/dL of fibrinogen; however, its use is not recommended for the reasons stated above.47 Cryoprecipitates contain 1,500 to 1,700 mg/dL of fibrinogen and can be used as an alternative, when other strategies are not available for correcting fibrinogen figures in the patient with cirrhosis. In addition to high fibrinogen concentrations, cryoprecipitates contain complete coagulation factors, including factor VIII, XIII, and vWF; cryoprecipitates are prepared through the controlled thawing of FFP, and thus, have potential associated risks, similar to those described for FFP.48,49
Fibrinogen concentrate is a pasteurized concentrate made from human plasma; it is available in a simple vial that contains 900 to 1,300 mg of lyophilized fibrinogen concentrated in powder for its reconstitution.50 Fibrinogen concentrate is an attractive alternative to allogenic blood products due to purification and viral inactivation during manufacturing, importantly minimizing the risk associated with the use of blood products. This also enables the rapid administration of a standardized quantity of fibrinogen, without causing hemodilution or volume overload. Fibrinogen administration should be guided through TEG or thromboelastometry.
Complications related to hypercoagulability in the patient with cirrhosis, and its managementRecommendation 12. The incidence of deep vein thrombosis in patients with cirrhosis is similar to or greater than that observed in patients without cirrhosis.
As previously described, as a consequence of the anticoagulation paradigm in the cirrhotic patient, prophylactic measures for deep vein thrombosis are not carried out. The risk for venous thromboembolism (VTE) in the patient with cirrhosis is the same or more frequent than in patients without cirrhosis.51,52
A significantly lower incidence of VTE has been described in patients with cirrhosis that receive prophylaxis (0.5%), compared with a group of cirrhotic patients that did not receive said treatment (1.8%) (p=0.05). Retrospective studies have shown a prevalence of VTE in subjects with cirrhosis similar to that in the general population. In a case-control study conducted in Denmark that included 99,444 patients with VTE and 496,872 controls, the patients with cirrhosis of the liver had a higher relative risk for developing deep vein thrombosis, compared with the control group or patients with liver disease and no cirrhosis. In a meta-analysis that included 695,012 patients with cirrhosis, compared with 1,494,660 controls in 11 studies, there was an increased risk for VTE in patients with cirrhosis (odds ratio [OR]: 1.703; 95% confidence interval [CI]: 1.333, 2.175; p<0.0001).53
Recommendation 13. The use of prophylactic anticoagulation is recommended in patients with cirrhosis at risk for developing VTE. The risk of bleeding complications due to prophylactic anticoagulation in patients with cirrhosis is similar to that in patients without cirrhosis.
Underutilization of prophylaxis for deep vein thrombosis and pulmonary embolism has been shown in patients with cirrhosis. The prevalence of bleeding does not increase in cirrhotics that receive prophylaxis for VTE. Several studies have reported an almost doubled increase in the risk for developing VTE in patients with liver disease, compared with the risk in control groups. Patients with cirrhosis related to steatohepatitis and those with cirrhosis related to hepatitis C have a higher risk for developing VTE. Mortality at 30 days after deep vein thrombosis in patients with cirrhosis (7%) is higher than in patients without cirrhosis (3%).54 Patients with cirrhosis and atrial fibrillation have been excluded from studies on prophylactic anticoagulation. In an observational study conducted in Taiwan that included 10,336 patients with cirrhosis and atrial fibrillation, the use of warfarin in patients with cirrhosis was associated with both a lower risk for developing events of vascular ischemia and clinical benefit, compared with a control group of noncirrhotic patients with atrial fibrillation that received prophylactic treatment.55 Anticoagulation has been shown not to increase the risk for gastrointestinal bleeding in patients with cirrhosis, even in those patients undergoing band ligation for esophageal varices.56 Because anticoagulation does not increase the risk for bleeding in the patient with cirrhosis, prophylaxis for VTE is recommended in cirrhotic patients in the same way that it is recommended in patients without cirrhosis.57–60
Recommendation 14. There is an increase in the incidence of PVT in relation to liver decompensation events in patients with cirrhosis.
In patients with cirrhosis, increased pressure of the portal and splenic venous circulation, as well as the presence of microthrombi, favor the risk for developing PVT.27 The incidence of PVT reported in a case series on 855 patients undergoing LT was 13.8%; 1.6% of those patients presented with thrombosis in the intrahepatic branches and 5.8% had complete obstruction of the main trunk of the portal vein.60 The incidence of PVT in noncirrhotic patients is low. In a statistical analysis conducted on 247,728 necropsies performed in Japan, Okuda et al. reported an incidence of PVT of 0.054% in patients without cirrhosis and 6.59% in patients with cirrhosis.61 An epidemiologic study on 779 LT recipients, between 1987 and 1996, reported an incidence of PVT of 8.1%. In a study on 461 patients with cirrhosis of various etiologies, Yasir et al.62 reported an incidence of PVT of 9.8%. Most of the patients (71.5%) presented with decompensated cirrhosis. PVT was not related to a particular cirrhosis etiology. A gradual increase in the incidence of PVT has recently been observed in patients with chronic liver disease. Cool et al.63 reported a 1.5% prevalence of PVT in an observational study that utilized a database containing information on 3,045,098 patients discharged from hospitals in the United States, within the time frame of 1998 to 2014. An increase in prevalence from 0.7 to 2.4% was observed, with a change in the annual percentage of 9%. A logistic regression analysis showed that PVT significantly increased the risk for AKI (OR 1.75, p < 0.001) and hepatorenal syndrome (OR 1.62, p < 0.001). It has also been associated with an increase in mortality (OR 1.12, p = 0.001).
Recommendation 15. The presence of PVT in patients with cirrhosis increases the technical difficulty in performing LT and should be routinely evaluated before the procedure.
In 1985, Shaw et al.64 reported the first successful cases of LT in the context of PVT in the recipient. PVT was considered an absolute contraindication for LT due to the risk for intraoperative mortality and the technical difficulty during the surgery. Advances in the surgical technique, broader experience with the surgical procedure, and better anesthesia management have increasingly made LT in patients with cirrhosis and PVT a routine procedure.
PVT in cirrhosis is associated with greater surgical complexity and with the development of postoperative rethrombosis. PVT in the patient undergoing LT has been associated with longer surgery duration, more transfusions, a higher reintervention rate, and a lower survival rate.65 In addition, portal rethrombosis, the use of dialysis, and infectious complications are increased in patients with PVT.66 In a 2011 study, Kim et al. found that patients with PVT and undergoing living-donor LT with grafts from the right lobe had a higher need for transfusions, longer stays in the intensive care unit, and a higher probability of bleeding, compared with the patients without PVT. Greater resource use was more pronounced in patients with occlusive PVT, compared with patients with nonocclusive PVT.67
An analysis from the United Network of Organ Sharing (UNOS) showed that recipients with PVT prior to transplantation have a higher risk for developing thrombosis of the hepatic artery and graft loss.68 At the transplant centers with experience in LT in patients with PVT, a multidisciplinary focus can lead to excellent results in both the short and long terms, even in patients with high-grade PVT. Innovations in the surgical techniques have reduced the threshold for performing LT in candidates with PVT, but those patients, particularly the subjects with occlusive thrombosis extending to the mesenteric veins, continue to have suboptimal results. Therefore, in cases of extensive occlusion, transplant can be contraindicated, unless the patient undergoes vascular stenting.69,70
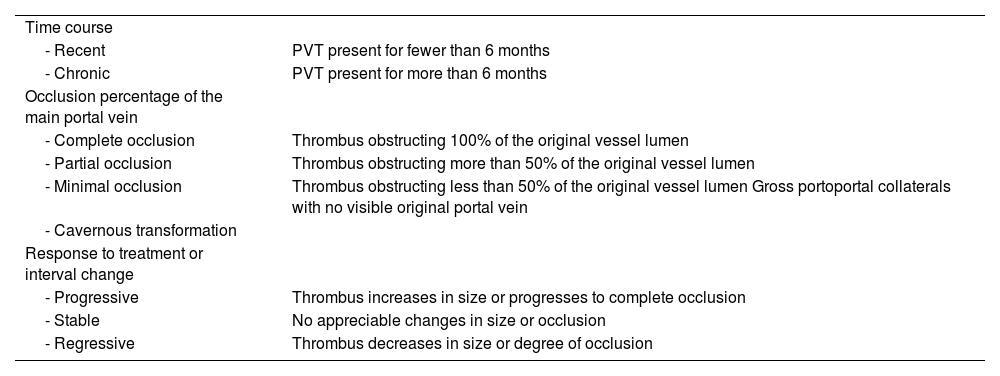
Recommendation 16. Characterizing PVT according to its location, extension, percentage of intraluminal occlusion, and chronicity is recommended.
There is a discrepancy in the literature, with respect to the terminology and classification systems of PVT due to the diversity of clinical manifestations, causes, and therapeutic options. Thus, patients with PVT need to be categorized in a standard manner to enable comparisons to be made between studies. Progression time is a factor that has been associated with the probability of recanalization in patients with PVT. In a prospective study on patients with recently diagnosed PVT, Plessier et al. showed that the probability of recanalization was significantly reduced if PVT had been present for more than six months. In fact, patients with PVT for more than six months had a higher risk for developing cavernous degeneration of the portal vein, despite having been treated with anticoagulation therapy.71
According to these findings, the cutoff point of six months has been justified for distinguishing patients with “chronic” PVT (> 6 months) or “recent” PVT (< 6 months). The term “recent” enables a more accurate definition of progression time to be made than the term “acute” due to the fact that not all patients present with symptomatology at the time of diagnosis.61 Multiple classification systems have been developed for classifying PVT, primarily in patients on the waiting list for LT, taking into consideration location, extension, progression, and treatment response.72,73
Northup et al. have recently proposed a standardized nomenclature for describing PVT for use in the clinical context and for research purposes. Said classification takes into account progression time, the percentage of occlusion of the main portal vein, cavernous transformation of the portal vein, and treatment response and/or thrombus progression (Table 3).27
Standard recommended nomenclature for describing PVT in the clinical and research context.
| Time course | |
| - Recent | PVT present for fewer than 6 months |
| - Chronic | PVT present for more than 6 months |
| Occlusion percentage of the main portal vein | |
| - Complete occlusion | Thrombus obstructing 100% of the original vessel lumen |
| - Partial occlusion | Thrombus obstructing more than 50% of the original vessel lumen |
| - Minimal occlusion | Thrombus obstructing less than 50% of the original vessel lumen Gross portoportal collaterals with no visible original portal vein |
| - Cavernous transformation | |
| Response to treatment or interval change | |
| - Progressive | Thrombus increases in size or progresses to complete occlusion |
| - Stable | No appreciable changes in size or occlusion |
| - Regressive | Thrombus decreases in size or degree of occlusion |
PVT: portal vein thrombosis.
Recommendation 17. A detailed evaluation for identifying primary hypercoagulability disorders in patients with cirrhosis and PVT is not recommended.
Multiple factors for developing PVT coexist in the cirrhotic patient. The main risk factors in patients with cirrhosis are the underlying liver disease and the severity of portal hypertension. Previous decompensation of liver cirrhosis and thrombocytopenia are factors that can predict the risk of PVT, suggesting the role portal hypertension severity plays in the pathophysiology of PVT. Despite the fact that the development of PVT is significantly associated with the severity of liver disease at baseline, there is some controversy, given that a multicenter study on 1,243 cirrhotic patients with no PVT that were surveilled for 47 months showed that the progression of the liver disease was not associated with PVT.74
The decrease in portal venous flow velocity determined through Doppler ultrasound has been associated with an increased risk of presenting with PVT. In a cohort of 100 patients with cirrhosis, Stine et al. showed that portal venous flow velocity was the most important independent risk factor for predicting the development of PVT. Venous flow velocity below 15 cm/s had a highly significant association with the development of PVT (hazard ratio [HR] 6.00, 95% CI 2.20-16.4, p=0.0001).75 In patients with cirrhosis related to viral infection, the development of collateral vessels has been shown to be a factor that predicts the development of PVT.76
Hypercoagulability, hemodynamic changes, and endothelial lesion are the three main factors related to the development of thrombosis. However, the role of hypercoagulability in PVT in patients with cirrhosis has been controversial. Numerous studies have identified a significant decrease in the level of natural coagulation inhibitors in the cirrhotic patient. Several studies have attempted to clarify the relation between the procoagulant imbalance in patients with cirrhosis and the risk for developing PVT. Despite the fact that there is a significant decrease in serum protein C, protein S, and antithrombin levels in patients with PVT, only the decrease in portal venous flow velocity has been independently associated with the development of PVT.77
In a prospective study on 151 cirrhotic patients with PVT (n = 20) and without PVT (n = 131), Tang et al. reported a significant decrease in serum levels of protein C, protein S, and antithrombin (p = 0.001, p = 0.05, and p = 0.001, respectively), with a progressive descent into greater severity of cirrhosis. However, no significant association was found between the decrease in natural anticoagulants with the development of PVT.78
Recommendation 18. In patients with cirrhosis of autoimmune etiology and PVT, evaluating primary hypercoagulability disorders, according to clinical judgement, is recommended.
Autoimmune liver diseases are chronic inflammatory diseases with a very broad clinical spectrum that includes acute liver failure, the development of advanced hepatopathy and/or hepatocellular carcinoma (HCC). Cases of autoimmune hepatitis (AIH) associated with thrombotic disorders have been reported that have a high rate of positive anticardiolipin antibodies, compared with patients with liver diseases due to other etiologies.79 Studies that included small subgroups of patients found a higher prevalence of PVT in patients with AIH.80,81
In a trial conducted at the Hospital Clínico, in Barcelona, a cohort of 37 patients with AIH and indication for LT were analyzed. Pretransplant PVT was documented in 30% (n = 11) of the patients, compared with 11% in the rest of the population of the 2,011 patients transplanted within the time frame of 1998 to 2016 at the same center (p = 0.02). Upon only including the patients with cirrhosis, PVT presented in 55% of the group with AIH, a significantly higher percentage than the 12% in the transplanted cohort (p < 0.001). In the PVT cases, the presence of a prothrombotic disorder was evaluated through IgG and IgM anticardiolipin antibody determination, deficiency of protein C and protein S in plasma, factor V Leiden mutation, and prothrombin 20210 gene mutation. A prothrombotic panel was obtained in 13 of the subjects and antiphospholipid syndrome was documented in only one case. Interestingly, in that small study, of the 11 subjects with pretransplant PVT, five were started on early anticoagulation for one to six months. None of the patients that received the therapy developed PVT, compared with 2/6 patients that did not receive the therapy (one patient at nine months and the other at 20 months posttransplantation). Those study results suggest the possible contribution of prothrombotic factors to PVT in the population with cirrhosis of autoimmune origin, as well as the potential benefit of early anticoagulation therapy in that group of patients, but more studies are needed to confirm those findings.82
Recommendation 19. Carrying out studies for early detection of HCC in patients with cirrhosis and PVT is recommended.
The prevalence of PVT is higher in patients with concomitant cirrhosis of the liver and HCC and varies from 35 to 40%.1,2 In an Israeli study, 38.4% of the cases with a recent PVT diagnosis were reported to be associated with the detection of concomitant HCC.83 Tumor invasion into the portal vein with thrombosis is a frequent complication of HCC, with reported incidences of 36 to 44%.84,85 Upon detecting PVT, the presence of HCC should be investigated, defining whether or not PVT is associated with the tumor, given that the prognosis and subsequent management must be adjusted to the different scenarios.
PVT can be diagnosed through HCC surveillance ultrasound carried out every six months, with 80 to 100% sensitivity and specificity that varies according to operator experience.86 Nevertheless, to detect and confirm HCC, dynamic studies should be performed (multiphase tomography and/or magnetic resonance imaging), according to the international guidelines.87,88 These last two imaging modalities can evaluate the extension of the thrombosis, carry out portosystemic collateral mapping, and diagnose whether the PVT is associated with tumor invasion of the portal vein.
Recommendation 20. Starting anticoagulation (preferably with unfractionated heparin) in patients with acute PVT and suspected intestinal ischemia is recommended.
In patients with cirrhosis and portal hypertension that develop PVT, intestinal ischemia is much less frequent, compared with patients without cirrhosis, due to the development of previously formed portosystemic collaterals.61 The main symptom of acute PVT is abdominal pain (90%) that ranges from nonspecific symptoms of dyspepsia to acute abdomen with intestinal ischemia, especially when the mesenteric veins are affected. Intestinal ischemia should be suspected when there are clinical symptoms of hematochezia or rectal bleeding, peritoneal irritation, leukocytosis, and metabolic acidosis.89
Anticoagulation is essential in all patients with acute PVT and suspected intestinal ischemia and should immediately be started subcutaneously or parenterally. Treatment goals are to recanalize thrombosed veins and prevent the development of bowel infarction and complications of portal hypertension. Starting immediate anticoagulation is recommended, whether with unfractionated heparin infusion or low molecular weight heparin (LMWH).90,91 In the case of intestinal ischemia with the possibility of surgical intervention, kidney failure, or elevated risk for bleeding, unfractionated heparin is preferred, given that coagulation can be corrected in less time due to its short half-life (one to two hours), using LMWH later.
Intestinal ischemia should be evaluated by a multidisciplinary team (surgery, hepatology, intensive care, interventional radiology, and hematology), in addition to fasting and prophylactic antibiotics. The surgical approach is recommended when there is bowel infarction.
Recommendation 21. The use of direct-acting oral anticoagulants (DOACs), instead of vitamin K antagonists (VKAs), in patients with compensated cirrhosis that require long-term anticoagulation is recommended.
The benefit of anticoagulation treatment in patients with cirrhosis and PVT is a controversial topic. Even though the spontaneous recanalization of PVT has been described in a considerable number of patients, when PVT is partial, an increased possibility of thrombosis progression has also been observed (48-70% of patients in a two-year follow-up). When starting anticoagulant treatment, the following points should be taken into account92,93:
- -
Whether the patient is on the LT waiting list or if the patient is potentially transplantable.
- -
Regarding thrombosis extension, if the thrombosis is recent and the vessels can be identified or if the thrombosis is chronic, with cavernous degeneration of the portal vein.
- -
In patients with cirrhosis and recent thrombosis in intrahepatic small portal branches or minimal main portal vein thrombosis (< 50% obstruction of the lumen), surveillance with serial imaging studies every three months with no anticoagulation is recommended. If the thrombus progresses, starting anticoagulation therapy is suggested.
- -
In patients with cirrhosis and recently appearing main or mesenteric PVT, whether occlusive or partial (> 50% obstruction of the lumen), anticoagulation is indicated to prevent thrombosis progression that could affect the possibility of LT in the future.
- -
Anticoagulation or interventional treatment is not recommended in patients that have chronic thrombosis and total occlusion of the portal vein with cavernous formation, and present with established collaterals; therefore, management should be centered on the complications of portal hypertension.
Treatment of PVT in candidates for LT: PVT has a greater impact in the context of LT because it can become a contraindication for the transplant. Patients with extensive PVT, especially when the superior mesenteric vein (SMV) is affected, may require a non-physiologic anastomosis during LT, which has been associated with higher posttransplant morbidity and mortality. Thus, in patients with PVT that are on the waiting list or are possible candidates for transplantation, anticoagulation treatment is indicated, for the purpose of enabling physiologic porto-portal reconstruction, recanalizing the portal vein, or at least preventing thrombus progression and extension into the SMV.94
Treatment of PVT in patients that are not candidates for LT: extension of PVT to the SMV with intestinal ischemia is an unquestionable indication for anticoagulation treatment.95
Pharmacologic treatment: anticoagulation treatment with enoxaparin 1 mg/12 h or 1.5 mg/24 h is recommended. Maintaining LMWH for four weeks after the acute phase is suggested, and if there are no complications, it can be substituted by oral anticoagulants (VKAs or DOACs). Oral anticoagulants and heparin are considered safe in patients with cirrhosis, with bleeding rates similar to those in the general population. The risk for bleeding due to varices does not increase if adequate primary or secondary prophylaxis is carried out. Thus, before starting anticoagulation, gastroscopy should be performed to rule out esophageal varices and begin prophylaxis according to the endoscopic findings.
Unfractionated heparin, LWMH, and VKAs have historically been used in patients with PVT. Therapy with a VKA can be affected by prolonging the baseline INR in patients with cirrhosis, creating uncertainty as to the therapeutic goal. On the other hand, a disadvantage of LWMH is its subcutaneous application, which makes its long-term use difficult.
In patients with compensated cirrhosis, the safety profile of the DOACs appears to be similar to that in patients without cirrhosis. The use of DOACs in patients with cirrhosis is based on small cohort studies. Indications for their use in cirrhotic patients has expanded to include atrial fibrillation, deep vein thrombosis, and PVT.96,97 DOACs (dabigatran, apixaban, rivaroxaban, and edoxaban) appear to be a good therapeutic option, given that they enable better drug adherence, thanks to their oral administration and the fact that there is no need to adjust the dose according to PT. However, more studies on this population are needed to establish their safety and efficacy. They should be used with precaution in patients with significant portal hypertension. VKAs are preferably used in cases of decompensated cirrhosis and renal insufficiency.98–100
Recommendation 22. Considering performing local or systemic thrombolysis, associated or not with a transjugular intrahepatic portosystemic shunt (TIPS), in patients with persistent intestinal ischemia despite anticoagulant therapy, is recommended.
Thrombolysis (local or systemic) has been proposed as treatment for achieving recanalization in patients with recent PVT, and its results are similar to those of anticoagulation. Routine thrombolysis is not recommended because it is associated with a higher risk of morbidity and mortality than anticoagulation.
In cases of expansive portomesenteric thrombosis, with signs of intestinal ischemia and a risk of bowel infarction and death, thrombolysis combined with anticoagulation can be an alternative for achieving recanalization. A recent case series (with the highest number of cases currently reported) included 22 patients with a poor prognosis and high risk of bowel infarction that underwent systemic thrombolysis (alteplase) with a stepwise protocol, achieving recanalization in 86% of the cases. Complication rates were 9%, which is low, compared with previously published results.101 TIPS is an efficacious complementary therapy for reducing portal hypertension and also for recanalizing the portal vein, enabling sustained portal vein permeability. Direct access of the portal vein is attainable through the TIPS, making it possible to perform thrombolysis and angioplasty of the affected vessels, for the purpose of recanalizing portal flow. In a study that included 70 consecutive patients with cirrhosis of the liver and PVT that underwent TIPS placement, complete recanalization was achieved in 57% of them; permeability was maintained during follow-up in 95% of the patients. Transjugular thrombectomy and local fibrinolysis and/or TIPS are treatment alternatives that should be considered only in very well selected cases, especially those in which intestinal ischemia persists, despite anticoagulation.
Recommendation 23. Recanalization of the portal vein followed by TIPS is considered in patients that will undergo transplantation or in patients with recurrent variceal bleeding and/or refractory ascites.
Cirrhotic patients with PVT who are candidates for LT and have complications of portal hypertension that are refractory to habitual treatment, should be evaluated for undergoing portal recanalization±TIPS, through interventional radiology (angiography), to enable physiologic anastomosis to be performed between the graft and the portal vein of the recipient. Portal recanalization with TIPS should be considered in patients that have PVT progression, despite anticoagulation, and in patients with chronic PVT or cavernoma with severe complications of pulmonary hypertension (PHT), e.g., in bleeding due to varices or refractory ascites. When TIPS placement is considered, having detailed staging of the PVT is crucial (thrombus evolution time course, identifiable portal branches, presence of cavernoma). TIPS can be technically more feasible in patients with recent thrombosis and with an identifiable vessel, even if the thrombosis causes complete blockage. Nevertheless, in cases with chronic PVT and/or cavernous transformation of the portal vein, portal recanalization with TIPS might be necessary; it is a procedure that often requires percutaneous transsplenic or transhepatic access.102 Small retrospective studies have shown that the transsplenic approach for accessing the thrombosed portal vein is superior to the transhepatic approach, with a high success rate (60 of 61 cases).103 In general, anticoagulation after TIPS placement is not necessary, but it should be individualized, taking into account the persistence of thrombus remnants secondary to recanalization and/or to recognizing an underlying prothrombotic disease.
Coagulation management in patients with acute-on-chronic liver failure (ACLF) or liver transplantationRecommendation 24. In the patient with cirrhosis and ACLF, conditions that favor hypocoagulability (sepsis, AKI, and endothelial dysfunction) and hypercoagulability (platelet activation, venous stasis, inflammation, and dehydration) occur simultaneously.
There is a mixed fibrinolytic profile in patients with cirrhosis and acute decompensation, with the presence of either hyperfibrinolysis or hypofibrinolysis. The presence of hypofibrinolysis associated with sepsis, organ failure, and mortality, secondary to defective clearance of intraorganic microthrombi, has been shown in the short term in this population.104 There is less platelet aggregation and secretion in patients with cirrhosis and AKI, which conditions an increase in the tendency toward bleeding. In addition, factor VIII increases and protein C, protein S, and antithrombin decrease, and when combined with greater thrombin production, this favors hypercoagulability. Factor XIII is decreased in the presence of AKI, increasing the tendency for hemorrhage.
AKI is a frequent complication in patients with cirrhosis, which is associated with a greater risk for bleeding. Intagliata et al. showed a significant decrease in factor XIII levels in patients with decompensated cirrhosis and AKI, suggesting that factor XIII deficiency in those patients can increase the risk for bleeding.105 In patients with decompensated cirrhosis, AKI is associated with characteristics of hypocoagulability and hypercoagulability, which potentially increases the risk for bleeding and thrombosis. In a population with decompensated cirrhosis and AKI, Zanetto et al. showed a decrease in platelet aggregation, an increase in factor VIII, a decrease in protein C, protein S, and antithrombin, which, together with greater thrombin generation, favors a state of hypercoagulability. In contrast, factor XIII was reduced in those patients, conferring a risk of bleeding. Lastly, despite the hypofibrinolytic and hyperfibrinolytic changes present in AKI, a larger plasmin-antiplasmin complex indicated a hyperfibrinolytic state. After AKI was resolved, fibrinolysis remained hyperactivated.106
Decompensated cirrhosis and ACLF respond in the same manner to sepsis that is associated with hemostatic changes, due to the inflammatory response of the host to infectious agents that lead to overexpression of inflammatory mediators. This, together with the microorganisms and their derivatives, is believed to favor changes that condition massive formation of thrombin, and in turn, of fibrin. This conditions anticoagulant physiologic pathway deterioration, due to the overproduction of the plasminogen activator inhibitor-1 by dysfunctional endothelial cells, conditioning fibrinolysis suppression, and most likely, the thrombin-mediated activation of the thrombin-activatable fibrinolysis inhibitor.
Recommendation 25. Patients with ACLF tend to present with a prolonged coagulation time and delay in clot formation and firmness.
According to the results of a prospective study specifically designed for characterizing the thromboelastography profile in patients with ACLF, compared with patients with acute decompensation, individuals with ACLF frequently present with a pattern of hypocoagulability, with a delay in the start of clot formation and its velocity and a decrease in clot firmness. These alterations worsen after hospital admission and are correlated with the presence of systemic inflammation, thus linking to greater short-term mortality. On the other hand, said alterations persist during the follow-up of patients with ACLF, and in contrast, tend to normalize in patients with acute decompensation without ACLF.107
Hypocoagulability has been observed in patients with decompensated liver cirrhosis without ACLF. In fact, Kleinegris et al.108 evaluated plasma thrombin generation and blood clot formation (rotational thromboelastometry [ROTEM]) in 73 decompensated patients and reported delay in clot formation and reduced firmness in the thromboelastography study, which was associated with an increase in cirrhosis severity. Likewise, it is important to consider other factors that contribute to coagulation alterations in patients with ACLF and that favor the tendency for hemorrhage, such as the frequent renal insufficiency that is present in that group of patients and that implies a greater risk for bleeding, mainly due to acquired platelet dysfunction.109
Recommendation 26. Baseline TEG parameters have a greater prognostic capacity than INR in patients with ACLF.
TEG is a comprehensive, dynamic study of coagulation that evaluates the viscoelastic properties of non-centrifuged blood, from initial clot formation to final complete firmness in blood. It is considered a more reliable test for evaluating coagulation than other standard coagulation tests, as well as for guiding transfusions in patients requiring major surgery, including LT.107 TEG tends to be normal in patients with compensated cirrhosis of the liver. In some studies, it is considered an additional prognostic tool in patients with ACLF that correlates better with short-term survival, compared with the results of standard coagulation tests.107
Coagulation management in patients with acute liver failureRecommendation 27. In patients with acute liver failure (ALF) that will undergo an invasive procedure, VETs are of greater utility for evaluating bleeding risk and reducing transfusion requirements than CCTs (PT, PTT, INR, platelets).
ALF is a syndrome characterized by the development of hepatic encephalopathy and coagulopathy within 26 weeks from the onset of acute liver disease.110,111 Patients with ALF have a “rebalanced” hemostatic system, with characteristics similar to those observed in patients with cirrhosis. They present with hypercoagulability and hypofibrinolysis, in addition to an imbalance in the vWF/ADAMTS-13 axis, elevated levels of procoagulant microparticles and factor VIII. These alterations increase the risk for both intrahepatic and systemic thrombosis.112,113 In ALF, elevated PT/INR does not signify a higher risk for bleeding, given that it only measures the activity of procoagulant factors, but it continues to be useful as an indicator of prognostic outcomes and the need for LT.114
Clinical studies have shown a discrepancy between the results of CCTs and VETs, despite abnormal values of the former.115 In 51 patients with ALF, Hawkins and Seetharan et al. observed that despite having an elevated INR, two-thirds (63%) had VETs with no alterations, and four (8%) had hypercoagulability. They also reported that the number of thrombotic complications was higher than the number of bleeding events.116,117
Agarwal et al. evaluated 20 patients with ALF and their results were similar. They observed no correlation between PT values and the VET profile, finding hypocoagulable tracings in 20%, normal tracings in 45%, and hypercoagulable tracings in 35%. There were no significant bleeding complications, and no transfusions were required.118 Based on recent evidence on a balanced hemostasis, prophylactic blood-product transfusion is not justified and can expose patients to adverse effects, such as volume overload, infections, and transfusion reactions. Coagulation should be corrected only in cases of invasive procedures with a high risk for bleeding (insertion of intracranial pressure [ICP] monitors, lumbar puncture, etc.) and in the presence of significant active bleeding.119–121 In conclusion, VETs are useful, but they do not describe the true hemostatic balance in ALF because they do not provide information about the anticoagulation system of protein C and they lack sensitivity to vWF.122 These tests may eventually have a role in the evaluation of coagulation but no high quality clinical evidence has yet to appear. In the future, their standardization will most likely enable a more complete way to manage coagulation disorders in this group of patients.
Coagulation management in patients with liver transplantationRecommendation 28. In patients with cirrhosis that will undergo LT, viscoelastic methods are more useful for evaluating the risk for bleeding, compared with CCTs (INR, PTT, platelets).
VETs have been used in LT since 1985 and are part of the standard protocol in many institutions.117,123–126 Coagulation disorders in the patient that will undergo LT are complex and can differ, according to the underlying disease and conditions of the donor, among other factors. LT is an intervention that involves various phases. The most severe coagulation disorders are produced in the anhepatic and reperfusion phases. In addition, ischemia and tissue trauma can trigger alterations similar to coagulopathy induced by trauma and hyperfibrinolysis. This procedure is not exempt from thrombotic events, which contribute to a substantial increase in mortality, resulting in the challenging necessity of maintaining hemostasis and preventing thrombosis in these patients.127 According to different clinical studies, VETs reflect the complex coagulation dynamics better than CCTs, evaluating pretransplantation coagulopathy, dilutional coagulopathy, and hypofibrinogenemia or hyperfibrinolysis in real time, and thus serve to enable a reasonable blood-product resuscitation strategy to be carried out in LT,128–134 albeit high quality prospective studies are still needed to evaluate their true utility.
Recommendation 29. In patients with cirrhosis that will undergo LT, VETs reduce blood-product transfusion, compared with CCTs (INR, PTT, platelets).
In 2010, Wang et al. conducted a randomized prospective study that included 28 patients that were to undergo LT. The patients were assigned to transfusion strategies, according to CCT and VET results. The VET group had almost 50% less need for FFP transfusions, as well as less blood loss, showing that the use of VETs was safe and beneficial, even though the sample size was too small to achieve statistical significance.131 Smart et al. conducted a retrospective study at a single center that included 34 patients that underwent LT, utilizing ROTEM for guiding transfusion, comparing them with 34 controls with CCT-guided transfusion therapy. The ROTEM group had less blood loss and less need for plasma but had more transfusions of cryoprecipitates.135
Álamo et al.134 described a case-control study conducted on 303 LT recipients. Patients with a high risk for bleeding during transplantation were found to have a lower use of transfusion products, a decrease in postoperative complications and renal insufficiency, better graft preservation, and a lower prevalence of dysfunction and primary failure of the graft. Many studies have been conducted to correlate the results of patients with LT in the postoperative period and compare the different VETs, obtaining very diverse values, depending on the tests employed. The available evidence suggests that the implementation of VETs, compared with CCTs, implies improvement in current methods, with the important advantage of reducing the need for blood transfusions. There are still limitations, such as the experience of the surgeon, anesthesia management, donor characteristics, as well as the heterogeneity characteristic of the patient with cirrhosis, hindering the application of standard transfusion algorithms.136
Recommendation 30. Prothrombin complex concentrate administration in LT surgery is superior to the use of FFP, for correcting coagulation during the perioperative period.
Prothrombin complex concentrates are products produced by the exchange of ions through chromatography of large quantities of plasma after removing antithrombin and factor XI. Different techniques in the process lead to the production of concentrates of three factors (II, IX y X) or four factors (II, VII, IX, X), with a concentration approximately 25 times higher than that in normal plasma.137 They can also contain natural coagulation inhibitors, such as protein C and protein S.138–142 Studies have suggested that the administration of prothrombin complex concentrates is an effective method for normalizing a prolonged PT in patients with cirrhosis of the liver.
There are reports since 1976 on the use of the prothrombin complex concentrate. The current indication for its use is bleeding and as perioperative prophylaxis of acquired bleeding or bleeding in congenital deficiency of one or four vitamin K-dependent coagulation factors, including the deficiency observed in patients with cirrhosis.143
During LT the use of prothrombin complex concentrate leads to a decrease in the need for transfusion, with no thromboembolic complications related to its use. There are no available prospective data on the efficacy of coagulation factor substitution for LT. Therefore, each transplant center must have its own guidelines. The decision to use CCTs should be based on guidelines and be individualized.
The clinical strategies for reducing blood loss include the use of blood products for correcting coagulopathy, such as FFP, platelet concentrates, and cryoprecipitates. A big disadvantage of FFP is volume overload, which can lead to an increase in central venous pressure and portal venous pressure that can cause a higher risk for bleeding.
Massive transfusion during LT is associated with an increased mortality risk, multiorgan failure, and reduced graft survival. During the transplant, the use of prothrombin complex concentrates, in addition to correcting coagulopathy, includes lowering volume administration, ambient air storage conditions, rapid reconstitution, immediate availability, lack of blood group specificity, and better safety profile.
Recommendation 31. The use of tranexamic acid during LT surgery significantly reduces the amount of bleeding and need for transfusion.
Primary hyperfibrinolysis is one of the main causes of increased blood loss during transplantation and indicates evidence for further goal-guided therapy with antifibrinolytic drugs. They are available as direct plasminogen inhibitors: tranexamic acid, epsilon-aminocaproic acid, and plasmin inhibitors (aprotinin).144–146
Tranexamic acid has been analyzed during LT in four prospective, controlled, double-blind studies that were compared with two studies with placebo. The results showed that tranexamic acid significantly reduced intraoperative blood loss and the use of blood products. There was also less bleeding, compared with placebo. However, despite a decrease in fibrinolysis, tranexamic acid at a dose of 2mg/kg/h did not provide a decrease in the need for transfusions. In two large studies, Savate et al. examined three antifibrinolytics. The first study included 124 patients; 42 received tranexamic acid, 42 received epsilon-aminocaproic acid, and 40 patients received a placebo. In the tranexamic acid group (10mg/kg/h), fibrinolysis, measured by TEG, was lower and packed red blood cell transfusion was significantly reduced, compared with the other two groups. There were no differences in the need for FFP, platelets, or cryoprecipitates between the three groups.
In the second study on 127 patients, tranexamic acid and aprotinin were compared. Sixty-four patients received tranexamic acid at 10mg/kg/h and 63 received aprotinin at 2×106kgIU, followed by an infusion of 0.5×106kgIU/h. There were no significant differences in the need for transfusion, thromboembolic events, reintervention due to bleeding, and mortality. The ranges were similar in the two groups. Tranexamic acid has been used at various doses and regimens. Low doses of 2 mg/kg/h suppress fibrinolysis, and higher doses (10-40mg/kg/h) significantly reduce intraoperative blood loss and the need for transfusions.143–149
In a double-blind, randomized, placebo-controlled study, Boylan et al. concluded that high doses of tranexamic acid significantly reduced blood loss and the need for blood-product transfusions, with a marked decrease in platelet and cryoprecipitate requirements.150
In 2007, Molenaar et al. conducted a review and meta-analysis of controlled studies on the use of antifibrinolytic drugs in LT. A total of 23 studies were identified, with a total of 1,407 patients, showing that tranexamic acid and aprotinin reduced the need for transfusions, compared with placebo. There was no increase in the risk for hepatic artery thrombosis, thromboembolic events, or perioperative mortality for any of the drugs analyzed. An intravenous dose of 10mg/kg/h before surgery, followed by 1mg/kg/h during surgery, is recommended.151
Recommendation 32. Synthetic fibrinogen administration effectively corrects bleeding problems associated with hypofibrinogenemia during the perioperative period of LT, compared with the use of cryoprecipitates.
Low levels of fibrinogen are recognized as an independent risk factor for increased bleeding in patients with cirrhosis. There are a larger number of greater bleeding events in cirrhotic patients with fibrinogen levels below 60mg/dL. In patients with cirrhosis admitted to the hospital, for each decrease in fibrinogen of 100mg/dL, mortality increases up to 29%. Maintaining levels of 100-120mg/dL has been proposed by many societies and groups of experts, in the acute blood loss scenario.
Noval-Padillo et al. conducted a study to evaluate clot stability and fibrin-based clot firmness (FIBTEM test) in thromboelastometry (ROTEM). Synthetic fibrinogen (Haemocomplettan) was administered to reach a maximum clot firmness, based on the weight of the patient and surgical bleeding. The study included 20 patients undergoing LT and they were compared with a group of 59 patients from the previous year. Synthetic fibrinogen was administered in 45% of the 20 patients. There was a 53% decrease in the use of packed red blood cells and a 65% decrease in the use of FFP. Platelet transfusion was reduced by 50%. Likewise, 20% of the transplanted patients did not receive blood-product transfusions, compared with 3.5% from the previous period. The incorporation of synthetic fibrinogen in the treatment of hemostatic disorders resulting from LT reduces the use of allogenic blood products.152
In LT, plasma levels of fibrinogen are low in many patients during surgery. Fibrinogen administration to correct the hypofibrinogenemia has a positive impact on surgical bleeding. However, there is little information in the literature on fibrinogen administration, therefore its use for normalizing fibrinogen levels must be adjusted to recommended ranges or guided by thromboelastometry.153–156
Treatment guided by ROTEM with coagulation factor concentrates was evaluated in an LT study, in which the fibrinogen concentrate was administered with a target FIBTEM (analysis of the contribution of fibrinogen in clot formation) MCF (maximum clot firmness) of 6 mm and the prothrombin complex concentrate was administered with a target EXTEM (analysis of the extrinsic coagulation pathway in the thromboelastography) MCF of 35 mm. Allogenic-product transfusion was low, with an average of 2 U of packed red blood cells and no units of FFP and platelets. The advantages of the use of fibrinogen over FFP are the decrease in pathogen transmission, as well as the reduced risk for transfusion-associated lung injury (TRALI) or transfusion-associated circulatory overload (TACO). The concentration of fibrinogen can easily be monitored by ROTEM, and replacement therapy, guided according to clot firmness, reduced transfusion, with respect to the units of packed red blood cell units, FFP, and platelets, by more than 50%. In addition, the number of transplants with no transfusion increased from 3.5% to 20%.157
Ethical considerationsThe current project is the result of the initiative of a group of collaborating specialists in the area of hepatology. The development of these guidelines is based on clinical information from clinical trials, obtained from meta-analyses, systematic reviews, randomized controlled clinical trials, cohort studies, case-control studies, ecologic studies, and case series. The information obtained was classified and analyzed by a committee of experts in clinical research, according to levels of evidence. We declare herein that this project involves no management of personal information of patients, by not including study subjects for analysis or the collection of clinical information. No patients can be identified through images or clinical data included in the reviewed projects. Likewise, drafting a statement of informed consent or undergoing evaluation by an ethics or biosafety committee were not necessary. This project meets the current bioethical research regulations and abides by the General Health Law and its Research regulations, as well as the Guidelines of the International Conference on Harmonization (ICH) on Good Practice Guidelines (GPC).
Financial disclosureNo financial support was received in relation to the formulation of these guidelines.
Conflict of interestThe authors declare that there is no conflict of interest.