Liver resection has been associated with high morbidity and mortality, and the most serious complication is liver failure. Patient evaluation is limited to risk scales. The 50-50 criteria and bilirubin peak>7mg/dl have been used as mortality predictors.
AimThe aim of this study was to determine the risk factors associated with morbidity and mortality for liver resection in our population.
Material and methodsA retrospective study was carried out on 51 patients that underwent liver resection. Sociodemographic variables, pathology, and the surgical act were analyzed, together with morbidity and mortality and their associated factors.
ResultsFifty-one patients, 23 men and 28 women, were analyzed. They had a mean age of 51.4±19.13 years, 64.7% had concomitant disease, and their mean MELD score was 7.49±1.79. The mean size of the resected lesions was 7.34±3.47cm, 51% were malignant, and 34 minor resections were performed. The Pringle maneuver was used in 64.7% of the cases and the mean blood loss was 1,090±121.76ml. Morbidity of 25.5% was associated with viral hepatitis infection, greater blood loss, transfusion requirement, the Pringle maneuver, lower hemoglobin and PTT values, and higher MELD, INR, bilirubin, and glucose values. A total 3.9% mortality was associated with hyperbilirubinemia, hyperglycemia, and greater blood loss and transfusions.
ConclusionsThe main risk factors associated with the morbidity and mortality of liver resection in our population were those related to the preoperative biochemical parameters of the patient and the factors that occurred during the surgical act.
La resección hepática se asocia con una alta morbilidad y mortalidad, siendo la insuficiencia hepática la complicación más grave. La evaluación de los pacientes se limita a analizar las escalas de riesgo. Los criterios 50-50 y el pico de bilirrubina >7mg/dl han sido utilizados como predictores de mortalidad.
ObjetivoDeterminar los factores de riesgo asociados a la morbilidad y mortalidad asociada a resecciones hepáticas en nuestra población.
Material y métodosEstudio retrospectivo en 51 pacientes sometidos a resecciones hepáticas. Se analizaron variables sociodemográficas; la patología y el acto quirúrgico; la morbilidad y mortalidad, y los factores asociados a estas.
ResultadosSe analizaron 51 pacientes, 23 hombres y 28 mujeres, con edad promedio de 51.4±19.13 años, patología concomitante en el 64.7%, MELD promedio de 7.49±1.79. Se resecaron lesiones con un tamaño de 7.34±3.47cm, un 51% de ellas malignas, 34 resecciones menores y se realizó la maniobra de Pringle en un 64.7% de los casos, con una pérdida sanguínea promedio de 1,090±121.76ml. Se encontró una morbilidad del 25.5% asociada a la presencia de HV, a la mayor pérdida sanguínea, a las transfusiones, a la realización de la maniobra de Pringle, a los valores de hemoglobina y TPT menores, a los valores de MELD, de INR, de bilirrubina y glucosa mayores. Se encontró una mortalidad del 3.9% asociada a la hiperbilirrubinemia, hiperglucemia, mayor pérdida sanguínea y transfusiones.
ConclusionesLos factores de riesgo asociados a la morbilidad y mortalidad en las resecciones hepáticas de nuestra población se asocian principalmente a los parámetros bioquímicos preoperatorios del paciente, y a los factores que ocurren durante la intervención quirúrgica.
Historically, liver resection has been associated with perioperative mortality of 10 to 20% and with morbidity after hepatectomy of 20 to 40%. Advances in perioperative management and surgical techniques have improved the mortality ranges, but morbidity has remained high.1–7
Current efforts to evaluate the postoperative course of the patients have been almost exclusively limited to preoperative assessment, primarily including biochemical parameters (serum bilirubin, prothrombin time, and albumin levels) and clinical risk scales, such as the Child-Turcotte-Pugh (CTP) and the model for end-stage liver disease (MELD). Preoperative parameters can be useful to determine the selection criteria for surgery and postoperative risk stratification. Post-hepatectomy liver failure is the most serious complication, defined as the increase in the international normalized ratio (INR) and hyperbilirubinemia at or after postoperative day 5, providing a severity grade. Its incidence varies from 1.2% to 32%.1,8–19
The “50-50 criterion” has been suggested for predicting post-hepatectomy liver failure and death in intensive care units, and has been defined as concomitant prothrombin time (PT) <50% and serum bilirubin >50μmol/l (2.92mg/dl). Patients presenting with this criterion have a 59% risk for death and it is regarded as an indicator of post-hepatectomy liver failure. In contrast, some researchers have defended a bilirubin peak >7mg/dl as a more specific predictor of post-hepatectomy mortality.7
Risk factor identification is important for reducing the incidence of morbidity and mortality associated with this procedure. The aim of the present study was to determine the risk factors associated with morbidity and mortality in relation to liver resection in our population.
Materials and methodsA retrospective, cross-sectional, analytic study was conducted on 51 patients with complete case records that were above 18 years of age and underwent liver resection within the time frame of January 1, 2008 and May 31, 2015, at the hepatobiliary surgery services of the Unidad Médica de Alta Especialidad, Hospital de Especialidades No 14 Centro Médico Nacional “Adolfo Ruiz Cortinez”, Instituto Mexicano del Seguro Social, Veracruz, Mexico and the Hospital de Alta Especialidad de Veracruz, Veracruz, Mexico. The study protocol was authorized by the research and ethics committees of the hospitals.
The data collected on each patient included the sociodemographic variables of age, sex, and body mass index (BMI), the presence of concomitant disease (diabetes mellitus, high blood pressure, cirrhosis of the liver, and viral hepatitis), preoperative chemotherapy, and the MELD score utilizing creatinine, total bilirubin, INR, and dialysis during the past week.1 Preoperative laboratory values were also determined: hemoglobin, hematocrit, leukocytes, platelets, glucose, blood urea and nitrogen (BUN), urea, creatinine, PT, partial thromboplastin time (PTT), INR, alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), total bilirubin, direct bilirubin, indirect bilirubin, total protein, albumin, and globulin. The analyzed variables related to the lesion were: size, location, benignity or malignancy, and primary lesions vs metastatic ones. The variables corresponding to surgery were: resection extension (more than three segments was considered major resection),1 the Pringle maneuver and the time it took to perform it, surgery duration, intraoperative blood loss and the number of packed red blood cell units transfused, the postoperative progression of the patient taking into account admission to the intensive care unit and the number of days spent there, liver failure, the 50-50 criterion, bilirubin peak >7mg/dl, surgical reintervention, days of hospital stay, and morbidity and mortality.
Statistical analysisThe statistical analysis of the continuous and discrete variables was measured in means and standard deviation. The categorical variables were measured in frequencies and percentages. The preoperative, intraoperative, and postoperative variables correlated with morbidity and mortality were identified through bivariate analysis. The categorical variables were evaluated using the chi-square test and/or Fisher's exact test with odds ratio (OR) and relative risk. The continuous variables were evaluated using the Student's t test for the parametric variables and the Mann-Whitney U test for the nonparametric variables. The statistically significant laboratory parameters for morbidity and mortality were categorized into normal and abnormal values, carrying out the OR to establish the relative risk based on the alteration of those parameters. ROC curves were plotted to establish areas under the curve with a power of prediction above 0.70, sensitivity, specificity, and cut-off points in the laboratory parameters that were statistically significant. They were then re-categorized from the cut-off points and the ORs and relative risks were calculated. Statistical significance was set at a p below 0.05 and the SPSS version 21 software was used for the analysis.
ResultsA total of 51 patients were analyzed, 23 of whom (45.1%) were women and 28 (54.9%) were men. Their mean age was 51.47 ± 19.13 years, mean weight 70.1 ± 10.31 kilograms, mean height 1.62 ± 0.07 meters, and a BMI of 26.65 ± 4.19kg/m2. Thirty-three (64.7%) of the patients presented with concomitant disease, 29 of whom (56.9%) had diabetes mellitus, 30 (58.8%) had high blood pressure, 5 (9.8%) had viral hepatitis, 8 (15.7%) presented with cirrhosis of the liver, and 13.7% received preoperative chemotherapy. The mean MELD score was 7.49 ± 1.79 and all the patients that presented with cirrhosis of the liver were classified as Child-Pugh A.
The means of the preoperative laboratory results were hemoglobin 13.5 ± 1.25mg/dl, hematocrit 39.37 ± 3.86%, leukocyte count 7,654.06 ± 3,241.43, platelets 245,111 ± 87,575.81, glycemia 95.91 ± 17.39mg/dl, blood urea and nitrogen 19.19 ± 16.61mg/dl, urea 29.14 ± 14.76, and creatinine 0.98 ± 0.37. The mean coagulation test results were PT 12.86 ± 1.88, PTT 27.77 ± 3.19, and the mean INR was 1.05 ± 0.13. The mean liver function test results were ALT 82.56 ± 217.94, AST 57.6 ± 88.34, ALP 40.95 ± 103.37, and as means, total bilirubin was 0.92 ± 1.14, direct bilirubin was 0.59 ± 1.09, indirect bilirubin was 0.4 ± 0.26, total protein was 6.97 ± 1.02, albumin was 4.02 ± 0.73, and globulin was 3.23 ± 1.05.
The mean size of the resected lesions was 7.34 ± 3.47cm, the majority of which were located in the right hepatic lobe (54.9%). Malignant tumors were found in 26 (51%) cases, of which 16 (31.4%) were primary tumors and 10 (19.6%) were metastatic tumors. Thirty-four (66.7%) of the resections performed were minor, 16 in the right hepatic lobe and 18 in the left hepatic lobe. A total of 17 (33.3%) were major resections, 12 right hemihepatectomies and 5 left hemihepatectomies. The Pringle maneuver was used in 64.7% of the resections, with a mean duration of 18.54 ± 7.33min, mean surgery time was 211.27 ± 57.76min, mean blood loss was 1,090 ± 1,217.26ml, and mean packed red blood cell unit transfusion was 2.29 ± 2.51.
In regard to postoperative progression, 22 (43.1%) patients were admitted to the intensive care unit with a mean stay of 1.37 ± 2.24 days. Eight (15.7%) patients had post-hepatectomy liver failure, 4 (7.8%) patients had a positive 50-50 criterion, and 8 (15.7%) patients had a bilirubin peak >7mg/dl. Three cases required surgical reintervention with a reported mean hospital stay of 8.62 ± 5.74 days. The incidence of morbidity was 25.5% and corresponded to liver failure in 7 patients and to hernia of the wall, bleeding, surgical wound infection, and a cerebral vascular event (n = 1 each). The reported mortality was 3.9% (n = 2).
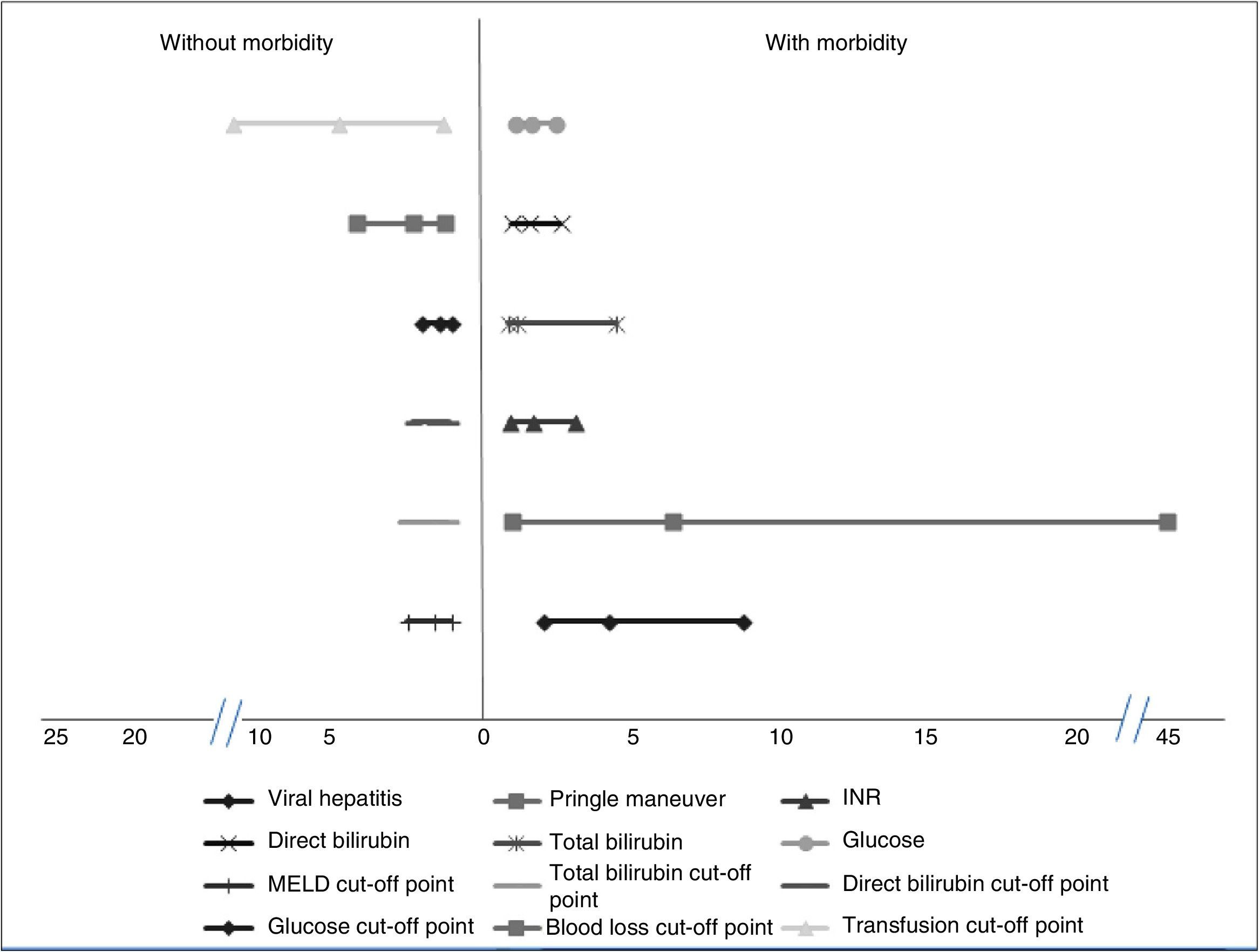
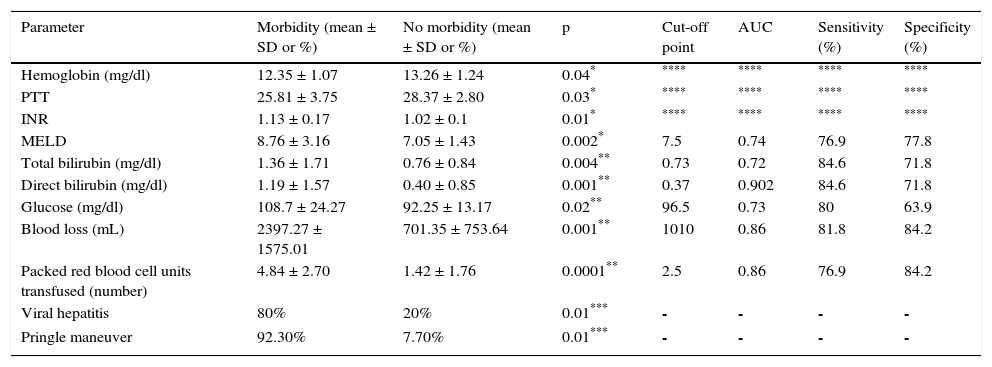
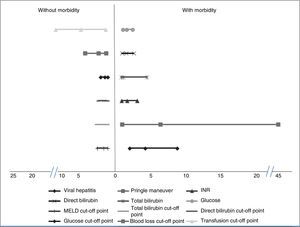
Factors associated with morbidityThe patients that had the highest morbidity rate had significantly lower hemoglobin values and shorter PTT than those that did not present with morbidity. The MELD score with a worse prognosis was also reported in the patients that presented with morbidity, as well as increased levels of INR, total bilirubin, direct bilirubin, and glucose. In addition, the patients with greater morbidity had greater blood loss and a higher mean transfusion requirement. Among the comorbidities, the patients with viral hepatitis had a higher complication rate. Significantly higher morbidity was also found in the patients that had the Pringle maneuver. Table 1 shows the corresponding data. Analyses of the statistically significant factors were done through ROC curves and showed the following cut-off points: MELD score 7.5 points (p = 0.004), total bilirubin 0.73mg/dl (p = 0.004), direct bilirubin 0.37mg/dl (p = 0.00), glucose levels 96.5mg/dl (p = 0.02), packed red blood cell units 2.5 (p = 0.00), and intraoperative blood loss 1,010ml (p = 0.001). Table 1 shows the sensitivity, specificity, and area under the curve (AUC) of the statistically significant factors.
Parameters associated with morbidity.
| Parameter | Morbidity (mean ± SD or %) | No morbidity (mean ± SD or %) | p | Cut-off point | AUC | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|---|
| Hemoglobin (mg/dl) | 12.35 ± 1.07 | 13.26 ± 1.24 | 0.04* | **** | **** | **** | **** |
| PTT | 25.81 ± 3.75 | 28.37 ± 2.80 | 0.03* | **** | **** | **** | **** |
| INR | 1.13 ± 0.17 | 1.02 ± 0.1 | 0.01* | **** | **** | **** | **** |
| MELD | 8.76 ± 3.16 | 7.05 ± 1.43 | 0.002* | 7.5 | 0.74 | 76.9 | 77.8 |
| Total bilirubin (mg/dl) | 1.36 ± 1.71 | 0.76 ± 0.84 | 0.004** | 0.73 | 0.72 | 84.6 | 71.8 |
| Direct bilirubin (mg/dl) | 1.19 ± 1.57 | 0.40 ± 0.85 | 0.001** | 0.37 | 0.902 | 84.6 | 71.8 |
| Glucose (mg/dl) | 108.7 ± 24.27 | 92.25 ± 13.17 | 0.02** | 96.5 | 0.73 | 80 | 63.9 |
| Blood loss (mL) | 2397.27 ± 1575.01 | 701.35 ± 753.64 | 0.001** | 1010 | 0.86 | 81.8 | 84.2 |
| Packed red blood cell units transfused (number) | 4.84 ± 2.70 | 1.42 ± 1.76 | 0.0001** | 2.5 | 0.86 | 76.9 | 84.2 |
| Viral hepatitis | 80% | 20% | 0.01*** | - | - | - | - |
| Pringle maneuver | 92.30% | 7.70% | 0.01*** | - | - | - | - |
AUC: area under the curve; MELD: model for end-stage liver disease; INR: international normalized ratio; PTT: partial thromboplastin time; SD: standard deviation.
The OR and relative risk analyses identified viral hepatitis (OR: 4.17) (p = 0.01) and the Pringle maneuver (OR: 6.35) (p = 0.01) as risk factors for presenting with complications. Normal glucose levels were associated with not having complications (OR: 1.53) (p = 0.04). We observed a trend for normal INR, total bilirubin, and direct bilirubin (OR: 1.61) (p = 0.057), (OR: 1.86) (p = 0.056), and (OR: 1.52) (p = 0.052), respectively, to be factors associated with the absence of morbidity. Likewise, levels under the cut-off point obtained in the ROC curves were associated with the absence of complications: MELD (OR: 1.71) (p = 0.04), total bilirubin (OR: 1.86) (p = 0.001), direct bilirubin (OR: 1.72) (p = 0.001), glucose (OR: 1.55) (p = 0.01), blood loss (OR: 2.42) (p = 0.001), and blood-derived transfusions (OR: 4.96) (p = 0.001) (fig. 1).
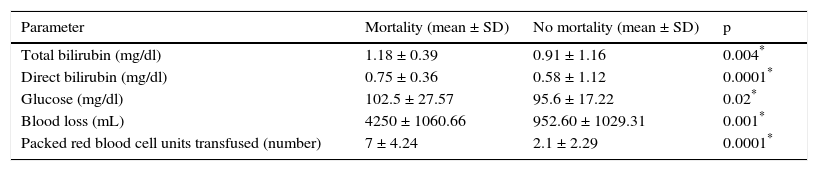
Factors associated with mortalityPerioperative mortality in the present study was associated with higher preoperative values of total bilirubin, direct bilirubin, and glucose. Blood loss and a greater number of transfusions were associated with mortality. Table 2 shows the corresponding data. Neither the 50-50 criterion nor the bilirubin peak > 7mg/dl was associated with mortality (p = 0.67 y p = 0.29, respectively).
Parameters associated with mortality.
| Parameter | Mortality (mean ± SD) | No mortality (mean ± SD) | p |
|---|---|---|---|
| Total bilirubin (mg/dl) | 1.18 ± 0.39 | 0.91 ± 1.16 | 0.004* |
| Direct bilirubin (mg/dl) | 0.75 ± 0.36 | 0.58 ± 1.12 | 0.0001* |
| Glucose (mg/dl) | 102.5 ± 27.57 | 95.6 ± 17.22 | 0.02* |
| Blood loss (mL) | 4250 ± 1060.66 | 952.60 ± 1029.31 | 0.001* |
| Packed red blood cell units transfused (number) | 7 ± 4.24 | 2.1 ± 2.29 | 0.0001* |
SD: standard deviation.
The ROC curve analysis found that total bilirubin had an AUC of 0.87 (95% confidence interval [95% CI] 0.75 – 0.99) (p = 0.07) with 100% sensitivity and 78% specificity when there were values above 0.88mg/dl. Direct bilirubin had an AUC of 0.85 (95% CI 0.71 – 0.99) (p = 0.09), 100% sensitivity and 75.6% specificity, with a cut-off point of 0.49mg/dl. The number of packed red blood cell units transfused had an AUC of 0.91 (95% CI 0.78 – 1.00) (p = 0.04), corresponding to 100% sensitivity and 82% specificity when 3.5 or more packed red blood cell units were transfused. Blood loss had an AUC of 0.97 (95% CI 0.92 – 1.00) (p = 0.02), 100% sensitivity, 93.6% specificity, and a cut-off point of 3,250ml.
The relative risk analysis found no significant differences in the comparison of abnormal total bilirubin, direct bilirubin, and glucose levels. When the cut-off points of the variables were compared, having received a number of packed red blood cell units lower than the cut-off point (OR: 1.22 0.92 – 1.61) (p = 0.04) was shown to be a protective factor. The rest of the variables showed no statistically significant difference.
DiscussionOur study results reflected the fact that morbidity and mortality in liver resection in this population were mainly associated with risk factors related to the clinical status of the patient prior to surgery and to factors involved in the intraoperative period.
The incidence of morbidity in our study was 25.2%, similar to that published in the literature, in which ranges vary from 14 to 43% in multicenter studies, as well as those from single centers, with a variable number of patients that was both higher and lower than ours.1,4,5,10,12,19 The most common complications after a liver resection in order of frequency are biliary leaks, infections, collections, bleeding, and liver failure.1,5,10–12 In our study, liver failure was the most frequent complication (13.7%), being somewhat higher than the published figures that range from 0.5% to 2%.5,10
The Pringle maneuver was shown to be a decisive variable in relation to morbidity. However, in some studies there is only an association when the maneuver takes more than 20min, thus being associated with cellular edema, lymphatic system drainage disruption, pleural effusion, abscesses, hemoperitoneum, and transitory liver failure, which was not the case in our results.20,21 Glucose and bilirubin levels and coagulation alterations, as well as the use of blood products, are associated with a higher complication rate.1,4,5,10–12,19–21 Some risk factors that are associated with morbidity, such as the risk factors of chronic degenerative diseases (diabetes mellitus, high blood pressure, and cirrhosis of the liver), were not found to be associated with complications in our study. The malignant strain of resected lesions has sometimes been associated with a greater risk for morbidity, 1,5,20 but that was not the case in our study.
There are different scales that evaluate the biochemical parameters of the patient to arrive at a survival prediction, such as the MELD scoring system, which was created for patients that were candidates for transjugular portosystemic intrahepatic shunts. Few studies associate morbidity and mortality with liver resection and in the existing ones there is a positive predictive value for the presence of complications with values above 8 and a predictive value for mortality with values above 10.1,5 In our results, patients that had a higher preoperative MELD score (8.7), had a higher complication rate, but we found no similar association in relation to mortality.
Our relative risk and odds ratio analysis showed there was a risk for presenting with complications when the Pringle maneuver was used and the patient was a carrier of viral hepatitis. Viral hepatitis has been associated with both an incidence of surgical site infections and a reactivation of the virus, which can lead to severe liver damage and patient death after a liver resection.22,23 Normal range biochemical parameters are protective factors for the presence of morbidity, but not for mortality, which had no significant association in relation to abnormal values or the cut-off point. Other studies state that patients with an increased INR are at greater risk for death. In our findings, altered INR values conferred no risk for presenting with mortality, but normal values inferred protection for morbidity.5 Hyperbilirubinemia was a risk for morbidity similar to others, unlike the results of larger studies.1,5,8 We also found that the number of packed red blood cell units transfused in our population had a significant risk for death when it exceeded 3.5 units (OR 1.22). Nevertheless, our risk was lower and the number of units was also lower than those reported by Lancaster et al.19 in which there was an OR of 11.63 with the transfusion of more than 4 packed red blood cell units.
The mortality of our series was acceptable in accordance with current studies in which it ranges from 0.5% to 6%. We also found that the factors associated with it (hyperbilirubinemia, hyperglycemia, and the use of blood products) have previously been described by other authors.1 The 50-50 criterion was not applicable in our study, nor did the bilirubin peak > 7mg/dl on day five show predictive value for mortality in our population, contrasting with other studies that consider it an important predictor.1 This could be due to the small size of our sample and the low mortality rate.
There are very few Mexican case series in the literature that describe the complication and mortality rates of liver surgery in our country.24–27 One of them is over 20 years old and reports on 67 patients over a 10-year period.27 The complication rate varies from 16 to 33%,24–26 with a recent mortality rate (after 2009) below 13%,24 and therefore, with respect to our study, it is difficult to establish similarities or differences at the national level. Our results are from a small case series with a retrospective review of the medical records, which could explain why no significant association was found between the current mortality criteria that include the 50-50 criterion and the bilirubin peak above 7mg/dl. Our results reflect the parameters of international standards of efficacy and safety and we found factors traditionally associated with perioperative complications and death in liver surgery.
ConclusionsThe risk factors associated with morbidity and mortality in liver resection in our population are mainly associated with the preoperative biochemical parameters of the patient and with the factors that occur during the surgical act.
Ethical responsibilitiesProtection of persons and animalsThe authors declare that no experiments were performed on humans or animals for this study.
Data confidentialityThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Financial disclosureNo financial support was received in relation to this study/article.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Martínez-Mier G, Esquivel-Torres S, Alvarado-Arenas RA, et al. Morbilidad, mortalidad y factores de riesgo de la cirugía hepática en los departamentos de cirugía hepatobiliar de Veracruz, México. Revista de Gastroenterología de México. 2016;81:195–201.