Esophageal cancer is the ninth cause of neoplasias and the sixth cause of cancer mortality. Surgical treatment of those tumors with gastric or colonic replacement has advanced significantly, but has been a risk factor for the development of metachronous cancer in reconstructed gastric tubes.1,2 Treatment based on total gastrectomy with colon ascension is an efficacious therapeutic option, but there is a high risk for severe complications.
A 34-year-old woman had a past medical history of distal esophageal peptic stricture that was refractory to treatment with esophageal dilations, as well as ulcerated acute esophagitis and Barrett's esophagus with high-grade dysplasia. In 2006, she underwent transhiatal esophagectomy with gastric pull-up with no complications and the definitive histopathologic study reported extensively ulcerated acute and chronic esophagitis, Barrett's esophagus, and complete intestinal metaplasia of the gastric mucosa. Numerous control endoscopies were carried out over a 2-year period, after which the patient did not continue with the follow-up sessions.
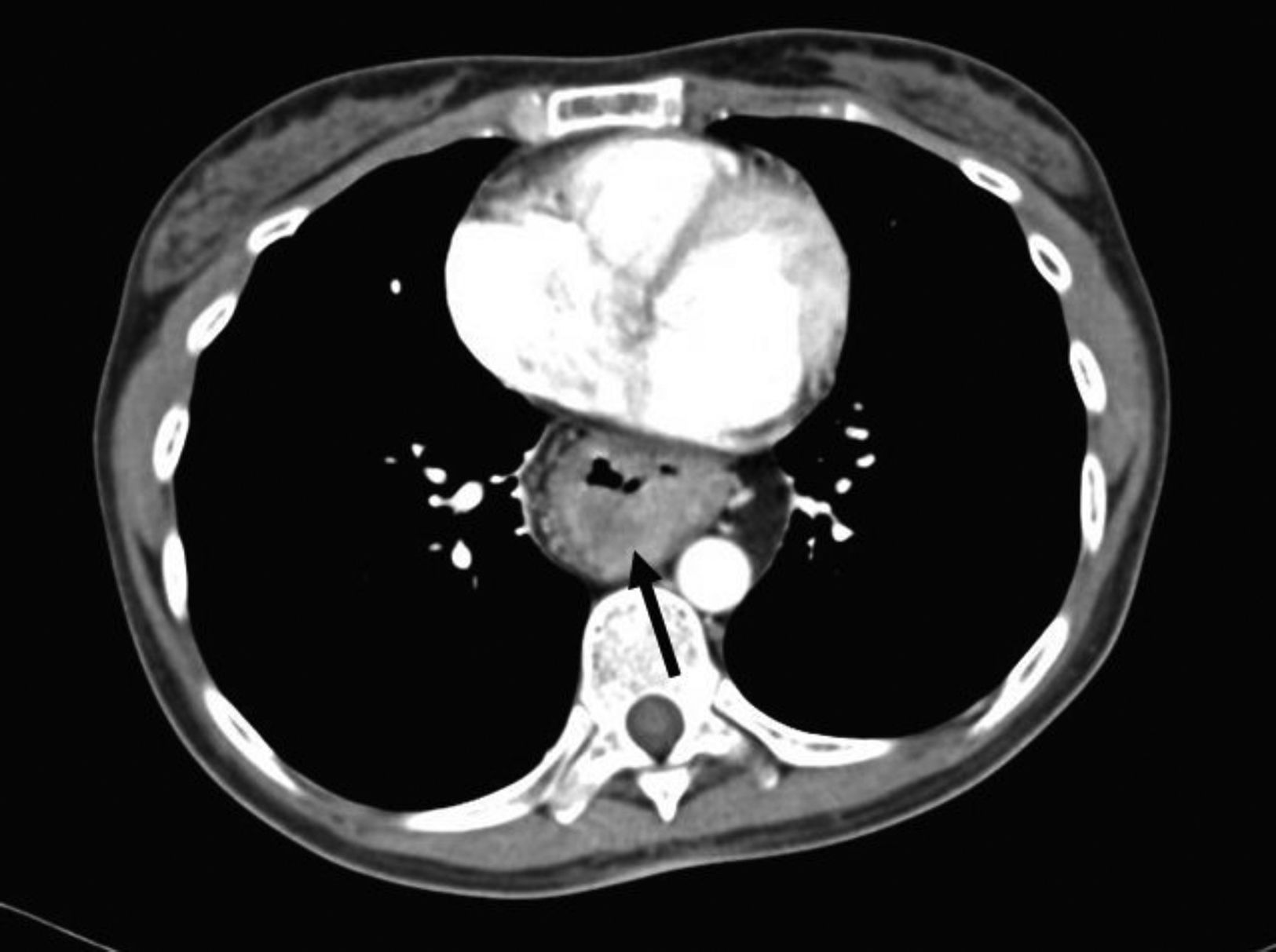
The patient sought medical attention again at our hospital center for illness that began in January 2016 with asthenia, adynamia, and melena. A computed tomography scan showed thickening of the mucosa of the intrathoracic gastric chamber (fig. 1) and panendoscopy revealed severe esophagitis, partial stricture of the gastroesophageal junction, and a tumor that extended from the greater curvature to the antrum, with friable and indurated mucosa. The histopathologic study from the biopsy reported diffuse, moderately differentiated gastric adenocarcinoma with the presence of signet ring cells.
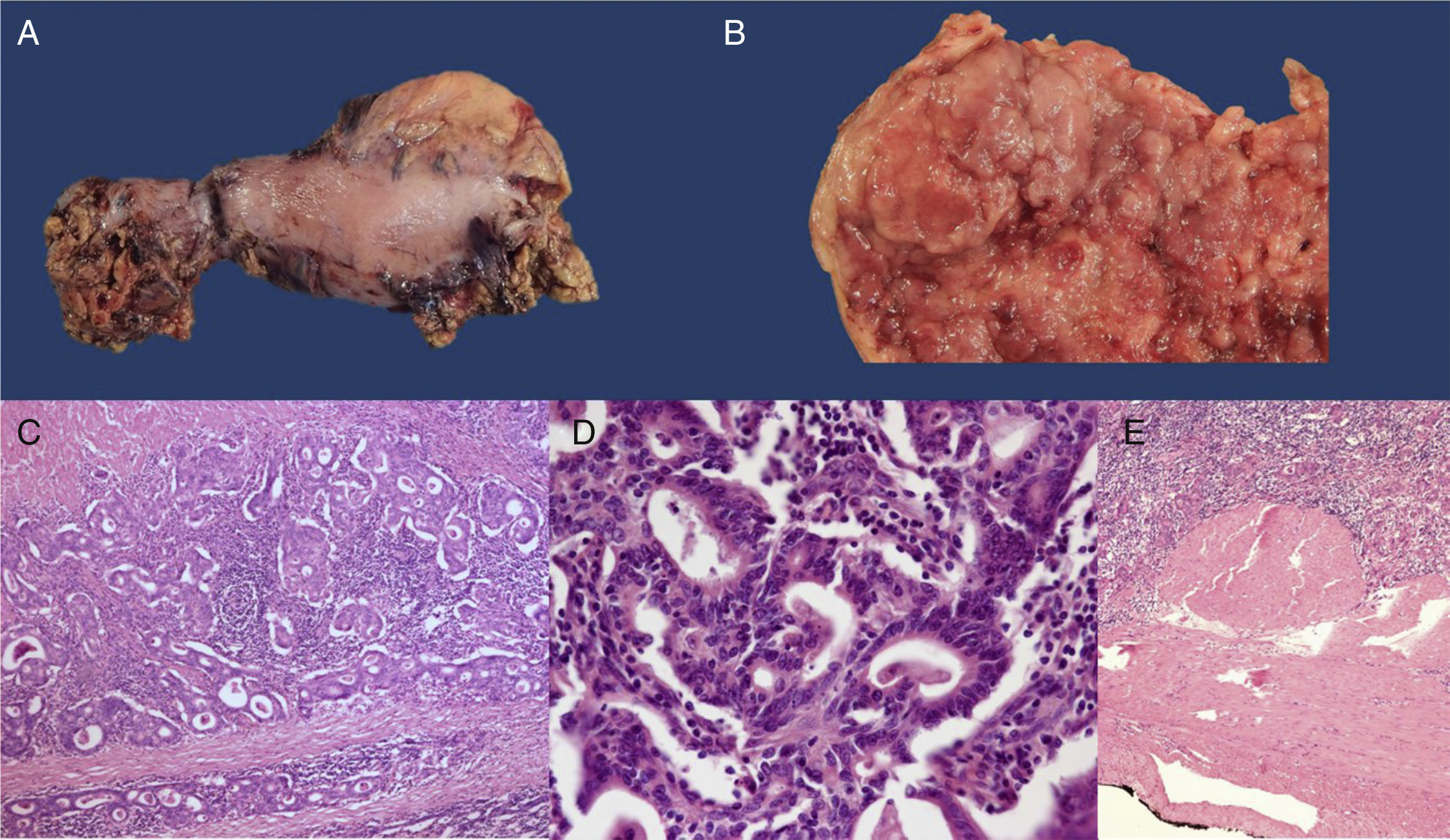
Therapeutic surgery consisting of right transthoracic gastrectomy with retrosternal colon ascension was proposed. Employing a cervical, right anterolateral and supraumbilical transthoracic approach, total gastrectomy was performed with dissection of the ascending colon, cecum, and 4cm of the ileum. The middle colic artery and its branches were spared and hand-sewn end-to-end ileotransverse anastomosis was carried out. There were no complications during the surgery and the definitive histopathologic study reported intestinal-type gastric adenocarcinoma with lymphovascular invasion reaching the subserosa: AJCC clinical stage IIA (pT3N0M0) (fig. 2). At present, the patient is tolerating an oral diet and receiving adjuvant chemotherapy.
A) Surgical specimen from the total gastrectomy. B) View of the surgical specimen showing fungal tumor in the resected gastric tube (x5). C) H&E-stained histology section, showing invasive adenocarcinoma (x 20). D) H&E-stained high power image (x100) showing the formation of tubules consistent with intestinal-type gastric adenocarcinoma. E) Panoramic H&E mount showing that the tumor reaches the subserosa without invading it (T2).
The development of carcinoma of the reconstructed gastric tube following esophagectomy has occasionally been found in survivors of esophageal cancer. Approximately 200 cases have been reported in British and Japanese studies.1–3 Different studies have shown that the accumulated incidence of gastric tube cancer at 5 years is 2.4% and it varies from 5.7 to 8.6% at 10 years.3,4 There is a mean 62.8-month interval between esophagectomy and the development of gastric tube cancer.5 Adjuvant radiotherapy to the mediastinum has been signaled as a possible long-term cause of carcinogenesis. On the other hand, different studies have confirmed biliary and pancreaticoduodenal reflux as a risk factor for the development of gastric tube cancer. There are reports that 89.5% of those cancers present with intestinal metaplasia in the mucosa surrounding the tumor.3,4 In addition, the majority of gastric tube cancers are found at the lower middle third of the gastric tube, reflecting the anatomic association biliary reflux has with the disease.4
The colon is usually a second alternative for esophageal substitution. Whether to use the right or left colon is based on the length of the colon required and the vascular anatomy encountered. It is important to carry out an angiogram prior to surgery to rule out vascular abnormalities. A maximum benefit of the ascending colon is the ileocecal valve, which prevents colonic and gastric content reflux into the cervical esophagus, together with the facility of ileoesophageal anastomosis.6
The ascension of the substitute can be carried out through the posterior mediastinum, along the route of the resected esophagus or through the retrosternal tunnel. No association between the reconstruction route and incidence of intestinal metaplasia in the substitute has been observed.4 The retrosternal route is indicated in patients with a history of posterior mediastinal surgery and has the advantage of simple, rapid drainage in case of leaks or strictures in the anastomoses, as well as protection from adjuvant radiotherapy. The experience and skill of the surgeon should also be taken into account when making the treatment decision.
Outcome for this disease depends on its stage at the time of diagnosis, and it tends to be poor in symptomatic-stage tumors. Up to 89.5% of early-stage gastric tube carcinomas are diagnosed through annual endoscopy screening, demonstrating that said intervention reduces the risk for cancer, improves quality of life, and is cost-effective.1–3,7,8
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Medina-Franco H, Mejía-Fernández L, Montante-Montes de Oca D. Adenocarcinoma gástrico tipo intestinal en tubo gástrico reconstruido posterior a esofagectomía transhiatal. 2018;83:352–353.