Infliximab (IFX) is a chimeric monoclonal antibody that works against tumor necrosis factor-alpha (TNF-α) and is used to treat autoimmune diseases, such as rheumatoid arthritis, psoriasis, and inflammatory bowel disease (IBD)1. IFX has been associated with rare side effects, including infections, skin reactions, autoimmunity (cutaneous vasculitis, lupus-like syndrome, systemic lupus erythematosus, interstitial lung disease), neurologic complications, congestive heart failure, and malignancy1–5.
IFX-related skin manifestations are infusion reaction, vasculitis, cutaneous infections, psoriasis, eczema, and skin cancer3. We describe herein the case of a pediatric patient with fistulating Crohn’s disease that developed IFX-related Henoch-Schönlein purpura.
A 15-year-old girl diagnosed with fistulating Crohn’s disease was followed at the Department of Pediatric Gastroenterology. Although she had been taking a 5-ASA agent, azathioprine, and intermittent steroids for two years, she experienced no recurrence of disease activity or fistulas. She was started on IFX (Remicade) (5 mg/kg/dose, 0-2-6-8-weeks), but was admitted to the hospital with purpuric lesions on her legs, 10 days after the fifth dose of IFX.
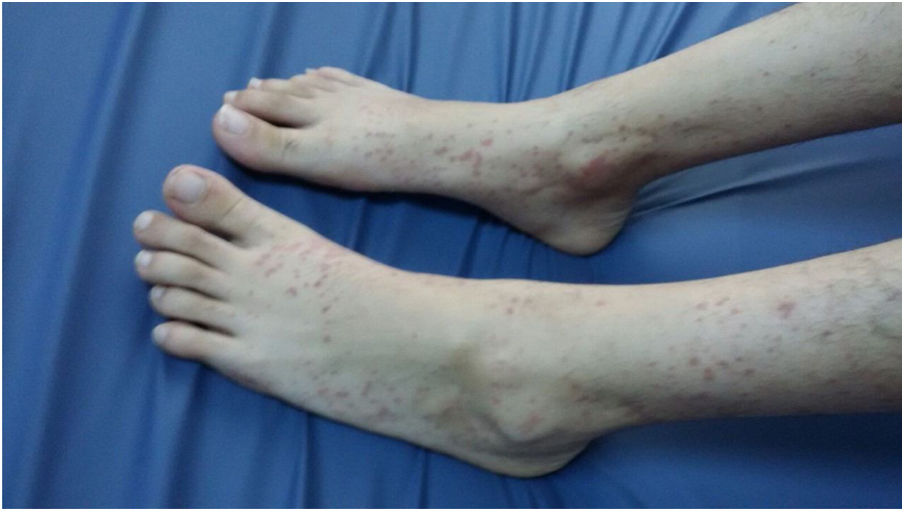
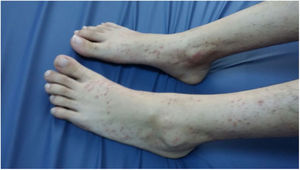
She had not taken any other drugs or herbal products. Upon physical examination, she weighed 50 kg (10–25th percentile), her height was 160 cm (25–50th percentile), and her body temperature was 36 °C. She had purpuric lesions on her legs (Fig. 1), her abdomen was soft and non-tender, with no distension, and the cardiovascular and respiratory system examinations were unremarkable.
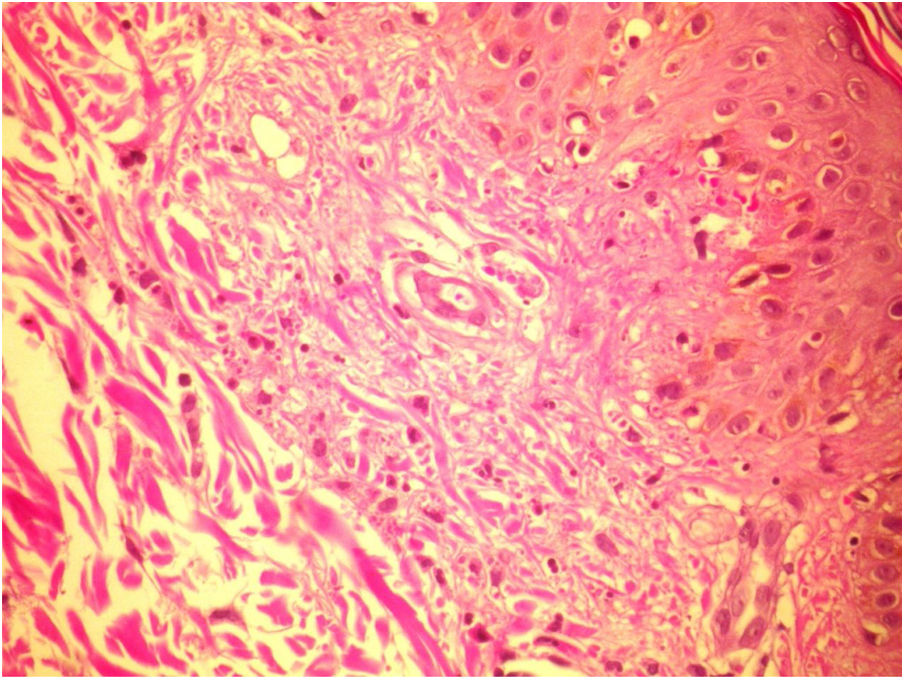
Laboratory work-up revealed no thrombocytopenia or coagulation disorder. Serologies for hepatitis A, B, and C, EBV, parvovirus, HSV, and CMV were negative. Protein electrophoresis, cryoglobulin, ASO, rheumatoid factor, serum amyloid A, and C3 and C4 levels were within normal limits. Anti-neutrophil cytoplasmic autoantibody was weakly positive. The histopathologic examination of the skin biopsy specimens revealed leukocytoclastic vasculitis (Figs. 2 and 3). Because vasculitis was considered a side effect of IFX, the treatment was suspended. The cutaneous lesions resolved completely, 3 days after IFX therapy was discontinued. No re-challenge with anti-TNF-α therapy was carried out because the patient was lost to follow-up.
Anti-TNF agents (IFX, etanercept, and adalimumab) have been associated with an increasing number of cases of autoimmune diseases1–6. Although evidence supports a good safety and tolerability profile for those agents, reports of adverse reactions, including infections, skin reactions, autoimmunity (cutaneous vasculitis, lupus-like syndrome, systemic lupus erythematosus, interstitial lung disease), and malignancy, are on the rise1–5.
In their recent prospective study, Mälkönen et al.7 reported that 48% of the children with IBD presented with IFX-related skin lesions, that frequently were psoriasis-like skin manifestations. In a study on adult IBD patients, Hellström et al.8 found that anti-TNF therapy was frequently associated with the new onset of skin reactions (especially scaling eczema), most commonly in patients with Crohn’s disease.
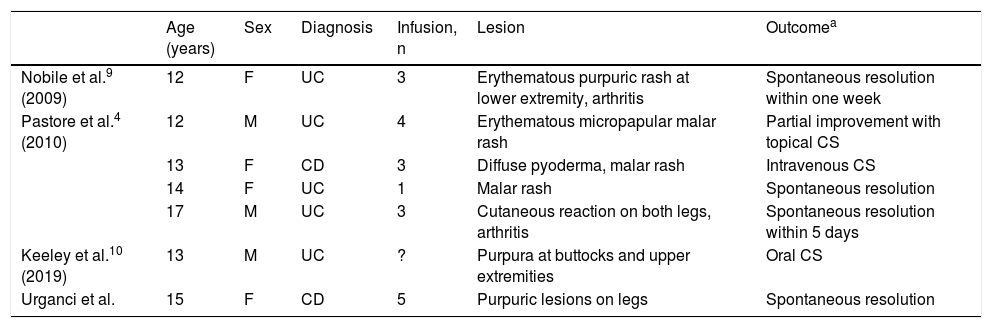
In reports on adult patients, leukocytoclastic vasculitis is described as the most common type of vasculitis, and purpura as the most frequent cutaneous lesion associated with TNF inhibitor use1,2,5. A total of 125 cases of vasculitis related to TNF inhibitors have been reported in adults. Sokumbi et al.1 described 8 patients with vasculitis (5 treated with IFX, 2 with etanercept, and 1 with adalimumab), Fujikawa et al.2 described 3 patients (IFX: 2 and etanercept: 1), Song et al.3 described one patient treated with IFX, and Ramos-Casals et al.5 described 113 patients (etanercept: 59, IFX: 47, adalimumab: 5, other agents: 2). The clinical characteristics of six pediatric cases4,9,10 with IFX-related Henoch-Schönlein purpura are shown in Table 1.
Clinical characteristics of reported pediatric cases with infliximab-related Henoch-Schönlein purpura.
| Age (years) | Sex | Diagnosis | Infusion, n | Lesion | Outcomea | |
|---|---|---|---|---|---|---|
| Nobile et al.9 (2009) | 12 | F | UC | 3 | Erythematous purpuric rash at lower extremity, arthritis | Spontaneous resolution within one week |
| Pastore et al.4 (2010) | 12 | M | UC | 4 | Erythematous micropapular malar rash | Partial improvement with topical CS |
| 13 | F | CD | 3 | Diffuse pyoderma, malar rash | Intravenous CS | |
| 14 | F | UC | 1 | Malar rash | Spontaneous resolution | |
| 17 | M | UC | 3 | Cutaneous reaction on both legs, arthritis | Spontaneous resolution within 5 days | |
| Keeley et al.10 (2019) | 13 | M | UC | ? | Purpura at buttocks and upper extremities | Oral CS |
| Urganci et al. | 15 | F | CD | 5 | Purpuric lesions on legs | Spontaneous resolution |
CD: Crohn’s disease; CS: corticosteroids; UC: ulcerative colitis.
Ramos-Casals et al.5 reported that one-quarter of the patients with anti-TNF agent-related vasculitis had extracutaneous involvement, such as interstitial lung disease. Sokumbi et al.1 found that peripheral neuropathy and renal vasculitis were the most frequent cases of extracutaneous involvement. Nobile et al.9 and Pastore et al.4 described pediatric cases with arthritis. We detected no systemic involvement in our case.
Vasculitic reactions occur on average within 18 weeks of treatment (range 6–38 weeks)1,4. Henoch-Schönlein purpura developed 10 days after the fifth dose of IFX therapy in our patient.
Although the pathogenesis of vasculitis associated with anti-TNF agents is still unknown, several possible mechanisms, such as the development of antibodies against anti-TNF agents leading to immune complex-mediated type III hypersensitivity1,2,11 and a switch from predominant Th1 cytokine response to the Th2 response associated with an antibody-mediated immune mechanism11, have been proposed.
Pastore et al.6 examined the incidence of serious adverse events in patients at a pediatric referral center, in relation to chronic rheumatologic and gastroenterologic inflammatory disorders. They concluded that anti-TNF-α therapy was generally well tolerated, and that serious adverse events frequently associated with IFX were rare and non-fatal11. In patients with mild reactions, such as isolated cutaneous lesions or immunologic alterations, cessation of anti-TNF therapy might be sufficient. A short course of steroids could also be considered. In our patient, the cutaneous lesions resolved completely after IFX therapy was discontinued, as occurred in the cases reported in the studies by Nobile et al.9 and Pastore et al.4 Treatment with intravenous or topical steroids was not required.
In conclusion, beyond the clinical benefits of IFX, clinicians must be aware of the drug’s side effects. Baseline clinical and immunologic evaluation, before starting anti-TNF therapy, should be carried out in those patients, as well as close follow-up.
Ethical considerationsInformed consent was requested of the patient to receive the treatment and participate in the research described above.
The present work complies with the current regulations on bioethical research and given the retrospective nature of a single case report, no authorization by the institutional ethics committee was required.
In the event that the patient could be recognized or identified through the images or data in the article, the author declares that the informed consent of the patient was obtained for the publication of her data/images.
Financial disclosureNo financial support was received in relation to this article.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Urganci N, Sakar M, Yalcín O, Kalyoncu D. Púrpura de Henoch-Schönlein inducida por infliximab para enfermedad de Crohn: reporte de un caso y revisión de la literatura. Rev Gastroenterol Méx. 2022;87:110–112.


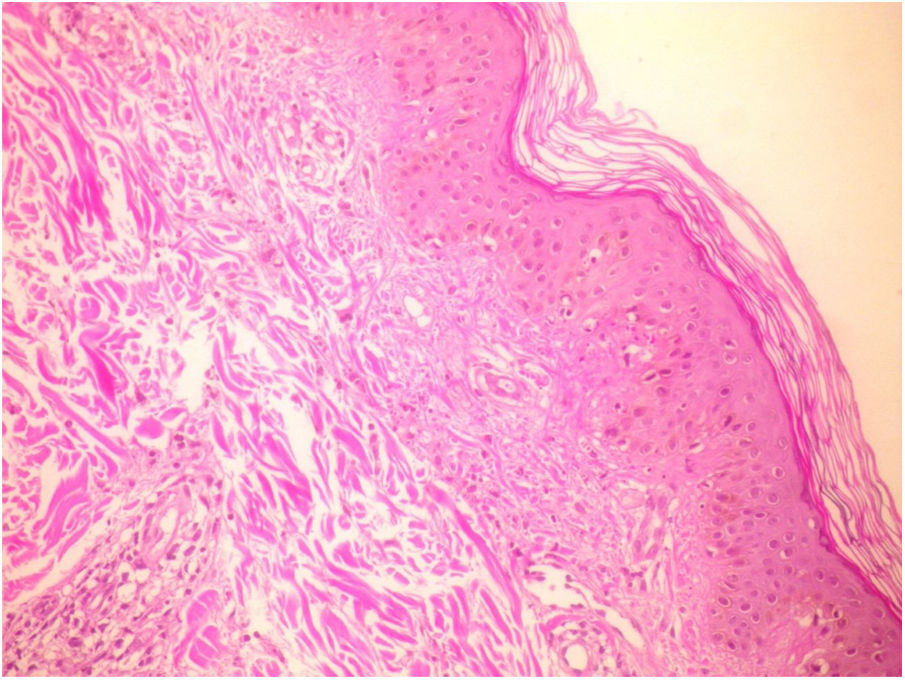
![Biopsy of skin lesions showing leukocytoclastic vasculitis (hematoxylin and eosin stain [H&E], ×20). Biopsy of skin lesions showing leukocytoclastic vasculitis (hematoxylin and eosin stain [H&E], ×20).](https://static.elsevier.es/multimedia/2255534X/0000008700000001/v2_202202050626/S2255534X21001109/v2_202202050626/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w97o/wdEXW47bqlyT1CqG6R0=)
![Biopsy of skin lesions showing leukocytoclastic vasculitis (hematoxylin and eosin stain [H&E], ×40). Biopsy of skin lesions showing leukocytoclastic vasculitis (hematoxylin and eosin stain [H&E], ×40).](https://static.elsevier.es/multimedia/2255534X/0000008700000001/v2_202202050626/S2255534X21001109/v2_202202050626/en/main.assets/thumbnail/gr3.jpeg?xkr=ue/ImdikoIMrsJoerZ+w97o/wdEXW47bqlyT1CqG6R0=)

