Fecal incontinence is a disabling condition with devastating consequences for the patients. Medical and surgical options are not very satisfactory, reason by which regenerative medicine has been considered in this field. In the present research, we analyzed functional and histologic effects after implanting pluripotent stem cells (PSCs) in a murine model with sphincterotomy.
Materials and methodsFemale Wistar rats were subjected to sphincterotomy and divided into three groups. Group 1 (control group) was treated with 300μL of balanced saline solution and group 2 (late treatment) and group 3 (early treatment) received 50,000 PSCs resuspended in 300μL of balanced saline solution. All animals were evaluated through high-resolution anorectal manometry 24hours before and after sphincterotomy and every month for three months. Finally, the rats were euthanized and histopathologic sections from the anal canal were obtained.
ResultsAll groups showed a decrease in resting anal pressure and squeeze anal pressure 24hours after sphincterotomy. At the third month, higher anal pressures in the groups treated with PSCs were detected. Regarding the histologic effects, the microscopic architecture was restored and there was a significant decrease in the inflammatory response in the groups treated with PSCs.
ConclusionPSCs implantation improves anal tone, as well as histologic structure, presenting better regenerative results when implanted as early treatment.
La incontinencia fecal es una condición incapacitante con consecuencias devastadoras para los pacientes. Las opciones médicas y quirúrgicas no son muy satisfactorias, razón por la cual la medicina regenerativa ha sido considerada en este campo. En esta investigación se analizaron los efectos funcionales e histológicos posteriores al implante de células madre pluripotentes (CMP) en un modelo murino con esfinterotomía.
Material y métodosRatas hembra Wistar fueron sometidas a esfinterotomía y divididas en tres grupos. El grupo 1 (grupo control) fue tratado con 300μl de solución salina isotónica, el grupo 2 (tratamiento tardío) y el grupo 3 (tratamiento temprano) recibieron 50,000 CMP resuspendidas en 300μl de solución salina isotónica. Todos los animales fueron evaluados por medio de manometría anorrectal de alta resolución 24 h previo y posterior a la esfinterotomía y cada mes durante tres meses. Finalmente, las ratas fueron sacrificadas y se obtuvieron cortes histopatológicos del canal anal.
ResultadosTodos los grupos mostraron una disminución en las presiones anales de reposo y esfuerzo 24 h después de la esfinterotomía. Al tercer mes, se obtuvieron presiones anales más elevadas en los grupos tratados con CMP, observando una diferencia estadísticamente significativa en la presión anal de esfuerzo. En cuanto a los efectos histológicos, se observó restauración de la arquitectura microscópica, así como una disminución significativa de la respuesta inflamatoria en los grupos tratados con CMP.
ConclusiónEl implante de CMP mejora el tono anal y la microarquitectura histológica después de una esfinterotomía, presentando mejores resultados regenerativos cuando se implantan de manera temprana.
Fecal incontinence (FI) is a disabling condition that affects a large number of patients and has devastating consequences for quality of life.1 It is also considered a main public health concern in aging societies.2 FI prevalence is highly variable and is reported to range from 5 to 15%. One of the major causes of this variability is related to the fact that many patients are embarrassed by the condition and do not discuss it with their physicians. In general, FI is defined as the continuous or recurrent uncontrolled passage of fecal material (more than 10ml) for at least one month in a person older than three years of age.3
Anal sphincter disruption is one of the most common causes of FI and occurs as a result of obstetric anal sphincter injury or anorectal surgery or trauma. In aging populations, it is due to the spontaneous apoptosis of muscle cells in the anal sphincters.2,4,5 Muscle injury can be isolated or multiple, either in the smooth muscle of the internal anal sphincter or the striated muscle of the external anal sphincter or puborectal muscle. Anorectal manometry is a useful tool for evaluating sphincter function in FI.6,7
There are several medical and surgical treatment options. The therapeutic alternatives include drugs that slow down peristalsis or increase stool consistency, special diets, and biofeedback, among others, but they only improve fecal continence in 50-60% of patients.8–10 Surgical options include anal sphincter repair, implantation of an artificial anal sphincter, colostomy, and neuromodulation therapy.9,11 Unfortunately, neither the medical nor the surgical options are very satisfactory, which is why regenerative medicine has been considered in this field.
The replacement or regeneration of dysfunctional anal sphincter through stem cell (SC) therapy and tissue engineering techniques holds great promise,2 given its known capacity for multilineage differentiation and self-renewal. It has also been reported that those cells exert a therapeutic effect via the secretion of bioactive factors that direct other SCs and progenitor cells to the area of injury.12
Several studies have shown the possible application of different types of mesenchymal stem cells (MSCs) in restoring anal sphincter functions due to their antiapoptotic, anti-scarring, neovascularization, and immunomodulatory properties.2,13–16 Unfortunately, the number of cells that can be generated from a single donor is limited, because of the restricted long-term proliferation capacity of the MSCs. Extended culture time also increases the risk for inducing chromosomal aberrations and the loss of their differentiation potential,17 highlighting the subsequent restriction of their differentiation capacity. In addition, MSCs can be unsuitable due to chronic health conditions of the patient. Thus, we suggest the use of pluripotent stem cells (PSCs), given that they have a greater differentiation capacity than MSCs, as well as long-term retention of their pluripotent state in culture. Moreover, mouse PSCs do not express MHC class I and class II molecules,18 which is advantageous for their use in different recipient species.
To the best of our knowledge there are no experimental studies using PSCs in anal sphincter injury. Our research group has demonstrated the ability of PSCs to decrease the inflammatory response and restore histologic integrity after ocular trauma,19 and we have proposed the possibility of using those cells in anal trauma. Therefore, the present study aimed to analyze functional and histologic effects after implanting mouse PSCs in a murine model with sphincterotomy.
Materials and methodsMurine model with sphincterotomyFemale Wistar albino rats aged between 10 and 12 weeks were housed in metabolic cages in a climate-controlled environment at a temperature of 21°C with a light/dark cycle of 12/12h and were allowed free access to food and water at all times. To establish a sphincterotomy model in rats, first, under isoflurane inhalation anesthesia (dose-response), surgical skin preparation was performed by shaving the perineum area and scrubbing it with iodine solution. After that, the perineal region was infiltrated with 1ml of 2% lidocaine with 1:80,000 epinephrine, and under direct vision, anal canal injury up to the dentate line was performed by incising the external and internal anal sphincters 2-3mm deep. The skin was closed with interrupted 4-0 absorbable Vicryl sutures. After the lesion was made, the animals received tramadol 3mg/kg and enrofloxacin 5mg/kg. The physiology of the anal sphincters was evaluated through manometry 24h pre and post-lesion. For sphincterotomy model standardization, the anal canal injury was confirmed through histopathologic analysis.
The guidelines of the Norma Oficial Mexicana for the use and care of laboratory animals (NOM-062-ZOO-1999) and the disposal of biological residues (NOM-087-ECOL-1995) were followed.
Pluripotent stem cell cultureR1 mouse embryonic stem cells (ATCC, Virginia, USA) were seeded in culture dishes at a density of 50,000 cells/cm2 on mitotically inactivated mouse embryonic fibroblasts (MEFs), using mouse embryonic stem cell basal medium (ATCC) supplemented with 15% fetal bovine serum (FBS) (ATCC), 0.1mM 2-mercaptoethanol (Invitrogen, California, USA), and 1,000 U/ml of mouse leukemia inhibitory factor (EMD Millipore, Darmstadt, Germany). Culture dishes were incubated at 37°C in a humidified 5% CO2 and 95% air incubator. PSCs were separated from the MEF monolayer before implantation, emphasizing that 4 to 8 cell passages were used.
Prior to implantation, morphologic and phenotypic characteristics of the PSCs were determined through optic microscopy and immunofluorescence, as previously reported,20 to confirm the pluripotent state of the cells.
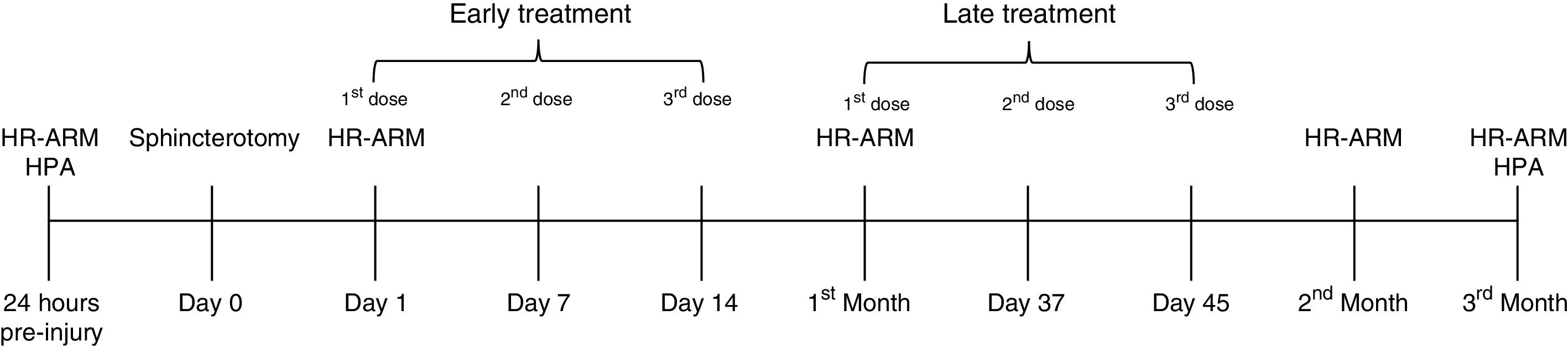
Implantation of mouse pluripotent stem cellsSince it is known that there is a different response according to treatment time (early and late treatment after injury), we randomly divided 43 female Wistar albino rats into three groups: one control group (n=13), and two experimental groups (n=15). Of the experimental groups, group 2 corresponded to the animals receiving late treatment and group 3 to those receiving early treatment after injury. All members of the groups were subjected to sphincterotomy, as described above, and subsequently received an intramuscular injection of balanced saline solution (BSS) or PSCs into the anal sphincter, as indicated according to group (fig. 1). Group 1 (control group) received 0.3ml of BSS at 30, 37, and 45 days after injury, group 2 (late treatment) had 50,000 PSCs re-suspended in 0.3ml of BSS at 30, 37, and 45 days post-lesion, and the rats in group 3 (early treatment) were treated with 50,000 PSCs re-suspended in 0.3ml of BSS at 24hours, and 7 and 14 days post-lesion. The quantity of PSCs and treatment timing were established according to previous works, in which there were no secondary complications or side effects.19
Experimental methodology, 24h prior to sphincterotomy, high-resolution anorectal manometry (HR-ARM) and histopathologic analysis (HPA) were performed. After sphincterotomy (day 0), at days 1, 7, and 14 post-injury, the early treatment group received its experimental treatment, and the late treatment group was treated at the first month, and on days 37 and 45 post-injury. Anal pressures were evaluated over a period of 3 months. HR-ARM was performed one day after injury and 1, 2, and 3 months after injury. Finally, 3 months after injury and treatment commencement, the animals were euthanized, and the anal canal of each animal was excised to perform the HPA.
High-resolution anorectal manometry (HR-ARM) was performed using the ManoScan 360 HR ® assembly with 12 circumferential sensors spaced at 0.6-cm intervals (Sierra Scientific Instruments, USA). Measurements of the anal sphincter pressures (resting and squeeze) were assessed on reactive rats that were confined to an acrylic plastic immobilizer during the measurement process. Resting anal pressure (RAP) was regarded as tone sustained for at least 3seconds and squeeze anal pressure (SAP) was measured when there was an increase in the baseline pressure from a spontaneous reactive stimulus. Maximum SAP was the highest pressure reached, even if it was not sustained. The manometric data were analyzed using the ManoView analysis software (Sierra Scientific Instruments Inc.). Anal pressures were recorded 24h prior to sphincterotomy, 24h post-injury, and then each month for three months (fig. 1), using a balloon connected to a digital recorder.
Histopathologic analysisThree months after sphincterotomy and treatment commencement, the rats were euthanized, and the anal canal of each rat was excised. The collected samples were fixed in 10% neutral-buffered formalin (Sigma-Aldrich, Darmstadt, Germany) and subsequently embedded in paraffin. Thin sections (3μm) were obtained using a microtome (Ecoshel, USA) and mounted on slides. Finally, the histopathologic sections were stained with Masson's trichrome and were viewed under a light microscope (TiU Eclipse, Nikon, Japan). Degrees of anatomic integrity were designated as mild, moderate, or severe microarchitectural alterations, considering sphincter mechanism integrity and collagen fiber arrangement, as well as the interweaving of collagen with muscle fibers.
A granulomatous inflammatory response is known to be a particular type of chronic inflammation characterized by focal collections of macrophages, epithelioid cells, and multinucleated giant cells, which is why we assessed the chronic inflammatory response, using the following criteria for the number of granulomas per field: mild (1-3 granulomas), moderate (4-6 granulomas) and severe (> 7 granulomas).
Statistical AnalysisFor the statistical analysis of the anal pressures, the differences between the treated groups and the period of treatment for each group were established using the one-way analysis of variance (ANOVA) and the Bonferroni test. The chi-square test was used for the histologic analysis. A p <0.05 was considered statistically significant.
The Institutional Ethics Committee for Animal Research of the Escuela Militar de Medicina approved the protocol. All experiments were examined and approved by the appropriate ethics committee and were performed following the ethical standards stipulated in the 1964 Declaration of Helsinki.
ResultsAnal sphincter pressuresAs mentioned above, a sphincterotomy was performed on all the rats, with no complications (fistulae, abscesses, surgical wound infection, or sepsis).
For sphincterotomy standardization, we obtained a pre-injury RAP of 59±12.7mmHg and a SAP of 120.39±52.6mmHg. At 24h after injury, the RAP and SAP decreased to 38.14±10.35 and 69.02±31.73mmHg, respectively.
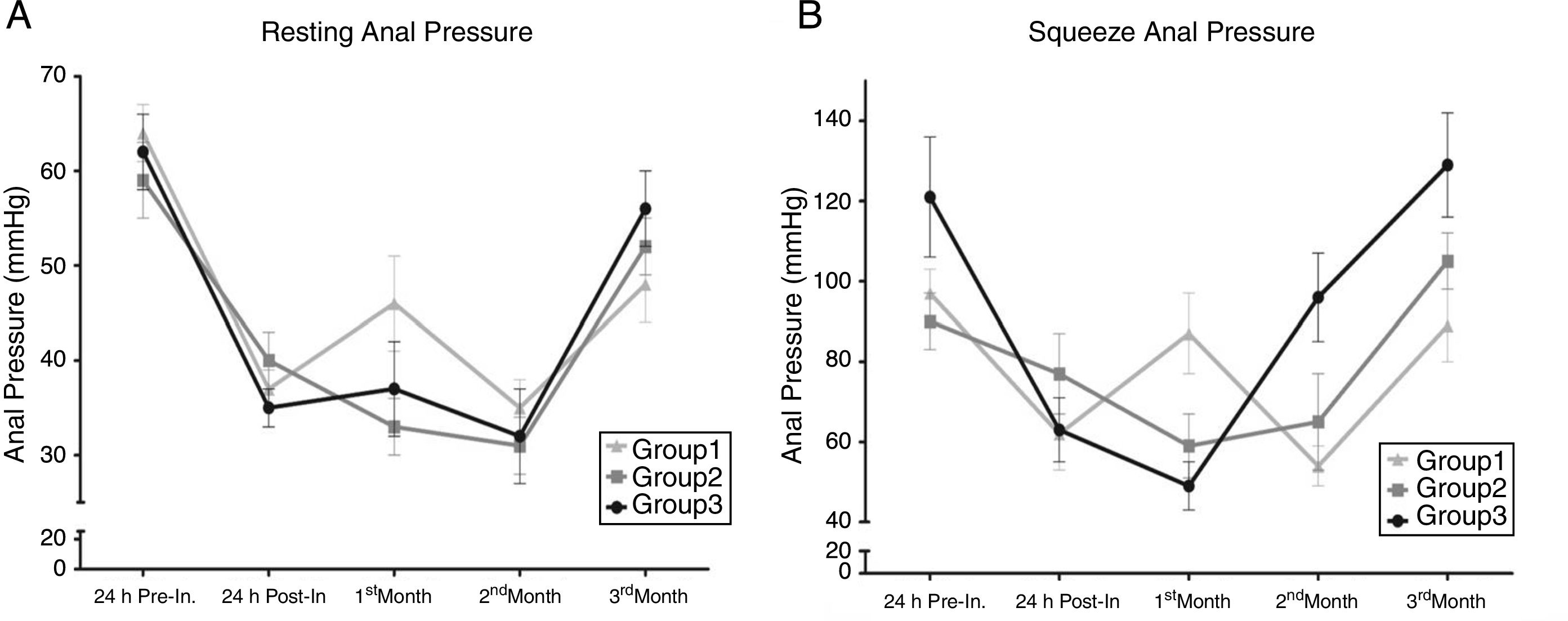
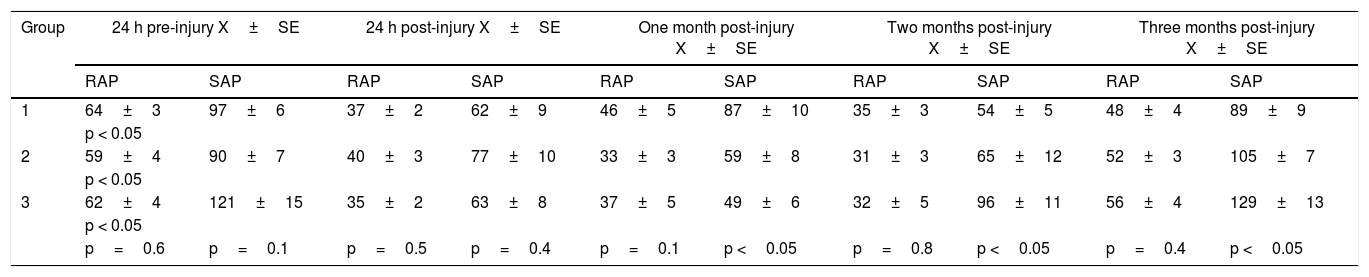
The results of the treated groups are summarized in Table 1. The physiologic results showed a decrease in RAP and SAP in all groups 24h after injury. However, one month after injury, there was an increase in both RAP and SAP in group 1, but a slight decrease in groups 2 and 3. At the second month, all groups presented with an insignificant reduction in RAP, but groups 2 and 3 showed a significant increase in SAP, in contrast to the decrease in the control group. Finally, at the third month, the groups presented the following RAP: control group 48mmHg, groups 2 and 3 treated with PSCs, 52mmHg and 56mmHg, respectively. Although we detected a higher RAP in the treated groups, the difference between groups was not statistically significant. On the other hand, there was a significant difference in relation to SAP. The control group had 89mmHg and groups 2 and 3 had 105mmHg and 129mmHg, respectively, and the difference in the higher pressures in the treated groups (fig. 2) compared with the lower control group value was statistically significant (p <0.05).
Anal sphincter pressures (mmHg).
| Group | 24 h pre-injury X±SE | 24 h post-injury X±SE | One month post-injury X±SE | Two months post-injury X±SE | Three months post-injury X±SE | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| RAP | SAP | RAP | SAP | RAP | SAP | RAP | SAP | RAP | SAP | |
| 1 | 64±3 | 97±6 | 37±2 | 62±9 | 46±5 | 87±10 | 35±3 | 54±5 | 48±4 | 89±9 |
| p < 0.05 | ||||||||||
| 2 | 59±4 | 90±7 | 40±3 | 77±10 | 33±3 | 59±8 | 31±3 | 65±12 | 52±3 | 105±7 |
| p < 0.05 | ||||||||||
| 3 | 62±4 | 121±15 | 35±2 | 63±8 | 37±5 | 49±6 | 32±5 | 96±11 | 56±4 | 129±13 |
| p < 0.05 | ||||||||||
| p=0.6 | p=0.1 | p=0.5 | p=0.4 | p=0.1 | p <0.05 | p=0.8 | p <0.05 | p=0.4 | p <0.05 | |
Resting anal pressure (RAP) and squeeze anal pressure (SAP) were not significantly different between groups (p>0.05) before injury and 24h after injury. However, RAP was higher three months post-injury in the animals treated with pluripotent stem cells compared with those treated with balanced saline solution. In contrast, SAP was significantly greater in the treated groups, with a higher SAP in group 3 (p<0.05).
Anal Pressures. A) Resting anal pressure (RAP). B) Squeeze anal pressure (SAP). RAP and SAP were not significantly different between groups (p>0.05), 24hours pre-injury and post-injury. However, RAP was higher 3 months post-injury in the animals treated with PSCs, compared with those treated with BSS (p>0.05). In contrast, SAP was significantly greater in the treated groups, with a higher SAP in group 3 (p<0.05).
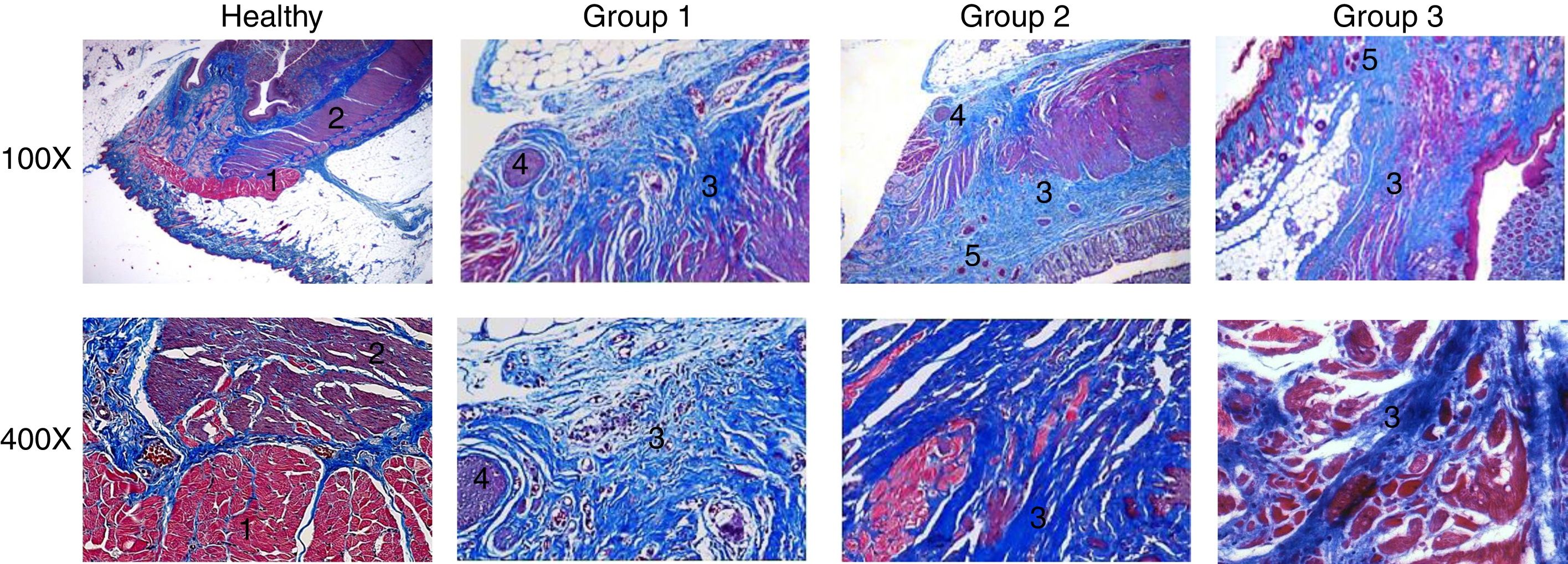
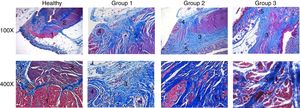
Regarding the histologic results (fig. 3), anatomic integrity and inflammatory response were assessed. The normal histology of a healthy anal canal consists of rings of muscle tissue with well-defined muscle fibers, in addition to a voluntary external sphincter and an involuntary internal sphincter. The external sphincter is composed of skeletal muscle fibers1 and the internal sphincter is formed by smooth muscle.2
Histopathologic analysis. Histopathologic sections stained with Masson's trichrome. Comparison of the microstructure between healthy tissue and the injured tissue from the groups that underwent the different treatments. Groups 1, 2, and 3 showed severe, moderate, and mild microarchitectural alterations and inflammatory response, respectively. 1: skeletal muscle fibers; 2: smooth muscle; 3: collagen fibers; 4: granulomas; 5: capillaries.
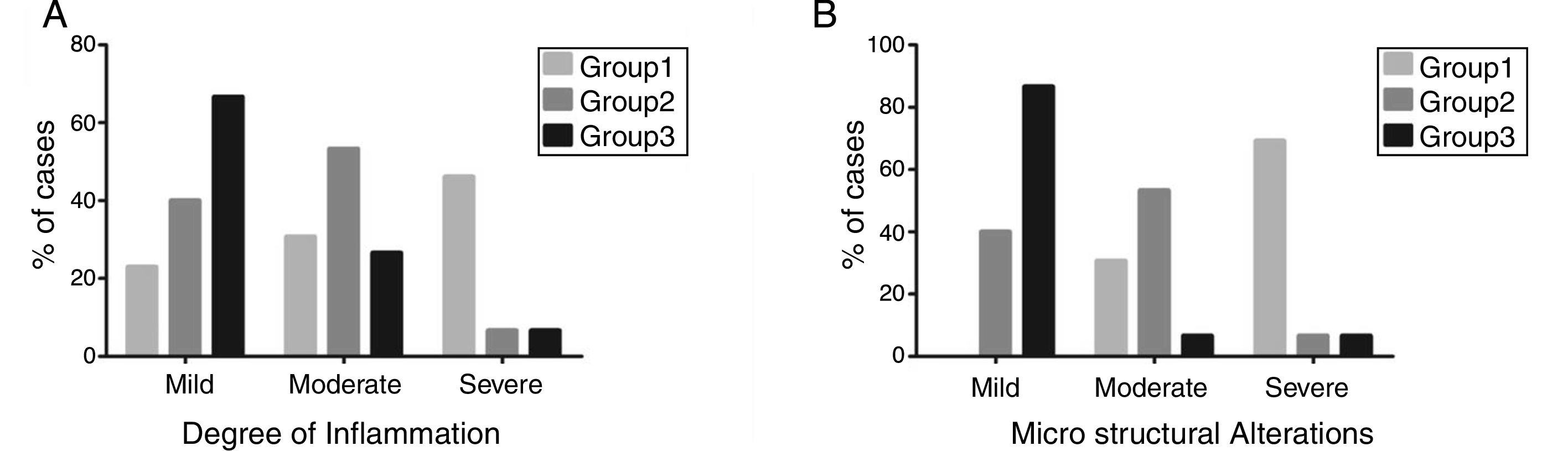
Most of the rats in group 1 had severe microarchitectural alterations (69.23%) and a severe inflammatory response (46.15%), showing a total loss of the architecture in both sphincters, with an irregular pattern of collagen fibers3 interspersed with skeletal and smooth muscle. There was an increase in collagen deposition that produced an intense blue stain, indicating scar tissue in the area of the defect. Abundant granulomas were also observed.4 Most of the group 2 animals presented with moderate microarchitectural alterations (53.3%) and moderate inflammatory response (53.3%), evidenced by thick and disorganized bands of mature collagen,3 albeit with fewer collagen fibers than observed in group 1. There was also abundant capillary neoformation,5 as well as a moderate number of granulomas.4 In group 3, most of the rats had mild architectural distortion of the anal canal (86.6%), as well as a mild inflammatory response (66.6%). There was also an apparently more organized presence of collagen fibers3 interspersed with muscle fibers, and capillary neoformation5 was still evident. Figure 4 shows that group 3 had the better results, in which most of the cases presented with mild inflammation and mild microstructural alterations.
Analysis of the degree of inflammation and microstructural alterations. A) Degree of inflammation. B) Microstructural changes. A significant decrease in the level of inflammation, as well as restoration of the anatomic integrity, were shown in the groups treated with pluripotent stem cells.
Regenerative medicine employing SCs is becoming a therapeutic option for chronic degenerative diseases, as well as for symptomatic benign disease processes,21 such as FI. Some studies propose cell therapy as an alternative treatment for anal sphincter injury14,15 and most of them use MSCs. There is no evidence of the use of PSCs evaluated through HR-ARM in FI. In our research, we implanted mouse PSCs as early and late treatment in rats with sphincterotomy, assessing physiology and histopathology through HR-ARM and bright light microscopy, respectively.
There is no well-established SC dosage or administration route, given that SC research is extensive and includes different types of SCs, such as adipose-derived SCs, bone marrow SCs, and embryonic stem cells (ESCs), among others. Thus, different SC doses and administration routes have been used, the former from 10,000 up to 500,000. For example, Kim et al. stated there was a significant effect on mice with emphysematous lung with the intravenous injection of only 50,000 MSCs.22 Bottai et al. reported that two doses of 500,000 ESCs intravenously injected into the tail vein in mice with a contusive lesion of the spinal cord promoted motor function recovery.23 Salcedo et al. demonstrated a significant increase in anal pressure with a single dose of 500,000 MSCs injected into the anal sphincter and six doses of 500,000 MSCs intravenously injected in rats with sphincterotomy.15 In previous works, we have reported that intravitreal injections of 50,000 pluripotent ESCs are very useful in the treatment of ocular trauma, restoring the microarchitecture and decreasing the inflammatory response.19
In relation to the different administration routes, Sudulaguntla et al. stated that the intravenous route is the easiest, but its main disadvantage is that only an approximate 3% of normal cardiac output will flow per minute through the left ventricle. It is also very limited by the transpulmonary first-pass attenuation effect seen on the cells. Moreover, there are some major cell types, such as skeletal myoblasts, that have the disadvantage of emboligenic potency when delivered systemically.24 Based on the abovementioned information and our own experience, in the present study we decided to inject 50,000 PSCs into the anal sphincter.
With respect to the anal pressure results, it should be mentioned that the manometry parameters and structure of the anal canal of normal rats are similar to those of humans, because rats have a resting pressure and rectoanal reflex in manometry, and external and internal anal sphincters.25 In the present study, sphincterotomy standardization showed a pre-injury RAP of 59±12.7mmHg and a SAP of 120.39±52.6mmHg in healthy rats, results that are similar to those described by Hosokawa et al., who reported a max RAP of 70.86±10.39mmHg in adult rats. Our results also coincide with those of Kang et al., who stated that the mean resting pressure of a healthy human anal canal is 51.4mmHg determined through water-perfused anorectal manometry and 51.9mmHg through HR-ARM, and the average maximum squeeze pressure determined through water-perfused anorectal manometry is 146.3mmHg and 137.7mmHg through HR-ARM. Nevertheless, HR-ARM is likely to provide better physiologic information and require a shorter measurement time compared with water-perfused anorectal manometry.25,26
Concerning recorded pressures after injury, we detected a significant decrease of more than 50% in relation to normal levels in the RAP and SAP 24h post-injury. Those results are similar to the percentages reported by Cerdán et al. and Chowcat et al. In their studies on humans, they reported a significant reduction in sphincter pressures, dropping by 50% in relation to normal levels after sphincterotomy.27,28
After the first month post-injury, the control group presented an increment in the RAP and SAP, probably due to the inflammatory response. However, the experimental groups showed a decrease rather than an increase. It is known that SCs possess immunosuppressive and immunomodulatory properties, and multiple animal studies have demonstrated the ability of PSCs to limit the inflammatory response.29 Bottai et al. injected ESCs into the tail veins of rats after inducing a contusive lesion of the spinal cord, reporting a significantly decreased inflammatory response in animals that received ESCs, observing that the number of invading macrophages and neutrophils was significantly reduced.23
In relation to the time to evaluate the effects of the PSC implantation, Salcedo et al. performed a sphincterotomy on rats and 24h after injury each animal received 2 million MSCs, either IM into the anal sphincter or IV via the tail vein. Anal sphincter pressures were recorded 10 days after injury, evaluating an early recovery phase, and they reported a significant increase in the anal pressures.14 In another similar study, they reported that both intramuscular and intravenous treatment of MSCs after an injury caused an increase in anal pressure sustained at five weeks.15 In our study, we evaluated the anal pressures at three months, given that 5 weeks is not long enough to assess muscle fiber restoration and its subsequent function recovery. As mentioned before, at the first month post-injury a decrease in anal pressures rather than an increase was reported, but three months post-injury a significant increase in both pressures was shown in the groups treated with PSCs. Even though statistical significance was not achieved in relation to the RAP, the groups treated with PSCs presented higher pressures. However, in regard to the SAP, there was a statistically significant increment in the groups treated with PSCs (group 2, from 59mmHg at one month post-injury to 105mmHg at the third month, and group 3 from 37mmHg at one month post-injury to 129mmHg at the third month).
It is important to mention that our results also agree with those obtained by Inoue et al. who reported no difference in the RAP between the 2 groups before injury. Moreover, 24h after injury they also reported a significant decrease in RAP in both groups, and finally showed an improvement in RAP in the group treated with adipose-derived SC sheets. In our results, there was no statistical significance in RAP values between groups prior to sphincterotomy, and 24h after injury we also reported a significant decrease in RAP in both groups. Likewise, we showed an improvement in RAP in the groups treated with PSCs. Nevertheless, Inoue et al. developed their sphincterotomy model by removing the left semicircle of the external and internal anal sphincters in the rats and evaluated RAP using a catheter with a microminiature silicon strain gauge sensor mounted at one end.30
Tissue damage can result from various stimuli, including infections, autoimmune reactions, toxins, radiation, or mechanical injury. In our study, we caused a mechanical injury, disrupting the muscle fibers. The repair process typically involves two distinct phases: a regenerative phase, in which injured cells are replaced by cells of the same type, and a phase known as fibroplasia or fibrosis, in which connective tissue replaces normal parenchymal tissue. Repair of damaged tissues must then occur by replacing non-regenerated parenchymal cells with connective tissue, which in time leads to significant fibrosis and scarring and can compromise function. Thus, the development of new therapeutic strategies that limit the progression of fibrosis without adversely affecting the overall repair process would represent a significant technologic advance.31 It has also been reported that the major impediment to optimal muscle healing after any injury is fibrosis, which is defined as an abnormal and unresolvable chronic overproliferation of extracellular matrix components, interfering with muscle regeneration and causing muscle function loss.32 That could be related to our physiologic results, wherein the groups treated with PSCs presented higher pressures, while demonstrating a minimal sphincter defect, as well as decreased collagen staining showing new muscle fibers.
The histologic results of our study coincide with those of Salcedo et al. We observed severe microarchitecture alterations in group 1 (control group) and moderate and mild microarchitecture changes in groups 2 and 3, respectively. Salcedo et al. reported that after 5 weeks of treatment, a significant sphincter defect was seen in non-treatment and saline groups, characterized by an increase in collagen deposition, in contrast to the group treated with MSCs, which showed a minimal sphincter defect and less collagen staining.15 Our histologic results also concur with those of Lorenzi et al., who developed a sphincterotomy in rats, repaired both anal sphincters, and treated them with saline or MSCs injections, in accordance to study group. Thirty days later, histologic analyses were carried out. Histologic examination revealed a significant decrease in muscle tissue at the repair site after sphincter injury in the group that received saline injections, whereas new muscle fibers were identified in the group that received MSCs.33 We also observed new muscle fibers in our study group 3, which could be related to a regeneration process.
Likewise, our results coincide with those reported by Inoue et al. In their control group, four weeks after sphincterotomy, the procedure site was replaced by collagen fibers alone and the sphincter muscle stump was clear. In contrast, in the group treated with adipose-derived SC sheets, the sphincter muscle stump was indistinct, indicating a possible relation to anal sphincter muscle regeneration.30 In our case, three months after sphincterotomy and treatment commencement, we detected regeneration data characterized by organized collagen fibers interspersed with muscle fibers and capillary neoformation.
Concerning the inflammatory response, granulomatous inflammatory response is a particular type of chronic inflammation characterized by focal collections of macrophages, epithelioid cells, and multinucleated giant cells. A continuous turnover of macrophages is known to take place within any lesion, with dying cells being replaced by new recruits from the circulation or through local mitosis. Thus, a granulomatous inflammatory reaction frequently results in tissue damage during the active phase, and in fibrosis during the healing process.34
Ennis et al. stated that the optimal regenerative healing environment is possible when inflammation is minimized but not absent, which is the reason why treatments aimed at immune suppression can improve healing. Moreover, it is known that MSCs can “sense” the degree of inflammation in the microenvironment and respond by releasing growth factors and cytokines to reduce the inflammatory process, using real-time biochemical cues.35 That could have occurred in our study: inflammation decreased in the groups treated with PSCs, enabling muscle fiber restoration.
Restoration results recover some degree of the original structure and function of the muscle. It is essential to understand that the regeneration process requires the survival of the basement membrane and tissue SCs, the cellular events of inflammation, revascularization, and innervation, and the process of myogenesis where the new muscle is formed.36 In that respect, given that SCs were applied to the injured area in our study, we provided and modulated some of the necessary factors for stimulating a satisfactory regeneration process.
In the present work, we also evaluated the best time for PSC administration, comparing early treatment with late treatment. Salcedo et al. reported that the chemokine stromal derived factor-1 (SDF-1) and monocyte chemotactic protein-3 (MCP-3) are maximally upregulated at 24h after a direct anal sphincter injury. They decline shortly thereafter and are not present 3 weeks after injury.15 We found there were better regenerative results when PSCs were implanted as early treatment.
Likewise, it is important to mention that research in humans is currently being developed, such as the works of Sarveazad et al. and Andjelkov et al., who treated patients with sphincter defects using adipose-derived stromal SCs. Sarveazad et al. reported that two months after surgery, the presence of scattered islands at the repair site were shown through endorectal sonography, compared with the control group,37 and Andjelkov et al. stated that after three months, all anal fissures had healed,38 which could be compared to the cicatrization process shown in the histopathologic analysis in our study.
In conclusion, the animals with sphincterotomy treated with an early implantation of PSCs showed a better regeneration process, characterized by histologic and physiologic restoration. There was a decrease in collagen staining, showing new muscle fibers, followed by improvement in anal canal function, presenting higher anal pressures.
The current work reflects the advances made in regenerative medicine in relation to cell therapy and its use in regenerative surgery to improve treatment for FI and other colorectal conditions. Nevertheless, further studies are needed on dosage, biodistribution, the integration of PSCs in the anal sphincter, and long-term follow-up, before moving on to the next stage.
Ethical disclosuresProtection of human and animal subjectsAll procedures involving animals were approved by the Ethics Committee for Animal Research of the Escuela Medico Militar (SIDM-09). The guidelines of the Norma Oficial Mexicana for the use and care of laboratory animals (NOM-062-ZOO-1999) and the disposal of biological residues (NOM-087-ECOL-1995) were followed.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Financial disclosureNo sponsorship of any kind was received to carry out this article.
Conflict of interestThe authors declare that they have no conflict of interests.
The authors wish to thank Lt. Cor. M.V.R. Alejandro Camacho Ibarra, veterinarian at the Escuela Militar de Medicina for his important contributions to the present research.
Please cite this article as: Vázquez-Zapién GJ, Ordoñez-Gutiérrez ME, Minero-Alfaro JI, Guerrero-Guerrero VH, Mora-Mendoza I, Mata-Miranda MM. Efectos funcionales e histológicos posteriores al implante de células madre pluripotentes en un modelo murino con esfinterotomía. Revista de Gastroenterología de México. 2019;84:165–173.