Inflammatory bowel disease (IBD) has a high economic burden due to its chronicity. Treatment has evolved, thanks to the understanding of IBD pathogenesis and the advent of biologic therapy, albeit the latter increases direct costs. The aim of the present study was to calculate the total cost and cost per patient/year of biologic therapy for IBD and IBD-associated arthropathy in Colombia.
MethodsA descriptive study was conducted. The data were obtained from the Comprehensive Social Protection Information System of the Department of Health for the year 2019, utilizing the medical diagnosis codes of the International Classification of Diseases related to IBD and IBD-associated arthropathy as keywords.
ResultsThe prevalence of IBD and IBD-associated arthropathy was 61 cases per 100,000 inhabitants, with a female-to-male ratio of 1.5:1. Joint involvement was 3%, and 6.3% of the persons with IBD and IBD-associated arthropathy received biologic therapy. Adalimumab was the most widely prescribed biologic drug (49.2%). Biologic therapy had a cost of $15,926,302 USD and the mean cost per patient/year was $18,428 USD. Adalimumab had the highest impact on healthcare resource utilization, with a total cost of $7,672,320 USD. According to subtype, ulcerative colitis had the highest cost ($10,932,489 USD).
ConclusionBiologic therapy is expensive, but its annual cost in Colombia is lower than that of other countries due to the government’s regulation of high-cost medications.
La enfermedad inflamatoria intestinal (EII), tiene una alta carga económica debido a su curso crónico. El tratamiento ha evolucionado gracias al entendimiento de la patogénesis de la EII y al advenimiento de la terapia biológica (TB), esto a expensas de un aumento de los costos directos. El objetivo del estudió fue hacer una estimación del costo total, costo paciente/año de la TB para la enfermedad inflamatoria intestinal y artropatía asociada en Colombia.
MétodosEstudio descriptivo. Se obtuvo los datos del Sistema Integral de Información de la Protección Social del Ministerio de Salud durante el año 2019, utilizando como palabras clave los diagnósticos de la Clasificación Internacional de Enfermedades relacionados con el diagnóstico con EII y artropatía asociada.
ResultadosLa prevalencia de EII y artropatía asociada fue de 61 casos por 100.000 habitantes, relación mujer: hombre 1,5: 1. El compromiso articular fue 3%. 6,3% de las personas con EII y artropatía asociada recibían terapia biológica. Adalimumab fue él más formulado (49,2%). La TB representó un costo de USD $ 15.926.302. Costo promedio paciente / año fue USD $ 18.428. Adalimumab fue el biológico que consumió más recursos con un costo total de USD $ 7.672.320. Según el subtipo, CU representó el mayor costo (USD $10.932.489).
ConclusiónLa TB representa un alto costo, el costo terapia por año es menor al compararla con otros países, lo que puede ser explicado por la regulación de medicamentos de alto costo por parte del gobierno nacional.
Inflammatory bowel disease (IBD) is a chronic inflammatory condition of unknown etiology, associated with genetic, immunologic, and environmental factors. It results in a dysregulation of the innate and adaptive immune system of the mucosa of the gastrointestinal tract, characterized by bouts of activity, with a high burden of morbidity and mortality, as well as a negative impact on quality of life.1 It is associated with a wide variety of extraintestinal manifestations, one of which is IBD-associated arthropathy, which can present in up to 30% of patients.2,3
Thanks to advances in the understanding of IBD pathogenesis and the progress made in biotechnology, the focus of treatment of the disease has changed. Biologic therapy (BT) for IBD arrived on the scene in 1998, when the Food and Drug Administration (FDA) first approved an anti-TNF medication (infliximab) as treatment for Crohn’s disease (CD). Three other anti-TNF agents then arrived, in the following order: adalimumab, certolizumab, and golimumab.4 Another series of medications directed at different therapeutic targets has been developed, such as IL-12/23 inhibitors (ustekinumab), anti-integrin antibodies (natalizumab), and a monoclonal antibody more specific to the gastrointestinal tract that suppresses lymphocyte migration to the inflamed intestine by blocking α4β7 integrins (vedolizumab).5
The biologics approved for the treatment of ulcerative colitis (UC) in Colombia are adalimumab, golimumab, infliximab, and vedolizumab.6 Adalimumab, certolizumab pegol, infliximab, and ustekinumab are approved for CD.7
In recent years, the total costs of IBD have shifted from hospitalization and surgery to medication-associated expenditure, due to the increased use of biologic agents. The rise in incidence of IBD worldwide, added to higher life expectancy, will increase the economic burden of IBD in the future.8,9
There are no published data in Colombia on the frequency of the use and costs of biologic therapy in patients with IBD. The aim of the present work was to approximately determine the epidemiology and the use and cost of BT, regarding IBD, based on official information from the Department of Health and Social Protection in Colombia.
Materials and methodsA descriptive cross-sectional study analyzed the databases of the Comprehensive Social Protection Information System (SISPRO, Spanish acronym),10 a tool created by the Department of Health that enables the collection and storage of data from the General System of Social Security in Health. Those data, which are given to the Health Service Provider Institutions during each event of outpatient or hospital care, are collected from the Health Services Individual Provision Register (RIPS, Spanish acronym). All national hospitals and health centers are legally obligated to send that information to the records of the SISPRO, using the diagnostic code of the International Classification of Diseases (ICD-10). Said register contains the consolidated data of the entire population that requires services within the social security system in Colombia. Those databases are accessible to the public and the data for carrying out the present study were obtained by consulting the dynamic tables of the Colombian Health Department. The demographic information available from the SISPRO register was obtained on patients diagnosed with CD, UC, and IBD-associated arthropathy, using the ICD-10 codes: K500, K501, K508, K509, K510, K511, K512, K513, K518, K519, M076, M074, and M075, for the period between January 1, 2019 and December 31, 2019.
Statistical analysisDescriptive statistics were employed to summarize population characteristics. The categorical variables were expressed as absolute values and percentages. Prevalence in Colombia was calculated through a 5-year age group analysis, and by sex, for each of the 32 administrative departments of Colombia. The denominator was the population estimated by projections of the National Administrative Department of Statistics (DANE, Spanish acronym), based on the latest national census carried out in 2018.11
To evaluate the prescription of medications not included in the Health Benefits Plan (PBS, Spanish acronym) but financed by the health system, the My Prescription (MIPRES, Spanish acronym) platform was reviewed. It is a technologic tool that enables healthcare professionals to report the prescription of medications or health technologies covered by the health system but not covered by the PBS.12 Data were collected on the use of biologic therapies approved for IBD and IBD-associated arthropathy in the entire country (utilizing the abovementioned ICD-10 codes) and their distribution, according to 5-year age groups, sex, and department.12 Medication prices are regulated in Colombia by the National Commission of Prices and Medicines and Medical Devices, together with the delegation of the national government. It is in charge of the prescription and regulation policy, with respect to prices of medicines and medical devices that are marketed nationally, fixing a maximum selling price and regulating the price per unit of essential medicines not locally available, establishing the same regional price for each biologic. The cost of BT was obtained from the databases of the Health Department’s Medicine Price Information System (SISMED, Spanish acronym),13 where the sale price of the drug per presentation unit is entered each quarter. The average price for each biologic agent was calculated for each quarter of 2019. For the calculation per person/year, we considered the recommended dose for IBD. According to subtype, the calculation was made with the induction dose, if necessary, as well as the maintenance dose for one year. For the biologics whose dose is based on weight, such as infliximab and ustekinumab, 80 kg was the average weight.
Only original molecules were considered in the analysis, taking into account that we only had the biosimilar, infliximab. The year 2019 was chosen for the development of our study because it was the year that the entire population affiliated with the Colombian healthcare system, and prescribed a biologic agent for IBD, was registered on the MIPRES platform. In addition, it was the last year before the COVID-19 pandemic, which produced changes in the pattern of prescription and availability of antirheumatic treatments, as described in two Latin American studies.14,15
Ethical considerationsThe present research was carried out following the international ethical guidelines of the Worldwide Medical Association Declaration of Helsinki, as well as the 1993 Resolution No. 008430 of the Colombian Health Department, which classifies this type of study as “low-risk research”, given that it involves no clinical interventions. The aim of the study is primarily scientific and of interest to the medical and scientific community and can contribute to the acquisition of new knowledge. No experiments were conducted on animals or humans in this study, nor did it present any physical or psychologic risk for the patients involved. Informed consent was not requested because no personal data that could identify patients were published in the article. The study was conducted by clinically and ethically suitable persons.
The work was approved by the Research and Ethics committee of the Pontificia Universidad Javeriana and the Hospital Universitario San Ignacio in Bogotá (Act number: FM-CIE-0414-20).
ResultsThe estimated population in Colombia in 2019 was 49,395,678, of which 35,195,630 were above 18 years of age. A total of 26,197 persons diagnosed and treated for IBD and IBD-associated arthropathy were identified, resulting in the prevalence calculation in the general population of 53 cases per 100,000 inhabitants. A total of 21,515 persons were above 18 years of age and the prevalence of IBD in the adult population was 61 cases per 100,000 inhabitants. Sixty percent of the cases were women, with a female-to-male ratio of 1.5:1. Prevalence by sex was 62 per 100,000 in women and 44 per 100,000 in men.
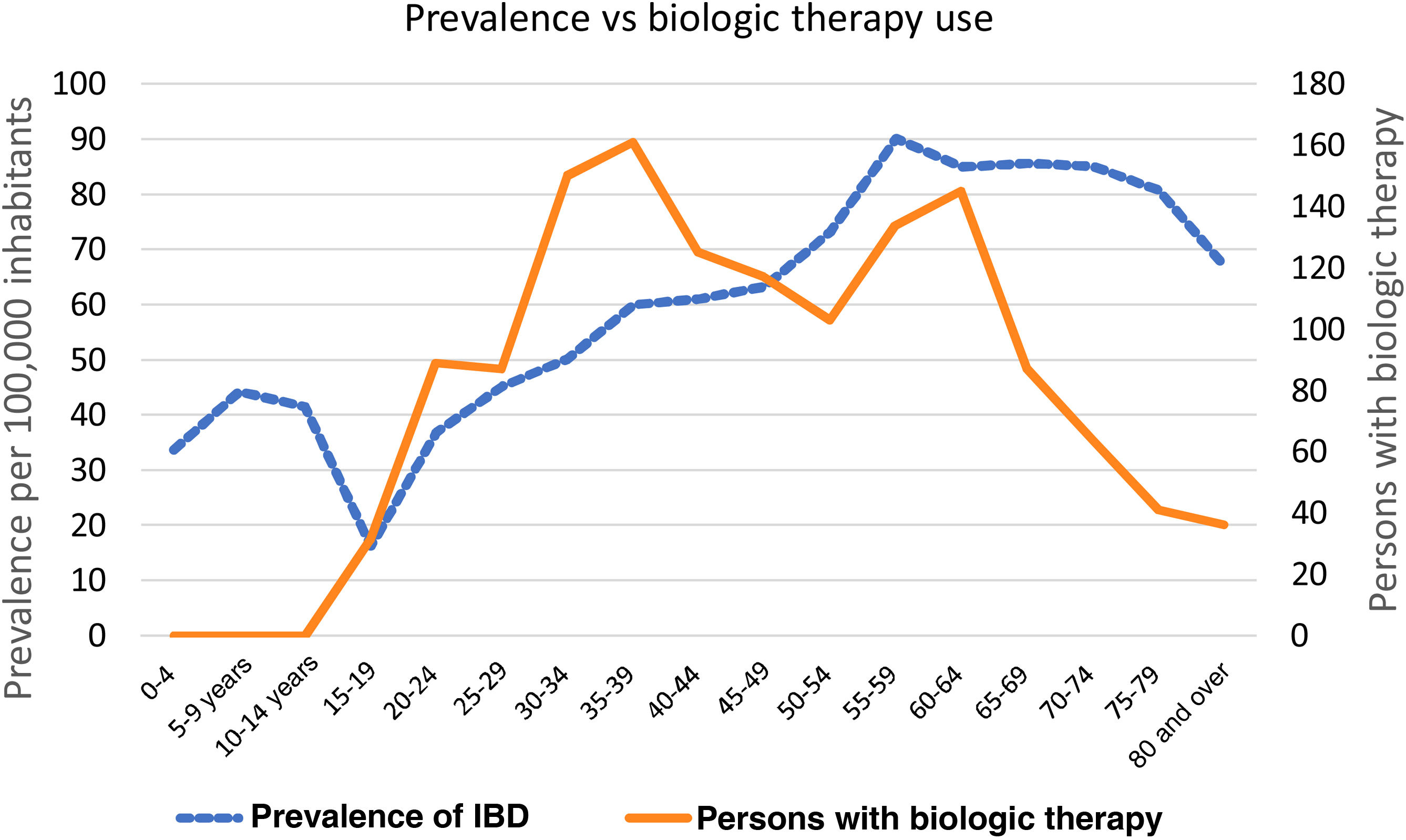
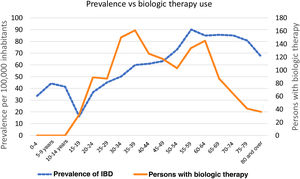
The prevalence of IBD per 5-year age group increased progressively from the 30 to 34-year age group. The 55 to 59-year age group had the highest prevalence (90 cases per 100,000 inhabitants), followed by the 65 to 69-year age group (86 cases per 100,000 inhabitants) (Fig. 1).
According to IBD subtype, prevalence in the general population was 15 cases per 100,000 inhabitants for CD, 36 cases per 100,000 inhabitants for UC, and 1.4 cases per 100,000 inhabitants for IBD-associated arthropathy. Prevalence in the population above 18 years of age was 14 cases per 100,000 inhabitants for CD, 45 cases per 100,000 inhabitants for UC, and 2 cases per 100,000 inhabitants for IBD-associated arthropathy. The highest prevalence by 5-year age group was the 35 to 39-year age group for CD, the 65 to 69-year age group for UC, and the 70 to 74-year age group for IBD-associated arthropathy. By sex, there was a higher number of women for UC, CD, and IBD-associated arthropathy, at 62, 60, and 67%, respectively.
The analysis of the number of IBD patients with joint involvement showed it was present in 3% of the cases and was associated with UC in 1.4% and CD in 0.7%.
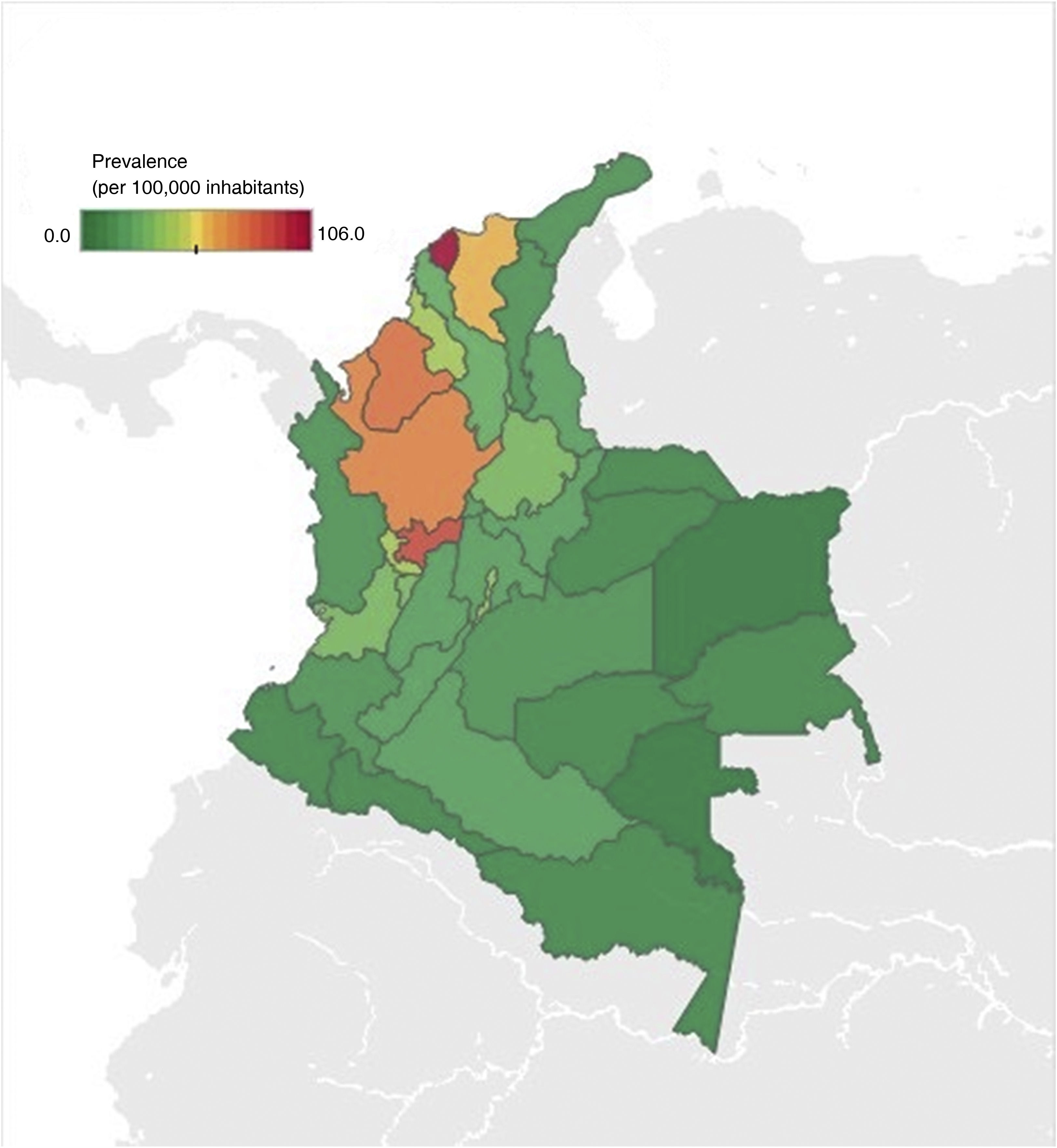
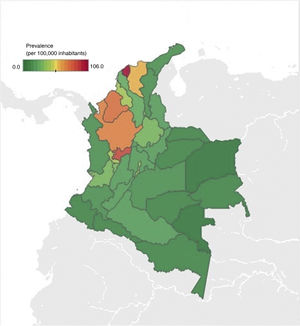
With respect to geographic distribution, prevalence ranged from 0 to 106 cases per 100,000 inhabitants (Fig. 2). Antioquia, Atlántico, Caldas, Bogotá, Córdoba, and Magdalena were the departments with the highest prevalence, corresponding to around 64% of the cases diagnosed with IBD and IBD-associated arthropathy. The departments with less prevalence of the disease were the Amazonia region (Amazonas, Putumayo, Guainía, Guaviare, and Vaupés) and the islands of San Andrés and Providencia.
A total of 6.3% (n = 1370) of the patients with IBD were receiving BT. According to IBD subtype, 5.8% (n = 918) of the patients diagnosed with UC, 8.3% (n = 429) of the patients with CD, and 3.4% (n = 23) of the patients with IBD-associated arthropathy were receiving BT.
Of the patients receiving BT, 56% were women. The 5-year age group that was most widely prescribed biologics was the 35 to 39-year group, followed by the 30 to 34-year, 60 to 64-year, and 55 to 59-year age groups (Fig. 1).
With respect to healthcare affiliation, the contributory scheme covered 90% (n = 1,237) of the prescribed biologics.
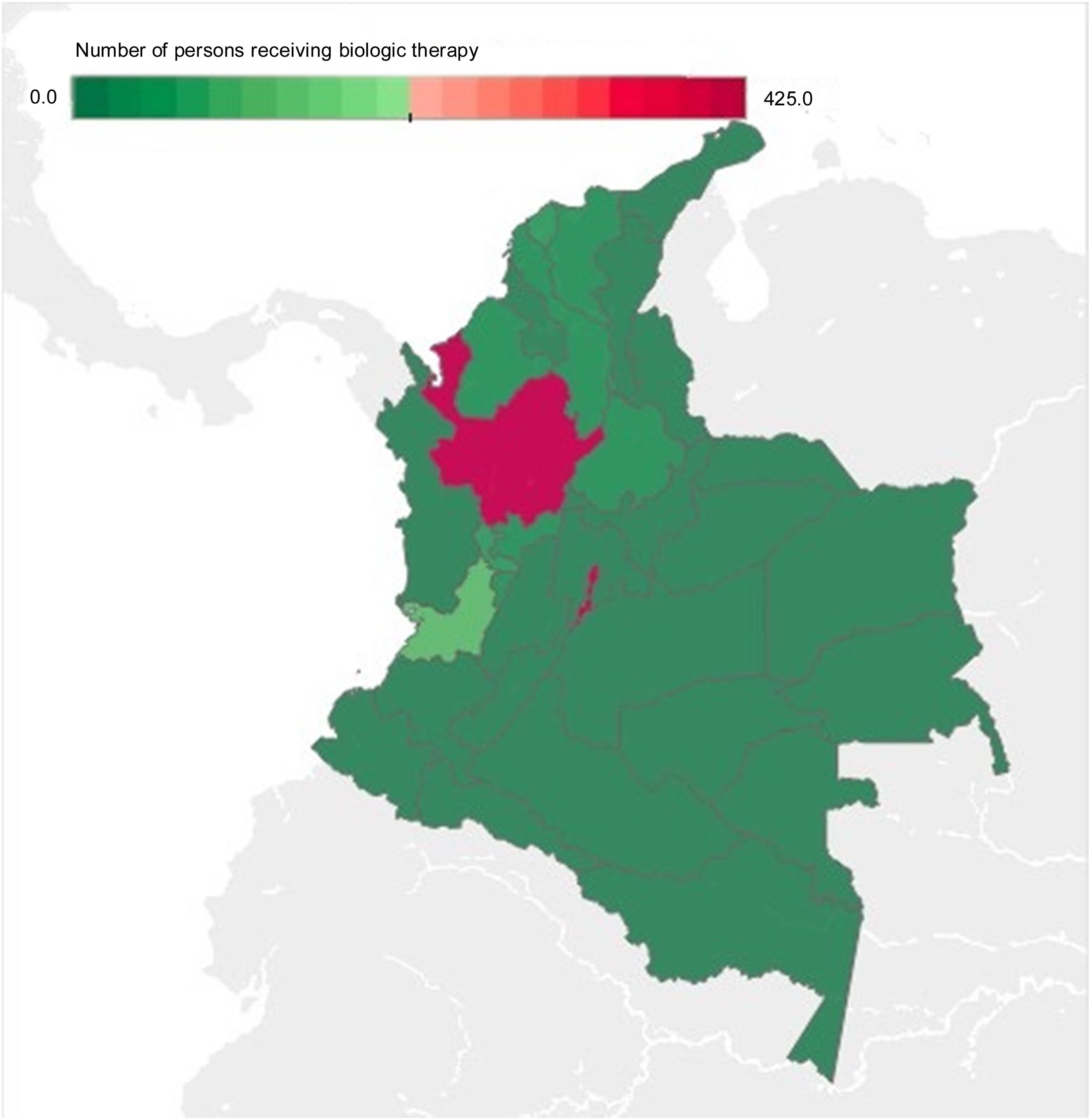
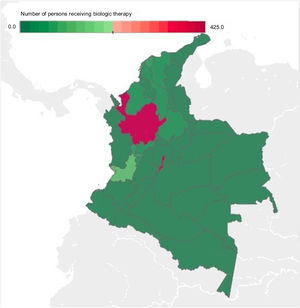
The departments with the highest number of patients receiving BT were Bogotá, Antioquia, and Valle del Cauca, with an absolute value of 428, 415, and 126, respectively, corresponding to 71% of BT prescription for IBD and IBD-associated arthropathy in Colombia for 2019. The departments that had no BT prescription were the regions of Amazonia (Amazonas, Putumayo, Guainía, Guaviare, and Vaupés), Orinoquía (Arauca, Casanare, and Vichada), and Chocó, as well as the islands of San Andrés and Provincia (Fig. 3).
The most widely prescribed biologic was adalimumab (49.2%), followed by infliximab (28.2%), vedolizumab (19.2%), and golimumab (2.7%), corresponding to 99.3% of the biologics prescribed in 2019 for IBD and IBD-associated arthropathy. The behavior was the same, according to IBD subtype, in which the most prescribed biologic for UC was adalimumab, at 44.1% (n = 405), followed by infliximab, at 28.3% (n = 260), and vedolizumab, at 23.7% (n = 218). For CD, the most prescribed biologic was adalimumab, at 60% (n = 257), infliximab, at 27.7% (n = 119), and vedolizumab, at 10.4% (n = 45). For IBD-associated arthropathy, the most widely used biologic was adalimumab, at 56.5% (n = 13), followed by infliximab, at 34.8% (n = 8).
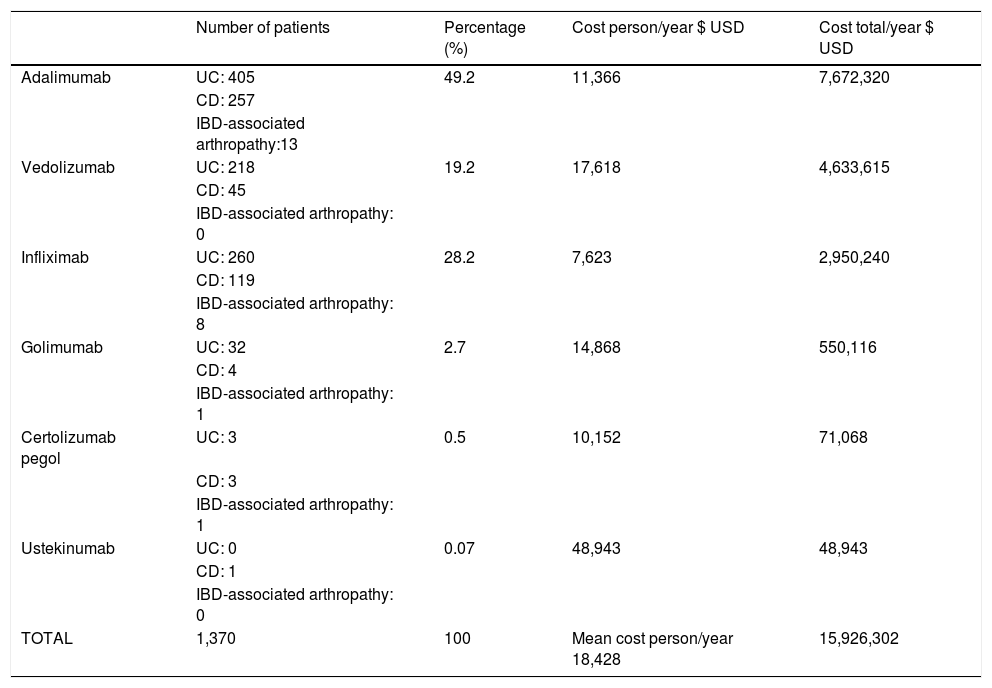
The cost of BT for IBD and IBD-associated arthropathy for the healthcare system in 2019 was $15,926,302 USD. The average cost per patient/year was $18,428 USD. Ustekinumab was the most expensive therapy per patient/year ($48,943 USD), followed by vedolizumab ($17,618 USD). Adalimumab was the therapy with the highest impact on healthcare resource utilization, at a total cost of $7,672,320 USD, followed by vedolizumab at $4,633,615USD, accounting for 77% of the total cost of BT for IBD and IBD-associated arthropathy (Table 1).
Annual cost of biologic therapy for IBD treatment and IBD-associated arthropathy.
| Number of patients | Percentage (%) | Cost person/year $ USD | Cost total/year $ USD | |
|---|---|---|---|---|
| Adalimumab | UC: 405 | 49.2 | 11,366 | 7,672,320 |
| CD: 257 | ||||
| IBD-associated arthropathy:13 | ||||
| Vedolizumab | UC: 218 | 19.2 | 17,618 | 4,633,615 |
| CD: 45 | ||||
| IBD-associated arthropathy: 0 | ||||
| Infliximab | UC: 260 | 28.2 | 7,623 | 2,950,240 |
| CD: 119 | ||||
| IBD-associated arthropathy: 8 | ||||
| Golimumab | UC: 32 | 2.7 | 14,868 | 550,116 |
| CD: 4 | ||||
| IBD-associated arthropathy: 1 | ||||
| Certolizumab pegol | UC: 3 | 0.5 | 10,152 | 71,068 |
| CD: 3 | ||||
| IBD-associated arthropathy: 1 | ||||
| Ustekinumab | UC: 0 | 0.07 | 48,943 | 48,943 |
| CD: 1 | ||||
| IBD-associated arthropathy: 0 | ||||
| TOTAL | 1,370 | 100 | Mean cost person/year 18,428 | 15,926,302 |
CD: Crohn’s disease; IBD: inflammatory bowel disease; UC: ulcerative colitis; USD: United States dollar.
According to subtype, UC had the highest cost for the healthcare system ($10,932,489 USD), followed by CD ($4,760,040 USD), and IBD-associated arthropathy ($233,770 USD).
DiscussionIBD has become a worldwide disease, increasing in traditionally low incidence regions, such as Asia, South America, and Southeast Europe, due to rapid modernization and westernization of the population.16
We found a prevalence in the Colombian adult population of 61 cases per 100,00 inhabitants and a female-to-male ratio of 1.5:1. A systematic analysis of the global burden of the disease carried out in 195 countries between 1990 and 2017, estimated approximately 7 million cases of IBD worldwide. The highest prevalence rate was found in North America, with 422 cases per 100,000 inhabitants, and the lowest prevalence rate was seen in the Caribbean, with 6.7 cases per 100,000 inhabitants.17 As occurred in our study, there was a predominance in women, at 60% of cases.
In our study, the prevalence of CD was 15 cases per 100,000 inhabitants, the prevalence of UC was 36 cases per 100,000 inhabitants, and the prevalence of IBD-associated arthropathy was 1.4 cases per 100,000 inhabitants. Those data are similar to the results reported in a systematic review that evaluated IBD epidemiology in South America, in which prevalence varied from 15 to 24 cases per 100,000 inhabitants for UC and 2.4 to 14 cases per 100,000 inhabitants for CD.18
IBD-associated arthropathy is a subtype of spondyloarthritis that can cause axial, peripheral, and extra-articular involvement, and is the most common extraintestinal manifestation of IBD. Studies have documented joint involvement in up to 30% of patients with IBD.2,3 In a Brazilian population with IBD, Souza et al.19 found a prevalence of joint involvement of 14.4%, a higher percentage than what we reported. In a descriptive, cross-sectional study conducted in Colombia on the prevalence of IBD and associated joint involvement, according to SISPRO data collected between 2012 and 2016, that type of extraintestinal involvement was present in 3.77% of cases.20 Those low percentages are perhaps associated with an under-registration of the extraintestinal presentation, resulting from its lack of identification, added to a large percentage of asymptomatic patients that could have sacroiliitis, as demonstrated in a 1990 study on destructive lesions in small joints in IBD-associated spondyloarthritis, in which 10 to 50% of those IBD patients had no symptoms.21
With respect to the use of biologic therapy in 2019, we found that 6.3% of the patients diagnosed with IBD and IBD-associated arthropathy were receiving a biologic agent, and 56% of them were women, correlating with the greater prevalence in females reported for the disease. Unlike other countries with a higher prevalence of IBD, the use of biologic therapy in Colombia continues to be low. For example, in a retrospective cohort study from the United States, the number of patients that used biologics increased from 21.8% in 2007 to 43.8% in 2015 for CD and from 5.1% to 16.2% for UC.22 The Costs of Inflammatory Bowel Disease in the Netherlands (COIN) study evaluated the costs of IBD in said country, with a cohort of more than 2,000 patients, and found that 23% of the patients with CD were receiving BT, compared with only 4% of the patients with UC.9 A retrospective analysis conducted in Germany on the cost of BT in IBD, with data collected between 2013 and 2017 and a total of 57,296 patients diagnosed with IBD, reported that 8.22% of all the IBD patients received therapy with a biologic agent,23 a percentage similar to that in our study. The data available in Latin America obtained from a systematic review of the literature on the introduction of BT, based on cohorts in Mexico, Central America, the Caribbean, and South America, showed a clear variation in the use of anti-TNF agents in CD, from 1.51% in Mexico to 46.9% in Colombia, and an overall use of anti-TNF agents of 20 to 40% in patients with CD and a lower use of 0 to 16.2% in patients with UC. Only two studies described the introduction of anti-TNF agents in IBD in general; 13.4% in a Colombian study and 37.9% in a Brazilian study.18
All the biologics approved by the FDA and the European Medicines Agency (EMA) for the treatment of IBD are available in Colombia. In our study, 90% of the biologics prescribed correspond to the contributory healthcare scheme. A study conducted in Colombia on the prescription of medicines in patients seen at secondary and tertiary care hospitals analyzed a total of 38,863 prescriptions. A total of 54.7% were covered by the contributory scheme, in which 90% of medication consumption, according to the number of daily doses defined, was made up of 38 medicines covered by the subsidized scheme and 64 by the contributory scheme. That gap was maintained even when the medicines were organized by their total cost (42 subsidized and 82 contributory), associated with the greater access to medicines by the population with contributory coverage.24 Strikingly, medicine prescription was higher in the contributory scheme, notwithstanding the fact that medicines in Colombia are available to the entire population affiliated with the General Social Security System in Health. That corresponds to 99.6% of the universal insurance coverage, with a distribution of 24,399,839 persons in the contributory scheme and 24,745,934 in the subsidized scheme, according to the latest official figures of the Department of Health from June 2022.25
The most widely prescribed biologic in Colombia was adalimumab, followed by infliximab, vedolizumab, and golimumab. Four patients with CD received golimumab and three patients with UC received certolizumab pegol, despite the fact that those agents are not approved by the FDA or the INVIMA (the Colombian food and drug surveillance regulating entity) for those diagnoses. Our study showed that the number of patients receiving biologic treatment in Colombia was greater in CD (8.3%) than in UC (5.8%). In a descriptive study that included 165 patients diagnosed with IBD seen at the Clínica Universitaria Colombia (Bogotá) between 2013 and 2016, the patients with CD proportionately received more BT than the patients with UC, at 35 and 16%, respectively, the same as in our study. However, unlike our analysis, those authors found that infliximab was the most widely used biologic, followed by adalimumab and vedolizumab, and that in CD, infliximab and adalimumab were used with the same frequency and vedolizumab use was lower.26 In a Brazilian population, Arantes et al.27 reported 33.6% of CD patients received adalimumab and 20.2% received infliximab. In the patients with UC, there was no difference in the use of infliximab and adalimumab (5.4% for each of them). A German study reported that the most widely used biologic in IBD was adalimumab (40%), followed by infliximab (35%), vedolizumab (18%), and golimumab (6%), with no difference according to IBD subtype.23 Very similar data were found in our study, with respect to the percentages of the most widely used biologics in IBD. There are presently no Latin American studies that include the frequency of use of the more recently approved anti-TNF agents (e.g., golimumab and certolizumab pegol) or biologic products with different mechanisms of action (e.g., vedolizumab or ustekinumab).
The departments with the higher number of BT prescriptions were Bogotá, Antioquia, and Valle del Cauca, with 71% of BT prescriptions for IBD and IBD-associated arthropathy, which could be due to the concentration of specialists in the main cities of Colombia. The departments with no biologic prescription were the regions of Amazonia, Orinoquía, and Chocó, areas that are also associated with a lower prevalence rate of the disease, possibly attributable to lower population density and less urban development.
Regarding the cost of biologic medicines for the treatment of IBD and IBD-associated arthropathy, the average cost per patient /year in Colombia was $18,428 USD, which is lower than in other countries. That phenomenon is explained by the centralized price regulation of high-cost medicines carried out by the Colombian government. A retrospective study conducted between 2007 and 2015 in the United States found that BT in IBD generated a cost of $25,275 USD per patient/year in 2007 and $36,051 USD per patient/year in 2015, accounting for an increase in the percentage of the cost associated with BT of 72.9% for 2007 and 85.7% in 2015.22 Similar data were reported in a retrospective study on 928 patients, conducted in Manitoba, Canada, between 2004-2016, about the effect on the direct costs of medical care of anti-TNF therapy in IBD. A 732% increase in the average annual cost associated with biologic treatment was reported, in which the average cost per patient/year was $37,860 CAD ($28,773 USD).28 A 2010 retrospective study from the United Kingdom on the clinical outcomes and healthcare resource utilization with infliximab in CD showed a cost of £7,128 ($13,614 USD) per patient/year,29 similar to results in our study. The COIN study9 evaluated the costs of medical care expressed as average 3-month costs per patient, resulting in 1,625€ ($2,080 USD) (± 150€) for CD and 595€ ($761USD) [± 90€] for UC. Treatment with an anti-TNF agent was the main cost driver, accounting for 64 and 31% of the total cost of CD and UC, respectively.
Even though there is a considerable difference in costs, according to geographic zone, as seen in previous studies, the use of biologic treatment has been increasingly growing, consequently shifting from hospitalization and surgical costs to expenditure associated with medical treatment, in the majority of countries. Said effect is possibly associated with better disease control through biologic treatment, resulting in a decrease in complications, and thus, less need for hospitalizations and surgeries.9 Nevertheless, a recent systematic review on the cost of IBD showed that the cost of medications has increased, while at the same time, hospitalization costs do not appear to be decreasing.8 The discrepancy between the different randomized controlled trials and real-world evidence is concerning. Keeping in mind the market share growth for biologic agents and the rising incidence of IBD, the resulting increase in BT may lead to its becoming the greatest driver of direct IBD costs in Colombia.
Among the limitations of our study is the possible under-registration of patients with IBD and IBD-associated arthropathy, which could impact the prevalence data of the disease. However, with respect to the frequency of use and costs of the medications employed for the treatment of IBD and IBD-associated arthropathy, it is not likely that they are under-registered because they cannot be covered by health insurance, in any way other than by their prescription through MIPRES, and given the high cost of BT, it is highly unlikely they can be acquired out-of-pocket.
Author contributionsAll the authors contributed to the study conception and design. The preparation of the material and data collection and analysis were carried out by Daniel Fernández Ávila and Valentina Dávila. The first draft of the manuscript was written by Valentina Dávila and all the authors commented on previous versions of the manuscript. All the authors read and approved the final manuscript.
Financial disclosureNo financial support was received in relation to this study/article.
Conflict of interestThe authors declare that there is no conflict of interest.