Drug-induced liver injury (DILI) is a diagnosis based on the ruling out of potential liver diseases and consolidated by establishing causality through the temporal relation between a potentially hepatotoxic substance and altered liver biochemistry. Incidence fluctuates greatly worldwide, with very few reports of causal agents of DILI in Colombia.
A retrospective study on patients treated at the Centro de Estudios en Salud (CES), within the time frame of January 2015 and June 2020, was conducted to document the causal substances of DILI in patients with liver biopsy and to correlate the types of histologic patterns with the biochemical pattern of liver injury (R ratio).
ResultsOf the 254 adult patients with liver biopsy and no tumor etiology, 20 patients were identified as cases of DILI (7.87%). The two most frequently found causal substances were efavirenz, in three HIV-positive patients, and Moringa oleifera (moringa), in two patients. There was a statistically significant association between cholestatic patterns (p = 0.037) and mixed patterns (p = 0.031), in the comparison of the histopathologic categories and the R ratio.
ConclusionTo the best of our knowledge, there are no reports on DILI secondary to Moringa oleifera (moringa). The R ratio could be a useful tool, in relation to the histologic pattern of injury, in cases of mixed and cholestatic patterns.
El daño hepático inducido por medicamentos (DILI, por sus siglas en inglés), es un diagnóstico basado en la exclusión de potenciales hepatopatías, y que se consolida, estableciendo la causalidad entre una sustancia potencialmente hepatotóxica y la alteración del perfil bioquímico hepático. La incidencia de este fenómeno fluctúa en un amplio rango a nivel mundial, con muy poca documentación de los agentes causantes de DILI en Colombia.
Con el objetivo de documentar las sustancias causantes de DILI en pacientes con biopsia hepática y de relacionar los tipos de patrones histológicos con el perfil analítico de toxicidad (índice R), se realizó un análisis retrospectivo de los pacientes atendidos en la clínica CES (Centro de Estudios en Salud) entre enero de 2015 y junio de 2020.
ResultadosDe 254 pacientes adultos, con disponibilidad de biopsia hepática sin etiología tumoral, 20 pacientes fueron identificados como DILI (7.87%). Las dos sustancias más frecuentemente encontradas fueron efavirenz en tres pacientes HIV positivos, y en dos pacientes Moringa oleifera (Moringa). Se encontró asociación significativa entre los patrones colestásicos (p = 0.037) y mixtos (p = 0.031) en la comparación de las categorías histopatológicas y el índice R.
ConclusiónEn lo mejor de nuestro conocimiento, no hay reportes de casos de DILI secundarios a Moringa oleifera (Moringa). El índice R podría ser una herramienta útil de relación con el patrón histopatológico, para el caso de los patrones mixto y colestásico.
Drug-induced liver injury (DILI) is a generic term associated with liver biochemistry profile (LBP) alterations in a cause-effect relation to medications or chemical substances. DILI is the primary cause of the removal of a drug from the market1. Combining idiosyncratic and dose-dependent reactions, the estimated annual incidence varies widely, from 2.5 to 23 cases per 100,000 inhabitants2,3. The etiology of approximately 50% of all cases of acute liver failure is drug-induced4 and 40% of those patients end up having liver transplantation5, which is a large burden for any healthcare system.
The diagnosis of DILI is based on the ruling out of other potential causes and on the clinical capacity of establishing causality through the temporal relation of a potentially hepatotoxic substance and LBP alterations, guided by the R ratio, which is mathematically obtained from specific liver biochemical parameters of the patient6. Given that there is no gold standard for diagnosing DILI, the abovementioned requisites for making an accurate diagnosis are often difficult or impossible to meet7. Liver biopsy can be added to the measures for making the diagnosis, histopathologically ruling out other possible causes of LBP alterations and documenting the histopathologic characteristics suggestive of DILI, which are grouped into three patterns: necroinflammatory, cholestatic, and mixed8. At present, a possible statistical relation between the categories of the R ratio and the histopathologic patterns suggestive of DILI has not been studied.
Little is known about the causal agents of DILI in the Colombian environment due to the limited number of studies on the theme. The frequency of DILI and its types of causal agents vary widely due to many factors, including geographic location, type of population, local epidemiology, and type of hospital documenting the cases (primary, secondary, or tertiary care centers). Thus, the findings reported in other studies on the theme cannot be applied to our local population9–11. For the purpose of describing a possible relation between the R ratio and the histopathologic liver biopsy findings, as well as obtaining information on the medications and substances causing DILI in the local environment, the present study is a retrospective analysis of a case series of patients treated at a tertiary care hospital center in the city of Medellín, Colombia.
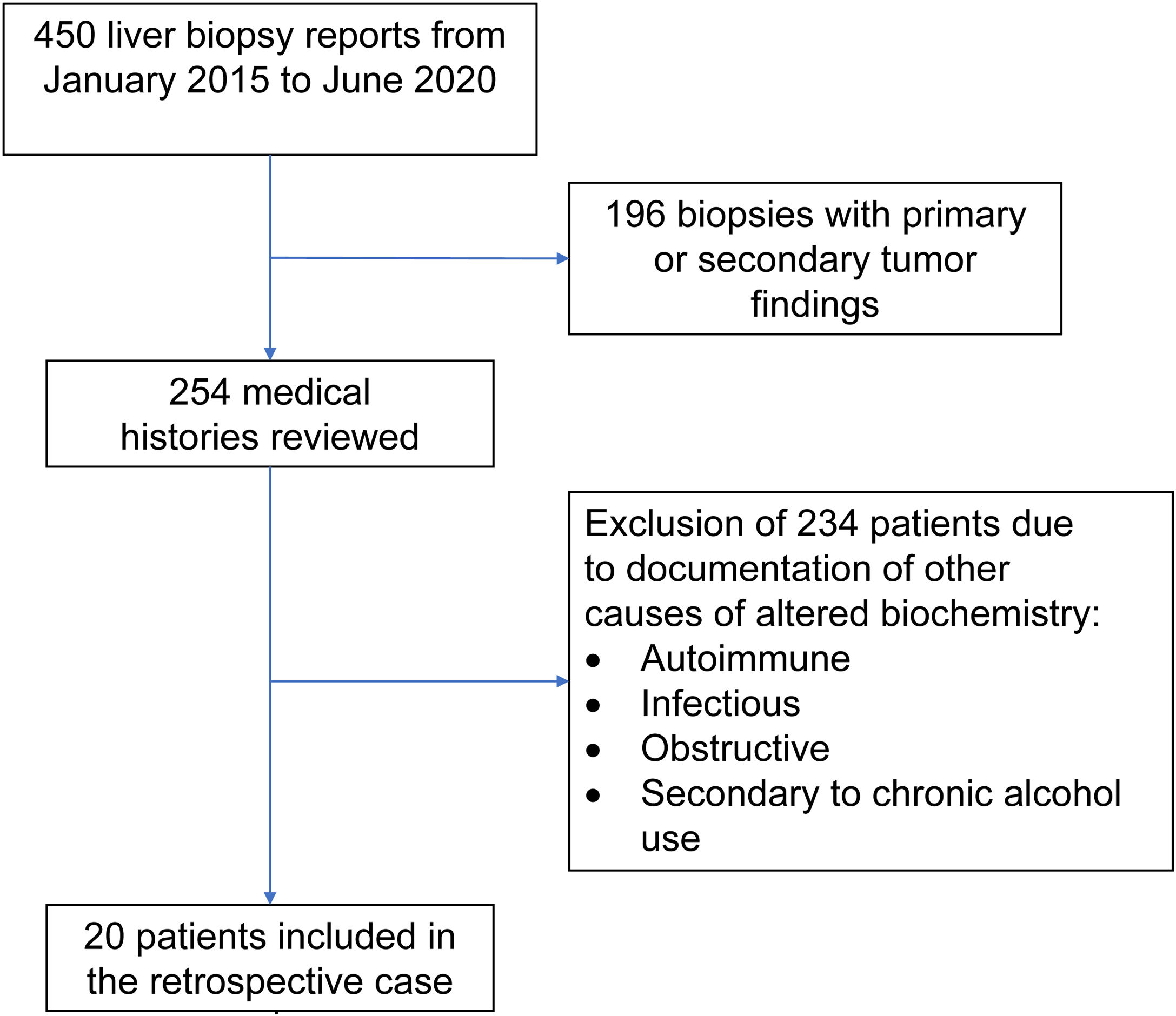
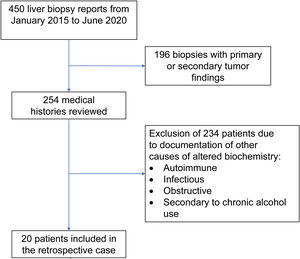
Materials and methodsOnce the study was approved by the ethics committee of the Centro para Estudios en Salud (CES), the list of liver tissue samples from adult patients and non-pregnant women, taken within the time frame of January 2015 and June 2020, was ordered and analyzed. A total of 450 biopsy reports corresponded to the same number of patients (Fig. 1). Patients that underwent procedures due to primary or secondary liver tumors were eliminated, leaving 254 medical histories. A retrospective analysis of those 254 cases was carried out, utilizing, as shown in Table 1, the inclusion and exclusion causality criteria as the evaluation method. The cases that were indicated for liver biopsy due to LBP alterations that were not associated with previous liver disease were selected, along with the cases in which metabolic, autoimmune, infectious, and obstructive liver diseases, and those secondary to chronic alcohol use, were ruled out with acceptable certainty during the hospitalization period. The diagnosis of DILI was considered a rule-out diagnosis, after making the differential diagnosis that included the abovementioned diseases, with documentation of the relevant histologic findings. Once the previously described criteria were applied, 20 patients were selected. A detailed review of their medical histories was carried out, together with laboratory data arrangement and a review of the histologic slides of the liver biopsies. The histopathologic findings were characterized, according to the classification proposed by Kleiner et al.12,13, into necroinflammatory pattern, cholestatic pattern, and mixed pattern. Neither the Roussel-Uclaf14 evaluation method of hepatotoxicity causality, nor other proposed methods15 were applied because of their limitations for use in retrospective studies16.
Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| Adult patients with liver biopsy performed at the Centro de Estudios de Salud | Pregnant women and patients under 18 years of age |
| ALT 2-times higher than the upper limit of normal | Previous diagnosis of liver disease |
| and/or | Previous biliary tree surgery, except for cholecystectomy |
| ALP 2-times higher than the upper limit of normal | Intrahepatic and extrahepatic bile duct imaging studies considered abnormal |
| and/or | Diagnosis of liver disease during hospitalization: |
| TB above the upper limit of normal | • Autoimmune |
| • Infectious | |
| • Obstructive | |
| • Secondary to chronic alcohol use | |
| • Neoplastic |
ALP: alkaline phosphatase; ALT: alanine aminotransferase; TB: total bilirubin.
The statistical analysis, including the tables and graphs, was carried out utilizing R version 4.0.3 (2020-10-10) software. The Shapiro-Wilk test was employed to compare the normal distribution data (ALP: alkaline phosphatase, ALT: alanine aminotransferase, TB: total bilirubin, and DB: direct bilirubin), and depending on those results, variance analyses or the Kruskal-Wallis test were conducted between groups. The evaluation of the relation between the histologic patterns and toxicity defined by specific biochemical parameters (R ratio) was performed using the Fisher’s test. Statistical significance was set at a p < 0.05.
Ethical considerationsThe present study meets the local and international norms for research in humans and was approved by the ethics committee of the Clínica CES. The authors declare that this article contains no personal information that could identify the patients, safeguarding their anonymity. No experiments on humans or animals were conducted in the present study and informed consent was not required for obtaining or publishing the data. No funds from any institution were received.
ResultsOf the 20 cases identified as DILI, 13 were women (65%) and 7 were men (35%), with ages ranging from 17 to 74 years (mean 37.5 years). A differential diagnosis of the possible causes of LBP alterations was not carried out in 50% (10 patients) of the patients with a final diagnosis of DILI. The most common approach for taking a liver biopsy was the ultrasound or tomography-guided percutaneous method in 14 cases (70%) and the surgical method in 6 cases (30%). Table 2 shows the general and specific histologic patterns of each case, together with the R ratio of the case at hospital admission, the substance identified as the cause, and other clinical variables. A single cause of DILI was not clearly registered in the medical histories of 7 of the 20 patients (35%).
Clinical and histopathologic characteristics of the patients that were identified as presenting with DILI
| Age | Sex | General pattern | Specific pattern | Substance | ALT | ALP | R | Type of toxicity defined by liver biochemistry | TB | DB |
|---|---|---|---|---|---|---|---|---|---|---|
| 74 | F | Necroinflammatory | Acute hepatitis | NC | 1025 | 446 | 7.24 | Hepatocellular | 13.64 | 11.38 |
| 50 | M | Necroinflammatory | Coagulative necrosis | NC | 582 | 157 | 11.68 | Hepatocellular | 1.83 | 1.4 |
| 34 | F | Necroinflammatory | Chronic hepatitis | NC | 90 | 240 | 1.18 | Cholestatic | 3.49 | 3.27 |
| 23 | F | Necroinflammatory | Chronic hepatitis | Efavirenz | 497 | 144 | 10.88 | Hepatocellular | 9.66 | 8.57 |
| 56 | F | Necroinflammatory | Chronic hepatitis | NC | 1830 | 184 | 31.34 | Hepatocellular | 0.97 | 0.65 |
| 30 | F | Necroinflammatory | Chronic hepatitis | Fluconazole | 216 | 2153 | 0.32 | Cholestatic | 0.28 | 0.21 |
| 30 | F | Necroinflammatory | Chronic hepatitis | Efavirenz | 586 | 91 | 20.29 | Hepatocellular | 2.16 | 1.51 |
| 65 | F | Necroinflammatory | Chronic hepatitis | NC | 1304 | 393 | 10.46 | Hepatocellular | 16.36 | 14.73 |
| 37 | F | Necroinflammatory | Chronic hepatitis | Ganoderma | 1015 | 142 | 22.53 | Hepatocellular | 7.01 | 6.43 |
| 52 | F | Necroinflammatory | Steatohepatitis | Methylprednisolone | 242 | 129 | 5.91 | Hepatocellular | 1.68 | 1.01 |
| 17 | F | Mixed | Mixed | NC | 303 | 153 | 6.24 | Hepatocellular | 2.37 | 2.27 |
| 51 | F | Mixed | Mixed | Amoxicillin/clavulanate | 496 | 348 | 4.49 | Mixed | 3.83 | 3.62 |
| 37 | M | Mixed | Mixed | Moringa | 710 | 152 | 14.72 | Hepatocellular | 1.39 | 1.17 |
| 32 | M | Mixed | Mixed | Efavirenz | 847 | 887 | 3.01 | Mixed | 5.89 | 5.37 |
| 35 | M | Cholestatic | Bland cholestasis | Anabolic steroids | 71.8 | 238 | 0.95 | Cholestatic | 24.14 | 20.9 |
| 38 | M | Cholestatic | Bland cholestasis | LIV 52 + protein | 43 | 190 | 0.71 | Cholestatic | 5.48 | 4.52 |
| 52 | M | Cholestatic | Bland cholestasis | Moringa | 438 | 1034 | 1.33 | Cholestatic | 0.31 | 0.2 |
| 38 | F | Cholestatic | Bland cholestasis | Azathioprine | 15.7 | 51 | 0.97 | Cholestatic | 8.3 | 7.73 |
| 35 | M | Cholestatic | Bland cholestasis | NC | 538 | 165 | 10.28 | Hepatocellular | 6.7 | 6 |
| 49 | F | Cholestatic | Bland cholestasis | Multivitamin | 574 | 214 | 8.45 | Hepatocellular | 0.57 | 0.27 |
ALP: alkaline phosphatase; ALT: alanine aminotransferase; DB: direct bilirubin; NC: not clarified; R: R ratio; TB: total bilirubin.
The duration of exposure to the substances identified as potentially hepatotoxic was not clear in the majority of the cases (65%). The mean of that datum in the medical histories, in which it was specified, was 3 months. Five of those patients (25%) had a previous diagnosis of HIV at hospital admission, ruling out opportunistic hepatic infections. Mean hospital stay of the cases was 12.5 days, ranging from 1 to 96. The clinical presentation in 2 patients was acute liver failure (10%). One of those patients was referred to another hospital, with the indication for liver transplantation due to the progressive increase of the international normalized ratio (INR), and the other patient died due to complications of liver failure. The clinical progression of the remaining patients (18 patients, 90%) had a trend towards improvement and they were released from the hospital and scheduled for outpatient follow-up.
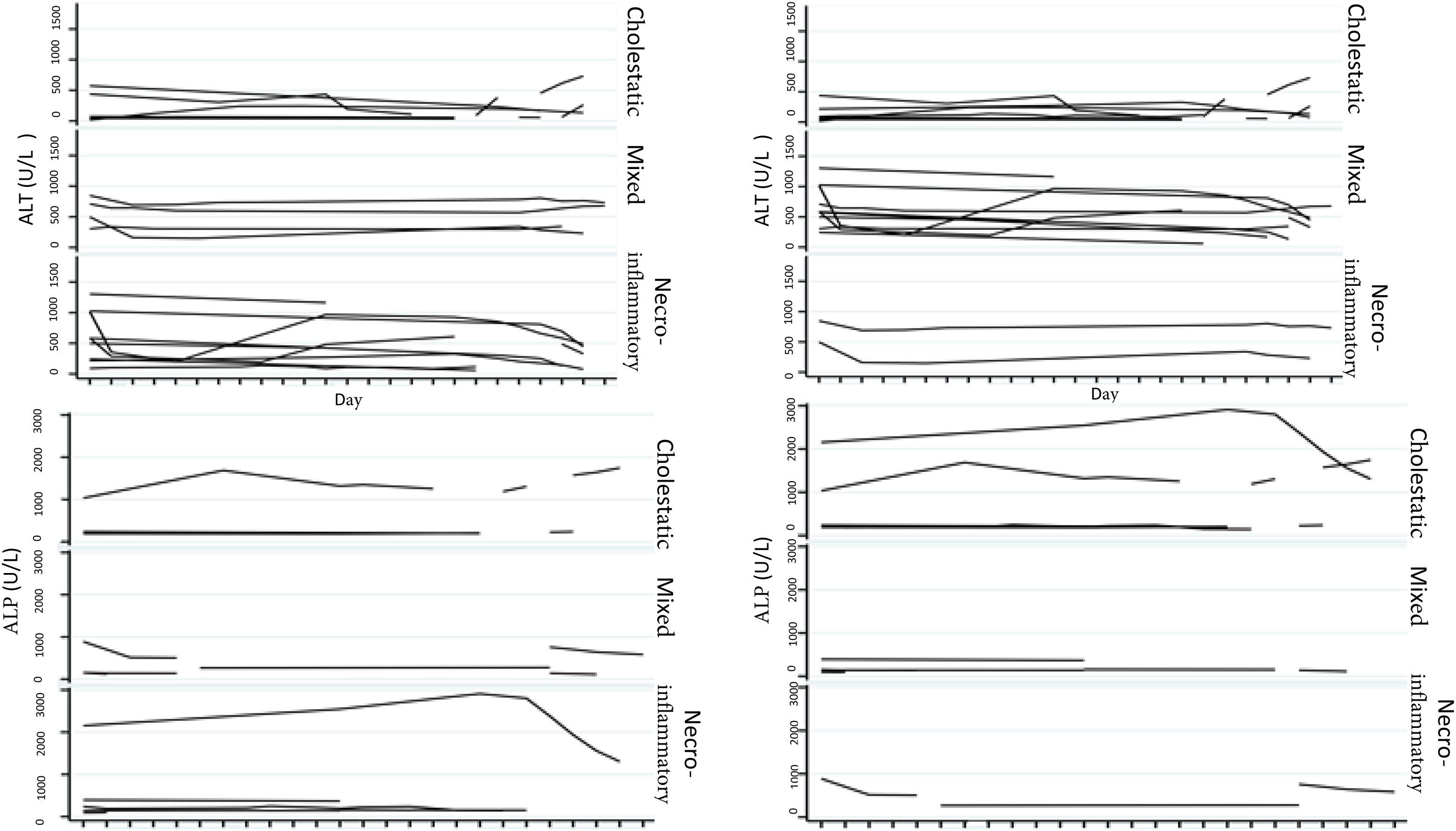
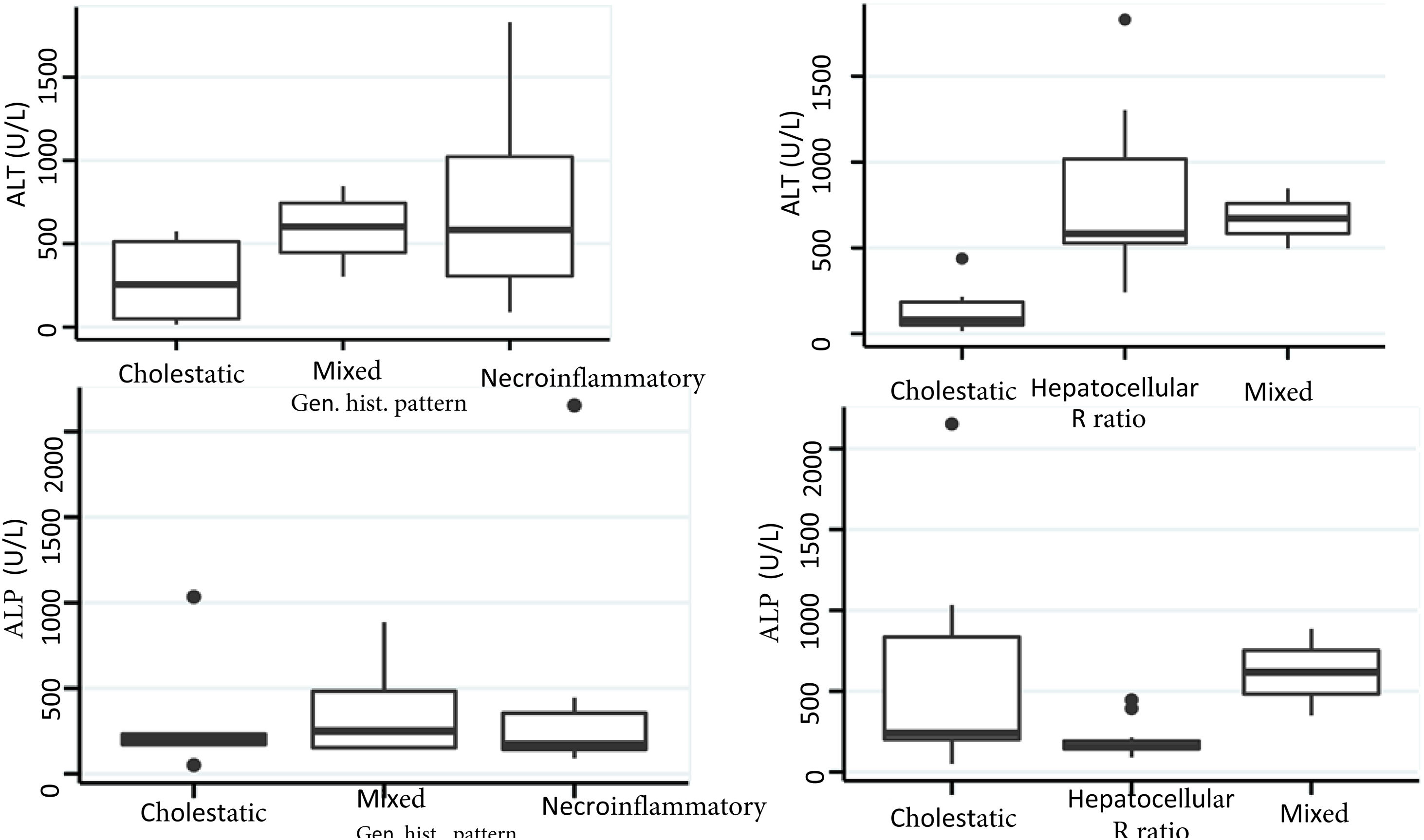
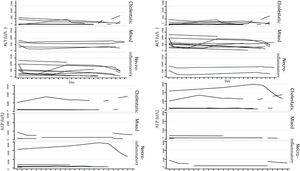
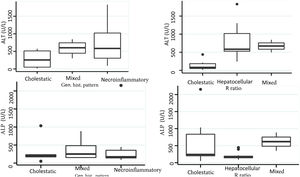
Biochemical liver profileThe periodicity of control LBP was not constant during patient hospital stay. The mean initial aspartate aminotransferase (AST) and ALT values were 538 IU/l and 571 IU/l, respectively, ranging from 2 to 40-times higher than the upper limit of normal of the laboratory. Regarding TB, it was above the laboratory range in 13 patients (76%), whereas that value was never altered in the remaining patients. The INR was higher than the range defining acute liver failure (>1.5) in only 4 patients (25%). The highest INR value in the entire case series was 2.8 and corresponded to the patient that died. The results of the variance analyses and Kruskal-Wallis tests utilized to compare ALT, ALP, TB, and DB with the histologic patterns were not statistically significant. The R ratio was calculated for each case17, as the type of toxicity defined by specific liver biochemical parameters, with the upper limit of normal for ALT of 33 IU/L and for ALP of 104 IU/L, using the first laboratory results from each patient. According to the R ratio, the biochemical alteration in 12 of the patients was hepatocellular (R > 5), 2 patients had a mixed pattern (R 2-5), and 6 patients had a cholestatic pattern (R < 2). The R ratio of the patient that died was 7.42. The altered LBP parameter margin was sufficiently broad and not very predictive of the clinical course of the patient, the same as their normalization trend (Fig. 2). There was a relation between the laboratory tests and outcome in only one case, specifically the progressive increase in the INR of the patient that died. The variance analyses and Kruskal-Wallis tests comparing ALT, ALP, TB, and DB with the type of toxicity defined by the biochemical parameters, or R ratio, were not statistically significant (Fig. 3).
The histopathologic findings were categorized as general and specific patterns, according to the classification proposed by Kleiner et al.12,13. The general necroinflammatory pattern was classified in 10 of the patients (50%), the cholestatic pattern in 6 of the patients (30%), and the mixed pattern in 4 (20%). Of the patients with the general necroinflammatory pattern, chronic hepatitis was the most frequent (70%) secondary pattern, and of the patients with the general cholestatic pattern, bland cholestasis was the only pattern identified (100%). In addition, 80% of the patients with the general necroinflammatory pattern were women and 66% of the patients with the cholestatic pattern were men. No pathologically important connective tissue deposits were observed in the trichrome staining. Regarding the concordance between the histopathologic classification and the toxicity defined by the biochemical parameters, based on the R ratio, it was 80% in the patients with the general necroinflammatory pattern, 50% in the patients with the mixed pattern, and 66% in the patients with the cholestatic pattern. In the comparison of the histologic pattern vs the toxicity defined by the biochemical parameters (Table 3), the Fisher test produced a statistically nonsignificant p value, when comparing the necroinflammatory pattern with the hepatocellular pattern (p = 0.17). In contrast, when the two types of cholestatic patterns (p = 0.037) and mixed patterns (p = 0.031) were compared, the results were statistically significant. The results of the variance analyses and Kruskal-Wallis tests performed to compare ALT, ALP, TB, and DB were nonsignificant (Fig. 2).
Comparison of the histopathologic pattern and the type of toxicity defined by liver biochemical parameters
| Type of toxicity defined by liver biochemistry | |||
|---|---|---|---|
| Histopathologic pattern | Hepatocellular | Non-hepatocellular | |
| R > 5 | R < 5 | p | |
| Necroinflammatory Yes | 8 | 2 | 0.16 |
| Necroinflammatory No | 4 | 6 | |
| Mixed | Non-mixed | ||
|---|---|---|---|
| 2 < R < 5 | R ≠ | p* | |
| Mixed Yes | 2 | 2 | 0.031 |
| Mixed No | 0 | 16 |
| Cholestatic | Non-cholestatic | ||
|---|---|---|---|
| R < 2 | R > 2 | p* | |
| Cholestatic Yes | 4 | 2 | 0.037 |
| Cholestatic No | 2 | 12 |
R: R ratio.
Fisher test.
DILI is a clinical condition of unknown incidence in the Colombian environment, whose profile of inducing substances should be characterized for each population. In our study, in which 254 clinical histories with no tumor etiology were reviewed, 20 patients presented with the clinical and histopathologic findings of DILI, accounting for 7.87% of the patients that underwent liver biopsy due to lesions not caused by a tumor. Given that only approximately 50% of the cases of DILI are biopsied18, the disorder is an under-evaluated problem that requires a national registry, similar to other initiatives for Latin America19, that would enable medications that have a higher risk for DILI in the Colombian population to be identified, along with supposedly innocuous substances sold as natural products, not subject to any regulation, that are potentially hepatotoxic. In 7 of the patients with histopathologic findings within the broad spectrum of DILI and its clinical diagnosis, as well as no other explanation for the altered LBP, a specific causal agent was not found. Whether that was due to the fact that it was not searched for, the patient did not provide the information, or because of the use of numerous medications, could not be determined.
Efavirenz and moringa were the two substances that were most frequently identified as causing DILI (Table 2). Efavirenz was used by HIV-positive patients, with necroinflammatory and mixed patterns, and moringa was used by male patients, with cholestatic and mixed histopathologic patterns. The clinical presentation of hepatotoxicity due to efavirenz in the three patients (2 women and one man) in our analysis was mild, unlike the greater severity reported in the few existing studies20,21. We have no explanation for the mild presentation described in our patients. Moringa oleifera (moringa) is a plant that belongs to the family Moringaceae. It is endemic to tropical and subtropical climates and is recognized for its use in traditional medicine, as well as for its nutritional value22. Its seeds, leaves, flowers, and roots are sold in the form of oil, powder, and preparations for infusions, with no restrictions in the majority of countries where it is produced and marketed23,24. Not only is it known for its supposed positive impact on the health of animals and humans, but also for its antioxidant effects and its protective effects against liver fibrosis in animal models25,26. To the best of our knowledge, there are no published cases of DILI secondary to moringa in humans, and we found no registers on the LiverTox® website, updated October 1, 202027, or local reports. The association between moringa and the LBP alterations in the 2 patients in our study was clear. Those patients did not regularly use any other medication, other causal pathologies were ruled out, and their biochemical parameters improved once the derivatives of the plant were suspended.
The substances most frequently found in our analysis as causes of DILI differed from those described in other international studies28,29. That could be due to the fact that only patients with liver biopsy were included in the present study, indicating the diagnostic difficulty of DILI in our case series and the specific epidemiologic profile in our country. In addition, many of the patients with DILI due to more common medications, such as antibiotics, are routinely identified and managed by the treating physician, with no formal register of the event or indication for liver biopsy. Therefore, the subgroup of patients with DILI and the indication for liver biopsy could be made up of a special population that is difficult to diagnose, within the universe of patients with DILI, as well as with a distinctive epidemiologic profile.
Liver biopsy is an extremely important diagnostic tool, in the context of patients with liver disease, that can provide useful information regarding the differential diagnosis and the severity of liver injury. Even so, it is only indicated in DILI, if other diagnostic possibilities have not been ruled out by other methods30. The histologic patterns found in DILI practically recreate all the non-neoplastic histologic patterns of liver disease, often making the analysis of the liver biopsy a source of frustration for the clinician that is expecting a concrete diagnosis of DILI by the pathologist. The classification into general and specific patterns is customary from both the histopathologic and diagnostic perspectives and is made by the semiquantitative or descriptive grading of the severity of the injury, especially with respect to the necroinflammatory pattern, commenting on the possibility of DILI when there are contextual clinical data. In our study, half of the patterns observed were the general necroinflammatory pattern, followed by the cholestatic pattern, and then the mixed pattern. The results of a prospective study conducted in the United States on DILI that included 249 patients, in which the mixed pattern was the most frequent, contrasted to ours, in which the necroinflammatory pattern was the most frequent13. The results of that study, in which the most frequent biochemical profile (R ratio) was hepatocellular, coincided with ours, but the frequency of the histologic pattern was different from our analysis. Costa-Moreira et al. conducted a retrospective study, spanning a 10-year period, on Portuguese patients with DILI that had liver biopsy. They identified 53 patients, with findings similar to ours, with respect to the preponderance of the necroinflammatory pattern, but with no statistically significant association regarding the histologic pattern and the R ratio31. In our analysis, the biochemical profile of toxicity recorded as the R ratio was associated with the cholestatic pattern and the mixed pattern, with statistical significance, indicating an interdependence in the presentation of those histologic patterns. Said interdependence means that the cholestatic and mixed histologic patterns have a strong and significant association with similar patterns, according to the R ratio. That must be validated in other studies, but could serve as a tool for pathologists, when DILI is suspected, by relating the expected histologic characteristics to the calculated R ratio, as a diagnostic support mechanism.
We are aware of the fact that our study is small and realize that more studies on DILI in the Colombian environment are needed to better comprehend the problem. Further studies should enable the identification of the substances in our geographic regions that are most commonly involved as causal agents, thus improving our understanding of DILI in our country. Ideally, those studies should include liver biopsies, but we recognize that said intervention is not a routine part of the diagnostic approach. In addition, substances that are marketed together with “natural” products and vitamin supplements have begun to be described as causal agents of DILI in other geographic locations32, and perceived as such, in many clinics. Nevertheless, their true burden, with respect to DILI, is unknown, given the lack of formal registers.
Contribution and authorshipArdila OM, Ortiz-Benjumea L, Guevara LG, and Arteta AA participated in the study design, patient selection, clinical history review, and histopathologic finding evaluation. All authors contributed to the data analyses and to the drafting and critical review of the article.
Financial disclosureNo specific grants were received from public sector agencies, the business sector, or non-profit organizations in relation to this study.
Conflict of interestThe authors declare that there is no conflict of interest.
Author contributionsArdila OM, Ortiz-Benjumea L, Guevara LG, and Arteta AA participated in the study design, patient selection, clinical history review, and histopathologic findings. All the authors contributed to the data analysis, drafting, and critical review of the article.
Please cite this article as: Ardila-Suárez OM, Oriz-Benjumea L, Arteta AA, Guevara-Casallas LG. Daño hepático inducido por medicamentos: relación entre el índice R y la histopatología. Rev Gastroenterol Méx. 2023;88:19–27.