Dietary fiber intake is one of the most influential and efficacious strategies for modulating the gut microbiota. Said fiber can be digested by the microbiota itself, producing numerous metabolites, which include the short-chain fatty acids (SCFAs). SCFAs have local and systemic functions that impact the composition and function of the gut microbiota, and consequently, human health. The aim of the present narrative review was to provide a document that serves as a frame of reference for a clear understanding of dietary fiber and its direct and indirect effects on health.
The direct benefits of dietary fiber intake can be dependent on or independent of the gut microbiota. The use of dietary fiber by the gut microbiota involves several factors, including the fiber’s physiochemical characteristics. Dietary fiber type influences the gut microbiota because not all bacterial species have the same capacity to produce the enzymes needed for its degradation. A low-fiber diet can affect the balance of the SCFAs produced. Dietary fiber indirectly benefits cardiometabolic health, digestive health, certain functional gastrointestinal disorders, and different diseases.
Una de las estrategias que más impacto y mayor eficacia tiene para la modulación de la microbiota intestinal es el consumo de fibra dietaria, que puede ser digerida por la propia microbiota generando numerosos metabolitos. Entre éstos, se encuentran los ácidos grasos de cadena corta (AGCC) con funciones tanto locales como sistémicas, que impactan en la composición y función de la microbiota intestinal y por lo tanto en la salud humana. El objetivo de esta revisión narrativa fue generar un documento que sirva como marco de referencia para conocer acerca de la fibra dietaria y sus efectos directos e indirectos.
Los beneficios directos de la ingestión de fibra dietaria, pueden ser dependientes o independientes de la microbiota intestinal. La utilización de la fibra dietaria por esta última, depende de varios factores y de sus características fisicoquímicas. La clase de fibra dietaria influye sobre la composición de la microbiota intestinal debido a que no todas las especies tienen la misma capacidad de producir enzimas necesarias para su degradación. El consumo de dietas con bajo contenido de fibra dietaria puede afectar el balance de los AGCC producidos. Los beneficios indirectos de la fibra dietaria impactan sobre la salud cardiometabólica, la salud digestiva, ciertos trastornos funcionales gastrointestinales y enfermedades diversas.
The gut microbiota is currently recognized as playing a relevant role in human health. Advances in its study have been made on how its composition can be modulated, as well as on the metabolic function of the different microbial species that colonize the gastrointestinal tract, to improve human health and potentially prevent or treat diseases in general.1 Some strategies can modulate the microbiota, such as the use of probiotics, prebiotics, and even fecal transplantation. One of the simplest and most efficacious is the intake of dietary fiber that is metabolizable by the gut microbiota itself, producing metabolites that include short-chain fatty acids (SCFAs), such as acetate, propionate, and butyrate, with both local and systemic functions. Through its impact on the composition and function of the gut microbiota, dietary fiber influences human health in general.
In February 2020, the Asociación Mexicana de Gastroenterología convened a multidisciplinary group of 17 specialists (clinical nutritionists, chemists with master’s degrees in nutrition, gastroenterologists, and pediatric gastroenterologists and nutritionists) to previously review, and then have an in-person discussion about the scientific evidence on the role of dietary fiber in the digestive physiology and health, in general, of children and adults. The aim of the present narrative review was to produce a document that serves as a frame of reference for a clear understanding of dietary fiber and its direct and indirect effects on health.
Dietary fiberOver time, there have been different definitions of dietary fiber, based on physiologic aspects or on methods for its analysis by the Association of Official Agricultural Chemists (AOAC). The Institute of Medicine, now known as the National Academy of Medicine and is part of the National Academies of the United States, proposed a definition of dietary fiber to distinguish endogenous fiber in foods, or “dietary fiber”, from the fiber that is extracted or synthesized, called “functional fiber”, the sum of which is total fiber.2 In 2009, the Codex Alimentarius Commission defined dietary fiber as “carbohydrate polymers with 10 or more monomeric units, which are not hydrolyzed by the endogenous enzymes in the small intestine of humans”,3 and is the definition that we use in the present technical position paper. Depending on the regulations of each country, including oligomers with 3 to 9 monomeric units in the definition is suggested.4
Dietary fiber can be classified according to its nature or origin, colligative properties, and fermentability.5 With respect to its nature or origin, it is classified as dietary (intrinsic or intact, that is found in foods) or functional (extracted or synthetic). Regarding its colligative properties, the fiber’s chemical structure defines two characteristics related to its mechanisms of action. The first is solubility: fiber can be soluble (with different degrees of solubility) or insoluble in water. The second is its gel-forming capacity: it can form a viscous or nonviscous solution, with the viscous solutions classified as having low, medium, or high viscosity. Regarding fermentability, fiber can be nonfermentable, partially fermentable (semi-fermentable), or completely fermentable. The physicochemical properties of the different types of fiber are not exclusive. The main properties are the capacity to react with water, to have a colligative property (solubility and gel formation or increased consistency or viscosity), to ferment, and to chelate. All those physicochemical properties sustain the functions of fiber in the organism. Among the characteristics of soluble fiber is the fact that it is more viscous and fermentable than insoluble fiber, it undergoes few changes, and it has a mechanical effect. However, different types of fiber have different combinations of those properties, and consequently, their effects on humans are different. Likewise, the same food can contain varying quantities of different types of fiber.
Tables 1 and 2 provide examples of fibers that can be grouped according to their degree of solubility and fermentability into:
- a)
Short-chain, highly fermentable, soluble fiber: it is made up of oligosaccharides, such as fructooligosaccharides (FOSs) and galactooligosaccharides (GOSs) that stimulate bifidobacteria production. It has a weak laxative effect and does not affect bowel transit time, although it produces much gas.
- b)
Long-chain, highly fermentable, soluble fiber: it stimulates bacterial growth in general, has a weak laxative effect, does not affect bowel transit time, and produces a moderate quantity of gases.
- c)
Mediumly fermentable, partially soluble fiber: it has a good laxative effect, accelerates bowel transit, stimulates bacterial growth in general, and produces a moderate quantity of gases.
- d)
Slowly fermentable insoluble fiber: it has a good laxative effect, stimulates bacterial growth, and produces a moderate quantity of gases.
- e)
Nonfermentable insoluble fiber: it has a good laxative effect, accelerates bowel transit, and only stimulates the growth of specific bacteria that degrade it, such as Xylanibacter and Prevotella.
Types of fiber, according to their functionality, and examples.
| Type of fiber | Examples |
|---|---|
| Dietary fiber | Lignin, cellulose, pectin, gums, β-glucans, and modified starch |
| Soluble fiber | Pectin, gums, β-glucans, wheat dextrin, Psyllium, and inulin |
| Fermentable fiber | Pectin, guar gum, β-glucans, wheat dextrin, inulin |
| Viscous fiber | Pectin, β-glucans, some gums (e.g., guar), Psyllium |
| Functional fiber | Resistant dextrin, Psyllium, fructooligosaccharides, polydextrose, isolated gums, isolated resistant starch |
| Insoluble fiber | Cellulose, lignin, some pectin, and some hemicelluloses |
| Nonfermentable fiber | Cellulose, lignin |
| Nonviscous fiber | Polydextrose, inulin |
Source: Slavin, 2013.7
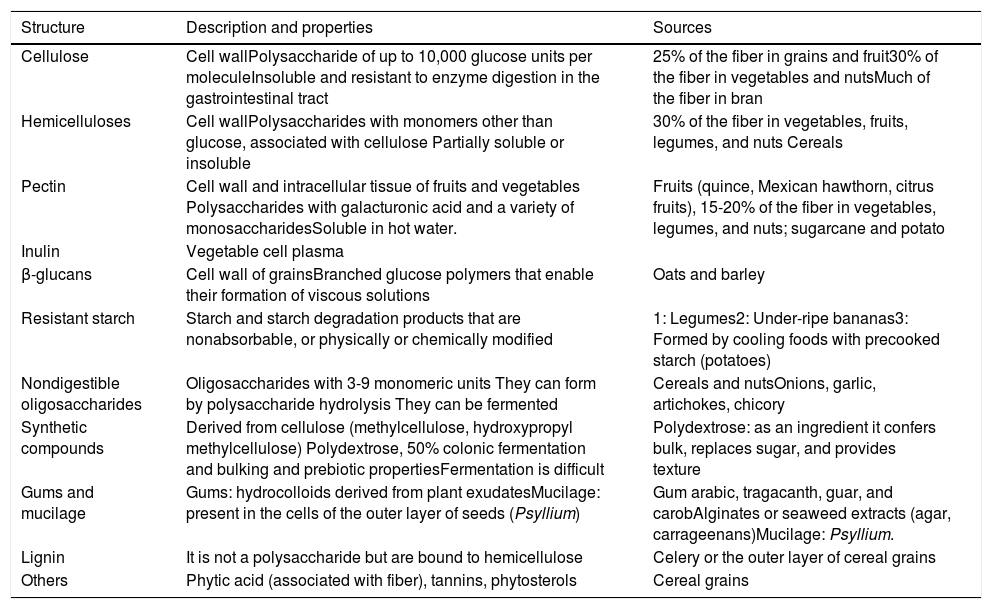
Types of fiber, properties, and sources.
| Structure | Description and properties | Sources |
|---|---|---|
| Cellulose | Cell wallPolysaccharide of up to 10,000 glucose units per moleculeInsoluble and resistant to enzyme digestion in the gastrointestinal tract | 25% of the fiber in grains and fruit30% of the fiber in vegetables and nutsMuch of the fiber in bran |
| Hemicelluloses | Cell wallPolysaccharides with monomers other than glucose, associated with cellulose Partially soluble or insoluble | 30% of the fiber in vegetables, fruits, legumes, and nuts Cereals |
| Pectin | Cell wall and intracellular tissue of fruits and vegetables Polysaccharides with galacturonic acid and a variety of monosaccharidesSoluble in hot water. | Fruits (quince, Mexican hawthorn, citrus fruits), 15-20% of the fiber in vegetables, legumes, and nuts; sugarcane and potato |
| Inulin | Vegetable cell plasma | |
| β-glucans | Cell wall of grainsBranched glucose polymers that enable their formation of viscous solutions | Oats and barley |
| Resistant starch | Starch and starch degradation products that are nonabsorbable, or physically or chemically modified | 1: Legumes2: Under-ripe bananas3: Formed by cooling foods with precooked starch (potatoes) |
| Nondigestible oligosaccharides | Oligosaccharides with 3-9 monomeric units They can form by polysaccharide hydrolysis They can be fermented | Cereals and nutsOnions, garlic, artichokes, chicory |
| Synthetic compounds | Derived from cellulose (methylcellulose, hydroxypropyl methylcellulose) Polydextrose, 50% colonic fermentation and bulking and prebiotic propertiesFermentation is difficult | Polydextrose: as an ingredient it confers bulk, replaces sugar, and provides texture |
| Gums and mucilage | Gums: hydrocolloids derived from plant exudatesMucilage: present in the cells of the outer layer of seeds (Psyllium) | Gum arabic, tragacanth, guar, and carobAlginates or seaweed extracts (agar, carrageenans)Mucilage: Psyllium. |
| Lignin | It is not a polysaccharide but are bound to hemicellulose | Celery or the outer layer of cereal grains |
| Others | Phytic acid (associated with fiber), tannins, phytosterols | Cereal grains |
Adapted from Gray.8
Importantly, the fermentation of dietary fiber gradually supplies energy, but the magnitude of the supply depends on the kind of fiber and the type of microbiota of the individual.6–8
In addition to the types of fiber described above, other substances function like dietary fiber. Those substances are synthetic carbohydrates, such as polydextrose, methylcellulose, carboxymethylcellulose, hydroxypropyl methylcellulose, curdlan, scleroglucan, and analogues. Some synthetic oligosaccharides, nonabsorbable polyols (sorbitol and mannitol), saponins, tannins, phytates, and substances of animal origin, such as chondroitin and chitosan, also act like fiber.9,10 Chitosan is a polysaccharide of natural origin composed of α-1,4-linked glucosamine residues and is a component of the exoskeleton of crustaceans and the cell walls of fungi.
Some types of fiber can have a prebiotic effect because, upon being fermented in the colon, they selectively promote the growth or activity of the microbiota, which has functional and beneficial effects for the host. However, not all dietary fiber is prebiotic.11 The primary prebiotics utilized in clinical studies are FOSs, GOSs, transgalactooligosaccharides (TOSs), xylooligosaccharides (XOSs), isomaltooligosaccharides (IMOSs), lactulose, hemicellulose (from sprouted barley), and inulin. Inulin is a fructan, or fructosan, a polysaccharide mainly composed of fructose units.12
Dietary fiber intakeDietary fiber intake recommendations are age-dependent. The daily requirement for children over one year of age is calculated as age plus 5 or 10 g, or as 0.5 g/kg/day, for children over 2 years of age.13 In adolescents and adults, the calculation is 14 g for every 1000 kcal.14 That quantity of dietary fiber is not scientifically sustained, and therefore is referred to as the “recommended daily intake”. There is great variability among the different institutions that make the recommendation. In Mexico, there are no systematically obtained complete tables of the fiber content in foods. The present nutritional value tables that refer to fiber content take data from other tables (generally not Mexican ones), in which the type of chemical analysis for measuring fiber is not registered. It is presumed that dietary fiber intake is greater in rural diets than urban diets because of the proportion of cereals, legumes, fruit, and vegetables they contain. The 2012 National Survey on Health and Nutrition (ENSANUT, the Spanish acronym) reflected deficient fiber consumption beginning at early ages.15 The 2016 midway ENSANUT showed that children 24 to 59 months of age with food insecurity ingested even less fiber that their counterparts that did not suffer from food insecurity. The daily fiber intake in adolescents was 23.7 g in males and 21.2 g in females, and in older adults was even less: 20.2 g in men and 17.9 g in women.16
The World Health Organization recommends several strategies for increasing dietary fiber intake. Standing out among them is the dietary orientation aimed at changing the behavior of persons with scant fiber intake or that have a health alteration that requires greater intake. Greater consumption of natural sources of fiber (fruits, vegetables, legumes, whole grains) is also suggested, as well as the use of fiber supplements, when the recommended quantity is not achieved through diet or if it could be beneficial for a specific health problem.17
The direct impact of fiberProperties of dietary fiber that influence the gut microbiotaThe use of dietary fiber by the gut microbiota depends on its source, type of molecules, bonds, chain length, particle size, and association with other compounds.18,19 It also depends on the abovementioned physicochemical properties of solubility, viscosity, and fermentability.1 The size of the particle determines its susceptibility to digestion, binding, water retention, and bowel transit time.20 Water retention can influence the capacity of bacteria to infiltrate and digest fiber, as well as the speed of transport through the intestine.21 Viscosity depends on the degree of hydration, particle size, and the pH.22 Highly fermentable fiber, of which the ß-glucans and pectins stand out, can also have high solubility and viscosity. The majority of soluble fibers are very viscous in the intestine. FOSs and pectins can be metabolized by bacteria in the ileum and ascending colon, unlike insoluble fiber, such as cellulose and hemicellulose, that is exclusively metabolized in the distal colon.1
Experimentally, soluble fiber, compared with insoluble fiber, has been shown to modify the colonization of intestinal bacteria, impacting the richness of the gut microbiota.23 Insoluble fiber intake produces a greater relative abundance of Bacteroidetes, Euryarchaeota and Ruminococcaceae, and at the genus level, of Prevotella, Phascolarctobacterium, Coprococcus, and Leeia. In contrast, soluble fiber intake produces a greater relative abundance of the phylum Proteobacteria and less abundance of Prevotellaceae, and with respect to genera, a greater abundance of Blautia, Solobacterium, Syntrophococcus, Weissella, Olsenella, Atopobium, and Succinivibrio.24 Thus, in a study with 7% pectin, a soluble fiber, there was an increase in Anaeroplasma, Anaerostipes, and Roseburia, whereas there was a decrease in Alistipes and Bacteroides spp.17
Some of the components of fiber, such as the arabinoxylan oligosaccharides, can increase the abundance of bifidobacteria in the ascending colon, lactobacilli in the ascending colon and transverse colon, and Clostridium coccoides and Eubacterium rectale in the descending colon.17 The viscosity of fiber increases the number of anaerobic bacteria and Clostridium spp., whereas the number of aerobic bacteria and the genus Enterococcus negatively correlate with viscosity.22 Inulin fermentation results in a greater proportion of lactobacilli and bifidobacteria, a lower proportion of Enterobacteriaceae, and greater butyrate production.24
The consumption of 12 g of inulin for 4 weeks in healthy adults with mild constipation induced an increase in the abundance of Bifidobacteriumand Anaerostipes spp. and a decrease in the population of Bilophila.25 Said effect could be attributed to the capacity of the genus Bifidobacterium to efficiently degrade FOSs and to the fact that Anaerostipes hadrusproduces butyrate.26 A similar effect occurred in patients with active ulcerative colitis that received 7.5 or 15 g of inulin enriched with oligofructose for 9 weeks, with an increase in the abundance of Bifidobacteriaceaeand Lachnospiraceae, and an increase in butyrate production.27
Resistant starch, which is a nondigestible fraction of cornstarch, raw potatoes, or unripe bananas, is also considered a dietary fiber. Its consumption is associated with an increase in the butyrate producers, Ruminococcus bromii, Faecalibacterium prausnitzii, and E. rectale. R. bromii is a key species for the fermentation of starches in the colon, as has been demonstrated in other studies.28,29
Fiber and short-chain fatty acid (SCFA) productionAnaerobic bacteria in the large intestine produce SCFAs, through dietary fiber fermentation.30 The principal SCFAs are acetate, propionate, and butyrate, at a ratio of 60:20:20. Lactate is the salt of a very common organic acid in the intestinal lumen that is also produced by bacteria, whereas other types of bacteria metabolize acetate, propionate, and butyrate. Thus, there are species that can be primary degraders of dietary fiber, whose products are degraded by other fermenting microorganisms that finally produce acetate, propionate, and butyrate.31,32
Acetate is mainly produced via acetyl-CoA. Propionate is synthetized via two pathways: the succinate pathway and the acrylate pathway, from hexose and pentose or lactate substrates and via the propanediol pathway that utilizes deoxyhexoses, such as fucose and rhamnose, as substrates.31 The Bacteroidetes phylum produces propionate, mainly through the succinate pathway. Butyrate can also be produced from peptides or amino acids, and not only derived from dietary fiber sources. Some butyrate-producing species correspond to the families of Ruminococcaceae and Lachnospiraceae, both Firmicutes, as well as to Erysipelotrichaceae and Clostridiaceae. In addition, F. prausnitzii (the family Ruminococcaceae) can utilize polysaccharides that come from starch, hemicellulose, inulin, and pectin, whereas E. rectale is able to utilize starch, arabinoxylans, and inulin to produce SCFAs.30
SCFAs perform different functions: they regulate both gene expression, by acting as inhibitors of histone deacetylases, and energy metabolism. They also act as signaling molecules that recognize specific receptors, thus promoting the regulation of the immune system and inflammation.30,31 The function of SCFAs varies, depending on the receptors in the host tissue that they can be assimilated in, giving rise to different physiologic effects.32 They carry out part of their function upon binding to the G protein-coupled receptors (GPCRs), also known as free fatty acid receptors, GPCR41 (or FFAR3), GPCR43 (or FFAR2), and GPCR109A. Acetate and propionate are potent GPCR43 activators, which are mainly expressed in colonocytes, adipose tissue, immune system cells, nervous system cells, and pancreatic cells and they are co-expressed with GLP-1 in the enteroendocrine cells. Thus, they are related to lipid or glucose metabolism, as well as to the immune system response.30,31
A diet that is low in dietary fiber can affect SCFA production. In contrast, dietary fiber intake, and its effect on SCFA synthesis, can stimulate the production and secretion of intestinal mucus. That substance, which protects the intestinal mucosa, arises due to the increase in bacteria that promote gene expression in caliciform cells or to the mechanical stimulus of dietary fiber itself.33,34
Dietary fiber and its relation to the brain-gut-microbiota axis (BGMA)There are different communication pathways in the BGMA. The main pathway is the vagus nerve, followed by enteric nervous system activity, upon producing molecules that act as neurotransmitters, such as gamma amino butyric acid (GABA), serotonin, melatonin, histamine, and acetylcholine. One of the main mechanisms that relates dietary fiber to the BGMA results from the direct influence of SCFAs and lactic acid, which also participate in the modulation of serotonin secretion.35 Butyrate is primarily produced from resistant starch. Because one of the functions of butyrate is to inhibit the histone deacetylases, it has been shown to be beneficial in different neurologic diseases, such as Parkinson’s disease, and to improve learning and memory in cases of dementia, including Alzheimer’s disease, depression, and addictions. It is also thought to be a substrate for the production of energy in the brain, although it is not known to what degree. Cerebral inflammation has been reported to decrease in in vitro and in vivo models of Parkinson’s disease.36
FOSs and GOSs increase brain-derived neurotrophic factor (BDNF) gene expression, the NR1 and NR2A subunits of the N-methyl-D-aspartate (NMDA) receptors, and plasma peptide YY.37 A study on mice showed that the microbiota modulated behavior, after recolonization of the mice with low concentrations of BDNF resulted in behavioral alterations.38 GOSs suppress the response to neuroendocrine stress through the hyposecretion of cortisol and increase the attentional vigilance to positive stimuli versus negative stimuli.39
The pharmacologic administration of sodium butyrate has been shown to have antidepressive effects.40 Some bacteria, such as Lactobacillus and Bifidobacterium spp., are associated with neurologic development, emotional responses, and GABA production. Streptococcus, Escherichia, and Enterococcus spp. are associated with serotonin synthesis and Bacillus spp. is involved in dopamine production.41 Likewise, changes in BDNF and NMDA concentrations due to bacterial metabolism can contribute to the structural and chemical imbalances associated with schizophrenia and other pyschopathologies.42–44
Direct effects of dietary fiber on the diversity and abundance of the microbiotaOne of the characteristics of the gut microbiota most consistently associated with a better health status is bacterial diversity.45 Said diversity is importantly affected when diets are low in fiber or carbohydrates that are available for the microbiota.46 The kind of dietary fiber influences the type of microbiota because not all species produce the enzymes necessary for fiber degradation.18
Different members of the gut microbiota are important dietary fiber degraders and include 130 glycoside hydrolases, 22 polysaccharide lyases, and 16 families of esterases, providing the flexibility to degrade different energy sources from the available fibers. The main species responsible for dietary fiber degradation are the phyla Firmicutes and Actinobacteria.34,47
In a Finnish study on pregnant women with overweight or obesity, the consumption of whole grains and vegetables correlated with diversity of the microbiota. The quality of diet, in general, in the same study, correlated with abundance of the genus Coprococcus of the family Lachnospiraceae, the species F. prausnitzii of the family Ruminococcaceae, and an unknown species of the family Barnesiellaceae.48 In general, differences in gut microbiota diversity between Western populations (Europe and the United States) and populations from Africa and Papua New Guinea have been demonstrated. Their diets also differ, with Western diets consisting of a high content of processed foods, meats, sugars, and saturated fats, whereas the African diet, especially in rural zones, is characterized by important consumption of vegetables, fruits, and whole-grain cereals.6,49–52
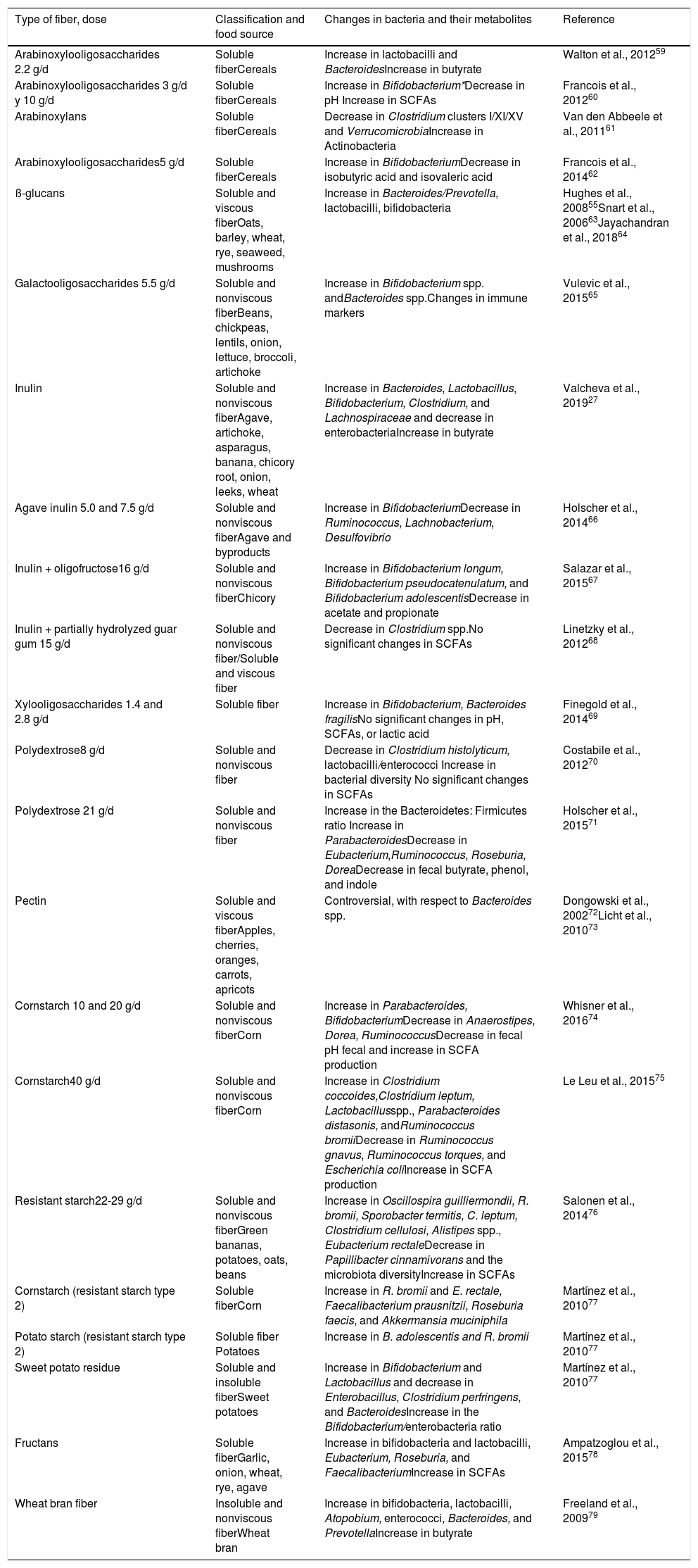
In addition to the bacterial abundance of the microbiota, the different metabolites it produces are determinants of the physiologic effects on the host.53 The consumption of soluble fiber, such as pectin and inulin, and insoluble fiber, such as hemicellulose, can increase the abundance of SCFA-producing bacteria.47,54–56 Butyrate is the main source of energy of the colonocytes and commensal bacteria, such as E. rectale and F. prausnitzii, participate in its production. That fatty acid participates in the regulation of proinflammatory and anti-inflammatory mechanisms.57 Under said premise, butyrate has been reported to induce malignant cell apoptosis, thus reducing the risk for colorectal cancer.58Table 3 lists the types of dietary fiber, the food source, and the changes produced in the gut microbiota and in SCFA production.27,55,59–79 Importantly, not all individuals produce the same quantities of SCFAs, upon consuming different sources fiber, because of the varying capacity of the intestinal bacteria to produce those metabolites.
Types of dietary fiber, food source, and changes induced in the gut microbiota and in short-chain fatty acid production.
| Type of fiber, dose | Classification and food source | Changes in bacteria and their metabolites | Reference |
|---|---|---|---|
| Arabinoxylooligosaccharides 2.2 g/d | Soluble fiberCereals | Increase in lactobacilli and BacteroidesIncrease in butyrate | Walton et al., 201259 |
| Arabinoxylooligosaccharides 3 g/d y 10 g/d | Soluble fiberCereals | Increase in Bifidobacterium*Decrease in pH Increase in SCFAs | Francois et al., 201260 |
| Arabinoxylans | Soluble fiberCereals | Decrease in Clostridium clusters I/XI/XV and VerrucomicrobiaIncrease in Actinobacteria | Van den Abbeele et al., 201161 |
| Arabinoxylooligosaccharides5 g/d | Soluble fiberCereals | Increase in BifidobacteriumDecrease in isobutyric acid and isovaleric acid | Francois et al., 201462 |
| ß-glucans | Soluble and viscous fiberOats, barley, wheat, rye, seaweed, mushrooms | Increase in Bacteroides/Prevotella, lactobacilli, bifidobacteria | Hughes et al., 200855Snart et al., 200663Jayachandran et al., 201864 |
| Galactooligosaccharides 5.5 g/d | Soluble and nonviscous fiberBeans, chickpeas, lentils, onion, lettuce, broccoli, artichoke | Increase in Bifidobacterium spp. andBacteroides spp.Changes in immune markers | Vulevic et al., 201565 |
| Inulin | Soluble and nonviscous fiberAgave, artichoke, asparagus, banana, chicory root, onion, leeks, wheat | Increase in Bacteroides, Lactobacillus, Bifidobacterium, Clostridium, and Lachnospiraceae and decrease in enterobacteriaIncrease in butyrate | Valcheva et al., 201927 |
| Agave inulin 5.0 and 7.5 g/d | Soluble and nonviscous fiberAgave and byproducts | Increase in BifidobacteriumDecrease in Ruminococcus, Lachnobacterium, Desulfovibrio | Holscher et al., 201466 |
| Inulin + oligofructose16 g/d | Soluble and nonviscous fiberChicory | Increase in Bifidobacterium longum, Bifidobacterium pseudocatenulatum, and Bifidobacterium adolescentisDecrease in acetate and propionate | Salazar et al., 201567 |
| Inulin + partially hydrolyzed guar gum 15 g/d | Soluble and nonviscous fiber/Soluble and viscous fiber | Decrease in Clostridium spp.No significant changes in SCFAs | Linetzky et al., 201268 |
| Xylooligosaccharides 1.4 and 2.8 g/d | Soluble fiber | Increase in Bifidobacterium, Bacteroides fragilisNo significant changes in pH, SCFAs, or lactic acid | Finegold et al., 201469 |
| Polydextrose8 g/d | Soluble and nonviscous fiber | Decrease in Clostridium histolyticum, lactobacilli/enterococci Increase in bacterial diversity No significant changes in SCFAs | Costabile et al., 201270 |
| Polydextrose 21 g/d | Soluble and nonviscous fiber | Increase in the Bacteroidetes: Firmicutes ratio Increase in ParabacteroidesDecrease in Eubacterium,Ruminococcus, Roseburia, DoreaDecrease in fecal butyrate, phenol, and indole | Holscher et al., 201571 |
| Pectin | Soluble and viscous fiberApples, cherries, oranges, carrots, apricots | Controversial, with respect to Bacteroides spp. | Dongowski et al., 200272Licht et al., 201073 |
| Cornstarch 10 and 20 g/d | Soluble and nonviscous fiberCorn | Increase in Parabacteroides, BifidobacteriumDecrease in Anaerostipes, Dorea, RuminococcusDecrease in fecal pH fecal and increase in SCFA production | Whisner et al., 201674 |
| Cornstarch40 g/d | Soluble and nonviscous fiberCorn | Increase in Clostridium coccoides,Clostridium leptum, Lactobacillusspp., Parabacteroides distasonis, andRuminococcus bromiiDecrease in Ruminococcus gnavus, Ruminococcus torques, and Escherichia coliIncrease in SCFA production | Le Leu et al., 201575 |
| Resistant starch22-29 g/d | Soluble and nonviscous fiberGreen bananas, potatoes, oats, beans | Increase in Oscillospira guilliermondii, R. bromii, Sporobacter termitis, C. leptum, Clostridium cellulosi, Alistipes spp., Eubacterium rectaleDecrease in Papillibacter cinnamivorans and the microbiota diversityIncrease in SCFAs | Salonen et al., 201476 |
| Cornstarch (resistant starch type 2) | Soluble fiberCorn | Increase in R. bromii and E. rectale, Faecalibacterium prausnitzii, Roseburia faecis, and Akkermansia muciniphila | Martínez et al., 201077 |
| Potato starch (resistant starch type 2) | Soluble fiber Potatoes | Increase in B. adolescentis and R. bromii | Martínez et al., 201077 |
| Sweet potato residue | Soluble and insoluble fiberSweet potatoes | Increase in Bifidobacterium and Lactobacillus and decrease in Enterobacillus, Clostridium perfringens, and BacteroidesIncrease in the Bifidobacterium/enterobacteria ratio | Martínez et al., 201077 |
| Fructans | Soluble fiberGarlic, onion, wheat, rye, agave | Increase in bifidobacteria and lactobacilli, Eubacterium, Roseburia, and FaecalibacteriumIncrease in SCFAs | Ampatzoglou et al., 201578 |
| Wheat bran fiber | Insoluble and nonviscous fiberWheat bran | Increase in bifidobacteria, lactobacilli, Atopobium, enterococci, Bacteroides, and PrevotellaIncrease in butyrate | Freeland et al., 200979 |
SCFAs: short-chain fatty acids.
Specialized bacteria, such as Clostridium, Lactobacillus, and Enterococcus, capable of adhering to the gastrointestinal mucosa, feed on mucus and bond to epithelial cells.80 Those types of bacteria have an important influence on the immune system and intestinal homeostasis.81 The gut microbiota also plays a significant role in maintaining mucosal integrity.47 In germ-free experimental models, mucin-producing caliciform cells decrease and the mucosal layer subsequently becomes thin.47,82Akkermansia muciniphila has been identified as the determinant in maintaining that mucosal barrier,83Bacteroides thuringiensis has been reported to produce a strong bacteriocin against Clostridioides difficile, and Bacteroides thetaiotaomicron has been shown to participate in metalloproteinase expression, for the conversion of prodefensin into defensin.84,85 The microbiota also participates in the maintenance of Paneth cell integrity, and consequently, in the correct production of antimicrobial peptides. The increase in taxa, such as Enterobacteriaceae, Pasteurellaceae, Veillonellaceae, and Fusobacteriaceae, and the decrease in Erysipelotrichales, Bacteroidales, and Clostridiales, produce defects in the formation of those antimicrobial peptides, which is associated with inflammatory bowel disease (IBD).86 There is an increase in Proteobacteria and a decrease in Firmicutes in the different entities in which there is inflammation of the mucosa and an increase in permeability.87,88
Indirect impact of fiberDietary fiber in cardiometabolic healthDiabetes mellitus (DM), obesity, dyslipidemia, high blood pressure, and metabolic syndrome are the most frequent cardiometabolic risk factors. Their appearance implies risks for future complications and even death, mainly when they present in a combined form. A proper diet contributes to maintaining health and a diet that is not adequate, balanced, or diverse is associated with the appearance of cardiometabolic alterations from infancy to adulthood.89,90 Less healthy dietary patterns, characterized by including little dietary fiber, correlate with greater cardiovascular risk.91,92 Two meta-analyses based on cohort studies found a lower mortality rate due to cardiovascular causes, in individuals that consume more dietary fiber.93,94
Dietary fiber intake can reduce postprandial glycemia,95 improve serum lipid levels,96 and prevent obesity and the accumulation of visceral fat.97 Dietary fiber, upon being metabolized by the gut microbiota, has been described to produce substrates that have a positive impact on the health of the host, particularly regarding cardiovascular health, because they reduce the risk for cardiovascular diseases and diabetes.98–100 In a systematic review and meta-analysis, from 19 studies, a lower risk for cardiovascular disease and coronary disease was associated with greater intake of total fiber, insoluble fiber, and fiber from cereals and vegetables. For each 7-gram increase of fiber intake per day, there was a RR of 0.91 (95% CI: 0.87-0.94) for coronary disease, as well as a reduced mortality rate (RR 0.59, 95% CI = 0.44, 0.78).98 In another similar study conducted by the American Society for Nutrition, fiber intake was associated with a low and intermediate risk for cardiovascular disease and diabetes.101 Likewise, fiber intake reduced total cholesterol, LDL cholesterol, and triglycerides,102 as was reported in vegans and vegetarians, who showed a better lipid profile than individuals that ate less fiber and more meat.103
Type 2 DM is associated with a decrease in bacteria that degrade dietary fiber. In studies utilizing animal models, the administration of soluble fiber, such as oligofructose and long-chain inulin, corrected the altered microbiota, or dysbiosis, reduced weight gain and low-grade inflammation, and improved glucose metabolism, intestinal permeability, and endotoxemia, partly related to the pathophysiology of DM.104 In a 16-week study that included a diet supplemented with functional fibers, there was improvement in the colonic microbiota, characterized by a significant increase in bifidobacteria, lactobacilli, and Bacteroides counts, as well as a decrease in the clostridia count, with a decrease in LDL cholesterol and total cholesterol.105 A rice bran extract induced the same decrease in postmenopausal women, in addition to reduced levels of TNF-α.106 In individuals with hyperlipidemia, a plant-based diet reduced blood pressure and LDL cholesterol.107 A decrease in inflammation (measured by C-reactive protein), overall inflammation, and cardiovascular risk has also been reported.108
The French Nutrition and Health Survey concluded that dietary fiber and whole grain intake was inversely associated with systolic blood pressure.109 The consumption of foods with a high glycemic index confers a greater risk for developing DM, compared with the effect of habitual dietary fiber and cereal intake.110 In fact, fiber intake ≥20 g/day reduces the risk for presenting with DM, most likely due to its effect on the proinflammatory state.111 Other mechanisms for reducing the risk of DM have been posited, such as the adsorption of glucose by fiber in the gastrointestinal tract, slower gastric emptying, and improved postprandial insulinemia. However, not all intervention studies have shown beneficial results,112 and the primary preventive effect of fiber in cardiovascular health has not been so obvious. That is possibly due to the fact that there are multiple genetic and environmental factors that are difficult to control, as well as to the small number of intervention studies, with discrepancies in the definitions employed, short intervention duration, and difficulty in conducting them.113 The conclusions of the most recent reviews and expert opinions on the topic coincide in underlining the fact that more studies with better quality methodologies are required.114
Dietary fiber, microbiota, and obesityAs mentioned above, dietary fiber intake can help prevent weight gain, visceral fat accumulation, and obesity.97 Fiber intake is associated with other beneficial lifestyle factors, such as the consumption of fruits and vegetables and exercise habits. High-fiber diets are typically lower in fat and energetic density and are useful for maintaining a healthy body weight. The results of more than 50 intervention studies were summarized in a review evaluating the relation between energy intake, body weight, and fiber consumption. An estimated fiber intake of 14 g per day was associated with a 10% reduction of energy intake and weight loss of 2 kg in 4 months. The changes observed in body weight and energy occurred, regardless of whether the fiber source was a naturally high-fiber food or a fiber supplement.115 In another review of more than 60 studies, the conclusion reached was that there is solid evidence that viscous dietary fiber intake (~7 g/day) helps reduce body weight and the fat mass, even in the absence of calorie restriction.116
Among the mechanisms by which fiber intake can aid in maintaining body weight is the gut microbiota. The gut microbiota affects the absorption of nutrients and energy homeostasis through hormones that regulate the deposit of fat into the adipocytes.117 Studies on animals have shown that dysbiosis of the microbiota can inhibit adenosine-monophosphate kinase (AMPK), which negatively affects fatty acid oxidation, promotes lipogenesis, cholesterol and triglyceride synthesis, and the deposit of fat, producing obesity.118 In addition, the gut microbiota has effects on the fasting-induced adipose factor (FIAF), modulates bile acid metabolism, modulates satiety, and regulates anorexigenic hormones, such as GLP1 and PYY, through the SCFAs.119
Studies on humans have shown that, throughout different populations, obesity and a higher BMI, in general, are associated with a low level of bacterial diversity.120–122 Studies that include rural and migrant populations suggest that the transition to a low-fiber diet, derived from the westernization of the populations, coincides with an increase in body weight, as well as a loss of gut microbiota diversity.123–125 The results of a longitudinal study showed that higher fiber intake was associated with greater microbiota diversity and concomitant lower weight gain in the long term.126 Those analyses sustain the premise that fiber intake, through its effect on bacterial diversity, could help regulate body weight.
Interestingly, dietary interventions with one type of fiber, despite resulting in certain benefits on metabolic health, have been shown to not necessarily increase bacterial diversity. In contrast, in studies on humans and in vitro models, variety was found in the structures of the fiber (through the consumption of different types of plants), which was associated with greater bacterial diversity.127 Thus, the consumption of a combination of different types and sources of fiber, as opposed to fiber intake per se, is posited to help increase microbial diversity, and in turn, regulate body weight.128
Fiber promotes the growth of genera, such as lactobacilli and bifidobacteria, inducing an environment that has traditionally been referred to as “healthier”. However, that is not completely clear, given that dysbiosis in obese persons has been found to be related to an increase in the phylum Firmicutes, the genus Clostridium, and in some species of Lactobacillus, signifying that not necessarily all members of the genus Lactobacillus, specifically in the context of obesity, have a positive connotation.129 Albeit there is no definition of what a normal microbiota is, fiber intake has a protective effect against body weight gain and the incidence of DM that is partially mediated by the gut microbiota.130 Strikingly, numerous studies have found great interindividual variability in the response to interventions with different kinds of fiber. Therefore, if those interventions can induce changes in the composition of the microbiota, the microbiota is thought to be able to determine how the fiber is metabolized, thus having an impact on the health of the individual.131 Studies on adults with overweight and obesity have shown that individuals with a specific gut microbiota profile can obtain greater benefit regarding body weight loss, after interventions that are rich in fiber. Individuals whose microbiota has a greater abundance of the genus Prevotella in relation to Bacteroides particularly appear to lose body weight after the intervention.132–134 Other genera associated with the degree of response to interventions with fiber, such as inulin, are Akkermansia, Butyricicoccus, Anaerostipes, and Bifidobacterium.135,136 Despite the fact that the characterization of the gut microbiota is not currently carried out systematically, the above evidence could have clinical implications, in which the incorporation of gut microbiota markers could aid in improving the efficiency of nutritional therapies.137
Dietary fiber and colon cancerExcess protein intake leads to fermentation in the colon, with the production of compounds that have been associated with colorectal cancer, but evidence confirming that is insufficient. A simple strategy to counteract adverse effects, if there were any, would be to reduce protein intake or administer synbiotics.138
Regardless of colorectal cancer etiology, mucosal biomarkers were reported to be reversed, with the administration of 55 g of fiber daily.139
Epidemiologic studies provide important information on fiber intake and colorectal cancer.140 In a meta-analysis of 11 prospective cohort studies, dietary fiber intake was inversely associated with the risk for both proximal and distal colon cancer.141 Several years earlier, a meta-analysis of 25 prospective studies had found that high total fiber intake, particularly fiber from cereals and whole grains, was associated with a lower risk for colorectal cancer.142 In a very recent meta-analysis of 22 studies, groups of adults with very high fiber intake were compared with those with very low fiber consumption. The results of the analysis suggest that dietary fiber intake could protect against rectal cancer, with a clinically relevant risk reduction.143 Fiber not only has an effect on cancer, but on inflammatory bowel disease, as well. In non-industrialized regions of Africa, in which its inhabitants consume more than 50 g/day of fiber, the prevalence of chronic inflammatory diseases is very low.140
Dietary fiber and constipationThe relation between fiber and bowel movement ease is related to certain properties of fiber, such as its capacity to retain water, increase fecal volume, increase intestinal propulsion, and reduce bowel transit time. Thus, it is important for maintaining normal bowel habit regularity. Fiber augments the food bolus, and the consequent distension of the intestine produces as increase in peristalsis.144 Said increase in the bolus is the result of liquid retention between the fiber and increased bacterial density, due to fermentation. Fiber supplementation of 20-30 g/day is the usual consideration for adult patients with chronic constipation.145 The use of different types of fiber for that purpose is similarly effective.146 Less fermentable fibers have greater water-holding capacity and greater resistance to bacterial degradation, compared with more fermentable fibers, which is important, because bowel transit is thought to become faster, the greater the added weight. However, in an in-depth review of different interventions, bowel transit time was reduced only in individuals with initial transit times above 48 h, regardless of the type of fiber. In the same study, cereal and vegetable dietary fibers had comparable effects on fecal weight, superior to those of fruit fibers.147
The beneficial effect of fiber on chronic constipation has been shown in cohort studies and in intervention studies.148 In a study conducted on nurses, there was a 36% reduction in constipation in the individuals in the highest dietary fiber consumption percentile, compared with those in the lowest percentile, corresponding to a 1.8% reduction in constipation for every extra gram of fiber consumed.149 The potential adverse effects of dietary fiber intake are flatulence, abdominal distension, and abdominal pain due to fiber fermentability, especially in FOS consumption, which can cause symptoms at doses as low as 10 g.150 Adaptations should be made in relation to the type of fiber the patient best tolerates, and fibers with low fermentability are most likely be better tolerated in patients with preexisting conditions associated with gas and bloating.
Dietary fiber, microbiota, and irritable bowel syndromeThe effect of fiber on the symptoms of irritable bowel syndrome (IBS) is variable and specific to the type of fiber, e.g., soluble fiber, like Psyllium, has shown beneficial therapeutic effects, but insoluble fiber, like wheat bran, has not. Second-line dietary therapy for IBS is a diet low in fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs). Those fermentable carbohydrates can contribute to the increase in the production of gas and exacerbate IBS symptoms.151 The majority of persons do not experience important symptoms after fiber consumption, and in those that do, intolerance to FODMAPs disappears over time, as the microbiota of the host adapts to their intake.152 Nevertheless, in some patients with visceral hypersensitivity, the use of low fermentability fibers, such as methylcellulose, or those of intermediate solubility, such as Psyllium plantago and ispaghula, is recommended.
The fructose, lactose, fructan, sorbitol, and fructooligosaccharide FODMAPs are found in fruits, onions, garlic, legumes, and wheat. After several non-controlled studies on the effects of FODMAPs on IBS symptoms, a randomized and blinded study demonstrated the improvement in the grading of symptoms of bloating, pain, and flatulence, with the implementation of a low-FODMAP diet.153 The same occurred in a study carried out in Mexico.154 According to another analysis, the response to a low-FODMAP diet was dependent on the structure of the patient’s microbiota, showing less response, the greater the dysbiosis index.155
Changes in the microbiota associated with a low-FODMAP diet have been demonstrated, and so whether its long-term use is adequate is still unclear. FODMAPs should be restricted in relation to adequate symptom control.156 The establishment of a low-FODMAP diet should always be carried out under the supervision of healthcare professionals trained in regard to these types of recommendations because such a diet could result in nutritional deficiencies and disorderly eating behaviors.150
With respect to the pediatric population, due to the scarcity of clinical trials on the use of fiber in children with IBS, a definitive conclusion cannot be reached. Healthcare professionals should be cautious when selecting the type and dose of fiber in children and adults with IBS, to not worsen their symptoms. The use of a low-FODMAP diet is not currently recommended for the pediatric population.157
The composition of the intestinal bacteria in patients with IBS differs from that of healthy subjects, with less abundance of the butyrate producers, Erysipelotrichaceae and Ruminococcaceae, than healthy children. Likewise, they have greater abundance of Methanobacteriales (methane-producing bacteria), Lactobacillus and Ruminococcus, and a decrease in Bifidobacterium, Faecalibacterium, Erysipelotrichaceae, and methanogens.158–160 When the microbiota of the patient with IBS is rebalanced due to the effect of treatment, the SCFAs it produces have a potentially beneficial effect, such as improving epithelial renewal, improving intestinal permeability, and reducing low-grade inflammation. However, more studies need to be carried out to determine the mechanisms through which fiber improves aspects of IBS pathophysiology. There are increasingly more tests demonstrating that not only the abdominal symptoms of IBS, but also the psychiatric comorbidity that presents in a considerable number of those patients, are explained by the gut microbiota.
Dietary fiber and inflammatory bowel diseaseThe glycoprotein and polysaccharide-rich layer of mucus that covers the surface of the intestinal mucosa is the first line of defense between intestinal cells and the gut microbiota,161 and in turn, is a source of nutrition for certain gut bacteria.162B. thetaiotaomicron has been shown to metabolize mucus glycans when there is a lack of dietary fiber, thinning the layer and resulting in the close contact of the bacteria with the epithelium.163 That could explain the damage that can be caused by the deficiency of fiber in IBD and colon cancer.164 SCFAs exert anti-inflammatory effects on macrophages and dendritic cells because they stimulate regulatory T cell differentiation.165 Patients with IBD have lower levels of SCFAs, including butyric acid and acetic acid, compared with healthy subjects. Butyric acid could provide protection against IBD.166 The scientific evidence for indicating fiber as treatment for ulcerative colitis and reservoiritis is still limited.167
Dietary fiber in colostomy managementLittle is known about the nutritional status and eating habits of persons with intestinal stomas and no universal dietary guidelines have been established. Many persons with stomas adjust their diet to avoid discomfort that interferes with daily life and makes them afraid to leave home, such as increased odor of gases or stool production, constipation, or leaks. Some patients avoid certain foods, especially fruits and vegetables.168 The increase in fiber and liquid intake is one of the more widely used measures in patients with stomas that suffer from chronic constipation, alleviating the condition in the majority of them.169 Soluble fiber supplements are frequently used if dietary measures are not sufficient, but their employment is empiric, given that there are no randomized comparative studies on the topic.170 The interaction between dietary fiber and the microbiota in patients with ileostomy and colostomy has not been specifically studied.
Dietary fiber and portosystemic hepatic encephalopathyThe decrease in the consumption of foods of animal origin and the increase in vegetable proteins reduces hepatic encephalopathy (HE), albeit the mechanism is not clear. When fiber is increased in the diet, its fermentation reduces the pH of the colon, favoring the excretion of ammonia, rather than its absorption, accelerating colon transit.171 In cirrhosis, protein consumption should not be reduced, but rather plant-based protein, naturally associated with dietary fiber, should be administered.172 The microbiota is capable of producing the majority of neurotransmitters found in the human brain and they obviously influence neurochemistry and behavior. HE is considered the prototypic brain-gut-microbiota axis disorder. Translational research indicates that certain bacteria and their manipulation can have an impact on the positive responses of brain function. The increase in fermentable fiber could reduce the absorption of ammonia in the portal system similarly to that of lactulose supplementation.173
The use of dietary fiber as the only therapeutic measure against HE had not been studied, but a fiber-rich diet enables the concomitant increase of protein intake. A Mexican study showed that supplementation with branched-chain amino acids plus a diet with a high content of fiber and proteins is a safe intervention in patients with cirrhosis, given that it helps increase muscle mass without elevating ammonemia or fostering the development of HE.174 Numerous well-designed studies have evaluated the benefit of different probiotics in the treatment of HE. Compared with placebo or no intervention, probiotics most likely improve recovery and can regulate ammonia levels in plasma, as well as quality of life, in patients with overt HE, although with no decrease in mortality.175 At present, there are no studies in the literature that describe the modification of the gut microbiota in cirrhotic patients with HE, as a response to specific diets.176 High quality clinical trials are needed to clarify the true potential of dietary fiber, the efficacy of probiotics, and their effect on the gut microbiota in HE.
ConclusionsDietary fiber can induce changes in intestinal health that are directly and indirectly mediated by the gut microbiota. The use of dietary fiber by the gut microbiota depends on various factors and characteristics of the fiber, such as its fermentability, solubility, and viscosity. The type of dietary fiber influences the composition of the microbiota, given that not all bacterial species degrade all types of fiber, which can be verified by changes at the level of the phylum, family, and species. Diets low in dietary fiber can reduce the production of SCFAs, affecting their different local and systemic functions. The indirect benefits of dietary fiber impact cardiometabolic and digestive health, including some functional gastrointestinal disorders, as well as different diseases. The recommended daily intake of dietary fiber in adolescents and adults is 14 g for every 1000 kcal, in general. In pathologic cases, treatment should be individualized, with very close follow-up.
Financial disclosureThe scientific agenda, discussion, and conclusions of the present review were autonomously determined and independently drafted by the members of the group summoned by the Asociación Mexicana de Gastroenterología (AMG).
For strictly logistical purposes, the AMG requested and received funding, with no conditions, from the Instituto de Nutrición and Salud Kellogg’s® (INSK). The authors received an honorarium for their participation.
Conflict of interestMTAA: has received honoraria for advisory board consulting services and for conferences for Takeda®, Alfasigma®, Mayoly-Spindler®, Sanofi®, Menarini®, Carnot®, Biocodex®, BioGaia®, and Abbott® Farmacéutica.
MPMG: has received honoraria for conferences Kellogg’s®, Abbott® Farmacéutica, Victus®, and Alfasigma®.
GAAA: has received funding for attending congresses and honoraria for conferences Nestlé® and Carnot®.
AMCB: has no conflict of interest.
RICS: has received honoraria for advisory board consulting services for Asofarma®, and is a speaker for Mayoly-Spindler®, Asofarma® y Chinoin®.
ACS: has received honoraria for conferences for Sanofi-Aventis®, Mead Johnson®, Takeda®, and Alexion Pharma®, and funding for clinical research studies from Alexion Pharma® and Sanofi-Aventis®.
ECA: is a member of the advisory board of Takeda® Mexico, Ferrer® Mexico, Asofarma® Mexico, Grunenthal® Mexico, and is a Speaker for Takeda® Mexico, Ferrer® Mexico, Asofarma® Mexico, Grunenthal® Mexico, Medtronic® Mexico, Asofarma® Central America and the Caribbean, Carnot® Mexico, and Siegfried Rhein® Mexico.
MFGC: has received honoraria for conferences for Grunenthal® Mexico.
VHR: has received funding to attend congresses and honoraria for conferences for Nestlé®.
MEIC: has received funding to attend congresses and has received honoraria for talks for Asofarma®, Takeda®, and Chinoin®.
JNMM: has no conflict of interest.
SMR: has received honoraria for conferences for Kellogg’s® and PepsiCo®.
EOO: has received funding to attend congresses and has received honoraria for conferences and the development of materials for VitaFlo Argentina®, Nestlé®, Danone®, Abbott®, and Kellogg’s®.
MRA: has received funding to attend congresses from Imed Orphan® and has received honoraria for advisory board consulting services for Abbott®.
RLRF: has received funding to attend congresses for Gilead Sciences®.
FZD: has no conflict of interest.
FZM: has received funding to attend congresses and has received honoraria for conferences and advisory board consulting services from Mead Johnson®.
RVF: has received funding to attend congresses, has received honoraria for conferences for Nestlé®, Sanofi®, Carnot®, BioGaia®, and Abbott® Farmacéutica, and has received funding for clinical research studies from Alexion Pharma® and Sanofi-Aventis®.
Please cite this article as: Abreu y Abreu AT, Milke-García MP, Argüello-Arévalo GA, Calderón-de la Barca AM, Carmona-Sánchez RI, Consuelo-Sánchez A, et al., Fibra dietaria y microbiota, revisión narrativa de un grupo de expertos de la Asociación Mexicana de Gastroenterología, Rev Gastroenterol México. 2021;86:287–304.