The incidence and prevalence of inflammatory bowel disease (IBD) has increased in recent years in several Latin American countries. There is a need to raise awareness in gastroenterologists and the population in general, so that early diagnosis and treatment of ulcerative colitis (UC) and Crohn's Disease (CD) can be carried out. It is important for all physicians to have homogeneous criteria regarding the diagnosis and treatment of IBD in Latin America. The Pan American Crohn's and Colitis Organisation (PANCCO) is an organization that aims to include all the countries of the Americas, but it specifically concentrates on Latin America. The present Consensus was divided into two parts for publication: 1) Diagnosis and treatment and 2) Special situations.
This is the first Latin American Consensus whose purpose is to promote a perspective adapted to our Latin American countries for the diagnosis, treatment, and monitoring of patients with UC and CD.
La incidencia y la prevalencia de la enfermedad inflamatoria intestinal (EII) se han incrementado en los últimos años en varios países de Latinoamérica. Existe una necesidad de concientizar a gastroenterólogos y a la población en general para poder tener un diagnóstico y tratamiento oportunos en la colitis ulcerosa crónica idiopática (CUCI) y enfermedad de Crohn (EC). Es importante que todos los médicos tengan un criterio homogéneo acerca del diagnóstico y el tratamiento de la EII en América Latina. La Pan American Crohn's and Colitis Organisation (PANCCO) es un organismo con el propósito de incluir a todos los países del continente americano pero se enfoca de manera específica a los países latinos. Este Consenso está dividido en 2 partes para su publicación: 1) diagnóstico y tratamiento, y 2) situaciones especiales.
Este es el primer Consenso latinoamericano cuyo objetivo es promover una perspectiva adaptada a nuestros países latinos para el diagnóstico, el tratamiento y la monitorización de pacientes con CUCI y EC.
Inflammatory bowel disease (IBD) is mainly comprised of ulcerative colitis (UC), Crohn's disease (CD), and indeterminate or unclassifiable colitis (IC). It is chronic and incurable, and presents with periods of relapse and remission. IBD etiology is unknown, but it has been postulated to be a multifactorial disease due to the genetic, immunologic, and environmental factors involved in its development. The Pan American Crohn's and Colitis Organisation (PANCCO) is an organization that aims to include all countries in the Americas and it is focused mainly on Latin American countries. The present Consensus is grouped into 2 parts: diagnosis and treatment, and special situations. This is the first Latin American Consensus whose purpose is to provide all physicians with homogeneous criteria regarding the diagnosis and treatment of IBD in Latin America, and thus improve the standard and quality of care given to patients.
AimTo promote a perspective adapted to our Latin American countries in relation to the diagnosis, treatment, and monitoring of patients with UC and CD.
MethodsThe following steps were involved in the strategy to reach the consensus:
- 1.
For the development of the first PANCCO guidelines, Dr. Jesús K. Yamamoto-Furusho coordinated and organized the contents of the consensus together with the PANCCO Steering Committee, made up of physicians from 6 Latin American countries: Argentina, Brazil, Chile, Colombia, Mexico, and Venezuela. Dr. Yamamoto-Furusho established and distributed each of the topics to the experts from these 6 Latin American countries. Each member was responsible for developing the relevant questions on each of the 12 subjects separately regarding the diagnosis, treatment, and special situations in both UC and CD. The questions were focused on current clinical practice and controversial issues. Participants were asked to answer the questions based on their experience and according to the literature (Delphi process). Task forces that reviewed the progress contained in the published literature were formed.
- 2.
In parallel, the members of the consensus conducted a systematic search of the literature for each of the issues by using Medline/Pubmed, the Cochrane database, EMBASE (Ovid), and LILACS.
The search strategy included the following MeSH terms for diagnosis: inflammatory bowel disease, Crohn's disease, ulcerative colitis, diagnosis, serum and fecal biomarkers, clinical indices, endoscopy, radiology (computed tomography [CT], and magnetic resonance enterography [MR enterography]). The MeSH terms for medical treatment included: 5 aminosalicylates (5-ASA), steroids, budesonide, thiopurines (azathioprine and 6-mercaptopurine), immunosuppressants (ciclosporin, tacrolimus, methotrexate), and biologic therapy (anti-TNF agents [infliximab, adalimumab, certolizumab pegol, golimumab] and anti-integrin therapies [natalizumab, vedolizumab]). The terms used for surgical treatment were: proctocolectomy, intestinal resection, pouch, ileoanal anastomosis, pouchitis, complications, toxic megacolon, and IBD surgeries.
We included all clinical practice guidelines, randomized controlled trials, controlled clinical trials, systematic reviews, meta-analyses, cohort studies, and case-control studies published in the last 15 years (2000-2014).
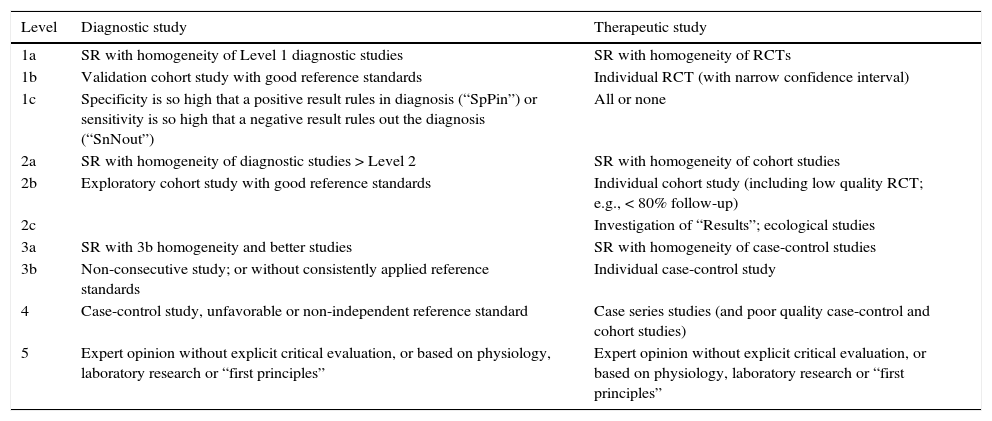
The level of evidence was classified (table 1) according to the Oxford Centre for Evidence-Based Medicine.
Table 1.Levels of evidence and degrees of recommendation based on the Oxford Centre for Evidence-Based Medicine.
Level Diagnostic study Therapeutic study 1a SR with homogeneity of Level 1 diagnostic studies SR with homogeneity of RCTs 1b Validation cohort study with good reference standards Individual RCT (with narrow confidence interval) 1c Specificity is so high that a positive result rules in diagnosis (“SpPin”) or sensitivity is so high that a negative result rules out the diagnosis (“SnNout”) All or none 2a SR with homogeneity of diagnostic studies > Level 2 SR with homogeneity of cohort studies 2b Exploratory cohort study with good reference standards Individual cohort study (including low quality RCT; e.g., < 80% follow-up) 2c Investigation of “Results”; ecological studies 3a SR with 3b homogeneity and better studies SR with homogeneity of case-control studies 3b Non-consecutive study; or without consistently applied reference standards Individual case-control study 4 Case-control study, unfavorable or non-independent reference standard Case series studies (and poor quality case-control and cohort studies) 5 Expert opinion without explicit critical evaluation, or based on physiology, laboratory research or “first principles” Expert opinion without explicit critical evaluation, or based on physiology, laboratory research or “first principles” RCT: Randomized controlled trial; SR: Systematic review.
- 3.
The revised statements on the subjects were written by those in charge of each subject, based on the responses of the task force, as well as evidence from the literature, and they were shown to all participants. The structure of each statement consisted of the recommendation based on study design and clinical evidence, and finally the levels of evidence and agreement were determined.
- 4.
All participants met in Washington DC, USA, in May 2015, to agree on the statements. The participants met under the coordination of Dr. Yamamoto-Furusho to reach an agreement on the final version of each statement. Technically, this was done by projecting the statements on a screen and reviewing them until a consensus was reached. Consensus was defined as the agreement of > 80% of the participants. A Consensus Statement was established and numbered for convenience purposes in the document.
- 5.
The final document of each subject was drafted by the person in charge of each topic or section. The Consensus Statements are written in bold type and followed by comments on the evidence and an opinion. The statements are meant to be read in the context of the qualifying comments and not in isolation. Dr. Yamamoto-Furusho edited the final text for style consistency and it was approved by the participants. In some areas, there are very few randomized controlled trials, resulting in a general low level of evidence. When this was the case, expert opinion was included.
The Montreal classification was used in the present Consensus to define disease distribution as follows:
E1 Proctitis Involvement is limited to the rectum (i.e., the proximal extent of inflammation is distal to the rectosigmoid junction).
E2 Left colitis Involvement is limited to the portion of the colon distal to the splenic flexure (analogous to “distal colitis”).
E3 Extended colitis Involvement extends to the splenic flexure and includes pancolitis.
Disease onsetSome evidence suggests that patients with UC stratified by age (A1: < 16; A2: 16-40; and A3: > 40 years of age) have different disease courses.
Active diseaseFor the purposes of this Consensus, the clinical activity of the disease was grouped into four categories, taking the Truelove and Witts criteria into account: inactive or in remission, mild, moderate, and severe.
RemissionRemission is defined as complete resolution of symptoms and/or endoscopic mucosal healing.
ResponseResponse is defined as clinical and endoscopic improvement, in other words, a decrease > 30% in the activity index, in addition to a decrease in rectal bleeding and endoscopy sub-scores.
RelapseRelapse is the exacerbation of symptoms in a patient with established UC that had been in clinical remission, either spontaneously or after medical treatment.
Early relapseThis consists of symptoms of disease activity in a period of < 3 months after achieving clinical remission.
Relapse patternRelapse can be rare (≤ one relapse/year), common (≥ 2 relapses/year), or continuous (persistent symptoms of active UC without a remission period).
Steroid-refractory ulcerative colitisPatients with active disease despite a dose of prednisone of up to 0.75mg/kg/day for a period of 4 weeks.
Steroid-dependent ulcerative colitis- 1.
Patients that are unable to reduce the steroid dose below the equivalent of 10mg/day of prednisone within the first 3 months of treatment, with no active recurring disease, or
- 2.
Patients that have a relapse in the first 3 months after steroid discontinuation.
These patients have active disease or relapse despite the administration of thiopurine therapy at the appropriate dose for at least 3 months (i.e., azathioprine 2-2.5mg/kg/day or 6-mercaptopurine 1-1.5mg/kg/day in the absence of leukopenia).
Refractory distal ulcerative colitisDefined as persistent symptoms caused by colonic inflammation confined to the rectum (proctitis) or to the left colon, despite the administration of oral and topical steroids and 5-ASA for 4-8 weeks.
Definitions in Crohn's diseaseActive diseasea. Crohn's Disease Activity Index (CDAI)
- 1.
Mild: 150-220 points
- 2.
Moderate: 220-450 points
- 3.
Severe: > 450 points
Remission: CDAI<150.
Response to treatment: CDAI score change; decrease of > 100 CDAI points.
Relapse: exacerbation of symptoms in a patient with CD that had been in clinical remission, either spontaneously or after medical treatment; a seventy-point increase in the CDAI.
Early relapse: exacerbation of symptoms in a patient with CD in remission for fewer than 3 months under medical treatment.
Relapse pattern: uncommon: ≤ one time per year; common: ≥ 2 times per year and continuous persistent symptoms of active CD without a period of remission.
Steroid-refractory disease: patients with disease activity despite administration of prednisone up to 0.75mg/kg/day for a period of 4 weeks.
Steroid dependent disease:- a.
patients are unable to reduce steroids below the equivalent of 10mg/day of prednisone (budesonide below 3mg/day) within the first 3 months after receiving steroids, without active recurrent disease, or
- b.
patients have a relapse in the first 3 months after steroid discontinuation,
- c.
the total duration of steroids should not exceed 3 months.
Recurrence: Lesions return after undergoing a surgical resection.
Morphologic recurrence: CD lesions after macroscopic resection of the disease, as established by the Rutgeerts score.
Endoscopic recurrence according to the Rutgeerts score:- 1.
0: no evident lesions.
- 2.
1: less than 5 aphthous lesions.
- 3.
2: more than 5 lesions with normal mucosa between lesions.
- 4.
3: diffuse aphthous ileitis with inflamed mucosa.
- 5.
4: ileal inflammation with nodules, ulcers, strictures.
Clinical recurrence: reappearance of symptoms after macroscopic resection of the disease once lesion recurrence has been confirmed.
Localized disease: intestinal involvement of CD under 30cm.
Extended disease: intestinal condition of CD extending over 100cm, regardless of location. It includes the sum of inflamed areas that alternate with uninvolved ones.
DiagnosesA. Clinical aspects and biomarkers1. The diagnosis of IBD should be based on the correlation of clinical, laboratory, endoscopic, and histologic aspects. The possible differential diagnoses must be ruled out. Level of evidence: 3. Level of agreement: 100%.
IBD refers to a group of disorders of unclear etiology, but common clinical and histopathologic aspects. The main diseases are UC and CD. UC is an inflammatory disorder of the colonic mucosa, which starts in the rectum, but may extend proximally and wrap around the colon. On the other hand, CD is a chronic disease that can cause inflammation from the mouth to the anus, with irregular distribution of lesions that can affect not only the mucosa, but also the entire thickness of the intestinal wall. The differential diagnosis of IBD and other inflammatory, infectious, or functional disorders, is often difficult. Biomarkers have been used only recently to aid in the diagnosis and management of IBD.1–4
Diagnosis can be made by taking a very detailed medical history that should include information on the onset of symptoms, previous crises, rectal bleeding, diarrhea, abdominal pain, weight loss, perianal lesions, and the presence of extra-intestinal symptoms. A family history of IBD, recent travel, the use of anti-inflammatory drugs, and infections (including tuberculosis) must also be evaluated.1,4
The diagnosis of IBD must be based on clinical, endoscopic, laboratory, and imaging data. Endoscopic evaluation is currently the baseline value test for IBD to detect and measure intestinal inflammation, but it is expensive, invasive, and uncomfortable for the patient. At least one in 3 patients presents with clinical and endoscopic activity with normal levels of C-reactive protein (CRP). There are simple, safe, and inexpensive tests adequately related to endoscopy that are welcome aids in the diagnosis and monitoring of IBD. They can be regularly used in place of other invasive tests, such as colonoscopy, especially when patients have symptoms.5,6 The possible differential diagnoses must be ruled out, and in cases of doubt when the inflammation is limited to the colon or is IC. The following are the recommended routine tests, according to need/site/local conditions:
- 1.
Physical examination.
- 2.
Laboratory tests: complete blood count, erythrocyte sedimentation rate (ESR), CRP, albumin, iron, ferritin, and stool examination (fecal calprotectin).
- 3.
Elimination of the possibility of human immunodeficiency virus (AIDS), tuberculosis, and other pathologies, such as intestinal infections, ischemia, etc. (blood and fecal tests).
- 4.
Ileocolonoscopy.
- 5.
Abdominal ultrasound (US).
- 6.
Magnetic resonance imaging (MRI) is preferred over computed tomography (CT) due to radiation exposure and it is performed with an enterography protocol.
- 7.
Radiologic examinations with barium (intestinal transit and barium enema) (when MRI or CT are not available).
- 8.
Capsule endoscopy (in cases where diagnosis has not been made, even after the previous tests).
2. The most widely used and reliable activity indices for Crohn's disease are the Crohn's Disease Activity Index (CDAI) and the Harvey-Bradshaw index (HBI). The most widely used indices recommended for UC are the Mayo score and the Truelove and Witts index. Level of evidence: 3. Level of agreement: 89%.
The most widely used and reliable activity indices for CD are the CDAI and the HBI. The most widely used indices recommended for UC are the Mayo score and the Truelove and Witts index.7
3. Anti-Saccharomyces cerevisiae antibodies (ASCAs) and antineutrophil cytoplasmic antibodies (ANCAs) are serologic markers that are useful for the differential diagnosis between UC and CD. They are not useful for IBD diagnosis. Level of evidence: 4. Level of agreement: 100%.
Despite the success achieved in the search for biomarkers for IBD, the serologic markers we have today are still very limited for IBD diagnosis. For example, when suspicion is high, marker negativity does not prevent appropriate imaging or endoscopy exams from being performed. However, when suspicion is low, marker positivity may lead the patient to have unnecessary invasive tests done. When suspicion is high and markers are positive, the patient also has to undergo radiographic and endoscopic examinations to obtain information on the extent, location, and severity of the disease. Therefore, since these markers are relatively sensitive and specific, they are of little use for diagnosis. In Latin America, only ASCA (IgA and IgG), perinuclear ANCA (p-ANCA), CRP, and ESR tests are generally performed. Just the first two are done for the differential diagnosis (they have reasonable specificity) and the others for evaluating inflammatory symptoms (nonspecific).8–12 In UC patients, we found a higher prevalence of atypical ANCA (x-ANCA) than of p-ANCA (50% vs 32%), along with a higher specificity (96% vs 92%) and positive predictive value (99% vs 96%).13 In addition, the x-ANCA pattern was associated with the presence of disease extent and arthralgia in Mexican UC patients.14
4. Acute phase reagents, such as CRP and ESR, are nonspecific and they should be performed if diagnosis of IBD is suspected. They are also useful in monitoring inflammatory activity in patients with IBD. Level of evidence: 2. Level of agreement: 89%.
CRP is synthesized in the liver and is a sensitive serologic marker for inflammation. During acute inflammation, CRP can increase greatly, up to one thousand times.15–17 A study carried out in 2002 showed that when the ELISA method was used for CRP, a cutoff value of 2.3mg/l had a sensitivity of 100% and a specificity of 67% in the differentiation of IBD from intestinal functional disorders.16 CRP appears to be the most sensitive serologic marker to detect IBD, but it is also increased in other conditions, such as active infections (tuberculosis, pneumonia, and other bacterial infections) and other inflammatory processes (rheumatoid arthritis, lupus, pancreatitis, myocardial infarction, and tumors), pregnancy, and the use of medication (such as oral contraceptives).6,15,18 CRP cannot differentiate between UC and CD. A review of the role of CRP in diagnosing gastrointestinal tract diseases has concluded that it should be used as an auxiliary tool to complement the clinical observation and physical examination, but it cannot replace them.15,17 A Mexican study found a significant correlation between serum high-sensitivity CRP and histologic activity (r2=0.39, p=0.01). Diagnostic utility was determined by ROC curves that showed a cutoff of ≥ 0.36mg/dl and an area under the curve of 0.73.19
5. Fecal markers, such as calprotectin, are sensitive and specific for documenting intestinal inflammation. They are also useful in monitoring patients with IBD. Level of evidence: 2. Level of agreement: 89%.
In addition to markers in the blood, there are fecal markers that assess inflammatory activity, but they are not specific for UC and CD, since they only indicate the presence of inflammation. They are important for differentiating between IBD and irritable bowel syndrome (IBS) and also for monitoring patients with IBD after diagnosis. There is a strong correlation between fecal calprotectin and excretion of labeled neutrophils, supporting the hypothesis that the increase in calprotectin is a result of leukocyte migration to the inflamed mucosa and the resultant leukocyte shedding in the intestinal lumen.20 Calprotectin is a calcium-linked protein, mainly derived from neutrophils. Excreted in feces, it is stable for up to a week. It is not specific for detecting intestinal inflammation, and it may be elevated with the use of NSAIDs and in enteric infections. It is important for the differential diagnosis of IBD and IBS, and it also has the ability to predict relapse and therefore is useful for monitoring patients in remission.21 Some studies have demonstrated that elevated excretion of fecal calprotectin is very sensitive (84%) and very specific (96%) for inflammatory diseases with a positive predictive value of 95% vs a negative predictive value of 85% for discriminating between IBD and IBS. Regular fecal calprotectin concentration was well established in many studies that showed an average value of 25mg/kg. Levels higher than 50mg/kg are considered high levels, whereas levels between 200 and 20,000mg/kg indicate the presence of inflammation. Cases with levels between 50 and 200 are inconclusive and thus cannot be considered significant for inflammation.22–30
Calprotectin appears to have great potential in pediatrics for differential diagnoses and the selection of patients that will need a colonoscopy to determine the etiology.22,23 Some studies also show it is capable of predicting disease relapse in patients with colitis or CD.23
6. Histologic examination may be of help in the diagnosis of IBD, as well as in verifying the degree of inflammation, and therefore, in therapeutic behavior. Level of evidence: 3. Level of agreement: 89%.
Histologic examination may be useful to complement IBD diagnosis, but it is of little value alone. In addition, granuloma occurs in no more than 25-30% of cases of CD. In UC, there is no specific lesion, but some microscopic alterations may be useful in confirming the diagnosis.1 Bitton et al. showed that basal plasmacytosis was the principal histologic predictor of relapse, regardless of maintenance therapy, among 74 patients with clinically and endoscopically quiescent UC.31
Azad et al. found that increased numbers of lamina propria neutrophils and eosinophils were associated with a higher risk for relapse over 12 months in patients with quiescent UC.32
Resolution of histologic inflammation in UC has also been associated with a higher likelihood of remaining symptom-free at 12 months after a course of corticosteroids, as well as with a reduction in hospitalization or colectomy rates.33,34
The prognostic value of histology has only been assessed in a single study on CD, which found that mucosal inflammation was not associated with more frequent clinical relapse, stricture formation, or surgery.35
7. Early diagnosis of IBD (UC and CD) has a major impact on the clinical course of the disease. Level of evidence: 4. Level of agreement: 100%.
Early diagnosis of IBD (UC and CD) has a major impact on the clinical course of the disease. This is the only way damage and sequelae in patients can be prevented.36–38 Even after the diagnosis is made, monitoring is very important to prevent other crises and subsequent complications.39
B. Endoscopic aspects8. If IBD is suspected, ileocolonoscopy is the procedure of choice for making the diagnosis and determining disease extension. Level of evidence: 2. Level of agreement: 100%.
Ileocolonoscopy represents the most important and powerful test in the diagnosis of suspected IBD, and it must be done quickly and before starting any medical treatment. In UC, endoscopic changes typically begin proximally to the anal margin and extend proximally and in a continuous, concentric, and confluent manner. The demarcation between normal and inflamed areas is usually clear and it may occur abruptly, especially in distal disease.40 The absence of macroscopic and microscopic rectal compromise has been described in children with ulcerative colitis before treatment.41,42 In adults, inflammation patches or a normal appearance in the rectum may be due to the use of previous topical therapy. Inflammation patches in the cecum43 are observed in patients with left colitis. When there is no rectal compromise or cecal patch involvement in a new diagnosis of colitis, the small intestine must be evaluated in addition to ileocolonoscopy. The absence of an appendiceal lesion is reported in more than 75% of patients with UC.44–46 This is associated with a better response to medical treatment and a high risk for pouchitis.44–46 Continued extension of inflammation from the cecum to the distal ileum is defined as reflux ileitis, observed in over 20% of patients with pancolitis, 47–48 and it is associated with a refractory course of the disease.49
The endoscopic feature of CD is the distribution of patchy inflammation with areas of inflammation interspersed with mucosa of normal appearance. CD ulcers tend to be longitudinal and may be associated with a cobblestone appearance in the ileum or the colon, with strictures, and with fistula openings. The rectum is compromised in an opposite and circumferential pattern and continuous inflammation is rare. Biopsies must be taken from the edges of ulcers and aphthous erosions to increase the possibility of finding granulomas, which are pathognomonic for CD.50
When there is severe active disease, in both CD and UC, the value of total colonoscopy must be considered, due to the high risk for perforation. Advanced age, serious disease, steroid use, female sex, and endoscopic dilation seem to be associated with an increased risk for perforation (0.3 to 1%).51,52 In this case, initial sigmoidoscopy is safe and ileocolonoscopy must be postponed until the clinical condition improves. However, a recent study suggests that the risk does not increase when carried out by experienced hands.53
9. For the diagnosis of CD and ulcerative colitis, multiple biopsies from 6 segments (terminal ileum, ascending, transverse, and descending colon, sigmoid colon, and rectum) should be obtained. Multiple biopsies involve at least 2 samples from each segment, including macroscopically normal segments. Level of evidence: 2. Level of agreement: 88%.
Biopsies of normal mucosa effectively exclude active IBD. For the diagnosis of IBD, multiple representative biopsies are necessary. If possible, at least 2 biopsies in 5 places of the colon, including the rectum and the terminal ileum, must be taken. Representative biopsies must be taken from areas that have high, as well as low, levels of inflammation to properly represent the intensity and spectrum of inflammation. Normal appearance mucosa biopsies must be taken.50 Biopsies from strictures, polypoid lesions, or other lesions may be taken, and they must be marked in separate vials. Biopsies must always be accompanied by detailed clinical information to assist the pathologist in providing an accurate diagnosis. It is important to remember that histologic activity may poorly correlate with clinical activity. 54
10. When diagnosis is doubtful, it is appropriate to repeat the endoscopy and histology. Level of evidence: 5. Level of agreement: 13%.
One of the disadvantages in diagnosing IBD is the difficulty in making the differential diagnosis. In 10% of adult patients, the diagnosis might be changed from UC to CD or vice versa, and diagnosis of IBD can continue to be ruled out during the first five years after symptom onset.55 Errors have been documented in the diagnostic classification of patients included in IBD genetic studies, showing that IBD misdiagnosis was not uncommon.56 Approximately 5% of patients initially diagnosed with IBD are later diagnosed with IC. About 80% of patients with IC at symptom onset, are then diagnosed as UC or CD during the first eight years of follow-up, through the re-evaluation of the clinical and demographic characteristics.
11. Endoscopic evaluation should be performed in cases of relapse, refractoriness, new symptoms, or when surgery is considered. Level of evidence: 5. Level of agreement: 89%.
Endoscopic re-evaluation is currently indicated to optimize treatment, take mucosal biopsies, and rule out infection caused by cytomegalovirus. This is often the case in pediatric IBD, in which the rate of change in management after endoscopic assessment is above 42% of cases.57
12. Ileocolonoscopy is the baseline value test in the diagnosis of postoperative ileocolonic recurrence in CD. It defines lesion severity and predicts clinical course. Level of evidence: 2. It is recommended 6-12 months after surgery, the period in which treatment decisions may be affected. Level of evidence: 2. Level of agreement: 90%.
In the natural history of CD, intestinal resection is inevitable in a substantial number of patients. Most patients develop disease recurrence at the anastomosis site or proximal to the anastomosis, and endoscopic recurrence predicts the development of clinical symptoms. Endoscopic follow-up data of patients after resection and ileocecal disease have shown that in the absence of treatment, postoperative recurrence is about 65 to 70% in the subsequent 12 months, and 80 to 100% within the 3 years after surgery.58 Identification and treatment of early recurrence in the mucosa may prevent clinical recurrence. Ileocolonoscopy is the baseline value in the diagnosis of postoperative recurrence. It defines the presence and severity of morphologic recurrence. Ileocolonoscopy is recommended 6 months after surgery, when it can affect treatment decisions.
13. There are no specific endoscopic lesions of ulcerative colitis or CD. The most characteristic endoscopic findings of ulcerative colitis are those of continuous involvement of the colon with a clear demarcation between inflammation and rectal compromise. Level of evidence: 2. The most useful endoscopic findings in CD are non-continuous lesions, deep ulcers, a cobblestone appearance of the mucosa, the presence of strictures and fistula, and perianal involvement. Level of evidence: 2. Level of agreement: 100%.
There are no specific endoscopic features of UC or CD. In the absence of extra-colonic disease, certain endoscopic findings may suggest the diagnosis of CD, instead of UC diagnosis.48–50,55,56,59 The most important feature is the detection of areas of inflammation interspersed between normal-appearing mucosa (skip lesions). Deep linear or serpiginous ulcers, multiple aphthous ulcers, and cobblestone mucosa support the diagnosis of colonic CD. The presence of ileitis, perianal disease, or fistula openings indicates CD. The pattern of mucosal involvement in UC is continuous, with a clear demarcation of inflammation in the majority of cases, and rectal involvement is almost always present.59–65 Strictures are rare in UC and CD and malignant disease diagnosis must be considered. Detailed information from colonoscopy studies is important, because once therapy is begun, inflammation may appear segmental, and often with no rectal compromise.66 There are other disadvantages in the differentiation of UC and CD, one of which is reflux ileitis.49
14. Endoscopic evaluation with biopsies of at least one site is essential in severe ulcerative colitis for diagnostic confirmation and for ruling out other causes. Level of evidence: 3. In most cases, flexible sigmoidoscopy is sufficient, and colonoscopy and laxatives can be avoided. Level of evidence: 5. Level of agreement: 80%.
When urgent diagnosis is needed in a patient presenting with bloody diarrhea that is suspected of having acute IBD, flexible sigmoidoscopy with mucosal biopsies is the appropriate initial examination, because it helps differentiate UC from other causes of acute colitis.67 Infectious colitis may be found in 38% of the cases of patients with symptoms of acute hemorrhagic colitis. However, stool cultures are positive in only 40-60% of those patients and a negative culture does not rule out infection. Endoscopy may be used in conjunction with microbiologic tests in these patients.68
15. Endoscopic dilation of strictures in CD is a safe and effective alternative to surgery in experienced hands and should be considered before surgery in selected patients. Level of evidence: 2. The best results are obtained in short strictures (under 4cm) and in anastomotic strictures. Level of evidence: 2. The possibility of a malignant stricture must be excluded. Level of evidence: 3. Level of agreement: 100%.
Intestinal strictures are a major cause of morbidity in CD and they require surgery. Traditional treatment is surgical resection and strictureplasty, but due to the high recurrence rate, new surgery is needed.69 There is increased evidence for endoscopic balloon dilation as a safe and effective procedure, especially in anastomotic strictures and ileocecal strictures.70–87 However, these studies are retrospective with an observational design, and there are few prospective studies with long-term follow-up.73,75,82,84,88 The success of endoscopic dilation varied between 86 and 93% from the technical point of view and clinical success was defined as the resolution of obstructive symptoms in 64-70% of the patients.84,88
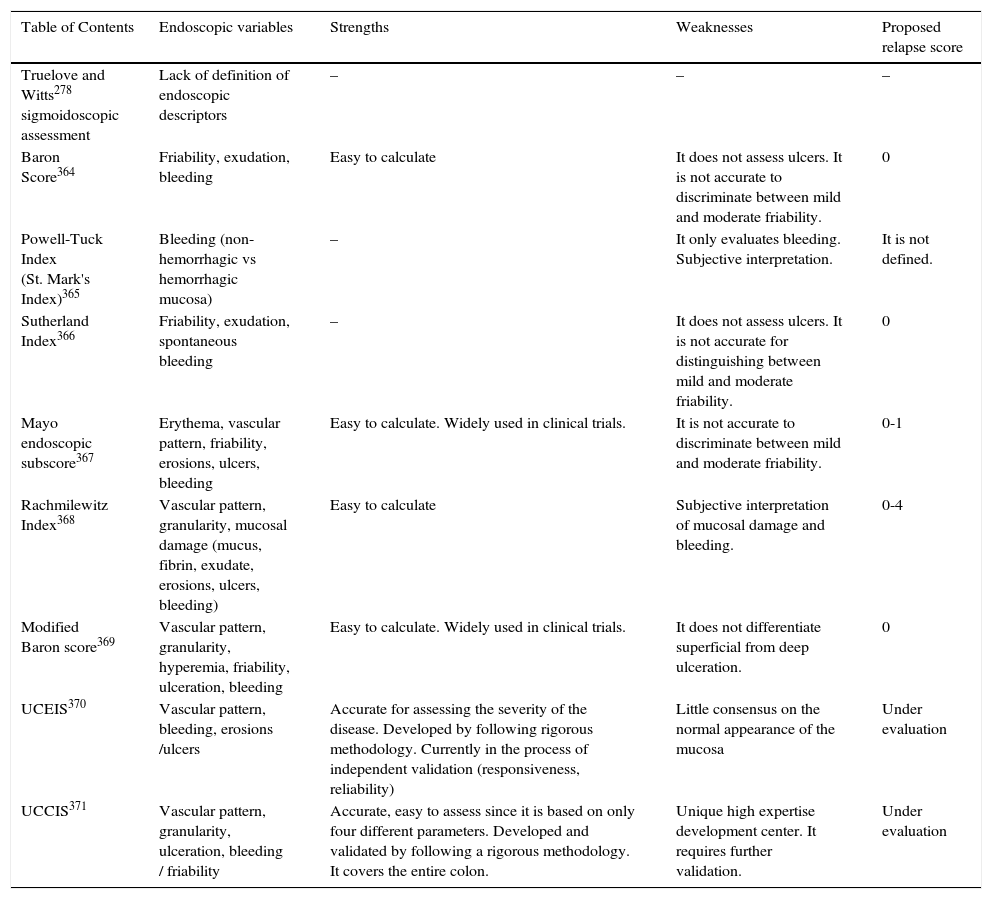
16. To evaluate endoscopic activity, we recommend using standardized and validated indices, such as the ulcerative colitis endoscopic index of severity (UCEIS) and the ulcerative colitis colonoscopic index of severity (UCCIS). Level of evidence: 2. The Mayo endoscopic subscore is used in randomized controlled studies, even though it is not validated. Level of evidence: 2. Level of agreement: 91%.
As shown in table 2.
Comparison of endoscopic score rates in ulcerative colitis (Ulcerative Colitis Endoscopic Index of Severity [UCEIS], Ulcerative Colitis Colonoscopic Index of Severity [UCCIS]).
| Table of Contents | Endoscopic variables | Strengths | Weaknesses | Proposed relapse score |
|---|---|---|---|---|
| Truelove and Witts278 sigmoidoscopic assessment | Lack of definition of endoscopic descriptors | – | – | – |
| Baron Score364 | Friability, exudation, bleeding | Easy to calculate | It does not assess ulcers. It is not accurate to discriminate between mild and moderate friability. | 0 |
| Powell-Tuck Index (St. Mark's Index)365 | Bleeding (non-hemorrhagic vs hemorrhagic mucosa) | – | It only evaluates bleeding. Subjective interpretation. | It is not defined. |
| Sutherland Index366 | Friability, exudation, spontaneous bleeding | – | It does not assess ulcers. It is not accurate for distinguishing between mild and moderate friability. | 0 |
| Mayo endoscopic subscore367 | Erythema, vascular pattern, friability, erosions, ulcers, bleeding | Easy to calculate. Widely used in clinical trials. | It is not accurate to discriminate between mild and moderate friability. | 0-1 |
| Rachmilewitz Index368 | Vascular pattern, granularity, mucosal damage (mucus, fibrin, exudate, erosions, ulcers, bleeding) | Easy to calculate | Subjective interpretation of mucosal damage and bleeding. | 0-4 |
| Modified Baron score369 | Vascular pattern, granularity, hyperemia, friability, ulceration, bleeding | Easy to calculate. Widely used in clinical trials. | It does not differentiate superficial from deep ulceration. | 0 |
| UCEIS370 | Vascular pattern, bleeding, erosions /ulcers | Accurate for assessing the severity of the disease. Developed by following rigorous methodology. Currently in the process of independent validation (responsiveness, reliability) | Little consensus on the normal appearance of the mucosa | Under evaluation |
| UCCIS371 | Vascular pattern, granularity, ulceration, bleeding / friability | Accurate, easy to assess since it is based on only four different parameters. Developed and validated by following a rigorous methodology. It covers the entire colon. | Unique high expertise development center. It requires further validation. | Under evaluation |
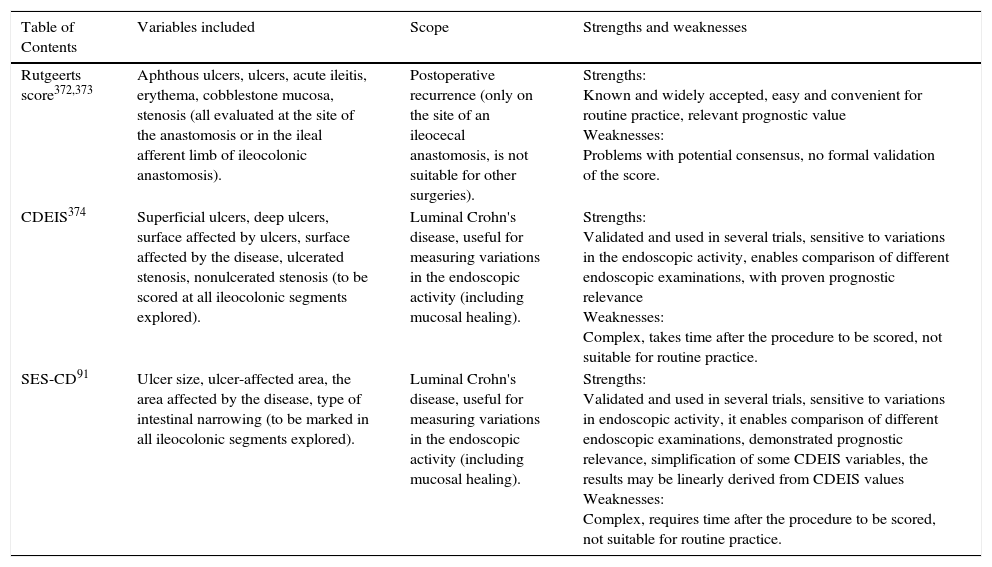
17. Recurrence of CD in the new terminal ileum after ileocecal resection should preferably be classified according to the Rutgeerts score. Level of evidence: 3. The CD endoscopic index of severity (CDEIS) (Level of evidence: 1) and simple endoscopic score for Crohn's disease (SES-CD) (Level of evidence: 1) are reproducible and validated measurement systems dedicated to measuring the intraluminal endoscopic activity of CD, but their clinical use is still to be defined. Level of evidence: 5. Level of agreement: 91%.
As shown in table 3.
Endoscopic scores more commonly used for Crohn's disease (Crohn's Disease Endoscopic Index of Severity [CDEIS], Simple Endoscopic Score for Crohn's disease [SES-CD]).
| Table of Contents | Variables included | Scope | Strengths and weaknesses |
|---|---|---|---|
| Rutgeerts score372,373 | Aphthous ulcers, ulcers, acute ileitis, erythema, cobblestone mucosa, stenosis (all evaluated at the site of the anastomosis or in the ileal afferent limb of ileocolonic anastomosis). | Postoperative recurrence (only on the site of an ileocecal anastomosis, is not suitable for other surgeries). | Strengths: Known and widely accepted, easy and convenient for routine practice, relevant prognostic value Weaknesses: Problems with potential consensus, no formal validation of the score. |
| CDEIS374 | Superficial ulcers, deep ulcers, surface affected by ulcers, surface affected by the disease, ulcerated stenosis, nonulcerated stenosis (to be scored at all ileocolonic segments explored). | Luminal Crohn's disease, useful for measuring variations in the endoscopic activity (including mucosal healing). | Strengths: Validated and used in several trials, sensitive to variations in the endoscopic activity, enables comparison of different endoscopic examinations, with proven prognostic relevance Weaknesses: Complex, takes time after the procedure to be scored, not suitable for routine practice. |
| SES-CD91 | Ulcer size, ulcer-affected area, the area affected by the disease, type of intestinal narrowing (to be marked in all ileocolonic segments explored). | Luminal Crohn's disease, useful for measuring variations in the endoscopic activity (including mucosal healing). | Strengths: Validated and used in several trials, sensitive to variations in endoscopic activity, it enables comparison of different endoscopic examinations, demonstrated prognostic relevance, simplification of some CDEIS variables, the results may be linearly derived from CDEIS values Weaknesses: Complex, requires time after the procedure to be scored, not suitable for routine practice. |
18. Mucosal healing in ulcerative colitis is associated with a lower risk for clinical relapse, hospitalization, colectomy, and risk for neoplasia associated with colitis. Level of evidence: 2. Level of agreement: 100%.
The aim of UC treatment is to heal the mucosa, which offers a better prognosis than symptom control. Mucosal healing may vary in its definition of mild erythema, granularity, and friability, 89 and include stricter definitions, such as normal mucosa with lack of any ulceration, with both microscopic and macroscopic healing.
19. Achieving mucosal healing through therapy for CD is associated with a decrease in relapse, hospitalization, and the need for surgery. Level of evidence: 2. Level of agreement: 100%. In the absence of formally validated definitions, mucosal healing may be defined as the absence of ulcers, and so use of the CDEIS is recommended. Level of evidence: 3. Level of agreement 100%. Early postoperative endoscopic recurrence (Rutgeerts score > i2) is associated with more frequent subsequent symptomatic and surgical recurrence. Level of evidence: 1. Therefore, medical treatment optimization must be considered. Level of evidence: 5. Level of agreement: 100%.
Increased evidence suggests that mucosal healing may change the natural course of CD, which decreases the frequency of relapses, hospitalization, and the need for surgery. Unfortunately, the definitions of mucosal healing vary greatly in the different clinical trials.90–102
20. In patients with suspected CD and negative ileocolonoscopy, capsule endoscopy could be the initial diagnostic modality (subject to availability) for the evaluation of the small intestine, in the absence of symptoms of obstruction or stenosis. Level of evidence: 2. Level of agreement: 91%.
CD often involves the terminal ileum, which can be treated with conventional ileocolonoscopy. However, in some patients, CD may affect the proximal small intestine, which is beyond the scope of ileocolonoscopy. In these patients, capsule endoscopy has a very high yield. It detects lesions better than resonance enterography, especially in early lesions. For this reason, when there is a suspected diagnosis of CD with negative ileocolonoscopy and no obstructive symptoms, video capsule endoscopy should be performed for diagnosis.41,42,50,103–109
21. Assisted enteroscopy is recommended in special cases for the evaluation of endoscopic findings, as well as biopsies for histologic evaluation. Level of evidence: 3. Level of agreement: 100%. If endoscopic therapy is indicated, including stenosis dilation, retained capsule removal, and treatment of bleeding, assisted enteroscopy must be performed by expert endoscopists. Level of evidence: 4. Level of agreement: 100%.
Deep enteroscopy is indicated in the diagnosis of CD when histology is required for the confirmation and exclusion of other pathologies. It is indicated in known CD, when needed to perform therapeutic endoscopy, such as stenosis dilation, the management of bleeding, masses, and polyps, and capsule placement and removal, among others.110–121
C. Imaging and histopathology22. The choice of CT enterography or MR enterography for the diagnosis of IBD must be in accordance with the availability of the method in the referral service. Level of Evidence: 4. Level of agreement: 100%. In emergency services, abdominal and pelvic CT or US should be used. Plain abdominal radiography has a role in clinical decision-making for specific emergency cases. MR enterography is the preferred study for patient follow-up. Level of evidence: 3. Level of agreement: 100%.
The availability of MR enterography or CT enterography is still limited to a few diagnostic centers, and they are secondary to the reduced availability of equipment and the interpretation expertise of the staff.122,123
In the emergency room, plain abdominal radiographs have been routinely used in the evaluation of patients with IBD, but are now used less frequently than US and CT, especially since the development of low-dose CT techniques.124–126
Although this method cannot properly evaluate disease activity, it can contribute to the assessment of the distribution and severity of colitis (extent of fecal waste, dilation, and wall thickening) and the location of small bowel obstruction (small dilation of the intestine). Plain abdominal x-ray together with chest radiograph may identify the perforation, but they are less sensitive than CT for detecting intra-abdominal abscesses and free gas.127,128
Abdominal US and plain x-ray should be considered for all IBD patients being evaluated for acute abdominal pain. CT should also be considered for patients with suspected perforation and negative or inconclusive first-line studies.129 In acute and severe colitis, plain abdominal x-ray is an acceptable first study to detect toxic megacolon (mean colonic dilation > 5.5cm in the transverse colon detected through imaging studies).130
In doubtful or selected cases, CT can also be used as the first imaging technique for tracking complications (e.g., perforation, abscess, thrombosis, ischemia) that require emergency surgery.131 Toxic megacolon is also predicted by the extension of the small intestine and gastric distension in most patients with severe colitis.129,131 Cross-sectional imaging can also be used, particularly MRI, to monitor therapeutic response, including CD of the small intestine and colon. However, there is a delayed timeline compared with the clinical or endoscopic changes in colonic CD. For the accuracy of other methods, this timeline is not well defined.129
23. When CD is suspected, CT abdominal and pelvic enterography or MR imaging with venous contrast and luminal distension (CT enterography or MR enterography) is recommended to evaluate the small intestine and colon, as well as disease extension. Level of evidence 5. Level of agreement: 100%.
24. Cross-sectional imaging techniques (MR enterography, CT enterography) enable the evaluation of disease activity and complications (inflammatory, stenosing, or fistulizing) in CD. They are important for monitoring progress and optimizing treatment. Level of evidence: 1. Level of agreement: 82%.
The use of intravenous (IV) contrast medium injection is required for evaluating the enhancement pattern of the intestinal wall and the mesenteric vessels.132–134 Intestinal distension is a fundamental requirement for any small intestine imaging method, since collapsed bowel loops can hide or simulate thickening of the wall and pathologic lesions.135 Biphasic contrast agents include various nonabsorbable iso-osmolar solutions (polyethylene glycol or mannitol solutions).129
CT enterography is faster, less demanding for radiologists, and provides good mid-terminal ileum distension, but it offers limited distension of the jejunum.136 Radiation exposure is the main limitation of CT, especially in patients undergoing repeated examinations.137
CT and MR have a similar diagnostic accuracy for IBD images. CT has greater availability and requires less time than MRI.137 Depending on the location and intensity of the disease activity, CT and MRI can detect signs of CD. For the initial presentation of terminal ileum location of CD, diagnostic accuracy is high and comparable between CT and MRI. At the location of the small intestine, MRI changes related to the presence of inflammation include wall thickening, wall hyper-enhancement after injection of the MR contrast medium, presence of edema of the wall, and presence of ulcers, as well as changes outside the wall, such as the presence of comb sign, fat stranding, and lymph node enlargement.138
In colonic CD, MRI can provide useful information on the extent of damage (wall thickening, presence of ulcers, wall depth, penetration, edema, loss of haustration, polyps, and extraluminal findings/complications), although mild disease may not be detected.139,140
Furthermore, these modalities have high accuracy for evaluating the penetrating phenotype and diagnosis of stenosis of the small intestine.129,141
Comparing the accuracy of MRI and CT for diagnosis in patients with suspected or established IBD, mainly CD, a high mean sensitivity and no significant difference for the diagnosis of IBD were detected on a per patient basis between the imaging modalities (93 and 88%, for MRI and CT, respectively). The calculated mean specificity per patient was also high, 93% for MRI and 95% for CT.142
25. Abdominal US in expert hands is a well tolerated, radiation-free imaging technique, particularly for examination of the small intestine and colon, and can guide interventional procedures (for example, drainage of abscesses). When used with contrast it may improve diagnostic precision. Level of evidence: 2. Level of agreement: 90%.
When abdominal US is used for the study of IBD, high-frequency linear probes (17.5MHz) will be needed to enhance spatial resolution and to allow proper evaluation of the diameter of the intestine and the recognizable pattern of the 5-layer wall.143
A systematic approach is recommended to look for abnormalities in the intestinal wall, including four scanning positions in the upper and lower right and left quadrants. The ileocecal region, sigmoid colon, as well as the upper and lower regions of the colon, are effectively visualized in most patients. The proximal ileum and jejunum may be difficult to assess due to multiple overlying bowel loops and deep pelvic location, whereas the study of the transverse colon is difficult due to its variable anatomy and accessibility of the rectum. Contrast-enhanced ultrasound (CEUS) may improve diagnostic precision and diagnostic confidence in detecting inflammatory activity.144,145
The guiding of interventional procedures is also a validated technique. For example, percutaneous or transrectal abscess drainage under US guidance has a high technical success rate of 96%.146
26. Conventional radiology (barium small bowel follow-through) is an alternative in the absence of CT and MRI for diagnosing superficial and transmural lesions. Small bowel transit study with or without enteroclysis is not accurate for evaluating disease activity and is not useful for mural and extramural complications, compared with MR enterography and CT enterography. Level of evidence: 3. Level of agreement: 82%.
27. Abdominal US, CT enterography, and MR enterography are highly accurate for evaluating penetrating complications and monitoring disease progression. Level of evidence 1. Level of agreement 91%.
For complex perianal fistulas, pelvic MRI or endoanal US are preferred. Level of evidence: 4. Level of agreement: 91%.
Abdominal US, CT, and MRI are highly accurate for evaluating penetrating complications (i.e., fistula and abscess) and monitoring disease progression. For deep fistulas, CT and MRI are preferable to US.129 Penetrating complications can be detected by US, with sensitivities that vary between 71 and 87% and specificities ranging from 90 to 100%.147 The diagnostic usefulness of MRI for intra-abdominal colon fistulas was determined, reporting sensitivity between 71 and 100% and specificity between 92 and 100%.148,149
Using a surgical reference standard, similar diagnostic accuracy was demonstrated between CT and US for the diagnosis of intra-abdominal fistulas complicating CD: sensitivity and specificity were 68 and 91% for CT, compared with 87 and 91% for US, respectively.147
The value of US for detecting abscesses showed sensitivity ranging from 81 to 100% and specificity from 92 to 94%. A comparison of US and CT, using a surgical reference standard, showed that abscesses were correctly detected in similar proportions, US 91% and CT 86%. However, overall precision was higher for CT (92%) than for US (87%).147
Both US and MRI are able to identify and classify fistulous tracts with good precision. MRI is the most precise diagnostic imaging method (80-100%) for perianal CD. It is recommended during the initial diagnosis, unless there is an immediate need for drainage of sepsis. Anal US is superior to clinical examination, with precision that varies between 50 and 100%. It is an alternative to MRI.129 In turn, these 2 procedures are superior to simple clinical evaluation for assessing the response to treatment, especially for the detection of residual abscesses. Significant changes in, or cessation of, surgical or medical therapy must also be taken into consideration. Although there are direct comparisons between MRI and endoanal US, MRI has shown greater clinical use for evaluating fistula healing, particularly during medical therapies.150–152
28. Cross-sectional imaging methods, CT enterography, and MR enterography, as well as conventional radiography (intestinal transit study with or without enteroclysis), are highly sensitive and specific for diagnosing stenosis of the small intestine. Level of evidence: 2. Level of agreement: 82%. The diagnostic precision of MR enterography and CT enterography for stenosis is based on the use of luminal contrast. CT enterography, abdominal US, and MR enterography can help differentiate between inflammatory or predominantly fibrotic stenoses. Level of evidence: 5. Level of agreement: 82%.
29. Biopsies of the gastrointestinal tract are a necessity, but histopathologic findings are not always conclusive for the diagnosis of IBD. Level of evidence: 5. Level of agreement: 82%.
Before starting any type of treatment, it is important to perform histologic examination in patients with suspected IBD. This facilitates proper diagnosis and excludes changes of morphology induced by certain medications. Histopathologic diagnosis cannot be established if the number of biopsies is low, the biopsy is not well determined or obtained from all the segments, or if there are not enough clinical, endoscopic, or histologic parameters necessary for making the diagnosis.
30. Samples sent for histologic analysis must be accompanied by the patient's clinical history, age, disease duration, the type and duration of comorbidity treatment, as well as by a description of the endoscopic findings. Level of evidence: 5. Level of agreement: 91%.
The diagnosis of IBD is based on a multidisciplinary approach, associated with clinical history, physical examination, laboratory work-up, typical endoscopic and histologic data, and radiologic findings. Histologic examination of endoscopic samples or resection specimens is a key step in the evaluation of affected patients. It can also be used for the differential diagnosis.50,153 The necessary information should include demographics, disease characteristics, disease duration, comorbidities, recent trips, endoscopic findings, and any treatment information.
31. For proper baseline evaluation of IBD, the material of the terminal ileum, as well as serial samples of the colon and rectum, must be collected in separate vials. At least 2 samples must be collected per segment. Normal and abnormal mucosal areas must be packaged in separate vials. Level of evidence: 1. Level of agreement: 100%.
In patients with suspected IBD, the histologic analysis of samples obtained from inflamed segments must be performed before starting treatment so that a proper diagnosis can be made. Diagnosis is based on the analysis of a complete series of colonoscopic biopsies.67 Rectal biopsies are needed to rule out or confirm rectal involvement and help distinguish it from other inflammatory lesions. Atypical distribution of lesions, such as peri-appendiceal inflammation, associated with left-sided colitis, can only be detected by this method.154 Terminal ileum biopsies must also be performed in order to confirm the suspicion of CD or make a differential diagnosis with backwash ileitis, which occurs in patients with UC. Samples must be collected in separate vials to facilitate diagnosis of discontinuous involvement of CD, as well as its location.155 Samples must be fixed immediately in 10% formalin. The use of filter paper or any similar product is not recommended. Correct inclusion in paraffin is essential for diagnosis (facilitated by staining of the fragments before processing), since it prevents tangential sections. Multiple cuts are recommended to detect focal changes.
32. The following microscopic criteria must be taken into account for ileum and colon CD (in endoscopic biopsies): focal chronic inflammation, discontinuous crypt distortion, and granulomas (unrelated to the crypt lesion). Level of evidence: 2. Level of agreement: 100%.
A wide variety of microscopic characteristics must be evaluated to help establish the diagnosis of CD. The variable increase in cellularity (lymphocytes and plasma cells) in the lamina propria must be considered (discontinuous) focal inflammation. Such inflammation can be seen in a biopsy sample. Focal inflammation is characterized as a localized increase in round cells with or without granulocyte infiltration, confined to one or more foci. This inflammatory process may occur against the normal background of round cells or may be associated with varying degrees of inflammation that may infiltrate the submucosa.54 The irregularity of the crypt (distortion and branching and shortening of the crypt) may occur, regardless of the degree of inflammatory process.54
Granuloma (collection of epithelioid histiocytes with boundaries that are not well defined) is considered the pathognomonic characteristic of CD, but only in the lamina propria. It is not related to crypt lesions. Noncaseating granulomas, small collections of epithelioid histiocytes, and giant cells, or isolated giant cells, can be seen in various types of infectious colitis. In samples of intestinal resection, the presence of transmural lymphoid aggregates, mainly outside the ulcerated areas, and granulomas non-related to crypt lesions, are typical characteristics of CD.54 A wide variety of microscopic characteristics must be evaluated to help make CD diagnosis. The irregular nature of inflammation can also be seen in the resolution of active UC in young people with UC (< 10 years) and in adult patients with untreated CD. 41,156,157
It has been suggested that the diagnosis of CD from surgical material or endoscopic biopsies be made when 3 histologic characteristics are present in the absence of granulomas, or when an epithelioid granuloma is present with other histologic characteristics, after exclusion of specific infections. The second characteristic can be focal inflammation or, preferably, architectural abnormalities.54
33. In the baseline histologic analysis, the pathologist must make the differential diagnosis of IBD and other intestinal diseases, including CD and ulcerative colitis. Discrimination between colonic CD and ulcerative colitis is not always possible. Level of evidence: 2. Level of agreement: 100%.
The diagnosis of IBD in general depends on the complex evaluation of several microscopic changes and their topographic distribution. Precise discrimination between CD and UC is not yet optimal among expert gastrointestinal pathologists, with correct diagnosis in 64% of cases with CD and 74% of cases with UC. An International Meeting of Expert Gastrointestinal Pathologists concluded that: 1) multiple biopsies are necessary to establish a precise diagnosis of IBD; 2) rectal biopsies alone are not diagnostic; 3) the overall diagnostic accuracy of endoscopic criteria and guidelines among pathologists may improve diagnostic accuracy, especially in CD. Several useful parameters that contribute to the diagnosis of CD in surgical specimens are not present in the samples collected by endoscopic biopsies (transmural inflammation, fibrosis, fistula); 4) most UC lesions are limited to the mucosa and submucosa, and can be detected in endoscopic biopsies.154,158 The macroscopic description of the resected specimen in UC is characterized by a continuous inflammatory process with proximal extension from the rectum. However, there may be a rare pattern without inflammation in the rectum or reflux ileitis.
34. The following must be considered microscopic criteria of UC: widespread distortion of crypt architecture, continuous inflammation of the mucosa with basal plasmacytosis, with or without association with cryptitis and crypt abscesses, and marked depletion of goblet cells. Level of evidence: 1. Level of agreement: 100%.
The chronic process with distorted architecture and inflammatory infiltration limited to the mucosa is a major microscopic characteristic of UC. The lack of fissures, the irregular and distorted architecture of the villi, and crypt branching and atrophy are most common in UC.54 Inflammatory infiltration is continuous with increasing severity toward the rectum. Cellularity is higher in the mucosa compared with the submucosa, and is comprised of lymphocytes, plasma cells, and of neutrophils that cause cryptitis (presence of neutrophils within the crypt epithelium) and crypt abscesses (presence of neutrophils within the crypt lumina).54 The distinction between the first attack of UC and infectious colitis can be made when there is a predominant presence of plasma cells between the base of the crypts and the muscularis mucosae (basal plasmacytosis) in UC (63% vs 6%). This rare characteristic can be seen in CD. Suppression of epithelial mucin is a minor diagnostic characteristic, which can also be detected in infectious colitis and CD.159 Other characteristics related to a chronic inflammatory process that can be seen are: inflammatory pseudopolyps, muscular hypertrophy of the mucosa, and rarely, submucosal fibrosis. An important observation related to the morphologic characteristics is that they may change, depending on patient age, disease duration, and prior treatment.54
The pathologist's report should contain a microscopic description based on a minimum of elements to justify the diagnosis of IBD. The use of a specific classification is not required.
35. Dysplasia (intraepithelial neoplasia) associated with colitis occurs only in areas of chronic inflammation, and can be divided into morphologic categories: negative, indefinite, and positive for low-grade or high-grade dysplasia. Confirmation of dysplasia by an independent expert GI pathologist is recommended. Level of evidence: 2. Level of agreement: 100%.
The concept of dysplasia is histologic neoplastic epithelium with no invasion160 and is the best and most reliable marker of increased risk for progression to neoplasia in patients with UC.161 Dysplasia may occur anywhere in the colon and is often multifocal, but it may also be an isolated focus. However, dysplasia must be considered related to IBD if it developed within areas of chronic inflammation.161,162 Dysplasia is stratified into 3 categories: negative for dysplasia, indefinite for dysplasia, and positive for dysplasia (low-grade and high-grade).160 The microscopic parameters used in the diagnosis of dysplasia include: overcrowding of glands, mucosal thickening, and elongation and distortion of the crypts, with excessive buds and enlargement. The surface and crypts are lined by tall columnar cells, in which there is some mucosal differentiation. Mucin tends to stay in columnar cells rather than in normal goblet cells. Nuclear alterations are similar to those seen in the tubular adenomas of patients without IBD (hyperchromatic and elongated nuclei and frequent overlay of nuclear stratification). Mitotic nuclei may be present in the upper part of the crypts and even on the surface (which is abnormal).160
There should be a second opinion on the histopathology report (review of plates and blocks of collected samples) to confirm the initial diagnosis of dysplasia made by the expert pathologist.162,163
There is greater agreement between gastrointestinal pathologists when dysplasia is high-grade or negative, but it is low for low-grade or indefinite dysplasia.164 The immunohistochemical detection of P53 is not useful in IBD for differentiating between regeneration and true dysplasia because of its high rate of false positives.54
TreatmentA. Conventional36. Treatment with topical aminosalicylates at doses of 1g/day is recommended as first choice for inducing remission in patients with mild-to-moderate active proctitis. Level of evidence: 1b. Level of agreement: 100%.
In a meta-analysis of 38 studies on patients with mild-to-moderate UC,165 10 studies compared rectal 5-ASA with placebo and demonstrated that topical 5-ASA drugs are more effective than placebo, with an OR for clinical remission of 8.30 (8 studies, 95% confidence interval [95% CI]: 4.28-16.12; p<0.00001) and an OR for endoscopic remission of 5.31 (7 studies, 95% CI: 3.15-8.92; p<0.00001). Rectal 5-ASA drugs were superior to rectal steroids in inducing symptomatic remission, OR of 1.65 (6 studies, 95% CI: 1.11-2.45; p=0.01). There were no differences between doses of 1-4g, regardless of whether suppository, enema, or foam was used.
Topical 5-ASA is more effective than oral 5-ASA for ulcerative proctitis.166 A recent randomized controlled study showed that 5-ASA suppository achieved endoscopic remission of 83.8% in 4 weeks, compared with 36.1% with placebo.167
A recent consensus168 suggests that it is preferable to use 5-ASA in suppositories for patients with ulcerative proctitis at doses not exceeding 1g/day. For patients with ulcerative proctosigmoiditis and active left colitis, it is preferable to use 5-ASA in enemas or foam.169,170
37. Treatment with oral aminosalicylates at doses between 3.0 and 4.8g per day or sulfasalazine 4.5g per day is recommended for induction of remission in patients with mild-to-moderately active UC, with any extension beyond the rectum. Level of evidence: 1a. Level of agreement: 100%.
There is clinical evidence that demonstrates the efficacy of oral aminosalicylates in mild-to-moderate UC. Two meta-analyses with 8 and 11 studies showed efficacy for induction of remission with a RR of 0.86 (95% CI: 0.81-0.91) and 0.79 (95% CI: 0.73-0.85), respectively.171,172 Regarding doses, 2.0g/day was superior to a dose of < 2g/day, but a nonsignificant difference was found between doses of 2.4 and 4.8g/day. However, a subgroup analysis in patients with moderate activity showed that these patients benefited from higher doses.173–175 It is worth mentioning that when the analyzed variable was endoscopic remission, doses of 3g/day or higher were more efficient.176 Sulfasalazine was as effective as the different salicylates used in inducing remission.177 Patients with UC should be evaluated within 4-8 weeks after beginning treatment with 5-ASA and if there is no symptomatic response, the need to modify treatment should be considered.168
No significant differences have been found between 5-ASA drugs and placebo in the incidence of adverse effects.178 However, 15% of patients do not tolerate these medications. Adverse effects include flatulence, abdominal pain, nausea, diarrhea, headache, clinical deterioration of UC, skin irritation, and thrombocytopenia. Idiosyncratic renal impairment has been described, so it is recommended to evaluate kidney function before and during treatment with these medications.179
38. Concomitant treatment with oral and topical aminosalicylates is superior to oral aminosalicylate as first-line treatment for inducing remission in patients with mild-to-moderately active UC, with any extension beyond the rectum. Level of evidence: 1b. Level of agreement: 100%.
A meta-analysis with 4 randomized controlled studies showed that the combination of oral and topical 5-ASA was superior to oral 5-ASA for the induction of remission of active UC with any extension beyond the rectum, with a RR of 0.65 (95% CI: 0.47-0.91).166 No significant difference in adverse events between the 2 groups was found, 22.3 and 26.9%, respectively, RR 0.77 (95% CI: 0.55-1.09). A recent consensus recommends that patients with UC under treatment with 5-ASA be evaluated if there is no symptomatic response in 4-8 weeks to determine whether treatment needs to be modified.168 In patients with mild-to-moderate UC that do not respond to treatment with oral aminosalicylates, the suggestion is not to switch to another class of 5-ASA drug, since, in terms of safety, no significant differences in clinical efficacy have been found among the diverse classes of 5-ASA drugs.180
39. One daily dose of oral aminosalicylates can be used to induce and maintain clinical remission in UC patients, and in turn, improve treatment adherence. Level of evidence: 1b. Level of agreement: 80%.
A meta-analysis with 3 studies showed no significant difference between using a single dose of 5-ASA and multiple doses per day to induce remission, with a RR of 0.95 (95% CI: 0.82-1.10).181 A recent additional study reported no difference in remission or safety rates between a single dose daily or twice daily with oral 5-ASA.182 In maintaining remission, a meta-analysis with 7 studies demonstrated no significant difference in the relapse rate by comparing a single dose daily with a conventional dose, RR 0.94 (95% CI: 0.82-1.08). Additionally, no significant difference was found regarding adverse events.183 Most patients prefer a single daily dose, which results in increased treatment adherence, especially during the maintenance phase.184,185
40. In patients with mild-to-moderate UC that achieve clinical remission with oral or topical aminosalicylates, continuing the same therapy for complete remission maintenance is recommended. The recommended dose of oral 5-ASA should be individualized for each case, and the recommended dose is at least 2g/day. Level of evidence: 1b. Level of agreement: 100%.
There is a high risk for relapse in subjects with UC, and so maintenance therapy is necessary in these patients. A Cochrane meta-analysis showed an OR of 0.47 (95% CI: 0.36-0.62), with a number needed to treat (NNT) of 6, in favor of oral 5-ASA, compared with placebo, for maintaining clinical remission.186 Both sulfasalazine and mesalazine are clearly more effective than placebo in preventing relapses of UC, with no significant differences between them.177 The ideal dose of sulfasalazine for maintenance is 2g daily. There is no proof that doses greater than 2g/day of mesalazine are more effective, but it should be mentioned that a very limited number of patients receiving higher doses have been studied.187 A meta-analysis of 7 studies for maintenance with rectal 5-ASA found a RR for relapse of 0.60 (95% CI: 0.49-0.73) and a NNT of 3. Compared with placebo, there was no difference for adverse events.188 A recent consensus suggests that rectal 5-ASA can be used daily or at a reduced frequency to maintain complete remission.168
41. In patients with moderate-to-severe UC of any extension, the use of oral systemic steroids as first-line treatment is indicated for inducing clinical remission. The use of oral systemic steroids as second-line therapy in the induction of remission of patients with mild-to-moderately active UC that are resistant to aminosalicylates is recommended. The use of oral systemic steroids for more than 12 weeks is not recommended. Steroids are not useful as remission maintenance therapy in UC. Furthermore, their prolonged use is associated with adverse events. Level of evidence: 1b. Level of agreement: 100%.
A meta-analysis with 5 randomized controlled studies showed that steroids are superior to placebo for induction of remission in patients with UC, RR 0.65 (95% CI: 0.45-0.93).189 A systematic review reported that there were no benefits with doses above 60mg/day. Therefore, it is suggested to use doses of oral prednisone between 40-60mg/day.165 Approximately 50% of patients using steroids experience adverse events such as acne, edema, mood swings, glucose intolerance, and dyspepsia, among others.190 A recent Canadian consensus recommends the evaluation of patients with UC under treatment with steroids for the induction of remission that have no symptomatic response, so that the need for treatment modification can be determined.168
42. Rectal steroids are suggested as second-line therapy for inducing complete remission in patients with mild-to-moderate ulcerative proctitis that do not respond to topical 5-ASA. Level of evidence: 1b. Level of agreement: 91%.
A meta-analysis on conventional steroids and rectal budesonide showed that rectal steroid therapy was superior to placebo in inducing clinical remission. However, a meta-analysis of 6 randomized controlled studies showed that rectal 5-ASA was superior to rectal steroids for inducing clinical remission, with an OR of 1.65 (95% CI: 1.41-2.88 p=0.0001).165 Therefore, in patients that do not respond to rectal 5-ASA, a reasonable second-line therapy may include the addition of rectal steroids. A recent study with budesonide foam demonstrated efficacy in inducing remission in patients with mild-to-moderately active ulcerative proctitis and proctosigmoiditis, compared with placebo.190
43. The use of novel oral steroids of low bioavailability, such as budesonide multimatrix (MMX), is indicated for inducing remission in patients with mild-to-moderately active UC of any extension that is aminosalicylate-resistant. This can be tried before the use of systemic steroids. Level of evidence: 1. Level of agreement: 91%.
Randomized controlled studies with oral budesonide MMX have shown it to be more effective than placebo, and to be as effective as oral 5-ASA for inducing clinical remission.191–193 However, this has not been demonstrated with other formulations of ileal release budesonide, such as Entocort® and Budenofalk®, whose effectiveness was lower than placebo and 5-ASA.194,195 Compared with conventional steroids, budesonide has fewer systemic adverse events (33% vs 55%)196 and it has not been associated with a significant decrease in bone mineral density.197
44. The use of IV systemic steroids, such as hydrocortisone 100mg every 6 to 8hours or methylprednisolone 60mg per day, is recommended to induce remission in patients with severe acute UC that require hospitalization. Level of evidence: 2b. Level of agreement: 100%.
In 1974, a publication by Truelove and Jewell showed that IV steroids are effective as a first-line treatment for acute severe UC, with clinical remission in 36 of 49 patients (73.5%), after 5 days of treatment.198 Subsequent studies have shown that IV steroids reduce morbidity and mortality in this patient population.199,200 With respect to the colectomy rate, no differences were found regarding the efficacy of the different types of steroids or the doses used. Consequently, it is not recommended to use an IV methylprednisolone dose above 60mg or its equivalent.199 A study that compared the use of an IV bolus of methylprednisolone every 12h with its continuous infusion, showed no significant differences regarding response or adverse events between the 2 regimens.201
45. Thiopurine immunomodulators are not recommended for inducing remission in patients with mild-to-moderate active cortico-resistant UC. Level of evidence: 1b. Level of agreement: 100%.
A meta-analysis of 4 controlled studies showed that azathioprine and 6-mercaptopurine were not effective for the induction of remission in patients with UC, in comparison with placebo or 5-ASA (OR 1.59, 95% CI: 0.59-4.29; p=NS).202 An analysis of the 2 studies compared with placebo203 found no significant benefit in endoscopic remission (RR 0.85; 95% CI: 0.71-1.01)204 or clinical remission (OR 1.44, 95% CI: 0.68-3.03, p=NS). An Italian study showed that azathioprine was more effective than 5-ASA in the induction of steroid-free complete remission in the group of patients with steroid-dependent UC (OR: 4.78; 95% CI: 1.57-14.5, p=0.006).205
46. The use of thiopurine immunosuppressants is recommended for maintaining remission in patients with cortico-dependent UC. Level of evidence: 1b. Level of agreement: 100%.
A meta-analysis of 4 randomized and controlled studies found that 44% of patients that receive azathioprine failed to maintain remission, compared with 65% of patients that received placebo, RR: 0.68 (95% CI: 0.54-0.86).206 Similar results were found in another meta-analysis of 3 studies, RR 0.60 (95% CI: 0.37-0.95).204 The quality of these studies was insufficient, with a reduced number of patients and heterogeneity. Among the adverse events associated with the use of thiopurine are bone marrow suppression, pancreatitis, hepatotoxicity, allergic reactions, and opportunistic infections, especially when the drug is combined with steroids or tumor necrosis factor alpha inhibitors.207 Additionally, there is risk for lymphoma (including hepatosplenic T-cell lymphoma)208 and non-melanoma skin cancer.209 The response to thiopurines should be evaluated in 10-12 weeks. Ideally, the levels of the thiopurine methyltransferase enzyme should be measured before starting the use of thiopurines, in order to identify patients at risk for myelosuppression. This does not replace the continuous monitoring of complete blood counts in these patients.207 The use of methotrexate is not recommended for inducing or maintaining complete clinical remission in patients with UC.
47. IV ciclosporin at a dose of 2mg/kg for inducing remission in patients with severe active UC refractory to IV systemic steroids is recommended in centers with experience in its use. Level of evidence: 1b. Level of agreement: 100%.
Ciclosporin is a calcineurin inhibitor, and it has traditionally been used as a second-line IV agent in patients with acute-to-severe UC that are refractory to IV steroids. In a randomized placebo-controlled study, 20 patients that had not responded to treatment with IV steroids for 7 days were given ciclosporin at a dose of 4mg/kg/day. Eighty-two percent of the patients responded, compared with 0% in the placebo group (p<0.001).210
Another randomized and controlled study made a comparison between ciclosporin doses of 4mg/kg/day vs 2mg/kg/day. No significant differences were found in terms of clinical response, and the main adverse event in the group with the highest dose was high blood pressure.211 A Cochrane review with 2 randomized and controlled studies found that in patients with severe UC, the lack of response to medical treatment was less frequent with ciclosporin, when compared with placebo, RR 0.18 (95% CI: 0.05-0.64).210 In ciclosporin-controlled studies, the portion of patients that avoided colectomy in the short term ranged from 64 to 90%. However, the long-term colectomy rate in responder subjects was 20% at one year, and 69% at 5 years.212,213
48. As a first-choice treatment of mild localized ileocecal CD, the use of ileal-release budesonide at a dose of 9mg/day is recommended. Level of evidence: 1a. Level of agreement: 82%. The benefit of mesalazine is limited. Level of evidence: 1b. Level of agreement: 82%. It is suggested that patients with colonic CD and mild activity can be treated with sulfasalazine. Level of evidence: 1b. Level of agreement: 82%.
A significant percentage of patients with CD have a mild behavior pattern of the disease. Budesonide at a dose of 9mg/day is the therapy of choice for inducing remission in patients with CD with mild activity and ileocolonic location. Clinical studies have shown that budesonide is superior to placebo (RR 1.96, 95% CI: 1.19-3.23) and mesalazine (RR 1.63, 95% CI: 1.23-2.16).214 Budesonide is preferred over prednisolone because it is associated with minor adverse events (RR 0.64, 95% CI: 0.28-0.95). Remission rate with budesonide is 51-60% in 8-10 weeks, according to several studies.215,216
A meta-analysis of 3 large clinical trials found no significant clinical efficacy with mesalazine in patients with ileocecal CD of mild-to-moderate activity.217 A more recent meta-analysis found a trend toward a beneficial effect related to the use of sulfasalazine, in comparison with placebo (2 studies), with a RR of failure to achieve remission of 0.83 (95% CI: 0.69-1.00). A systematic review and meta-analysis of randomized controlled trials reported no benefit with mesalazine (4 studies) RR: 0.91 (95% CI: 0.77-1.06).218
49. The use of oral systemic corticosteroids is recommended for inducing remission in patients with mild-to-severe, ileocecal or colonic, active CD. Level of evidence: 1a. Level of agreement: 100%.
Prednisolone is an appropriate option for mild-to-severe CD. A Cochrane systematic review with 2 studies showed that corticosteroids are more effective than placebo in inducing remission, with a RR of 1.99 (95% CI: 1.51-2.64, p<0.00001). The idea is to minimize prolonged exposure with steroids in patients with CD, given the lack of efficacy in maintaining remission.219
50. The use of oral systemic corticosteroids is recommended for inducing remission in patients with extensive small bowel CD. It is suggested to associate the use of thiopurines or methotrexate to maintain remission. Level of evidence: 5. Level of agreement: 100%.
CD is defined as extensive when the involvement exceeds 100cm in length, which is associated with nutritional deficiencies. In these patients, treatment with systemic steroids is recommended, sometimes associated with thiopurines or anti-TNF biologic therapy, depending on the severity of the disease.196 Nutritional support is important in the treatment of these individuals.220 The surgical option must always be considered in this group of patients.
51. In patients with CD that achieve remission with systemic corticosteroids, the use of thiopurines or methotrexate is recommended. Level of evidence: 1b. Level of agreement: 100%. The use of corticosteroids is not recommended as remission maintenance therapy. Level of evidence: 1a. Level of agreement: 100%.
A meta-analysis of 6 clinical studies compared azathioprine with placebo, and it found a remission rate of 71% vs 52%, respectively (OR 2.32; CI: 1.55-3.49, with NNT: 6, to prevent relapse), with a dose-response effect of 1mg/kg/day (OR: 1.2; 95% CI: 0.60-2.41), 2mg/kg/day (OR 3.01; 95% CI: 1.66-5.45), and 2.5mg/kg/day (OR 4.13; 95% CI: 1.59-10.71).221
One study compared intramuscular methotrexate at doses of 15mg weekly vs placebo, for the maintenance of remission in patients with CD. At 40 weeks, the remission rates were 65% vs 39% (p=0.04), respectively.222 A meta-analysis of 12 studies by Seow et al. showed that budesonide at doses of 6mg/day was not more effective than placebo in maintaining remission at 3 months (RR 1.25, 95% CI: 1.00-1.58; p=0.05), 6 months (RR 1.15, 95% CI: 0.95-1.39; p=0.14), or 12 months (RR 1.13, 95% CI: 0.94-1.35; p=0.19).214
B. Biologic Treatment52. Biologic therapy against TNF-alpha (anti-TNF-¿), such as infliximab, adalimumab, and certolizumab pegol, is indicated in patients with moderate-to-severe CD that have been refractory or intolerant to treatment with steroids and immunomodulators. Level of evidence: 1. Level of agreement: 100%.
The ACCENT 1 study evaluated the efficacy and safety of infliximab in patients with CD through a multicenter, randomized, double-blind, placebo-controlled, 54-week trial involving 573 patients with a CDAI score > 220. A total of 335 patients that responded to induction doses were randomized into 3 groups: group I with placebo; group II with infliximab at a 5mg/kg maintenance dose; and group III with infliximab at a 10mg/kg maintenance dose.
At weeks 30 and 54, the proportion of patients in remission was higher in infliximab groups II (39%) and III (45%), compared with group I (21%) (p=0.002). No difference was found in the remission rates between the groups receiving 5mg/kg and 10mg/kg of infliximab. At week 54, 29% of the patients were in clinical remission in the infliximab groups and had discontinued their treatment with corticosteroids, in comparison with 9% in the placebo group (p=0.004). This trial showed that the administration of infliximab was effective and safe in patients with CD.95
The CLASSIC I study was a multicenter, randomized, double-blind, placebo-controlled trial with different induction dose ranges. In this study, patients with moderate-to-severe CD that were naïve to anti-TNF therapy received induction therapy at weeks 0 and 2 with adalimumab 40/20mg, 80/40mg, 160/80mg, or placebo, and had follow-up at week 4.
The primary outcome measure of this study was the efficacy of induction therapy with adalimumab in patients with CD. A total of 299 patients were randomized at week 0 to receive induction with placebo, adalimumab 40mg/20mg, adalimumab 80mg/40mg, or adalimumab 160mg/80mg. A statistically significant difference (p=0.004) was found in the remission rates between the groups of patients that received adalimumab 80/40mg (24%), adalimumab 160/80mg (36%), and placebo (12%) at week 4. This study showed that the 160/80mg adalimumab induction dose was superior to the other adalimumab doses and placebo in patients with moderate-to-severe CD activity.223
The CLASSIC II study included patients that achieved remission after a 4-week induction in the CLASSIC I study, and also maintained remission for 4 additional weeks (weeks 0 and 4 of CLASSIC II) with adalimumab 40mg doses every 2 weeks, in the open-label phase. Patients were subsequently randomized for receiving adalimumab or placebo for another 56 weeks in a double-blind trial.
From a total of 256 patients, there was a significant difference in the remission rate between the groups treated with adalimumab 40mg every 2 weeks (79%) and adalimumab 40mg every week (83%), compared with placebo (44%) (p<0.05).
The results of this study support the efficacy of adalimumab in inducing and maintaining remission in infliximab therapy-naïve patients with moderate-to-severe CD. Adalimumab therapy resulted in an increase of clinical remission in 46% of the patients at week 56.224
The CHARM study, a phase 3, randomized, double-blind, placebo-controlled trial of 56-week duration, evaluated the efficacy and safety of adalimumab in maintaining response, as well as remission, in patients with moderate-to-severe CD. On the first visit, all patients received an open-label adalimumab 80mg dose, followed by a 40mg dose on week 2. At that week, 778 patients were randomized into 3 groups: adalimumab 40mg every 2 weeks, adalimumab 40mg every week, or placebo, and they continued until week 56. The percentage of patients that responded at week 4 and were in remission at week 56 was: 36% adalimumab 40mg every 2 weeks, 41% adalimumab 40mg weekly, and 12% placebo (p<0.001). The difference between adalimumab groups was not statistically significant (p=0.34). The results of this trial confirm that adalimumab is more effective than placebo in the maintenance of long-term remission in patients with moderate-to-severe CD that initially responded to adalimumab.225
The goal of the ADHERE study was to evaluate the long-term effect of adalimumab therapy at two years in an open trial, as an extension of the CHARM trial, and it included 467 patients. At week 60, clinical remission was 37.6, 41.9, and 49.8% in patients that received placebo, adalimumab 40mg every 2 weeks, and adalimumab 40mg every week, respectively. A total of 84.1% of patients that received adalimumab in the CHARM trial maintained remission until the end of the ADHERE trial.
This study showed that adalimumab therapy can maintain long-term remission and reduce the number of hospitalizations in patients with moderate-to-severe CD.226
The purpose of the GAIN study was to determine the efficacy of adalimumab in patients with CD that did not improve or that lost the ability to respond to infliximab. The trial included 325 patients with moderate-to-severe CD that were randomized to receive induction doses of adalimumab 160/80mg at week 0 and 2 or placebo for 4 weeks. At the end of week 4, 21% of the adalimumab group achieved clinical remission, compared with 7% of patients in the placebo group (p<0.001). Adalimumab therapy was superior to placebo for the induction of remission and response in patients with moderate-to-severe CD that did not tolerate infliximab or that lost the ability to respond to infliximab.227
The PRECISE 1 study was a 26-week, multicenter, randomized, double-blind, placebo-controlled trial. A total of 662 patients with CD were divided into 2 groups: 1) certolizumab pegol 400mg and 2) placebo. Among the patients with ≥ 10mg/l baseline CRP levels, 22% in the certolizumab group had a reduction of at least 100 points in the CDAI at week 26, compared with 12% in the placebo group (p=0.05). Certolizumab pegol treatment was associated with a modest benefit in response rates, but there was no improvement in remission rates in comparison with placebo in patients with moderate-to-severe CD.228
The PRECISE 2 study was designed as a multicenter, randomized, double-blind, placebo-controlled analysis. It evaluated the efficacy of certolizumab pegol as maintenance therapy in patients with moderate-to-severe CD. In this study, patients received an open-label induction dose of 400mg at weeks 0, 2, and 4. Patients that responded to the induction therapy at week 6 were randomized to receive 400mg of certolizumab pegol or placebo. Follow-up was conducted at week 26. In total, 213 patients had baseline CRP levels ≥ 10mg/l. A total of 62% patients in the certolizumab pegol group had clinical response, compared with 34% in the placebo group (p<0.001). The clinical remission rate was 48% in the certolizumab pegol group, compared with 29% in the placebo group (p<0.001). This study showed that the continuous administration of certolizumab pegol was superior to administration of placebo in 64% of patients with moderate-to-severe CD.229
53. Infliximab and adalimumab-based anti-TNF therapy is effective in closing fistulas and maintaining that closure in patients with CD. Antibiotic and surgical treatment may be required. Level of evidence: 1. Level of agreement: 100%.
The ACCENT II study196 evaluated the efficacy of infliximab in the treatment of fistulizing CD. In comparison with placebo, most patients that received infliximab 5mg/kg responded to treatment, defined as a decrease of 50% or more in fistula drainage (p=0.002). There was complete response (fistula closure) in 55% of the patients that received infliximab 5mg/kg, compared with 13% of the patients in the placebo group (p=0.001).
The ACCENT II trial230 included patients with CD and simple or complex fistula. It was observed that at week 54, 23% of the patients in the placebo group had response (defined as a decrease of 50% in fistula drainage), compared with 46% of the patients that received infliximab (p=0.001).
In a sub-study of the ACCENT II trial231 that evaluated patients with rectovaginal fistula, 71.4% of the patients that received infliximab achieved fistula healing, compared with 54.5% in the placebo group.
The French group GETAID232 conducted a retrospective and multicenter study on patients with enterocutaneous fistulas receiving anti-TNF treatment. It showed that only 33% of the patients under study achieved complete healing of the enterocutaneous fistula. In the multivariate analysis, the lack of healing was associated with multiple fistula tracts (hazard ratio [HR]: 5.80, 95% CI: 1.07-31.5, p=0.04) and with the presence of intestinal strictures (HR: 4.67, 95% CI: 1.05-20.82, p=0.04).
Adalimumab therapy has proven to be effective in the induction and maintenance of perianal fistula closure for a period of more than 2 years.225,226
A systematic review of the literature233 demonstrated that the combined treatment of pharmacologic therapy (anti-TNF and immunomodulators) and surgery is the strategy with the best healing rates, compared with pharmacologic therapy or surgery alone.
54. Infliximab and adalimumab-based anti-TNF therapies induce mucosal healing in patients with CD and are associated with steroid-free remission, fewer surgical procedures and hospitalizations, and quality of life improvement. Level of evidence: 2. Level of agreement: 100%.
Mucosal healing has been proposed as a goal in the treatment of IBD and has been associated with a more effective control of the disease, steroid-free remission, fewer surgical procedures, fewer hospitalizations, a better quality of life, and reduced steroid use.234,235
An expert consensus from the International Organization for the Study of Inflammatory Bowel Disease defined mucosal healing in UC as restoration of the normal appearance at endoscopy of a previously inflamed region, and the complete absence of ulcers, friability, blood, erosions, and macroscopic and histologic signs of inflammation.7,89,90,236–238
An endoscopic sub-study was conducted in the ACCENT 1 study,95 showing, at week 10, that 29% of the patients that received 3 infusions of infliximab achieved mucosal healing, compared with 3% of patients that had received only one infusion (p=0.006). At week 54, 50% of the patients that underwent infliximab scheduled treatment achieved complete healing, compared with 7% of the patients with episodic treatment (p=0.007). In the same study, patients that achieved mucosal healing with infliximab had a relapse-free period that lasted longer than those patients that did not achieve healing. No patients with mucosal healing at week 10 and week 54 were hospitalized, in comparison with patients that achieved healing only once (25%) and those that did not achieve healing at any of the weeks (46%). All this suggests that mucosal healing predicts a reduction in hospitalizations.
The MUSIC trial239 is an open-phase study used to evaluate endoscopic improvement in patients with active CD treated with certolizumab pegol. At week 10, 5% of the patients achieved mucosal healing.
The EXTEND study240 evaluated the efficacy of adalimumab in patients with CD. The mucosal healing was evaluated at week 52, and 24.2% of the patients treated with adalimumab achieved mucosal healing, compared with 0% of the patients treated with placebo (p<0.001).
55. Combination therapy of an anti-TNF agent (infliximab or adalimumab) with an immunomodulator (thiopurine or methotrexate) is superior to monotherapy with an anti-TNF agent or azathioprine in patients with IBD, regarding response and clinical remission, mucosal healing, and steroid-free remission. Level of evidence: 1. Level of agreement: 100%.
Several studies suggest that the combination treatment of anti-TNF agents with immunomodulators is more effective than monotherapy with either of these drugs. Nonetheless, there are doubts regarding the long-term efficacy of this therapeutic strategy and the safety of combining 2 immunosuppressants for an indefinite period of time, especially in young men.208,241,242 There is very little information about the effect of combination therapy with anti-TNF agents other than infliximab, such as adalimumab, certolizumab pegol, or golimumab.
A study conducted by Sokol et al.243 evaluated the difference between the effectiveness of infliximab therapy and combination therapy with an immunosuppressant (thiopurines or methotrexate). The combination therapy was shown to reduce the risk for relapse (OR: 0.50, 95% CI: 0.32-0.77) and it reduced the need for abdominal surgery (OR: 0.18, 95% CI: 0.05-0.63).
A multicenter, randomized, double-blind, 30-week trial called SONIC,244 which was extended for 20 weeks, was conducted between March 2005 and November 2008. A total of 508 patients diagnosed with CD were divided into 3 groups according to the therapy administered: 1) azathioprine monotherapy, 2) infliximab monotherapy, and 3) combination therapy with infliximab and azathioprine. At week 26, 56.8% of the patients that received combination treatment were in steroid-free clinical remission, compared with the patients that received monotherapy with azathioprine (30%, p<0.001) and the patients that received monotherapy with infliximab (44.4%, p=0.02). There was also a statistically significant difference in mucosal healing between patients that received combination therapy vs azathioprine (p<0.001) and infliximab (p=0.06). The incidence of adverse events was similar among the 3 groups, with no significant differences.
The UC SUCCESS study245 is a randomized, double-blind trial that evaluated the efficacy of infliximab and thiopurine combination therapy in patients with UC, within the time frame of November 2007 and February 2010. At week 16, a higher percentage of patients that received the combination therapy achieved steroid-free clinical remission, compared with those that received either azathioprine (p=0.32) or infliximab (p=0.017), alone. Mucosal healing was evaluated at week 16 and occurred in a higher percentage of patients receiving the combination therapy versus the monotherapy azathioprine group (p=0.001). A greater improvement was seen in the total Mayo score in patients that received the combination therapy, compared with patients that received azathioprine (p<0.001) or infliximab (p=0.028). No statistically significant differences among the three groups regarding adverse effects were reported by the patients.
In the COMMIT trial246 that evaluated the efficacy of the infliximab and methotrexate combination, no significant differences were found between those patients and the patients that received infliximab monotherapy, regarding steroid-free remission at week 50 (p=0.63).
Some studies have reported that using a concomitant immunomodulator may reduce the formation of anti-TNF antibodies, thus allowing for larger concentration quantities of the agent, resulting in increased therapeutic efficacy.
Ungar et al.247 demonstrated that combination therapy resulted in longer antibody formation-free survival (p=0.003) and a longer remission period in patients with no formation of anti-drug antibodies (ADA) than in those patients that developed ADA (p=0.01).
One study248 reported that the combination of anti-TNF therapy and immunomodulators may decrease ADA formation.
56. In CD patients with predictors of poor prognosis, it is recommended to start intensive therapy (Top-Down), which consists of beginning anti-TNF therapy associated with immunomodulators. Level of evidence: 2. Level of agreement: 100%.
Poor prognostic factors have been identified in CD patients. For example, young age at diagnosis (< 40 years of age), stenosing and fistulizing pattern including perianal disease, involvement of the upper digestive tract, extensive disease (over 70cm), and active smoking.249,250
In the Top-Down study,99 133 patients with newly diagnosed CD were randomized into 2 groups: the first group received early treatment with combined immunosuppressants (infliximab and thiopurines) and the second group received conventional treatment with corticosteroids.
On week 52, 61.5% of patients that received early treatment with immunosuppression were in clinical remission, compared with 42.2% of the patients that received conventional treatment (p=0.0278, 95% CI: 2.4-36.3). After week 52, no difference was found between the 2 groups. At week 104, mucosal healing was found in 73.1% of patients with the Top-Down approach, compared with 30.4% with the Step-Up protocol (p=0.002).
The study demonstrated that early therapy with combined immunosuppressant agents produced a greater ratio of patients in clinical remission, faster normalization of CRP levels and induction of remission in CD patients that had not been previously treated with corticosteroids, thiopurines, or biologic drugs, compared with patients that received conventional therapy.
Several cohort studies conducted at tertiary care hospitals and on other populations have reported that disease course in up to 50% of patients with IBD is benign. Accordingly, this percentage of patients will never require the use of immunosuppressive or anti-TNF therapy. Due to the possible side effects that anti-TNF therapy may have, many studies suggest that treatment be an accelerated Step-Up protocol for patients with poor prognosis, in order to receive the appropriate treatment in time to prevent long-term complications.
A recent German study251 on a prospective cohort evaluated the current treatment of patients with newly diagnosed IBD and the factors associated with the use of immunosuppressants and anti-TNF therapy. The use of steroids in the diagnosis, the location of the disease, and the need for surgery related to IBD were shown to be independent predictive factors for the use of immunosuppressants and anti-TNF therapy. Disease extension was the only predictive factor found in patients with UC.
57. The duration of anti-TNF therapy in patients with IBD has not yet been defined and the recommendation is to treat them on a case-by-case basis. It is suggested to maintain long-term anti-TNF therapy, since discontinuation is associated with disease relapse. Level of evidence: 4. Level of agreement: 100%.
There are few studies that evaluate how long therapy with anti-TNF agents should be maintained in CD patients, and there are no studies on UC patients.
The STORI trial252 was conducted in France and Belgium, from March 2006 to December 2009, on CD patients that had been receiving therapy with infliximab and an immunomodulator for one year, and that had been in steroid-free clinical remission for at least 6 months. The study's main aim was to determine the time of relapse after the discontinuation of infliximab treatment and to identify factors associated with a lower risk for relapse. Of the 115 patient total, 52 had relapses. Mean follow-up time was 28±2 months. There were 40 relapses within the first year and 7 in the second year. Of the patients that had relapse, 93% achieved remission after being treated again with infliximab and 98% had clinical response.
The factors associated with the relapse time were analyzed and CRP levels ≥ 5mg/l (p=0.0008, HR: 2.49, 95% CI: 1.41-4.39) and fecal calprotectin levels ≥ 300¿g/g (p=0.0002, HR: 3.22, 95% CI: 1.68-6.15) were shown to be associated with a greater risk for relapse.
The study demonstrated that the discontinuation of infliximab treatment was not a good strategy. Approximately 50% of the patients relapsed upon discontinuing treatment after one or 2 years. Another issue concerning the discontinuation of anti-TNF treatment was the increased rate of immunization, resulting in hypersensitivity reactions to infliximab, as well as loss of therapeutic effect.
Given the current lack of evidence, it is impossible to make any recommendation regarding the duration of treatment with anti-TNF agents.153 However, in clinical practice the decision to maintain treatment indefinitely or discontinue it must be made in accordance with the individual characteristics of each patient.
58. Anti-TNF biologic therapy (infliximab, adalimumab, and golimumab) is indicated in patients with a lack of response or intolerance to treatment with aminosalicylates, steroids, or immunomodulators in patients with UC with moderate-to-severe activity. Level of evidence: 1. Level of agreement: 100%.
Two randomized, double-blind, placebo-controlled studies evaluated the efficacy of infliximab as induction and maintenance therapy in adults with UC with moderate-to-severe activity that were refractory or intolerant to conventional treatment. A total of 364 patients that received treatment with either placebo or infliximab (5 or 10mg/kg) at weeks 0, 2, 6, and then every 8 weeks, were included. A follow-up was conducted for the patients at week 54 in ACT1, and at week 30 in ACT2. Clinical response at week 54 for patients in the ACT1 study was 19.8, 45.4, and 44.3% (p<0.001) and clinical response at week 30 for patients in the ACT2 study was 26, 47.1, and 60% (p<0.001) for the placebo, infliximab 5mg/kg, and infliximab 10mg/kg groups, respectively. Clinical remission rates at week 54 for patients in ACT1 were 16.5, 34.7, and 32% for the placebo, infliximab 5mg/kg, and infliximab 10mg/kg groups, respectively, and they were 10.6, 33.9, and 35.8% at week 30 for the patients in ACT 2. No significant differences were found between the 2 infliximab doses. These studies showed that infliximab was effective for clinical response and remission in patients with UC with moderate-to-severe activity despite the use of conventional treatment.253
The ULTRA1 study, an 8-week, multicenter, randomized, double-blind, placebo-controlled and phase 3 study included 576 patients with UC for evaluating the efficacy of adalimumab in inducing clinical remission in anti-TNF therapy-naïve patients with moderate-to-severe UC. Patients were randomized to receive treatment with adalimumab 160/80mg, adalimumab 80/40mg, and placebo.
Clinical remission at week 8 was 9.2, 10, and 18.5% in the placebo, adalimumab 80/40mg, and adalimumab 160/80mg groups, respectively (p=0.31). The effect of the treatment was more pronounced in patients that did not have extensive colitis, patients treated with immunomodulators without corticosteroids at the beginning of the trial, and patients that did not receive aminosalicylates at the beginning of the trial.
Adalimumab at a dose of 160/80mg was effective in inducing clinical remission in patients with moderate-to-severe UC that did not respond to or tolerate conventional therapies.254
The ULTRA2 study, a 52-week, randomized, double-blind, placebo-controlled, phase 3 trial, evaluated the efficacy of adalimumab in the induction and maintenance of clinical remission in 494 patients with moderate-to-severe UC that received concomitant therapy with oral corticosteroids or immunosuppressants. Patients were assigned to 2 groups: adalimumab 160mg at week 0, 80mg at week 2, and 40mg every 2 weeks until week 52 or placebo. At week 52, 17.3 and 8.5% of patients achieved clinical remission in the adalimumab and placebo groups, respectively (p=0.002). At week 52, clinical response was observed in 30.2% vs 18.3% for the adalimumab and placebo groups, respectively (p=0.002). Of the anti-TNF treatment-naïve patients, 22% in the adalimumab group and 12% in the placebo group achieved clinical remission at week 52 (p=0.029). Adalimumab treatment was demonstrated to be effective in maintaining clinical remission in patients with moderate-to-severe UC that did not respond to conventional treatment.240
Golimumab is a subcutaneously administered monoclonal anti-TNF-¿ antibody of human origin used in patients with moderate-to-severe UC that is approved by the US Food and Drug Administration (FDA) and the European Medicines Evaluation Agency (EMEA). In a multicenter, randomized, double-blind, placebo-controlled, phase 2 (dose/response) and phase 3 (confirmatory) study in 2007, called PURSUIT-SC, 1,064 patients with moderate-to-severe UC were analyzed (Mayo score 6-12, endoscopic subscore ≥ 2). The patients were randomized into groups in which they received golimumab at doses of 100mg/50mg (only phase 2), 200mg/100mg, or 400mg/200mg every 2 weeks. In phase 3, the cut-off point used was 6 weeks pending clinical response. The results of the 100mg/50mg, 200mg/100mg, and 400mg/200mg doses corresponded to the Mayo score of –1.0, –3.0, and –2.0, respectively, compared with –3.0 in the placebo group. In phase 3, the response rates at week 6 were 51.0% with the 200mg/100mg dose and 54.9% with the 400mg/200mg dose of golimumab in patients with UC, compared with 30.3% in the placebo group (p ≤ 0.0001). The rates of clinical remission and mucosal healing were significantly higher in the golimumab groups, compared with placebo (p ≤ 0.0014). Subcutaneous golimumab treatment induced clinical response, mucosal healing, and improvement in the quality of life of patients with active UC, compared with the placebo group.255
59. Infliximab therapy is an option for patients with severe UC refractory to IV steroids, as an alternative to avoid colectomy. Level of evidence: 1. Level of agreement: 100%.
Severe UC is a potentially life-threatening condition. The historic prevalence data show that 18.8% of the initial exacerbation of the disease is severe, and that 17.6% of all patients have a serious relapse in the course of their disease. Single-dose infliximab (5mg/kg) has been shown to be an effective rescue treatment for patients with severe steroid-refractory UC. A randomized controlled study included 45 patients (24 received infliximab and 21 placebo) initially treated with IV betamethasone.256 At 3 months, colectomy rates were significantly lower in the patients that received infliximab than in those that received placebo (7/24 vs 14/21, p=0.017; OR 4.9, 95% CI: 1.4-17). The long-term follow-up revealed a colectomy rate after three years of 12/24 (50%) patients that received infliximab and 16/21 (76%) that received placebo (p=0.012). However, the use of thiopurine therapy was not controlled and varied between groups.257,258
Conversely, the open-label CYSIF study randomized 111 thiopurine-naïve patients with severe UC (Lichtiger score>10), despite 5 days of IV steroids, to IV ciclosporin at doses of 2mg/kg/day for 8 days (150-250 ng/ml levels) followed by 4mg/kg/day oral therapy, or to infliximab at doses of 5mg/kg at weeks 0, 2, and 6. All patients that responded at day 7 received oral azathioprine, and their steroid doses were gradually reduced starting from day 8. Initially, the study was powered to demonstrate fewer treatment failures with ciclosporin than with infliximab between days 7 and 98 (lack of steroid-free remission at day 98, colectomy, or treatment interruption before day 98). Approximately 85% of patients in both groups responded to treatment on day 7. Treatment failure (the primary efficacy outcome) was reported in 60% of patients in the group that received ciclosporin, compared with 54% of patients in the group that received infliximab (treatment difference 6.4%, 95% CI: –12 to 24.8%, p=0.49). Colectomy rates at day 98 in the group that received ciclosporin vs the group that received infliximab were 18 and 21%, respectively (p=0.66). Serious adverse events were more common in the infliximab group (17/56 vs 9/55 in the ciclosporin group), with a total of 9 serious infections within the 98 days of the study, but the differences were not statistically significant.259 As in other studies, infliximab and ciclosporin were shown to be effective in reducing the colectomy rate in patients with severe UC that was refractory to IV steroids.260–263
60. The optimization of anti-TNF treatment is based empirically on doubling the dose or decreasing administration intervals. Level of evidence: 4. However, it would be ideal to measure the anti-TNF serum levels and anti-TNF antibody formation. Level of evidence: 2. Level of agreement: 100%.
The monitoring of serum drug levels and measurement of anti-drug antibody (ADA) levels that are developed in some patients have emerged as a tool to help clarify the mechanisms of loss of response, as well as to serve as a guide for the treatment in a large number of patients. This may help identify patients that would benefit from an increase in the dose of the agent and those that would hardly respond to this strategy.
A retrospective study264 evaluated the clinical usefulness of measuring the levels of infliximab and antibodies (ADA) in patients that had lost response or that had an incomplete response to infliximab. Only 18% of patients with positive antibodies did not respond to an increased dose of infliximab, compared with 86% of patients with negative antibodies and sub-therapeutic concentrations that significantly benefited from an increase in the dose (p<0.016).
A study265 that evaluated patients treated with infliximab found that 12 months after discontinuation of infliximab treatment, antibody titers became undetectable in the majority (13/16) of them. This is why the clinical value of measuring antibodies in months prior to the re-introduction of this drug is limited, since antibodies are undetectable, especially if the previous exposure was more than 12 months earlier.
The initial management of a patient with relapse during anti-TNF therapy determines the anti-drug antibodies and biologic drug levels, and deduces the treatment failure mechanism. Once it has been established that the symptoms are due to relapse, despite treatment adherence, there are several therapeutic options, the most common of which is dose intensification and administration interval reduction. The second most commonly used option is that of switching to another anti-TNF agent. A change in the class of drugs or surgery266 is recommended when there is a loss of response with optimal levels of anti-TNF and negative antibodies.
Detectable serum anti-TNF concentrations have been associated with clinical remission and endoscopic improvement. Conversely, undetectable levels of infliximab are associated with an increased risk for colectomy, a secondary loss of efficiency, worse clinical outcomes, or increased inflammatory activity.267–272
An observational study273 found that minimum concentrations of infliximab were significantly lower in patients that were positive for anti-infliximab antibodies, compared with patients that had negative antibodies (p=0.015). An increased ESR and CRP level were correlated with low serum infliximab levels (p=0.04 and p=0.002, respectively).
In their study, Baert et al.268 found that serum anti-infliximab antibody levels were inversely correlated with the duration of drug response (p<0.001). It was also found that patients with > 12¿g/ml had a significantly longer response duration than patients with lower concentrations (p<0.01). This was confirmed by the team of Maser et al.267 in a multivariate analysis, in which higher serum infliximab levels were found to be predictors of clinical remission (OR: 38, 95% CI: 9-60, p<0.001) and endoscopic improvement (OR: 23, 95% CI: 4-124, p<0.001) in those patients.
In conclusion, appropriate anti-TNF concentrations have been associated with clinical and endoscopic remission. Appropriate anti-TNF levels have been associated with an increased risk for colectomy, secondary loss of efficiency, worse clinical outcomes, or increased inflammatory activity.61. Vedolizumab biologic therapy is an alternative for patients with refractory CD or those that cannot tolerate treatment with corticosteroids, thiopurines (azathioprine or 6-mercaptopurine) and have failed with anti-TNF therapy. Level of evidence: 1. Vedolizumab treatment is effective for inducing and maintaining remission in biologic anti-TNF therapy-naïve patients with CD. Level of evidence: 1. Level of agreement: 100%.
Vedolizumab is a specific humanized monoclonal antibody that targets integrin ¿4ß7 (a variable surface glycoprotein expressed on the surface of circulating T and B cells), interacting with the MAdCAM-1 adhesion molecule, which is specifically expressed on the intestinal vasculature.
The GEMINI 2 study analyzed the efficacy of vedolizumab in CD patients, since its role and efficacy in this disease is unknown. Results for vedolizumab in patients with active CD showed that patients receiving vedolizumab at doses of 300mg had a remission rate of 15%, compared with a 7% rate (p=0.02) in those receiving placebo, at week 6 of the induction phase. In the maintenance phase (300mg every 4 or 8 weeks), patients that responded to induction therapy showed disease remission at week 52 of 36% (p=0.004) and 39% (p<0.001), compared with 22% of the patients that received placebo.274
The GEMINI 3 study evaluated the effect and efficacy of vedolizumab in patients with moderate-to-severe CD that had experienced at least one failure to one anti-TNF agent. A total of 315 patients were randomized to receive placebo or vedolizumab at a dose of 300mg daily at week 0, 2, and 6. The primary outcome measure was clinical remission at week 6, which was found in 15.2% of the vedolizumab group, compared with 12.1% of the placebo group (p=0.433). However, clinical remission at week 10 was evaluated as a secondary outcome. There was statistical difference in favor of the vedolizumab group, with 26.6% vs 12.1% in the placebo group (p=0.001).275
62. Vedolizumab biologic therapy is indicated for the induction and maintenance of remission in patients with moderate-to-severe ulcerative colitis that have failed conventional therapy. Level of evidence: 1. It may be used as first-line biologic treatment in these types of patients prior to the use of anti-TNF therapy. Level of evidence: 4. Level of agreement: 91%.
The GEMINI 1 study evaluated the efficacy and safety of vedolizumab in UC patients. Results for vedolizumab in patients with UC showed that 17% of the patients that received vedolizumab and 5% that received placebo had clinical remission (p<0.001). For maintenance therapy at week 52, patients randomly assigned to continue receiving vedolizumab (42% every 8 weeks and 45% every 4 weeks) were more likely to have clinical remission than those randomly assigned to switch to placebo (16%) (p<0.001).276
These findings were confirmed in a systematic review, which included 4 studies with a total of 606 UC patients. The overall induction rate of clinical remission was 52%, compared with 38% of the placebo group after week 6 of treatment. At week 52, 54% of the group had a clinical relapse of UC, compared with 84% of the placebo group. No increase in adverse effects was observed in the patients that received vedolizumab.277
C. Surgical Treatment63. Better results are obtained when both the gastroenterologist and colorectal surgeon carry out the treatment and surveillance of patients with severe UC from the time of their hospital admission. Level of evidence: 5. Level of agreement: 100%. Symptoms, physical examination, and signs of systemic toxicity should be closely monitored. In the event of any clinical deterioration, an emergency colectomy must be considered. Level of evidence: 1. Level of agreement: 100%.
Acute severe UC is a serious potentially life-threatening condition. Patients should be admitted to the hospital and both the gastroenterologist and colorectal surgeon should provide multidisciplinary management. From the moment the patient is admitted, he or she must be informed about the available surgical options if medical treatment fails.278,279 Severe UC diagnosis is based on the Truelove and Witts index, which is the simplest, best validated, and most widely used for the clinical classification of UC. Any patient with > 6 bloody stools/day and tachycardia (> 90 x min), or temperature (> 37.8°C), or anemia (Hb<10.5g/dl), or high ESR (> 30mm/h), has severe colitis. Only one criterion in addition to the frequency of 6 bloody stools/day is necessary to define a severe attack.199 Intensive medical treatment with IV steroids, imaging studies, and continuous laboratory and clinical monitoring are required. From the surgical point of view, hydroelectrolyte and nutritional support are needed, as well as blood transfusions, when required. During patient hospitalization, the necessary tests are carried out to confirm diagnosis. Enteric infections, such as cytomegalovirus, Clostridium difficile and other bacteria, are excluded through flexible sigmoidoscopy and biopsies. Plain abdominal films can warn about toxic dilation or perforation.153 In the event of any clinical deterioration, signs of toxicity, or abdominal warning signs (dilation or signs of peritoneal irritation) occurring during immunosuppressive therapy, a subtotal colectomy with ileostomy must be considered.280
64. The response of patients with severe UC to IV steroids should be evaluated at day 3. In refractory cases, other treatment options must be discussed with the patient, including rescue therapy or second-line therapy, such as IV infliximab or ciclosporin. Level of evidence: 1. Level of agreement: 100%. If there is no improvement after 5-7 days of second-line pharmacologic therapy, colectomy is recommended. Level of evidence: 2. Level of agreement: 100%. Delay in surgery is associated with an increased risk for postoperative complications and mortality. Level of evidence: 1. Level of agreement: 100%.
In severe UC, lack of response is considered if conditions deteriorate while the patient is undergoing medical therapy or if there is no improvement after an initial stabilization period. Corticosteroids are the cornerstone of first-line conventional therapy and include the IV administration of hydrocortisone 100mg/4 times a day or methylprednisolone 60mg/day. IV steroids administered for more than 7-10 days have no additional benefits and they present increased morbidity and mortality. Therefore, response should be evaluated at an early stage after the third day of treatment.199 It is often difficult to define the need for and timeliness of surgery in patients whose conditions seem to persist after an initial period of improvement. The Oxford criteria establish that patients with > 8 stools/day or 3-8 stools/day and CRP>45mg/ml after 3 days of IV steroid therapy have an 85% risk for colectomy during hospitalization.198
If there is no response to IV steroids, a second-line therapy or a rescue therapy with IV ciclosporin or infliximab is recommended.259,281 In Latin America, rescue therapy with infliximab is used more often. Reports on both the efficacy and safety of infliximab rescue therapy in patients with severe IV steroid-refractory UC are widely published. Järnerot et al. reported that infliximab infusion reduced the rate of colectomy in the short-term (90 days) in 29% vs 67% of patients that did not receive infliximab.256 Kohn et al. reported that infliximab reduced the rate of colectomy in hospitalized patients with severe UC by 34% with a single infliximab infusion vs 5.3% with 2 or more infusions at 60 days (p=0.001).282 It was recently reported that an accelerated regimen of infliximab infusion (24 days) reduced the rate of early colectomy (12 months) in patients with acute severe UC (6.7%, 1 out of 15), compared with the standard regimen of infliximab 5mg/kg infusion at week 0, 2, and 6, followed by maintenance every 8 weeks (40%, 14 out of 35). Nevertheless, it is worth noting that the long-term colectomy averages were the same as those of the pre-biologic period in those patients.283 These results allow for timely rescue therapy, so that elective colectomy can be performed under better conditions of reduced morbidity and mortality for the patient. It is recommended that surgeons avoid single stage total proctocolectomy with ileoanal reservoir in patients undergoing anti-TNF therapy.
65. The procedure of choice for emergency surgery in IV treatment-refractory UC, severe bleeding, dilation, or toxic megacolon is subtotal abdominal colectomy with end ileostomy and rectal stump or mucous fistula, depending on the surgeon's decision. Level of evidence: 4. Level of agreement: 100%. If laparoscopic surgery is available, a minimally invasive approach is possible in stable patients, which may have some advantages specific to that method. Level of evidence: 2. Level of agreement: 100%.
Medical treatment may be effective in a large percentage of acute severe UC cases. However, surgery delay is detrimental to patient results.284 A staged proctocolectomy with the initial performance of a subtotal colectomy is considered a first step in the surgical treatment of acute severe ulcerative colitis. A subtotal colectomy with ileostomy and Hartmann closure of the distal intestine, or creation of a mucous fistula, is a safe and effective approach, and it will cure the patient of colitis by allowing for recovery and dietary normalization. It will also give the patient time to consider the construction of an ileoanal reservoir, a definitive ileostomy, or in a few selected cases, an ileorectal anastomosis, in a second stage. Management of the rectal cuff is controversial. Most surgeons divide the rectum at the level of the promontory or they leave the distal part of the sigmoid colon anchored to the anterior abdominal wall, facilitating its subsequent identification and dissection, or they move the intestine through the abdominal fascia by enclosing it in the subcutaneous fat or as a mucous fistula. This last option is considered very safe because it does not remain inside the abdomen, although it gives the patient another stoma, which may be difficult to handle.285,286 If laparoscopic surgery is available, a minimally invasive approach is possible in stable patients, which may have some advantages specific to that method, mainly less pain, a shorter hospital stay, and fewer surgical site infections.287
66. Restorative proctocolectomy with ileoanal reservoir has become the elective surgical procedure most commonly performed for UC patients. Ideally, surgery can be carried out laparoscopically, if there is previous experience. Level of evidence: 1. Level of agreement: 100%.
Restorative proctocolectomy is the elective surgical procedure most frequently performed for UC patients that require elective surgery. This procedure can be performed openly by laparotomy or with minimally invasive techniques, with the appropriate means and experience. It is a relatively reliable and safe procedure.288,289 The surgical technique has 4 main steps. First, the colon is removed. Then, a pelvic dissection with resection of the rectum is performed; the pelvic nerves and the sphincters remain intact. Subsequently, the ileal reservoir is constructed, and finally, the reservoir is anastomosed to the anal canal. Ileoanal anastomosis has an acceptable morbidity average (19-27%), a very low mortality average (0.2-0.4%), and a very good quality of life result.280
Complications are similar to those of any major abdominal surgical procedure: in addition to the risks secondary to pelvic dissection, such as infertility or sexual dysfunction, and specific pouch complications, such as “pouchitis” (reservoir inflammation), anastomotic leak with pelvic sepsis, fistula, stenosis and “cuffitis” (inflammation of the rectal cuff), there is clear evidence that high-volume surgeons (> 10 reservoirs per year) in specialized referral centers have a lower average of complications and a higher average of preservation of complicated ileoanal reservoirs.290
When comparing the open approach with the laparoscopic approach, a 2009 Cochrane review of 11 studies including over 600 patients established similar days of hospital stay, reoperations, readmissions, and mortality averages between the two surgical approaches. The laparoscopic approach is associated with better cosmesis (less scarring), smaller total incision length, and longer operating times.291 Comparing the 2 procedures, retrospective data have shown that laparoscopy is associated with a shorter time for closing the ileostomy, intestinal continuity restoration, and secondarily, a decrease in the formation of adhesions, mainly adnexal ones (tubes and ovaries). Over 70% of the patients studied presented with no adhesions in the annexes.292
The impact of different medical therapies on this surgery, mainly with steroids and immunosuppressants, is widely documented. In general, the literature describes increased morbidity in patients with high doses of steroids.293,294 The impact of anti-TNF biologic therapies is more controversial, and further prospective, multi-center, and standardized studies are needed to establish a definitive position. Some studies show higher averages of short-term surgical complications. A retrospective study from the Mayo Clinic (USA) reported that prior exposure to infliximab increases the average of ileoanal reservoir surgery complications, suggesting that patients that received infliximab had a higher risk for anastomotic leaks and infectious and pouch-specific complications than the controls.295 Another study from the Cleveland Clinic (Ohio, USA) showed similar results in early complications and sepsis, concluding that prior exposure to infliximab could lead patients to a 3-stage ileoanal reservoir surgical procedure, given the high risk for complications and poor outcomes.296 However, other studies have shown contrasting results, demonstrating that high doses of steroids and reservoir surgery without defunctioning ileostomy were independent factors for a higher average of complications after a restorative proctocolectomy with ileoanal reservoir, but preoperative administration of infliximab (within 12 weeks) was not.296 The most significant analysis with a larger sample of patients was carried out in a national study in Denmark. It included 1,200 patients, 199 of whom had previously received infliximab (within 12 weeks of surgery). It was concluded that there was no significant increase in complications after surgery in patients previously exposed to infliximab.297
67. The J-reservoir is the treatment of choice due to its construction simplicity and its good long-term functional results. Level of evidence: 2. Level of agreement: 100%.
Various reservoir configurations have been performed (S, J, W). A meta-analysis that included 1,519 patients reported no significant differences in surgical outcomes, rates of pelvic sepsis, early failure of the reservoir, or mortality.298 However, the operating time was shorter and the surgical technique was simpler with the J-reservoir. At first, there was a higher number of stools (due to the smaller size of the pouch), but they decreased over time. In one study, the average number of stools per day was the same for patients with a J or W-reservoir. There was no difference between the 2 reservoirs regarding the averages of incontinence, urgency, leakage, and the use of antidiarrheal agents.299
Ileoanal anastomosis should not be carried out more than 2cm above the dentate line. One of the major complications for performing the ileoanal anastomosis technique is leaving a larger rectal mucosa cuff above the dentate line, which can lead to chronic inflammation (cuffitis) with reservoir dysfunction and risk for dysplasia or cancer. Ileoanal anastomosis can be performed manually or with a stapler. However, there are reports that indicate that manual anastomosis and mucosectomy result in a greater degree of incontinence, lower anal resting pressures, and permanent loss of rectoanal inhibitory reflex. The advantages of double-stapled anastomosis include: less technical difficulty because mucosectomy and the perineal phase of the procedure are avoided, less strain on the anastomotic suture line, and better functional outcomes. Sphincter injuries are also minimized and the anal transition zone and its nerve endings remain intact. This is reflected in a lower incidence of nocturnal enuresis. Furthermore, the subsequent incidence of cancer is the same as that after a mucosectomy with manual anastomosis. For these reasons, the double-stapled ileoanal anastomosis technique is currently the most widely used.300,301
Performing a temporary protective ileostomy during surgery of the ileoanal reservoir reduces the risk for anastomotic leaks (50%), septic complications, reservoir dysfunction, and mortality. The optimal time for ileostomy closure is 12 weeks or more, and the performance of a contrast enema before closure to display the reservoir morphology and rule out leaks is suggested.301–303
68. Proctocolectomy with definitive ileostomy is a surgical option in UC patients. Its main indications are in patients that do not choose to have an ileoanal reservoir or that have a significant risk for reservoir failure, such as anal sphincter dysfunction, prior anal and perineal disease, and other physiologic disorders secondary to comorbid conditions. Level of evidence: 5. Level of agreement: 100%.
Although restorative proctocolectomy with ileoanal reservoir is the most commonly performed surgical procedure in patients with UC, proctocolectomy with ileostomy is still carried out in patients with anal sphincter dysfunction, significant comorbidities or distal rectal cancer, or in elderly patients or those that choose not to have a reservoir.278
During the perineal time of this surgical technique, intersphincteric proctectomy to achieve good pelvic-floor closure and reduce the risk for problems in perineal wound healing is recommended.304 Complications can occur in 26% of patients and they may include small bowel obstruction, bladder function disorders, sexual dysfunction, infertility, and most frequently, stoma-related complications.305
Total proctocolectomy with continent ileostomy (Kock pouch) is a complex procedure with a high risk for re-operation. It is an alternative both in patients with UC that are not candidates for having an ileoanal reservoir and in those in whom this procedure has failed. Total proctocolectomy is mainly indicated in patients with poor sphincter function or a dysfunctional ileoanal reservoir, or in those that are dissatisfied with conventional ileostomy (Brooke). These few indications are the result of successful restorative proctocolectomy with ileoanal reservoir and the high average of early and late complications associated with continent ileostomy, such as sepsis and ileal pouch retention.306
69. Colectomy with ileorectal anastomosis should be considered in a highly selected group of UC patients (low morbidity and preservation of female fertility) as an alternative to ileoanal reservoir that must be assessed against the need for rectal surveillance and subsequent proctectomy in approximately 50% of cases. Level of evidence: 4. Level of agreement: 100%.
To perform a secure anastomosis, subtotal abdominal colectomy with ileorectal anastomosis may be an option in patients with a minimally affected rectum. In addition to anal-perineal disease and anal sphincter dysfunction,280 other contraindications of the procedure include severe rectal inflammation and a marked decrease in rectal capacity (compliance), in which the rectum cannot act as a reservoir. Therefore, it is necessary to take precautions in patients with dysplasia or carcinoma in potentially curative conditions.280
Unpredictable functional results after an ileorectal anastomosis in an inflamed rectum with diminished compliance, as well as the subsequent development of cancer, explain why surgeons refuse to perform ileorectal anastomosis in UC. In addition to the technical simplicity and low morbidity, among other advantages, this procedure avoids pelvic dissection and eliminates the risk for impotence in men and decreased fertility in women.307 Disadvantages are related to the long-term durability of the procedure. Some studies have shown a failure average of 12 to 53% with a 3.5-year follow-up. Finally, this results in the removal of the rectum due to persistent symptoms or the development of cancer.308 The risk for developing rectal cancer should be taken into account when this procedure is indicated. Although incidence seems low in the long term (0-8%), patients should be warned that they will require endoscopic follow-up with biopsies every year. Defecation urgency is the most common cause of failure of ileorectal anastomosis (22-23%).280
70. There is an increased risk for thromboembolic complications in patients that undergo surgery for IBD. Preventive and risk reduction measures are recommended for these patients. Level of evidence: 3. Level of agreement 100%.
UC patients have an increased risk for thromboembolic events.309 In a large review of surgical patients in the United States, incidence was 3.3% with a higher risk for death (4%). The use of antithrombotic agents, lower limb compression stockings, and early mobilization are recommended.310
71. Pouchitis (reservoiritis) is the most frequent late complication of ileoanal reservoir. Level of evidence: 2. Level of agreement 100%.
Long-term complications of reservoirs include: small bowel obstruction, fistulas, reservoir dysfunction, pouchitis, sexual dysfunction, and reduced fertility. Pouchitis is the most common, and it occurs in > 50% of patients at 10 years.311–313 However, no reservoir surveillance is required in asymptomatic patients. A follow-up protocol is required only in the case of risk factors such as dysplasia, cancer, or primary sclerosing cholangitis.280 Multiple evidence suggests that gut microbiota plays a key role in the occurrence and progression of pouchitis. Bacterial overgrowth causes dysbiosis with a predominance of some anaerobic bacteria in the reservoir, which causes an immune response with the development of IBD.314,315 Extensive UC, extra-intestinal manifestations, such as primary sclerosing cholangitis, non-smokers, high preoperative levels of p-ANCA, the use of preoperative steroids, and NSAID intake are considered risk factors for developing pouchitis.316
72. The diagnosis of pouchitis (reservoiritis) is made based on clinical, endoscopic, and histopathologic criteria. Level of evidence: 3. Level of agreement: 91%.
The diagnosis of pouchitis is not always simple, due to the lack of specific symptoms and signs. In patients with non-inflamed reservoirs, 4-7 stools/day with continence are expected. In patients with pouchitis, there may be various clinical occurrences, ranging from increased stool frequency, urgency, abdominal cramps, nocturnal enuresis, and incontinence. These symptoms are not specific and may be present in other inflammatory and non-inflammatory pouch disorders.317 However, a combined evaluation of symptoms and endoscopic and histologic characteristics is required to make the diagnosis and differential diagnosis of pouchitis.318 Several diagnostic instruments have been used for the diagnosis and classification of pouchitis. The most commonly used diagnostic instrument is the 18-point pouchitis disease activity index, based on the evaluation of symptoms, endoscopy, and histology. This instrument proposes that pouchitis be diagnosed in the presence of clinical symptoms of diarrhea>6 stools/day; endoscopic findings of > 4 signs of edema, granularity, friability, loss of vascular pattern, mucous exudates, or ulcerations; and histopathologic disorders of at least grade 4 on a 6-point index (polymorphonuclear leukocyte infiltration and ulceration percentage per low-power field). Endoscopy is the most valuable tool for the diagnosis and differential diagnosis of pouchitis. Early endoscopy is required in symptomatic patients to establish a differential diagnosis between pouchitis, cuffitis (active inflammation of the rectal cuff), stenosis, anastomotic leak, ischemia, irritable pouch syndrome, fistulas, abscesses, CD, cytomegalovirus, C. difficile, dysplasia, and cancer.319,320
73. Most patients with acute pouchitis (reservoiritis) respond to ciprofloxacin 500mg/2 times a day for 2 weeks, or metronidazole 1g/2 times a day. Level of evidence: 1. Level of agreement: 100%.
Patients with initial episodes of acute pouchitis respond well to antibiotic therapy. There should be suspicion of surgical complications, such as anastomotic leakage or fistulas, in patients with symptoms of pouchitis immediately after reservoir construction and ileostomy closure that do not respond to antibiotic therapy. Few randomized and placebo-controlled studies on the treatment or prevention of pouchitis have been published. In clinical practice, metronidazole, ciprofloxacin, tinidazole and rifaximin have been used in the treatment of acute pouchitis.321 First-line therapy includes a 14-day course of metronidazole (15-20mg/kg/day) or ciprofloxacin (1g/day). Side effects are less common with ciprofloxacin. Other antibiotics, including tinidazole, rifaximin, and amoxicillin-clavulanic acid, have also been analyzed and found to be effective in some patients in small studies.322
74. Budesonide is an alternative for patients with chronic pouchitis (> 4 weeks) that do not respond to antibiotic treatment. Level of evidence: 2. Level of agreement: 91%.
Patients with chronic pouchitis are those that have symptoms for more than 4 weeks. In some cases, patients do not respond to antibiotic monotherapy and an antibiotic combination therapy is indicated. The administration of ciprofloxacin 1g daily plus 2g of rifaximin or metronidazole 1g/day or tinidazole 1-1.5g/day for 4 weeks has proved to be effective in chronic refractory pouchitis.323,324 Patients with continuous relapses (> 4 episodes/year) after antibiotics have been removed are considered antibiotic-dependent. In a small case series, corticosteroids (including oral or topical budesonide) have been evaluated for the treatment of chronic antibiotic-refractory pouchitis, showing some efficacy.325 In another small case series, the use of anti-TNF agents, such as infliximab326 or adalimumab,327 demonstrated good clinical efficacy. The efficacy of these agents suggests the involvement of immune processes in the occurrence, relapse, and progression of chronic refractory pouchitis.
75. Administration for 9 to 12 months of the probiotic VSL#3 (18 x 10)11, composed of 8 bacterial strains, was shown to be effective in maintaining antibiotic-induced remission. Level of evidence: 2. Level of agreement: 82%.
Recurrent pouchitis is common after treatment and resolution of the initial episode. It is estimated that up to 19% of patients with acute pouchitis will develop refractoriness to treatment or frequent relapses.328
Randomized, controlled studies have reported that the probiotic VSL#3 (which contains 4 strains of Lactobacillus [L. casei, L. plantarum, L. acidophilus, and L. delbrueckii subsp. bulgaricus], 3 strains of Bifidobacterium [B. longum, B. breve and B. infantis], and one strain of Streptococcus salivarius) was effective in maintaining antibiotic-induced remission in patients with recurrent pouchitis. In the first randomized, controlled trial in Bologna, Italy, VSL#3 was administrated at a dose of 6g daily as maintenance therapy after having induced remission with ciprofloxacin and rifaximin. This 9-month study with 40 patients showed that 15% of patients in the probiotic group relapsed, compared with 100% of patients in the placebo group.329 The administration of probiotics has also been shown to be effective in preventing the onset of pouchitis.330
76. Patients with chronic pouchitis refractory to all medical treatments should be referred to the colorectal surgeon in order to evaluate the removal of the ileoanal reservoir. Level of evidence: 4. Level of agreement: 91%.
Patients with chronic pouchitis refractory to all medical treatments should be assessed by the colorectal surgeon to evaluate the functional mechanism of the reservoir and structural disorders of the pouch, such as stenosis, anastomotic sinuses or fistulas, and ischemia of the reservoir. In addition, the quality of life of the patient must be evaluated to determine whether the patient is a candidate for surgery to remove the reservoir. The averages of reservoir failure in UC range from 3 to 15%.331 Definitive ileostomy is not the only alternative. There are perineal or abdominoperineal procedures to rescue the reservoir with good averages of success: between 48 and 93%.332
Surgery for Crohn's disease77. Surgery is mainly indicated in complicated CD. Urgent indications are: bowel perforation, abscesses, small bowel obstruction, and more rarely, massive bleeding. In the chronic setting, surgery is indicated in medical treatment failure, bowel fibrosis, fistulizing abdominal CD, and associated dysplasia or neoplasia. Level of evidence: 2. Level of agreement: 100%.
Surgery is not considered a failure, and it is important in the management of CD. Approximately 80% of patients will undergo an intestinal resection within 20 years of the diagnosis.333
In urgency, indications for surgery can be obvious, such as in cases of bowel perforation and multiple abdominal abscesses. Some patients with obstruction features can sometimes be managed with conservative treatment, but acute obstruction is also a clear indication for surgery. Massive bleeding is a rare indication for surgery in CD, being more common in severe UC. Perianal abscesses need immediate drainage.334
In the chronic setting, the most common indication for surgery is medical therapy failure. The definition for failure is not easily recognized in clinical practice. One definition can be the “inability to control symptoms despite optimal medical therapy”.335 There is still controversy as to whether surgical rates are reduced in population studies after the introduction of biologic therapy. Failure of biologic therapy can occur and it is a common indication for surgery, mainly when there is associated fibrosis. Fibrosis causing obstructive symptoms is also a common indication for surgery in the chronic setting. Attention is needed in cases with suspicion for neoplasia, in which surgery is again promptly indicated. Surgery is also essential in combination with medical therapy in the management of perianal fistulizing CD.334
Proper timing for surgery can be better defined through joint evaluation between gastroenterologists and surgeons. It is essential for multidisciplinary teams to be involved in decision-making together with the patients.336 An extensive evaluation of endoscopic and imaging tests helps to define the need for an operation and the best timing for the procedure.
78. Strictureplasty (bowel preserving surgery) is a valid option in small bowel CD. Shorter strictures (< 10cm) can be managed by conventional strictureplasty (e.g. Heineke-Mikulicz technique). Extensive stenosis, when leading to short bowel syndrome, constitutes a good indication for non-conventional strictureplasties (e.g. Michelassi technique). Strictureplasties are contraindicated in the colon, and in cases with associated fistulas. Level of evidence: 2. Level of agreement: 82%.
The preservation of bowel length and prevention of intestinal failure secondary to short bowel syndrome are among the most important goals in the management of small bowel CD. Stenoses<10cm can be treated with conventional strictureplasties, such as the Heineke-Mikulicz technique (for shorter stenoses) or the Finney technique (indicated in longer stenoses, over 10cm in length). There is good evidence that performing intestinal sutures in inflamed tissue with associated fibrosis is safe and does not increase complications, compared with anastomosis.337
There is evidence of increased use of non-conventional strictureplasties, in cases of stenoses affecting longer segments of the small bowel, mainly in patients with previous resections or patients with multiple stenoses confined to a longer segment of the bowel. The results are promising, with preservation of nutrient absorption, but there is a possibility of increased functional problems after surgery. In cases with no previous resections, several short stenoses confined to a defined segment of the bowel are preferably managed with intestinal resection.147,338
Stenoses with associated fistulas and abscesses are better managed with resection. Poor nutritional status with low albumin is also a contraindication for strictureplasty. Special attention should be paid to the suspicion of neoplasia in certain stenoses in which resection is mandatory. Some cases with adenocarcinoma at the site of the strictureplasty have been reported, and long-term follow-up is essential to promptly diagnose and treat those patients.339
79. Ileocecal resection is among the most common surgical procedures in CD. A laparoscopic approach is the preferred option if proper expertise is available. The preferred anastomotic technique after ileocecal resection in CD is a wide-lumen stapled side-to-side (functional end-to-end) anastomosis. Level of evidence: 2. Level of agreement: 100%.
In cases of abdominal CD that require resection, ileocecal resection is the most common surgical procedure. In recent decades, there has been a significant increase in the development of surgical equipment, techniques, and recovery protocols. Currently, laparoscopic ileocecal resection is the procedure of choice in localized ileocecal CD with surgical indication. Patients with luminal, medical-refractory, or stenotic disease constitute optimal candidates for this approach, with favorable outcomes.
The advantages of the laparoscopic approach include lower complication rates, shorter hospital stay, less bleeding, faster recovery, and improved cosmesis. Several studies and 2 meta-analyses were published in the literature comparing conventional and laparoscopic techniques.340,341 The benefits of the laparoscopic approach are clear in terms of reduced postoperative morbidity and better recovery. Indeed, there is room for the laparoscopic approach in complicated CD, such as cases with inflammatory masses, penetrating disease, and previous resections. These cases can be managed with laparoscopy only in centers with adequate equipment and extensive expertise, as well as significant conversion rates. There is no evidence that in complicated disease, laparoscopic resection achieves better outcomes than the conventional approach, although the performance of these procedures is viable.342
Recurrence does not appear to be affected by the type of access to the abdominal cavity, with no difference in postoperative recurrence rates between the laparoscopic and conventional techniques. Moreover, laparoscopic procedures lead to lower adhesion formation rates than with the conventional approach, and make re-interventions in the abdominal cavity (which can occur over time in CD) easier.343
Other minimally invasive techniques, such as single-port operations, are also viable in localized ileocecal CD. However, the benefits of this approach over multiport laparoscopic procedures are still to be determined.
One meta-analysis demonstrated that there are lower anastomotic leakage rates and lower overall postoperative complications with stapled side-to-side anastomosis in comparison with the end-to-end technique.344 Stapled anastomosis is considered safer, quicker, and easier to perform, mainly when different diameters between the ileal and colonic bowel loops are observed. Recurrence rates do not appear to be affected by the type of anastomosis, with no difference between end-to-end and side-to-side ileocolonic anastomoses. One prospective study from Canada demonstrated this similarity in a follow-up of one year.345
80. Segmental colectomy can be performed when CD affects a single site in the colon. When the 2 extremities of the colon are affected, 2 associated segmental colectomies can be performed as an alternative to total colectomy or proctocolectomy. When the rectum is also affected and refractory to medical therapy or when perianal severe fistulizing CD is present, total proctocolectomy and ileostomy are indicated. Level of evidence: 3. Level of agreement: 100%.
Segmental colectomy is an alternative to total colectomy or proctocolectomy when CD affects the colon.346 It is a valid alternative for avoiding permanent stomas in total proctocolectomy, in accordance with patient preference. Recurrence rates are higher in segmental colectomy, compared with more extensive resections. However, intensive medical therapy to prevent recurrence, mainly with biologic agents, can be an option to reduce the recurrence rates. There is a lack of studies comparing recurrence rates between segmental colectomy and total colectomy/proctocolectomy in the biologic era.
The rectum plays a significant role in deciding on the extent of resection in colitis secondary to CD. In cases of significant proctitis and perianal fistulizing disease with other associated diseased segments in the colon, total proctocolectomy and ileostomy are indicated.347
In cases with 2 different segments of the colon affected by CD with a normal rectum and no perianal fistulas, total colectomy with ileorectal anastomosis can be indicated.348 Two associated segmental resections are an option in cases when both ends of the colon are affected.
81. Preoperative corticosteroids increase overall complication rates in abdominal surgery for CD. Thiopurines can be safely used in the perioperative period. There is controversy regarding the impact of preoperative anti-TNF agents on complication rates in abdominal intestinal resections for CD. Level of evidence: 2. Level of agreement: 100%.
Preoperative corticosteroids increase complications in abdominal operations for CD. Complication rates are proportional to the dosage of the medications. In elective situations, corticosteroids should be withdrawn for at least 6 weeks, when possible, to reduce postoperative complications.349 The same principle cannot be applied to thiopurines. These drugs were demonstrated to be safe when used in the perioperative period. They do not need to be withdrawn before the operations, and can be started as soon as oral diet is reintroduced.350
There is controversy regarding the impact of anti-TNF agents per se on postoperative complications in abdominal CD. Several studies demonstrated a possible negative effect, blaming biologic agents for increased infection and overall complications,351 but the majority of the studies did not show this association.352 Almost all studies are retrospective. There is only one prospective study that demonstrated that patients with higher serum levels of infliximab one week before the operations had an increase in overall complications and more hospital readmissions than patients with undetected levels of the drug.353 Therefore, if possible, anti-TNF agents can be withdrawn to possibly reduce complications.
And so the decision as to which surgical strategy should be used (e.g., primary anastomosis vs defunctioning stoma) should be individualized in each case. Confounding factors such as poor nutritional status and previous corticosteroids have to be taken into consideration. There is no clear evidence to support the proper timing for the reintroduction of biologics after surgery. A period of at least 4 weeks after surgery, when complications are ruled out, can be recommended.
82. Abdominal abscesses secondary to CD are preferably treated through percutaneous drainage guided by imaging studies, together with antibiotic administration. Delayed resection is preferable, whenever possible. Level of evidence: 2. Level of agreement: 100%.
In the absence of obstructive symptoms, abdominal abscesses secondary to active CD are preferably treated by percutaneous drainage associated with antibiotics, no oral intake, and observation. When there is no technical possibility of percutaneous drainage, or there are multiple abscesses, laparotomy with surgical drainage is advisable. Delayed resection is preferred when successful drainage is accomplished, with no active sepsis, and better clinical conditions. There is no consensus as to whether this strategy can be applied in patients with severe sepsis or in children.146,354,355
83. Perianal fistulizing CD requires multidisciplinary management. Examination under anesthesia with seton placement in combination with medical therapy (antibiotics, immunomodulators and/or anti-TNF agents) is considered the best approach. In the presence of persistent rectal inflammation, treatment optimization is needed. Level of evidence: 1. Level of agreement: 100%.
Perianal fistulas secondary to CD are one of the most important challenges in the management of IBD. Proper diagnosis with a combination of methods (in general, examination under anesthesia associated with pelvic MRI) is essential for the planning of therapeutic actions. Perianal abscesses need immediate drainage. Simple superficial fistulas can be treated with fistulotomies.356
Currently, the combination of surgical treatment (seton placement, stenosis dilation, and fistulous tract curettage) and medical therapy is considered the best approach for the management of complex perianal fistulas in CD. Anti-TNF agents in combination with immunomodulators are mostly used in these cases.230 There is evidence that the association of anti-TNFs with antibiotics can improve short-term results.357–359 There is lack of data in regard to the use of immunomodulators and antibiotics as main therapies in the management of this disease phenotype.358
There is no consensus on the best timing for withdrawing setons or repeating the examination under anesthesia. Each case should be individualized.358,359 Mucosal healing of the rectum is generally needed before seton withdrawal. In patients without mucosal healing after induction and a variable period of maintenance therapy with anti-TNF agents, treatment optimization is required. In patients with a healed rectum and fistula drainage persistence, additional surgical procedures, such as the rectal advancement flap or the ligation of intersphincteric fistula tract (LIFT), can be offered. In cases of severe fistulizing disease associated with fecal incontinence, severe stenosis, and complex recurrent abscesses, a proctectomy should be considered.358
84. After ileocolic resection, prophylactic treatment for recurrence is recommended. Smoking cessation is mandatory after surgery. Metronidazole is more effective than placebo for the prevention of recurrence in the short term. Azathioprine is also more effective than placebo and mesalazine in the preventive scenario. Anti-TNF agents are more effective than conventional therapy in recurrence prevention and are recommended mainly in patients at high risk for recurrence. Risk stratification is needed after surgery to guide prophylactic therapy. Colonoscopic evaluation with detection of recurrence after 6 postoperative months is recommended to optimize medical therapy. Level of evidence: 1. Level of agreement: 82%.
Endoscopic recurrence occurs in approximately 75% of patients one year after ileocolic resection for CD. It precedes clinical and surgical recurrences. Therefore, prevention of recurrence with adequate medical therapy is a real necessity for better disease activity control.360
Patients at high risk for recurrence are smokers, patients with previous resections, and patients with penetrating abdominal disease. All smokers must stop smoking after surgery. Patients that do not present with these characteristics are considered to be at low risk.361 Those patients at high risk for recurrence should at least be taking immunomodulators as preventive therapy for recurrence. Anti-TNF agents are recommended in patients with preoperative use of these drugs, as well as in those presenting with extremely severe disease. They are not recommended for all high-risk patients due to cost and availability limitations.362
Tailored therapy based on a colonoscopic examination 6 months after the surgical procedure is the best strategy for optimizing recurrence management. Current therapy can be maintained in patients with normal mucosa. Treatment optimization is necessary in patients presenting with recurrence. Patients on thiopurines can begin anti-TNF agents and those already on biologics may require dose optimization.362,363
Financial disclosureGlobal Takeda Laboratories provided the financial support in relation to the logistics and travel expenses for all the participants in the formulation of this Consensus. They received no honoraria.
AuthorshipAll coauthors contributed to this work in equal fashion.
Conflict of InterestProf. Jesús K. Yamamoto-Furusho has received honoraria from Abbvie, Takeda, Janssen, UCB, Almirall, Pfizer, Novartis, and Danone as a speaker, key opinion leader, and member of national and international advisory boards. He has received research funds from Bristol, Shire, and Pfizer and is President of the Pan American Crohn¿s and Colitis Organisation (PANCCO) and Treasurer of the Asociacion Mexicana de Gastroenterología.
Francisco Bosques-Padilla. Speaker and consultant for Abbvie, Janssen, Takeda, and Ferring.
Juan De-Paula. Speaker and consultant for Abbvie, Janssen, and Takeda.
María T. Galiano. Speaker and consultant for Abbvie, Janssen, and Takeda.
Patricio Ibañez. Consultant for Abbvie.
Fabián Juliao. Speaker and consultant for Abbvie, Janssen, Biotoscana, and Takeda.
Paulo Kotze. Speaker and consultant for Abbvie, Janssen, Takeda, UCB, and Ferring.
José L. Rocha. Speaker for Abbvie, Janssen, and Ferring.
Flavio Steinwurz. Speaker, consultant or investigator for Abbvie, Ferring, Gilead, Janssen, Pfizer, Takeda, and UCB.
Guillermo Veitia. Speaker for Abbvie, Janssen, Farma, and Takeda.
Cyrla Zaltman. Speaker and consultant for Abbvie, Janssen, Takeda, UCB, and Ferring.
The authors wish to thank Carlos Augusto Barrera-Ochoa for his help in the reference format of this manuscript.
Please cite this article as: Yamamoto-Furusho JK, Bosques-Padilla F, de-Paula J, Galiano MT, Ibañez P, Juliao F, et al. Diagnóstico y tratamiento de la enfermedad inflamatoria intestinal: Primer Consenso Latinoamericano de la Pan American Crohn's and Colitis Organisation. Revista de Gastroenterología de México. 2017;82:46–84.