Laparoscopic sleeve gastrectomy (LSG) is the most widely performed bariatric surgery worldwide but complications and failed procedures are on the rise.
AimsTo determine the reasons for failed LSGs and report the results of conversion to gastric bypass surgery, comparing the outcomes with those of primary gastric bypass surgery.
Materials and methodsPatients with failed LSG that underwent conversion to gastric bypass surgery through a robotic-assisted and laparoscopic (hybrid) technique were evaluated. Outcomes and follow-up related to weight loss failure (WLF) were compared with those in patients that underwent primary laparoscopic gastric bypass (pLGB) surgery.
ResultsRevisional surgery was performed on 13 patients due to WLF, on 3 patients because of refractory gastroesophageal reflux disease, and on 2 patients due to gastric stricture. There were no differences between the preoperative characteristics of the patients with WLF before undergoing conversion to gastric bypass and the patients that underwent pLGB surgery. At postoperative month 36, the percentage of excess weight loss (%EWL) was greater in the patients that underwent pLGB surgery, than in those with WLF that underwent conversion to gastric bypass (69.17±23.73 vs. 54.17±12.48, respectively; p<0.05). Refractory GERD, symptoms due to gastric stricture, and comorbidities all improved after the revisional surgery.
ConclusionRevisional surgery resulted in acceptable weight loss at 36 months of follow-up and favored comorbidity remission. In addition, it resolved symptoms of refractory GERD and gastric stricture.
La manga gástrica laparoscópica (MGL) es la cirugía bariátrica más realizada en el mundo. Sin embargo, sus complicaciones y fallas del procedimiento están aumentado.
ObjetivosDeterminar los motivos de falla de MGL y observar los resultados de conversión a bypass gástrico, comparándolos con bypass gástrico primario.
Material y MétodosSe consultaron pacientes con falla de MGL, operados de cirugía de conversión a bypass gástrico asistido por robot y laparoscópico (técnica híbrida). Los resultados y el seguimiento por falla de pérdida de peso (FPP) se compararon con pacientes operados de bypass gástrico laparoscópico primario (BGLp).
ResultadosTrece pacientes se operaron de conversión por FPP, tres por enfermedad de reflujo gastroesofágico (ERGE) intratable y dos por estenosis gástrica. No hubo diferencias entre las características preoperatorias de los pacientes con FPP y BGLp antes del bypass gástrico. Treinta seis meses después de cirugía, el porcentaje de exceso de peso perdido (%EWL) (54.17±12.48 vs. 69.17±23.73, respectivamente; p = <0.05) fue mayor en BGLp que en FPP. El ERGE intratable, síntomas por estenosis gástrica y las comorbilidades mejoraron después de la cirugía de conversión.
ConclusiónLa cirugía de conversión permite pérdida de peso aceptable a 36 meses de seguimiento y favorece la remisión de comorbilidades. Además, resuelve los síntomas de ERGE refractario y estenosis gástrica.
Obesity is a health problem and its comorbidities are difficult to control with medical treatment alone. Bariatric surgery is the only treatment that has shown successful weight loss and comorbidity resolution.1 In recent years, laparoscopic sleeve gastrectomy (LSG) has become the most widely performed procedure worldwide due to its technical simplicity, acceptable incidence of complications, effective weight loss, and resolution of comorbidities, without modifying the integrity of the digestive tract.2
The increase in the number of LSGs carried out is followed by an increase in the number of cases of failure or complications that mainly include weight loss failure (WLF) or weight regain, gastric stricture, and refractory gastroesophageal reflux disease (GERD). WLF is related to anatomic causes, such as incomplete resection of the gastric fundus and dilatation of the gastric corpus. In some cases, failure occurs in the absence of an anatomic alteration and is due to eating disorders or zero adherence to the indications of the multidisciplinary team. Weight regain is associated with absolutely no comorbidity improvement. Refractory GERD is caused by the disruption of the antireflux mechanisms or hiatal hernia undetected during surgery and 26% of the patients develop new symptoms of GERD after LSG.1,3 There is a 0.35% incidence of gastric stricture after LSG and it causes gastric obstruction, the inability to progress from a liquid to a solid diet, and vomiting.1
Some reports indicate that conversion surgery due to failed LSG is required in 5-11% of cases and Roux-en-Y gastric bypass surgery has been proposed as adequate treatment.4,5 However, results of revisional bariatric surgery are still scarce and the follow-up period in the majority of previous reports is 3 years or less. There is no consensus on the ideal procedure after LSG failure and the subsequent intervention decision is based on individual preference.5,6
The aim of the present study was to evaluate the indications for the conversion to robotic-assisted laparoscopic (hybrid technique) gastric bypass surgery in patients with failed LSG and its results. The results of patients operated on due to WLF were compared with patients that underwent primary laparoscopic gastric bypass (pLGB) surgery.
MethodsPatient selectionThe present study was approved by the Research and Ethics Committee of the Centro Médico Nacional “20 de Noviembre” (folio number 054.2018), following the indications of the Declaration of Helsinki. A retrospective study was conducted utilizing the database containing all the patients that initially underwent LSG, had procedure failure, and then underwent revisional surgery with conversion to gastric bypass surgery, within the time frame of January 2007 to October 2014. All the surgeries were performed by 2 surgeons certified in bariatric surgery (MRJ and BAR), as well as by surgeons with advanced specialty training. Before the primary surgery or the revisional surgery were performed, our patients underwent the same preoperative protocol and postoperative follow-up. The inclusion and exclusion criteria are listed below.
Inclusion criteria- •
Patients > 18 years of age.
- •
Attendance to medical consultations, informative seminar, and support group.
- •
BMI>35 with comorbidities (high blood pressure, diabetes mellitus, obstructive sleep apnea) or BMI>40 with no comorbidities.
- •
Negative pregnancy test.
- •
American Society of Anesthesiologists classification between 1 and 3, no contraindications from the preoperative evaluation by the multidisciplinary team (psychiatry, nutrition, endocrinology, and internal medicine).
- •
Ability to understand nutritional instructions and complete the preoperative studies. To be approved for primary surgery or revisional surgery, the patient must demonstrate dietary adherence and a decrease in excess weight of at least 7%.
- •
No contraindications for LSG based on clinical, endoscopic, and upper gastrointestinal series findings (LSG is not performed in cases of symptomatology and positive signs of reflux, esophagitis, or hiatal hernia). Eradication therapy was given in cases of Helicobacter pylori infection.
Exclusion criteria
- •
Uncontrolled mental disorder, such as anxiety, depression, etc.
- •
Schizophrenia or psychosis.
- •
Pregnancy planned within the next 18 months.
- •
Uncontrolled endocrinologic disease (Cushing syndrome, hypothyroidism).
- •
Uncontrolled eating disorder, such as bulimia nervosa, binge eating.
- •
Psychiatric hospitalization within the previous 2 years, attempted suicide.
The type of primary surgery to be performed was dependent on the consensus of the hospital multidisciplinary committee.
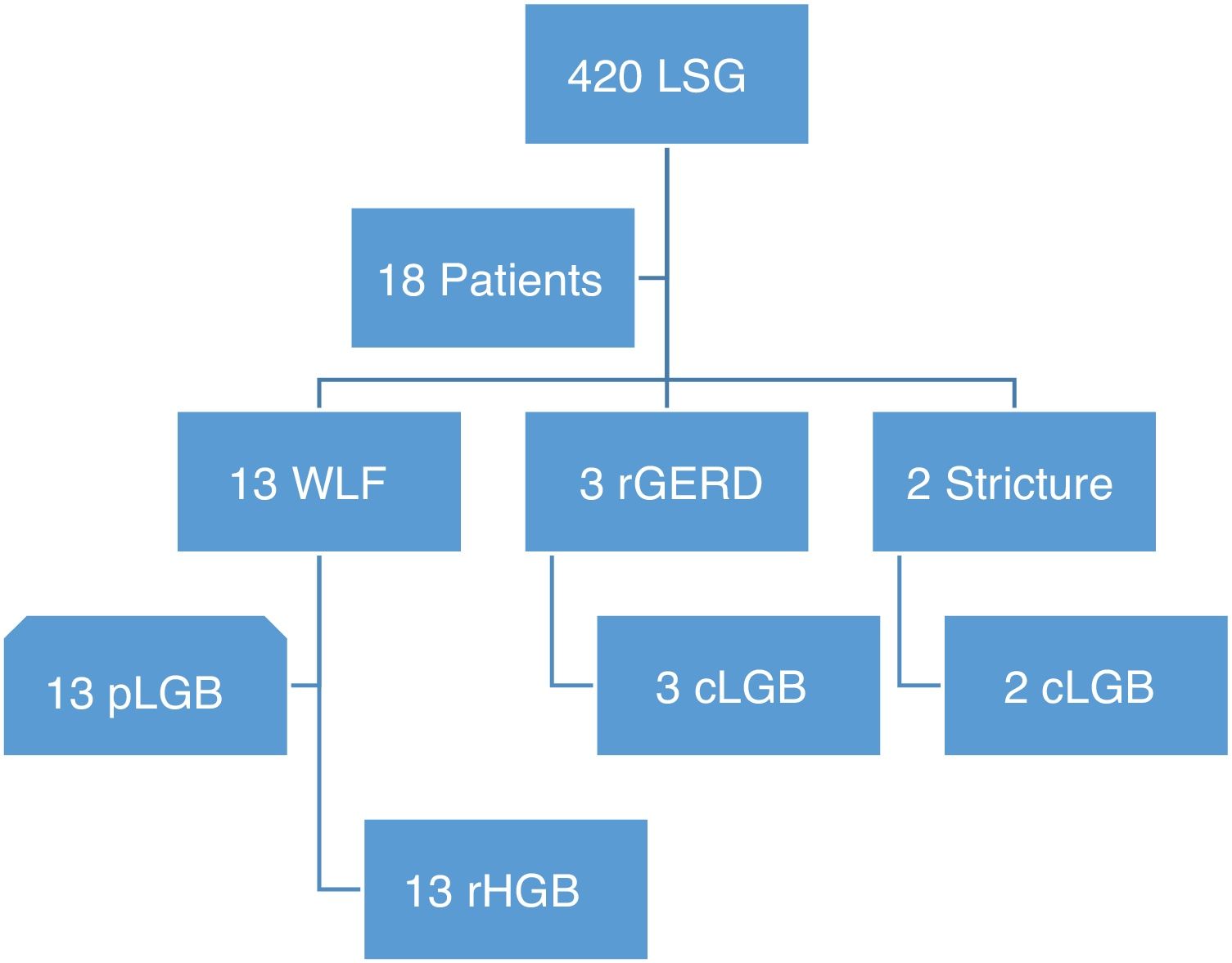
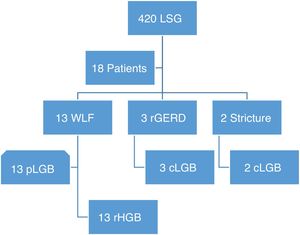
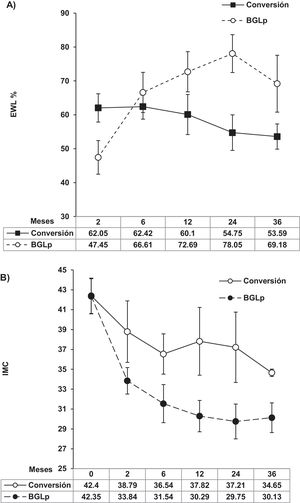
WLF was defined as insufficient weight loss, the percentage of excess weight loss (% EWL) < 50% 24 months after surgery, or BMI persistence > 35kg/m2, and no remission of comorbidities. Progressive weight regain was defined as weight regain > 25% EWL from the nadir. Refractory GERD was characterized by epigastric pain and reflux symptoms with no relief after 3 months of proton pump inhibitor use. Gastric stricture caused symptoms of dysphagia and persistent vomiting in the immediate postoperative period and imaging studies showed the stricture site, with or without esophageal dilatation. Patients with WLF were compared with patients that consecutively underwent pLGB, within the same study time frame, based on their age, weight, BMI prior to bypass surgery, and complete follow-up for at least 36 months (Fig. 1). According to the institutional medical insurance policy, only patients with WLF underwent robotic-assisted conversion surgery. Ideal weight was calculated in relation to the formulas established by the American Society for Metabolic and Bariatric Surgery.7 The percentage of total weight loss (%TWL) and %EWL were calculated based on weight prior to the LSG and at 2, 6, 12, 24, and 36 months after the conversion surgery or pLGB surgery.
Flowgram of patients converted to gastric bypass due to sleeve failure.
cLGB: conversion to laparoscopic gastric bypass; rGERD: refractory gastroesophageal reflux disease; LSG: laparoscopic sleeve gastrectomy; pLGB: primary laparoscopic bypass surgery; rHGB: robotic-assisted hybrid gastric bypass; WLF: weight loss failure.
The presence of comorbidities and their resolution after bariatric surgery were defined as remission when the glucose, blood pressure, and lipid parameters were normal, in the absence of medications. Improvement was defined as the significant reduction of comorbidities and decrease in the number of medications. No change was the absence of remission or improvement, just described.7
Early postoperative complications were those that occurred within the first 30 days after surgery and late complications were those that occurred after 30 days and within the following 12 months. The Clavien-Dindo classification was utilized for grading the complications.8
Surgical techniqueLSGSurgery was laparoscopic. The gastric antrum was spared, and gastric resection was performed with linear stapling that began 4-6cm before the pylorus. Calibration was carried out with a 32 Fr calibration tube and the staple line was reinforced with nonabsorbable material. Since 2010, in accordance with the International Sleeve Gastrectomy Expert Panel Consensus,9 calibration is carried out with 36 Fr tubes and the staple line is not reinforced.
Conversion to Roux-en-Y gastric bypass surgeryThe conversion of LSG to gastric bypass consisted of creating a 30-45ml “gastric reservoir”, resection of the stomach between the second and third gastric vessel arcade, a 100cm antecolic Roux alimentary limb, and a 100-150cm biliary limb. Laparoscopic and robotic-assisted surgeries were performed in the same manner. At our hospital center, conversion surgeries are carried out through robotic assistance as a hybrid procedure, as described by Jung et al.10 The gastric reservoir and the measuring of the intestinal segments were carried out via laparoscopy. The da Vinci Si® Surgical System was positioned over the head of the patient to perform the gastrojejunal anastomosis (GJA) and the jejunojejunal anastomosis with the linear technique. The side-to-side GJA was performed in the posterior gastric wall, utilizing a 34 Fr calibration tube, while the common channel of the enterotomy was closed with continuous suturing and insufflated to perform the hydropneumatic leak test. The side-by-side jejunojejunal anastomosis was performed proximal to the GJA in the omega shape and the enterotomy was closed with continuous suturing. Finally, the omega loop was divided between the 2 anastomoses. The mesenteric spaces and the Petersen space were routinely closed.
Liquid diet was begun on the second postoperative day. Routine imaging studies to search for leakage were not carried out. If no complications presented, the patient was released on the third day. All the patients had outpatient consultation at 1, 3, 6, and 12 months, and then annually, and they all received vitamin supplementation.
Statistical analysisThe quantitative results were expressed as mean and standard deviation with ranges and the categorical variables as number of cases (n) and percentages. The Student’s t test was utilized to appropriately compare the means of the quantitative variables and the quantitative variables were evaluated through the chi-square test, when appropriate. In all the cases, statistical significance was set at a p<0.05. All the calculations were made utilizing the GraphPad Prism 7 program.
ResultsIn our database, we identified 420 patients that underwent LSG at our hospital center, 18 of whom (4.28%) underwent conversion to gastric bypass due to a failed procedure. Thirteen patients (72%) presented with WLF, 3 (17%) due to refractory GERD and 2 (11%) because of gastric stricture. Their mean patient age was 47.38±7.32 years and 94% were women. Before the LSG, mean weight and BMI were 127.96 (100-160) kg and 50.21 (38.16-64.58) kg/m2, respectively. Endoscopic findings prior to LSG were: normal study (39%, n=7), hyperemic gastric antrum (27.7%, n=5), and Helicobacter pylori infection (33.3%, n=6). The patients with Helicobacter pylori infection received 14-day eradication therapy with 30mg of lansoprazole every 12h, 500mg of clarithromycin every 12h, and 1g of amoxicillin every 12h. Eradication was confirmed through a breath test in 65% of the patients and 35% underwent repeat endoscopy and histologic study. The upper gastrointestinal series showed no gastroesophageal reflux or hiatal hernia. Routine esophageal pH monitoring was not carried out prior to LSG.
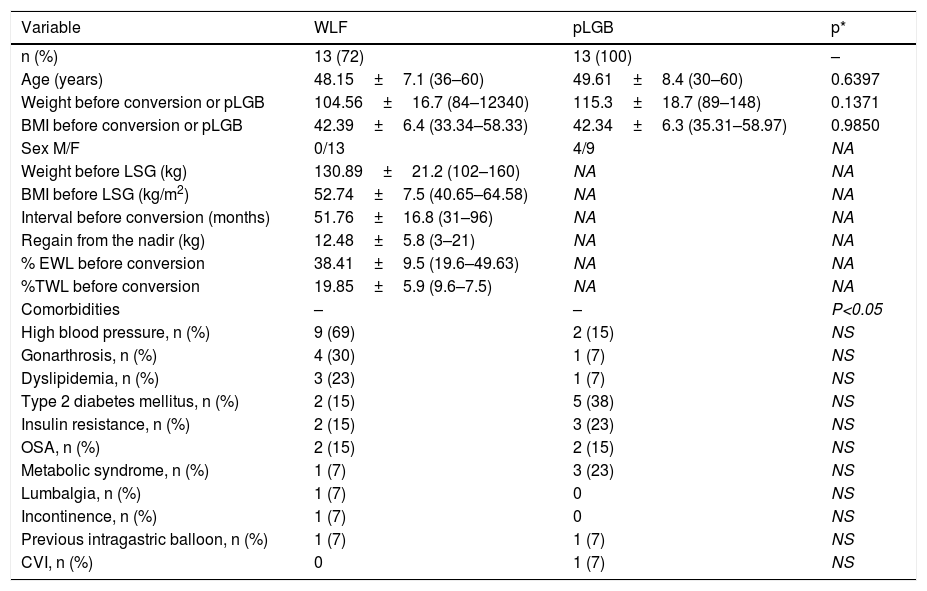
Patients with weight loss failure vs. primary laparoscopic gastric bypass surgerySixty-one percent of patients that underwent conversion surgery due to WLF presented with super obesity (BMI>50). Table 1 shows the baseline characteristics of the patients with WLF and pLGB. There were no differences between the two groups in relation to mean age, weight, BMI, and comorbidities before gastric bypass. All the patients completed 36 months of follow-up. No conversion surgery or primary gastric bypass surgery was performed until the patient demonstrated adherence to the nutritional indications. A total of 61.5% of the patients achieved an average 8.5% excess weight loss and the rest achieved only 7%. The endoscopic findings and those of the upper gastrointestinal series in patients with WLF showed: no gastric sleeve dilatation (53%), some degree of esophagitis with no Barrett’s metaplasia (24%), gastric sleeve dilatation (15%), and retained gastric fundus (8%). No patient presented with hiatal hernia.
Baseline demographic characteristics and comorbidities of patients with WLF and pLGB.
| Variable | WLF | pLGB | p* |
|---|---|---|---|
| n (%) | 13 (72) | 13 (100) | – |
| Age (years) | 48.15±7.1 (36–60) | 49.61±8.4 (30–60) | 0.6397 |
| Weight before conversion or pLGB | 104.56±16.7 (84–12340) | 115.3±18.7 (89–148) | 0.1371 |
| BMI before conversion or pLGB | 42.39±6.4 (33.34–58.33) | 42.34±6.3 (35.31–58.97) | 0.9850 |
| Sex M/F | 0/13 | 4/9 | NA |
| Weight before LSG (kg) | 130.89±21.2 (102–160) | NA | NA |
| BMI before LSG (kg/m2) | 52.74±7.5 (40.65–64.58) | NA | NA |
| Interval before conversion (months) | 51.76±16.8 (31–96) | NA | NA |
| Regain from the nadir (kg) | 12.48±5.8 (3–21) | NA | NA |
| % EWL before conversion | 38.41±9.5 (19.6–49.63) | NA | NA |
| %TWL before conversion | 19.85±5.9 (9.6–7.5) | NA | NA |
| Comorbidities | – | – | P<0.05 |
| High blood pressure, n (%) | 9 (69) | 2 (15) | NS |
| Gonarthrosis, n (%) | 4 (30) | 1 (7) | NS |
| Dyslipidemia, n (%) | 3 (23) | 1 (7) | NS |
| Type 2 diabetes mellitus, n (%) | 2 (15) | 5 (38) | NS |
| Insulin resistance, n (%) | 2 (15) | 3 (23) | NS |
| OSA, n (%) | 2 (15) | 2 (15) | NS |
| Metabolic syndrome, n (%) | 1 (7) | 3 (23) | NS |
| Lumbalgia, n (%) | 1 (7) | 0 | NS |
| Incontinence, n (%) | 1 (7) | 0 | NS |
| Previous intragastric balloon, n (%) | 1 (7) | 1 (7) | NS |
| CVI, n (%) | 0 | 1 (7) | NS |
Values in mean±standard deviation (ranges) and numbers (%).
BMI: body mass index; CVI: chronic venous insufficiency; F: female; M: male; NA: not applicable; NS: non-significant; OSA: obstructive sleep apnea.
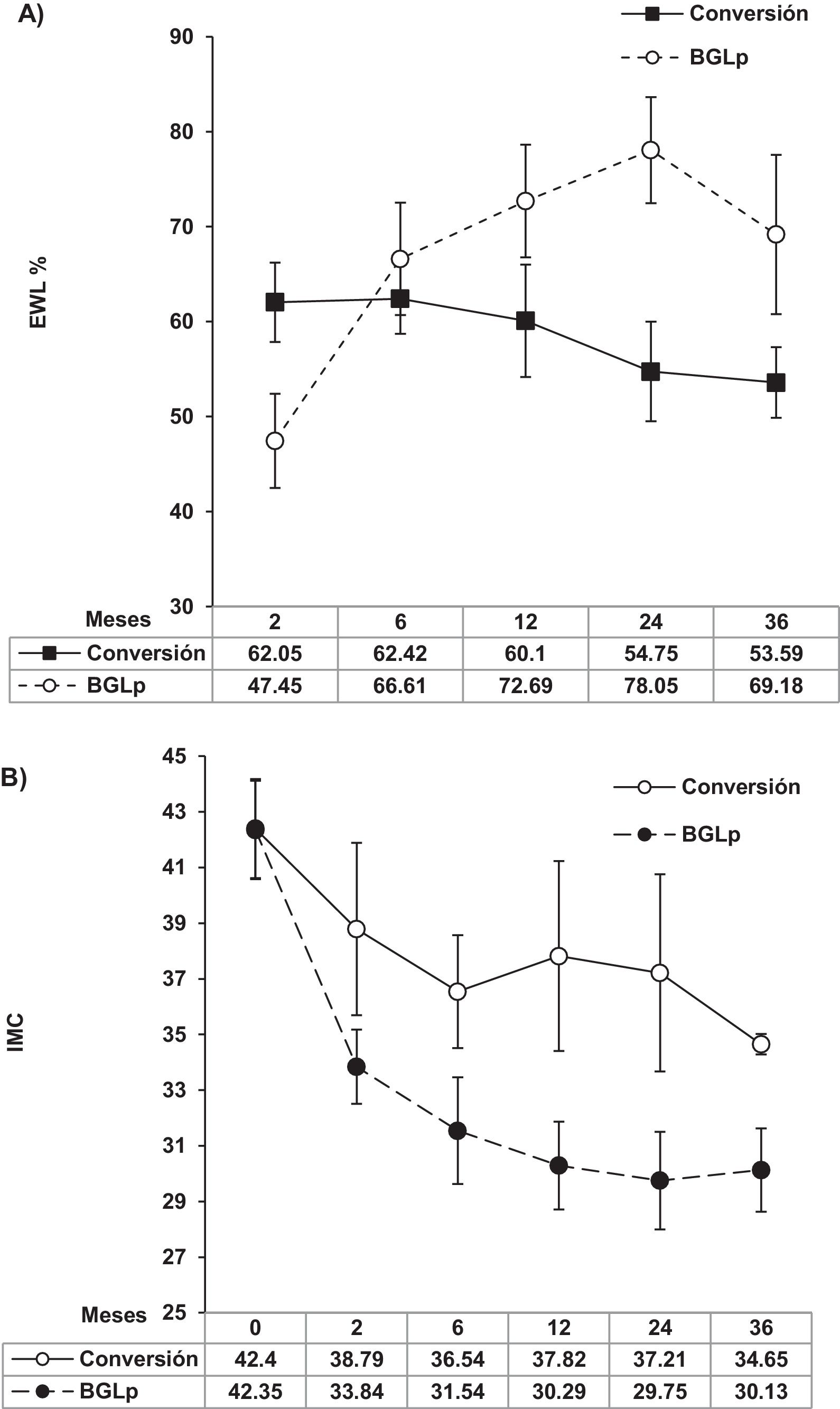
The mean interval of time before conversion surgery for patients with WLF was 52 months. Fig. 2 shows weight loss after gastric bypass surgery at 3 years of follow-up. The %EWL was higher in the patients that underwent pLGB than in the patients that had WLF (69.17±23.73 vs. 54.17±12.48, respectively; p≤0.05). The patients with WLF achieved a decrease of a mean 7.75 points in their BMI, whereas the decrease in the patients that underwent pLGB was 12.21 points. The mean %TWL was similar between the WLF group and the pLGB group (25.9±10.4 [18.62-33.33] vs. 26.8±9.1 [7.48-36.49]), respectively.
Surgery duration was longer in the robotic-assisted hybrid procedure than in the pLGB (200.62±69 vs. 168.46±38min, respectively; p=0.1837), nor were there differences in hospital stay between the two procedures (4.5±2 days for the robotic-assisted hybrid procedure vs. 6.07±7 days for pLGB; p=0.5658).
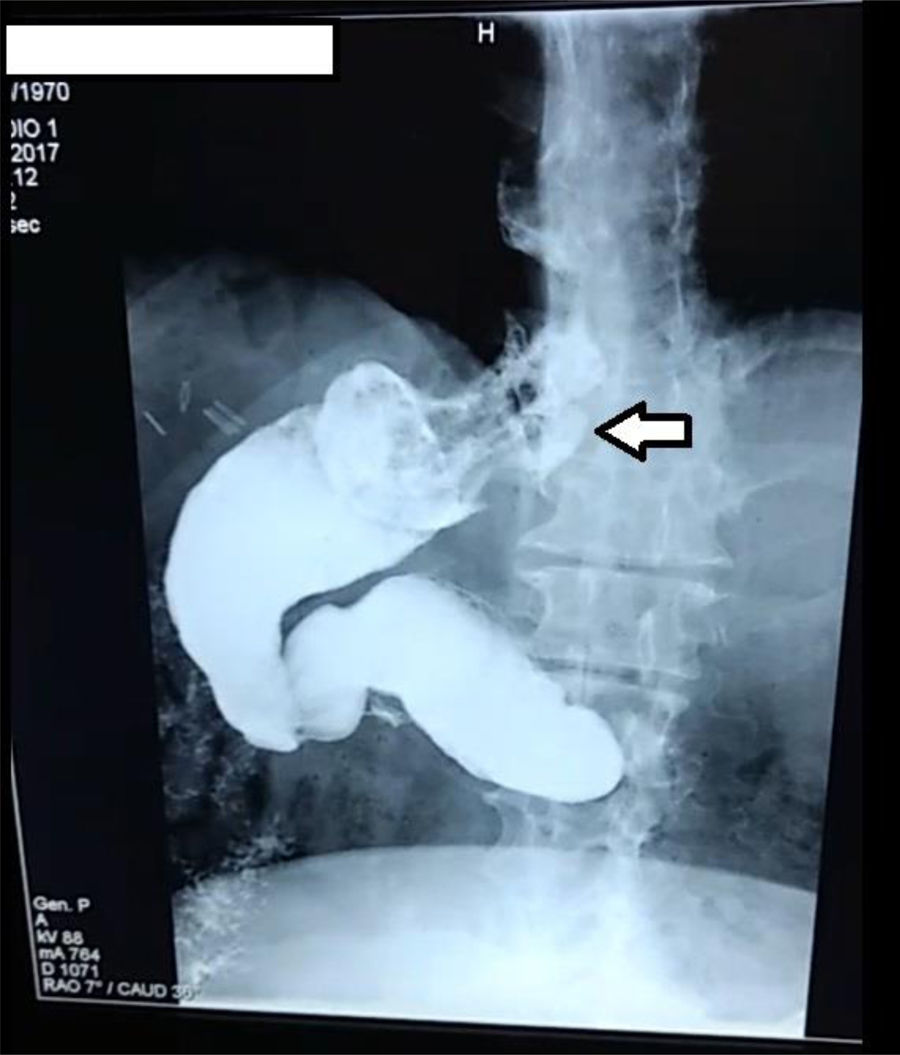
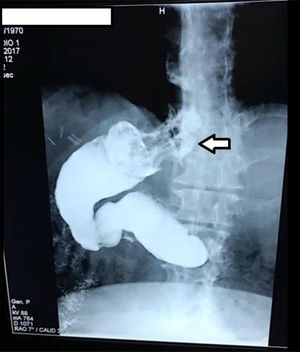
Patients with refractory gastroesophageal reflux disease and gastric strictureAfter LSG, 3 patients presented with refractory GERD (n=3/420, 0.71%) and 2 with gastric stricture (n=2/420, 0.47%). All the patients with refractory GERD were women, and one man and one woman presented with gastric stricture. The mean age of those patients was 45.4±8.2 (33-54) years and the interval in months before conversion was 17.2±8 (11-31) months. Before LSG, mean patient weight was 120.4±17.8 (100-143.6) kg and BMI was 43.6±4.3 (38.16-48.51) kg/m2 and before conversion surgery, mean weight was 73.4±16.8 (50-97) kg and BMI was 26.5±5.8 (23.1-34.38) kg/m2. The main symptomatology of the patients with refractory GERD was heartburn and regurgitation. They did not present with extraesophageal symptoms. Prior to conversion surgery, all the patients received 3 months of treatment with 20mg of oral omeprazole every 12h, with no symptom improvement. The pH monitoring study showed a mean DeMeester index of 28.93 (21.3-40.2). The findings of endoscopy and contrast-enhanced studies in those patients showed Los Angeles grade B esophagitis (n=2) plus hiatal hernia (75%) (Fig. 3) and Los Angeles grade C esophagitis (n=1) with gastric sleeve dilatation (25%). No patient presented with histologic Barrett’s metaplasia. After conversion surgery, the symptoms of GERD resolved and none of the patients required proton pump inhibitor therapy. Thirty-three percent of the patients completed 12 months of follow-up, achieving a BMI of 27.6kg/m2 and 84.6% EWL.
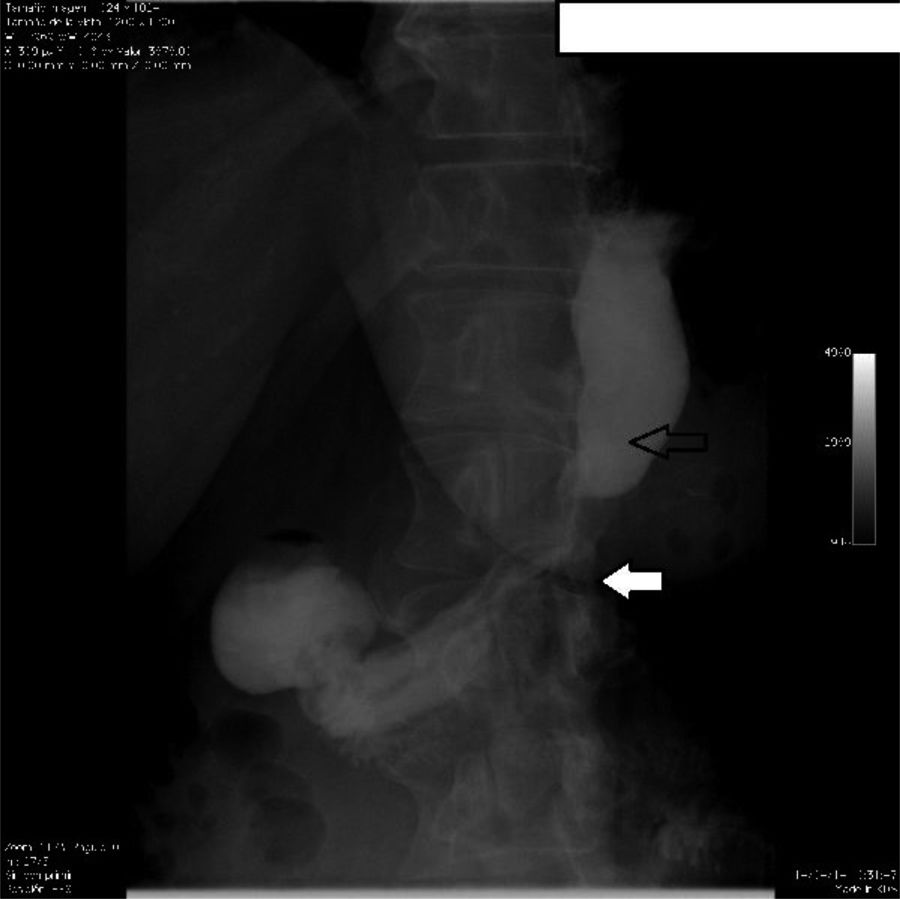
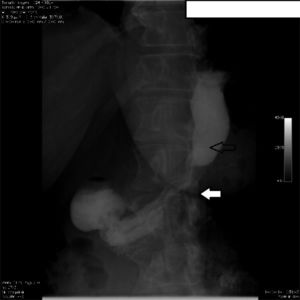
Upper gastrointestinal series and endoscopy showed stricture at the level of the incisura angularis in all the patients with gastric stricture. Before conversion surgery, they were treated with endoscopic balloon dilatations. Between 2 and 5 sessions were carried out unsuccessfully due to stricture grade (Fig. 4). After conversion surgery, 50% of the patients completed 36 months of follow-up, achieving a BMI of 34.28kg/m2 and 58.3% EWL. Gastric obstruction symptoms resolved in all the patients.
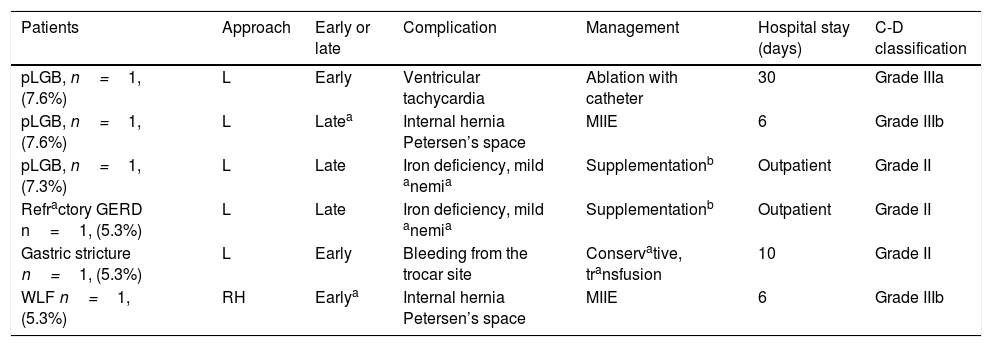
ComplicationsThere were no postoperative deaths in the study patients. Two patients that underwent conversion surgery and 2 that had primary surgery developed major adverse events and one patient in each group required reintervention due to internal hernia (Clavien-Dindo grade IIIb classification). None of the patients presented with complications associated with leakage, ulceration, or GJA stricture. Late complications were mild iron deficiency anemia due to a lack of adherence to the multivitamin dose indications and recovery was achieved with oral ferrous fumarate intake (Table 2).
Early and late complications after conversion surgery and pLGB.
| Patients | Approach | Early or late | Complication | Management | Hospital stay (days) | C-D classification |
|---|---|---|---|---|---|---|
| pLGB, n=1, (7.6%) | L | Early | Ventricular tachycardia | Ablation with catheter | 30 | Grade IIIa |
| pLGB, n=1, (7.6%) | L | Latea | Internal hernia Petersen’s space | MIIE | 6 | Grade IIIb |
| pLGB, n=1, (7.3%) | L | Late | Iron deficiency, mild anemia | Supplementationb | Outpatient | Grade II |
| Refractory GERD n=1, (5.3%) | L | Late | Iron deficiency, mild anemia | Supplementationb | Outpatient | Grade II |
| Gastric stricture n=1, (5.3%) | L | Early | Bleeding from the trocar site | Conservative, transfusion | 10 | Grade II |
| WLF n=1, (5.3%) | RH | Earlya | Internal hernia Petersen’s space | MIIE | 6 | Grade IIIb |
C-D: Clavien-Dindo; GERD: gastroesophageal reflux disease; L: laparoscopic; MIIE: minimally invasive intestinal examination; pLGB: primary laparoscopic gastric bypass; RH: robotic-assisted hybrid; WLF: weight loss failure.
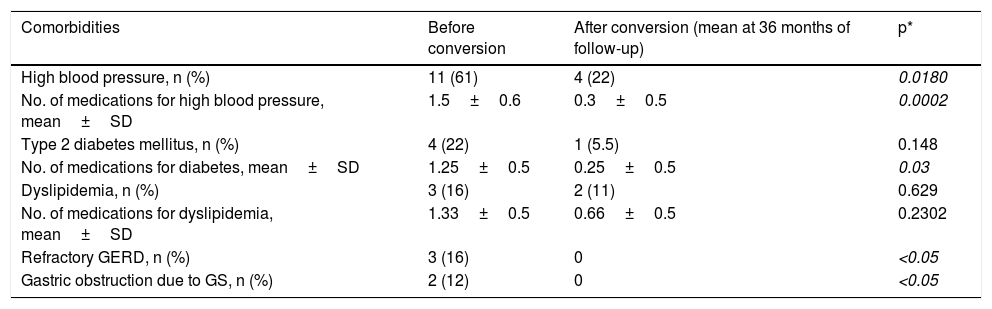
Comorbidity remission was achieved in only 11% (2/18) of the patients after LSG. The rest of the patients presented with some degree of improvement and therefore comorbidity recurrence was not an indication for conversion surgery. The number of comorbidity remissions and the significant decrease in medications increased after conversion to gastric bypass, especially in relation to high blood pressure and type 2 diabetes mellitus (Table 3).
Progression follow-up of comorbidities and medications after conversion surgery.
| Comorbidities | Before conversion | After conversion (mean at 36 months of follow-up) | p* |
|---|---|---|---|
| High blood pressure, n (%) | 11 (61) | 4 (22) | 0.0180 |
| No. of medications for high blood pressure, mean±SD | 1.5±0.6 | 0.3±0.5 | 0.0002 |
| Type 2 diabetes mellitus, n (%) | 4 (22) | 1 (5.5) | 0.148 |
| No. of medications for diabetes, mean±SD | 1.25±0.5 | 0.25±0.5 | 0.03 |
| Dyslipidemia, n (%) | 3 (16) | 2 (11) | 0.629 |
| No. of medications for dyslipidemia, mean±SD | 1.33±0.5 | 0.66±0.5 | 0.2302 |
| Refractory GERD, n (%) | 3 (16) | 0 | <0.05 |
| Gastric obstruction due to GS, n (%) | 2 (12) | 0 | <0.05 |
Results in mean±standard deviation.
GERD: gastroesophageal reflux disease; GS: gastric stricture; SD: standard deviation.
LSG began as the first stage of biliopancreatic diversion with duodenal switch (BPD/DS) in patients at risk due to super obesity. However, many patients achieved adequate weight loss, making the second stage unnecessary. LSG was the main restrictive procedure for super obesity.11,12 In previous years, LSG was considered the best option for those patients at our hospital center. After long-term follow-up, we found that more than 60% of patients with WLF had super obesity. We reviewed 13 studies with follow-up on gastric sleeve conversion to gastric bypass (Table 4). As with our patients, the patients with WLF in 4 studies had a BMI>50kg/m2 before LSG. The best primary procedure for super obesity has not been completely established and in recent comparative studies, gastric bypass surgery presents the best results.13
Previous studies conducted on LSG conversion surgery to GB.
| Study characteristics | Characteristics before conversion | Surgical techniqueComplications | Results and follow-up after conversion |
|---|---|---|---|
| Quezada et al.12016June 2005-April 2015N: 50Sex: 8/42 (F, 84%)Age: 39.8 ± 8.4 years | BMI before SG: 36.4 (34-40) kg/m2BMI before conversion: 35.4 (33.9-37.9) kg/m2Reasons for conversion: WLF (n=28, 56%), GERD (n=16, 32%), stricture (n=6, 12%)Interval before conversion: 49 (24-67) monthsIncidence of conversion from SG to GB: no data | GJA: ManualBiliopancreatic limb: 30-30 cmAlimentary limb: 150 cmComplications: GJA stricture (n=2), Pseudomembranous colitis (n=1), Total 3/50, 6% | Mean follow-up: 36 months (75%)BMI after conversion: 28.6 (24-36) kg/m2%EWL: 66.9 (26-90)GERD resolution: 63%Stricture resolution: 100% |
| Iannelli et al.32016October 2005-December 2013N: 40Sex: 9/31 (F, 77.5%)Age: 40.2 (20-61) years | BMI before SG: 47.5 (37.6-66) kg/m2BMI before conversion: 39.3 (26.3-52.7) kg/m2Reasons for conversion: WLF (n=29, 72.5%), GERD (n=11, 27.5%), comorbidity recurrence (n=1, 2.5%)Interval before conversion: 32.6 (8-113) monthsIncidence of conversion from SG to GB: 40/430 (9.3%) | GJA: ManualBiliopancreatic limb: 50 cmAlimentary limb: 150 cmComplications: open surgery (n=3), GJA stricture (n=4), intra-abdominal abscess (n=1), hernia (n=1), internal hernia (n=1), Total 9/40, 22.5% | Mean follow-up: 18.6 months (100%)BMI after conversion: 30.8 (20.8-44.1)%EWL: 64 (24.1-103)GERD resolution: 100%Comorbidity resolution: 100% |
| Casillas et al.42016February 2009-June 2014N: 48Sex 2/46 (F, 96%)Age: 44 (23-65) years | BMI before SG: 45.9 kg/m2BMI before conversion: 36.8 (27-52) kg/m2Reasons for conversion: GERD + WLF (n=16, 33.3%), GERD (n=14, 29.2%), WLF (n=11, 22.9%), stricture (n=4, 8.3%), GERD + recurrent DM2 (n=2, 4.1%), chronic gastrocutaneous leakage (n=1, 2%)Interval before conversion: 26 (2-60) monthsIncidence of conversion from SG to GB: 36/2794 (1.2%) | GJA: Linear staplingBiliopancreatic limb: 40 a 50 cmAlimentary limb: 100cmComplications: oral intolerance (n=6), postoperative bleeding (n=2), Stricture GJA (n=3), hiatal hernia recurrence (n=1), chronic abdominal pain(n=1), gastrocutaneous leakage persistence (n=1)total: 10/40, 20.8% | Mean follow-up: 24 (0-48) monthsBMI after conversion: no data%EWL: 16.4GERD resolution: 97%Stricture resolution: 100%DM2 improvement: 50%Fistula resolution: 0% |
| Malinka et al.52017January 2009-July 2013N: 32Sex: 10/22 (F, 68.7%)Age: 42.8 ± 11.5 (18-62) years | BMI before SG: 49.4 ± 5.3 (48-62) kg/m2BMI before conversion: 40.9 ± 5.6 (28.8-48)Reasons for conversion: GERD (n=21, 65.6%), WLF (n=11, 34.3%), comorbidity recurrence (n=1, 2.5%)Interval before conversion: 595 ± 369.9 daysIncidence of conversion from SG to GB: 32/239 (13.3%) | GJA: Linear staplingBiliopancreatic limb: 50 cmAlimentary limb: 150 cmComplications: none | Mean follow-up: 36 (87%) monthsBMI after conversion: 33.4 ± 5.9 (23.5-43.6) kg/m2%EWL: 52.0 ± 40.3 (-10-163.6)GERD resolution: 57%Comorbidity resolution: 100% |
| Carmeli et al.112015December 2006-November 2012N: 10 out of 19Sex: 3/7 (F, 70%)Age: 45.3 ± 16.3 years | BMI before SG: 44.5 ± 5.1 kg/m2 / 51.4 ± 11 kg/m2 (BPD/DS)BMI before conversion: 39.8 ± 5.7 kg/m2 / 43.3 ± 6.1 (BPD/DSReasons for conversion: WLF (n=10, 52.6%),WLF (n=9, 42.7% conversion to BDP/DS)Interval before conversion: 36.2 ± 17.4 monthsIncidence of conversion from SG to GB: no data | GJA: Manual in two planesBiliopancreatic limb: 50-70 cmAlimentary limb: 150 cmComplications: marginal ulcer marginal (n=1), total 1/10, 10%BPD/DS complications: Vitamin A deficiency (n=1), Anastomosis leak (n=1), total 2/9, 22% | Mean follow-up: 16 ± 9 months / 30.8 ± 23.5 months (BPD/DS)BMI after conversion: 30 ± 4.8 kg/m2 / 30.2 ± 6.7 kg/m2 (BPD/DS)%EWL: 66.6 ± 33.9%EWL with BD/DS: 80.3 ± 40.3 |
| Abdemur et al.122016January 2004-August 2014N: 30Sex: 7/23 (F, 73.6%)Age: 50.3 ± 13.8 years | BMI before SG: 40.7 ± 5 (34-50) kg/m2BMI before conversion: 33.3 ± 4 kg/m2Reasons for conversion: leak (n=12, 40%), GERD (n=9, 30%); WLF (n=7, 23%), stricture (n=2, 7%)Interval before conversion: 43.6 ± 27.5 (12 days to 80 months)Incidence of conversion from SG to GB: 30/1118 (2.7%) | GJA: Linear staplingBiliopancreatic limb: 30-50 cmAlimentary limb: 100cmComplications: JJA hematoma (n=1), Anastomotic ulcer (n=2), Re -Fuga (n=1), Subhepatic collection (n=1), Bowel obstruction (n=1), total: 6/30, 20% | Mean follow-up: 18.3 ± 15.8 (3-49) monthsBMI after conversion: 28.6 ± 4.8 (21.9-37.1) kg/m2%EWL: 76.5 ± 30.7 (28.8-116.3)GERD resolution: 66%Stricture resolution: 100%Leakage resolution: 91% |
| Poghosyan et al.142016March 2007-December 2014N: 34Sex: 8/26 (F, 76.5%)Age: 44.4 ± 12 years | BMI before SG: 53 ± 11.5 kg/m2BMI before conversion: 44.7 ± 9.8 kg/m2Reasons for conversion: WLF (n=31, 91.1%), GERD (n=3, 8.8%)Interval before conversion: 32 (7.8-69) monthsIncidence of conversion from SG to GB: 34/622 (5.4%) | GJA: Manual (n=5) or circular staplingBiliopancreatic limb: no dataAlimentary limb: no dataComplications: Bowel lesion (n=1), GJA leak (n=1), Trocar site hernia (n=2), Anastomotic ulcer perforation (n=1, ELAP due to abdominal pain (n=1), total: 6/32, 18.7% | Mean follow-up: 36 ± 23 (44%) monthsBMI after conversion: 40.9 ± 8.5 kg/m2%EWL: 63.1 ± 36.2GERD resolution: 100% |
| Langer et al.162010December 2002- September 2009N: 8Sex 4/4 (F, 50%)Age: no data | BMI before SG: 46.9 (39.8-72.3) kg/m2BMI before conversion: no dataReasons for conversion: WLF (n=5, 62.5%), GERD (n=3, 37.5%)Interval before conversion: 33 (15-70) monthsIncidence of conversion from SG to GB: 8/73 (11%) | GJA: Circular stapling (n=7), Linear stapling (n=1)Biliopancreatic limb: 80 cmAlimentary limb: 150 cmComplications: GJA leak (n=1), total: 1/8, 12.5% | Mean follow-up: WLF 25.2 (1-52) months, GERD 26 (2-62) monthsBMI after conversion: no data%EWL: 67 ± 31GERD resolution: 100% |
| Parmar et al.182017August 2012-August 2015N: 22Sex: 6/16 (F, 72.7%)Age: 51 (32-70) years | BMI before SG: 53.1 kg/m2BMI before conversion: 43.3 kg/m2Reasons for conversion: WLF (n=11, 50%), GERD (n=10, 45.5%)Interval before conversion: no dataIncidence of conversion from SG to GB: 22/399 (5.5%) | GJA: Linear staplingBiliopancreatic limb: 50cmAlimentary limb: 150 cmComplications: bowel obstruction due to internal hernia (n=1), marginal ulcer (n=4), abdominal pain and GERD persistence (n=1), abdominal pain (n=1), GERD persistence (n=1), total: 8/22, 36.3% | Mean follow-up: 16 monthsBMI after conversion: 40.8 (32.3-48.1) kg/m2 /%EWL: 46 (32.3-57.7)GERD resolution: 80% |
| Felsenreich et al.232016January 2003-December 2005N: 17Sex: 5/12 (F, 73%)Age: 40.4 (15-66) years | BMI before SG: 48.9 ± 9.4 kg/m2BMI before conversion: 39.8 ± 6.3 kg/m2Reasons for conversion: WLF (n=11, 64.7%), GERD (n=6, 35.3%)Interval before conversion: WLF 48 (24-84) months, GERD 24 (12-84) monthsIncidence of conversion from SG to GB: 17/53 (32%) | GJA: no dataBiliopancreatic limb: no dataAlimentary limb: no dataComplications: GJA leak (n=2), total: 2/17, 11.7% | Mean follow-up: 130 (120-152) monthsBMI after conversion: 35.5 ± 6.9%EWL: 53.5 ± 26.6GERD resolution: 100% |
| Yorke et al.242017May 2013-December 2015N: 18Sex: 5/13 (F, 77%)Age: 41.7 ± 10.6 years | BMI before SG: 50.5 ± 12 kg/m2BMI before conversion: 43.1 ± 9 (31.1 – 60.5) kg/m2Reasons for conversion: WLF (n=9, 65.3%), GERD (n=7, 26.1%), GB in 2 stages (n=2, 8.6%)Interval before conversion: 41.8 ± 12.5 monthsIncidence of conversion from SG to GB: 18/273 (6.5%) | GJA: Circular staplingBiliopancreatic limb 30-50 cmAlimentary limb: 100-110 cmComplications: iron deficiency (n=2), conversion to open surgery (n=2), surgical wound infection (n=3), postoperative bleeding and transfusion (n=1), anastomotic ulcer (n=2), total: 10/18, 55.5% | Mean follow-up: 21.1 ± 11.3 monthsBMI after conversion: 36.4 ± 9 kg/m2%EWL: 52.8 ± 32.7GERD resolution: 75% |
| El Chaar et al.252017January 2010-December 2014N: 9 out of 12Sex: F, 79.5%Age: 44 (23-62) years | BMI before SG: 39.2 (33.7-51.9) kg/m2BMI before conversion: 29.4 (25.5-34.3) kg/m2Reasons for conversion: GERD (n=6, 50%), WLF (n=3, 25%)Interval before conversion: 29 (20-41) monthsIncidence of conversion from SG to GB: 9/630 (5.5%) | GJA: no dataBiliopancreatic limb: no data Alimentary limb: no dataComplications: None | Mean follow-up: 3 monthsBMI after conversion: 24.4 (24.1-31.9) kg/m2%EWL: 75 (49-113)GERD resolution: ? |
| Gautier et al.262013June 2005-December 2010N: 18Sex: no dataAge: 40.9 (24-55) years | BMI before SG: 55 (38-72) kg/m2BMI before conversion: 40.9 (28-48) kg/m2Reasons for conversion: WLF (n=9, 50%), GERD (n=6, 33.3%), DM2 persistence (n=3, 16.7%)Interval before conversion: 23.8 (4.3-51) monthsIncidence of conversion from SG to GB: 18/114 (15.7%) | GJA: Manual in two planesBiliopancreatic limb: 70 cmAlimentary limb: 120-150 cmComplications: small bowel injury (n=1), total 1/18, 5.5% | Mean follow-up: 15.5 ± 1.9 (2.6-31.1) monthsBMI after conversion: 35.8 (24-42.6) kg/m2%EWL: 61.7 (34.2-103.2)GERD resolution: 100%DM2 resolution: 75% |
BMI: body mass index; BPD/DS: biliopancreatic diversion with duodenal switch; DM2: type 2 diabetes mellitus; ELAP: exploratory laparotomy; EWL: excess weight loss; GB: gastric bypass; GERD: gastroesophageal reflux disease; GJA: gastrojejunal anastomosis; JJA: jejunojejunal anastomosis; LAPE: SG: before sleeve gastrectomy; WLF: weight loss failure.
Conversion surgery is required in 5.5 to 20% of cases due to procedure failure or complications.5,8,14 In the studies reviewed, we found 6,745 LSGs performed, 264 conversions to LGB, between 8 and 50 cases per study, and 3.9% of the population affected. The most frequent reasons for conversion were WLF (9/13), refractory GERD (3/13), and leaks (1/13). The incidence of conversion ranged from 1.2 to 32%, and our results showed an incidence of 4.28%.
Restriction loss and increased food volume cause gastric sleeve dilatation.14 Inadequate gastric resection, sleeve dilatation, and neo-fundus formation are anatomic causes of WLF,5,15 but there are cases of WLF with no anatomic alteration. In our study, 53% of the patients had no anatomic alteration. Five patients that underwent conversion surgery due to WLF in the study by Langer et al., and one in the study by Casillas et al., did not present with gastric dilatation following LSG.4,16 Behavioral factors, such as poor adherence to lifestyle and dietary changes, were the probable cause of WLF.5
Lack of long-term follow-up and loss to follow-up are limitations affecting bariatric surgery results. Table 4 shows that the longest follow-up after conversion surgery was 10 years in only one study, whereas the others had poor follow-up duration of 3 to 36 months. The change in BMI after conversion was from 3 to 14.5 points, the best %EWL was 76.5%, and the lowest was 16.4%. In our results, the average %EWL 52 months after LSG was 38.4% vs. 53.5% at 36 months after conversion. A %EWL>50% at 2 years after bariatric surgery is considered successful and the conversion surgery was acceptable in our patients. Nevertheless, pLGB surgery achieved a higher %EWL in our study, as well as in others.5,15–17 Carmeli et al. compared weight loss after conversion to BPD/DS vs. gastric bypass, finding better weight loss with BPD/DS, which is a technique that requires a longer learning curve and results in more postoperative complications.11,18
Refractory GERD after LSG is caused by the disruption of antireflux mechanisms and by Laplace’s law (elevation of intragastric pressure). LSG herniation is more pronounced due to weight loss and visceral fat reduction.4,14 One of our patients with refractory GERD and no hiatal hernia prior to LSG, presented with very obvious gastric fundus migration (Fig. 3). GERD after LSG continues to be a subject of debate. A systematic review showed an increase in the prevalence of GERD in 4 studies and its improvement in 7.19 Hendricks et al. reported that the incidence of de novo GERD was higher than 3% and there was symptom exacerbation in 1% of the patients with a previous diagnosis. Four percent of 919 patients required conversion to Roux-en-Y gastric bypass.20 It is important to know that studies that examine the incidence of GERD after LSG have many limitations and design defects. First, there is no standardized surgical technique for LSG, and different techniques result in variable sleeve sizes (residual fundus and incisura stricture), influencing postoperative GERD. Second, the real incidence of GERD in patients after LSG is unknown, given that they do not undergo routine impedance testing or pH monitoring. Third, many patients develop nonspecific gastrointestinal symptoms, such as regurgitation, bloating, and chest pain, which are erroneously attributed to refractory GERD, with no objective confirmatory studies.18 The resolution of symptoms of refractory GERD after conversion to gastric bypass was effective in our patients. In the studies we reviewed, 12 showed effective GERD resolution, between 57 and 100%, with no need for more medication in 100 patients (Table 4).
The incidence of gastric stricture after LSG is 0.1% to 3.9%.19 It occurred in 0.47% of all cases in our study. Shnell et al. showed the effectiveness of endoscopic balloon dilatations with a 44% success rate.15 Our patients underwent from 2 to 6 dilatation sessions with no improvement of obstruction due to the long stricture segment at the level of the incisura. Conversion surgery resolved the symptoms of gastric stricture. In the studies reviewed, conversion surgery was performed in 3 studies due to gastric stricture in 12 patients, resolving the gastric obstruction.
The general tendency is that weight loss is slightly inferior and the resolution of comorbidities, such as type 2 diabetes mellitus, is lower with LSG.21 Comorbidity recurrence was not a reason for conversion in any of our patients, given that there was a degree of improvement and reduced medications after LSG. Thirty-six months after conversion, some of our patients achieved remission regarding high blood pressure and type 2 diabetes mellitus. In 4 studies of those reviewed, the recurrence of comorbidities, especially of type 2 diabetes mellitus, was the reason for conversion in 7 patients, after which remission between 50 and 100% was achieved.
There are different techniques for performing robotic gastric bypass surgery, including the totally robotic-assisted and the hybrid procedures. The type of technique depends on the docking of the robot and the approach utilized in the GJA. The totally robotic-assisted procedure is carried out by completing the GJA manually or with the robotic stapler (EndoWrist Stapler®). In hybrid procedures one part is performed laparoscopically and one part is robotic-assisted.10
In the studies reviewed herein, there was a higher incidence of complications in patients with conversion surgery than with the primary procedure (10-55% vs. 5-12%).5,22 The most frequent complications in the 264 conversions were: anastomotic ulcer (n=10), GJA stricture (n=7), GJA leak (n=5), bleeding (n=4), internal hernia (n=3), and abscess or collection (n=2). The manual and circular GJA techniques are associated with more complications of ulcer or stricture, compared with the linear technique (5 studies vs. 3 studies). The goal of robotic-assisted surgery is to make the surgery easier and improve results. At our hospital center, the learning curve was surpassed and there is greater experience with the hybrid procedure. We had no complications related to leaks, ulcers, or stricture in the GJA, but we did have a case of internal hernia (n=1). Therefore, we believe it is the safest technique in patients that require conversion surgery.
The limitations of our study include the lack of a standardized technique in LSG, the retrospective study design and consequent difficulty in randomization, and the small number of cases that underwent conversion surgery. A longer follow-up period is needed to determine whether the patients experience weight regain or if they achieve the long-term expected goals.
In conclusion, after the conversion from LSG to gastric bypass, acceptable weight loss was achieved, but was not better than that resulting from pLGB surgery. Refractory GERD symptoms, gastric stricture, and comorbidity resolution were better after conversion surgery. Definitive conclusions could be reached, given the small patient sample size. A larger number of failed LSGs and further studies are needed to determine the best option for those patients.
Ethical disclosuresProtection of human and animal subjects. The authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Declaration of Helsinki of the World Medical Association.
Confidentiality of data. The authors declare that they have followed the protocols of their work center on the publication of patient data, with absolute respect for patient anonymity.
Right to privacy and informed consent. The authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of that document.
Financial disclosureNo financial support was received in relation to this article.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Aguilar-Espinosa F, Montoya-Ramírez J, Gutiérrez Salinas J, et al. Conversión por técnica híbrida robótica a bypass gástrico en Y de Roux posterior a falla de manga gástrica: resultados a corto plazo. Revista de Gastroenterología de México. 2020;85:160–172.