Capsule endoscopy is part of the diagnostic approach to patients with suspected small bowel bleeding and data on its clinical impact are still limited in developing countries. The primary aim of the present study was to determine its impact on subsequent diagnostic and therapeutic decisions.
Material and methodsA retrospective study was conducted that included all the patients that underwent capsule endoscopy with the PillCam™ SB 3 Capsule system due to suspected small bowel bleeding treated at the Hospital Universitario Fundación Valle del Lili between January 2011 and December 2020.
ResultsA total of 158 patients met the inclusion criteria. Mean patient age was 63 years (interquartile range [IQR], 52-74), 53.6% of the patients were women, and high blood pressure was the most frequent comorbidity (43.7%). The main indication was overt bleeding (58.2%). Of all the capsule endoscopies carried out, 63.9% showed lesions that were potentially responsible for bleeding. Medical or surgical treatment was indicated in 63.3% of the case total. Rebleeding at 6 months occurred in 15 patients and there were 2 deaths due to gastrointestinal bleeding at 6 months.
ConclusionsCapsule endoscopy has a high impact on patients with suspected small bowel bleeding, with respect to clinical decision-making, as well as rebleeding, hospitalization, and mortality outcomes. The positivity rate of lesions potentially responsible for bleeding was similar to that reported in developed countries.
La videocápsula endoscópica hace parte del abordaje diagnóstico de pacientes con sospecha de hemorragia de intestino delgado; los datos sobre su impacto clínico todavía son limitados en países en vías de desarrollo. El objetivo principal del estudio es estimar su impacto en las decisiones diagnósticas y terapéuticas posteriores.
Material y métodosSe realizó un estudio retrospectivo que incluyó a todos los pacientes que se sometieron a videocápsula endoscópica con el sistema PillCam™ SB 3 Capsule por sospecha de hemorragia de intestino delgado tratados en el Hospital Universitario Fundación Valle del Lili entre enero de 2011 y diciembre de 2020.
ResultadosUn total de 158 pacientes cumplieron los criterios de inclusión. El 53.6% de los pacientes eran mujeres, con una media de edad de 63 años (rango intercuartílico, IQR 52-74), y la hipertensión arterial fue la comorbilidad más frecuente (43.7%). La principal indicación fue la hemorragia manifiesta (58.2%). El 63.9% del total de cápsulas endoscópicas mostraron lesiones potencialmente causantes de hemorragia. Se indicó tratamiento médico o quirúrgico en el 63.3% del total de casos. Se presentaron nuevas hemorragias a los 6 meses en 15 pacientes y se produjeron 2 muertes por hemorragia gastrointestinal a los 6 meses.
ConclusionesLa videocápsula endoscópica tiene un alto impacto en pacientes con sospecha de hemorragia de intestino delgado, tanto en la toma de decisiones clínicas como en los desenlaces de resangrado, hospitalización y muerte. La tasa de positividad de lesiones potencialmente causantes de hemorragia es similar a la reportada en países desarrollados.
Small bowel bleeding is suspected when the origin of the bleeding is not detected through upper and lower endoscopic studies. It accounts for 5 to 10% of cases and can manifest as overt bleeding (hematochezia, hematemesis, or melena) or occult bleeding (refractory iron-deficiency anemia and/or fecal occult blood).1 Small bowel bleeding is uncommon but can lead to an increase in the number of diagnostic procedures (up to 7 per patient), more frequent and prolonged hospitalizations, higher health costs, and more transfused blood product units.2,3Up to the end of the twentieth century, the approach was limited to direct surgical inspection and endoscopic methods, such as single or double balloon enteroscopy.4 They provide high diagnostic yield and therapeutic management but are invasive interventions that have risks (pancreatitis, bleeding, perforations, and sedation-related adverse effects).5 Capsule endoscopy (CE) was approved by the Food and Drug Administration (FDA) in 2001. Its current most relevant indication, according to both the American Gastroenterological Association and the European Society of Gastrointestinal Endoscopy, is the suspicion of small bowel bleeding.6,7CE is recommended, based on evidence showing that it is equivalent or superior to other imaging modalities, by enabling complete visualization of the small bowel in 79-90% of cases.4 The indication for specific medical or surgical management guided by CE findings has been described in 37-87% of cases.8 In a systematic review and meta-analysis of 227 studies (with 22,840 CE procedures), it was indicated for suspected small bowel bleeding in 66% of the evaluations, with a 59.4% detection rate of positive findings, and angiodysplasia was the most widely detected alteration (50%).9 There is little information available on the performance and impact of CE in low-resource or middle-resource countries.
The primary aim of our study was to determine the impact of CE on clinical decision-making by measuring the detection rate of lesions potentially responsible for bleeding and the indication percentage of additional diagnostic or therapeutic tests, based on those findings. The specific aims were to describe the sociodemographic and clinical characteristics of the patients, evaluate the diagnostic approach before CE, determine the frequency of the findings, and estimate the clinical outcomes at 30 days and 6 months.
Materials and methodsA retrospective study was conducted that included patients above 16 years of age that underwent CE as outpatients or hospitalized patients at the Hospital Universitario Fundación Valle del Lili, within the time frame of January 2011 and December 2020. The procedure was indicated for suspected overt small bowel bleeding (melena or hematochezia) or occult small bowel bleeding (detection of blood in feces or treatment-refractory iron-deficiency anemia), once upper gastrointestinal endoscopy and colonoscopy were performed, in which no cause of symptoms was found. SAP software was employed to search for the patients and their respective procedures. The variables reviewed were sociodemographic and clinical characteristics, as well as the tests performed for the diagnostic approach (collected from the clinical histories of the hospitalized patients and outpatients), outpatient or post-hospitalization follow-up, and CE findings (obtained from the registered results of the PillCam™ software (Medtronic) during the study period. The inclusion criteria were patients above 16 years of age that underwent CE indicated for gastrointestinal bleeding and a minimum follow-up time of 6 months. Patients with incomplete clinical histories, that were under 16 years of age, that underwent CE indicated for reasons other than bleeding, that had incomplete endoscopic study, and whose follow-up time was under 6 months were excluded. The indication for the procedure and treatment given based on the CE results was individualized by the different hospital specialties.
Description of the procedureThe Medtronic® PillCam™ SB 3 Capsule system was employed. The video camera measures 26.2 mm × 11.4 mm and weighs 3 g, with an effective visual field of 156 degrees, an estimated capture time above 8 hours, and a photogram rate of 2-6 per second. The patients (outpatients and hospitalized patients) previously fasted for 8 hours and had bowel preparation with 2 packets of polyethylene glycol 3350, diluted in two liters of water. Eight sensors were placed on the abdominal wall, connected to a data recorder located in the belt to be worn for the duration of the exam. The device was then swallowed with 20 cc of water. After 8 hours, or the time needed by the capsule to achieve a cecal image, the recorder was turned off and the images downloaded onto the PillCam Desktop Software system. The images were read and interpreted by a gastroenterologist, expert in CE, with 10 years of experience in managing the device and software. The findings were organized in their order of relevance for each of the segments of the gastrointestinal tract evaluated.
Statistical analysisThe patients were registered using the BD Clinic platform, which is password protected, with safe servers for consulting, downloading, and storing the collected data. Only the persons involved in the study had access to that database. Each enrolled patient was assigned a study ID, ensuring anonymity. To evaluate the quality of the data, 10% of the written data was randomly selected and compared with the digital data. The categorical variables were expressed as absolute and relative frequencies. The continuous variables were expressed as mean and standard deviation, if they had normal distribution, and as interquartile range (IQR), if they did not.
Ethical considerationsThis study was carried out according to the international agreements on biomedical research of the Council for International Organizations of Medical Sciences (CIOMS), in collaboration with the World Health Organization (WHO), and the 1993 Colombian Resolution 8430. All the procedures reviewed were performed after having received informed consent. Informed consent was not required for the present study, given that it is classified as “non-risk” and has a retrospective design. The authors declare that this article contains no personal information that could identify the patients.
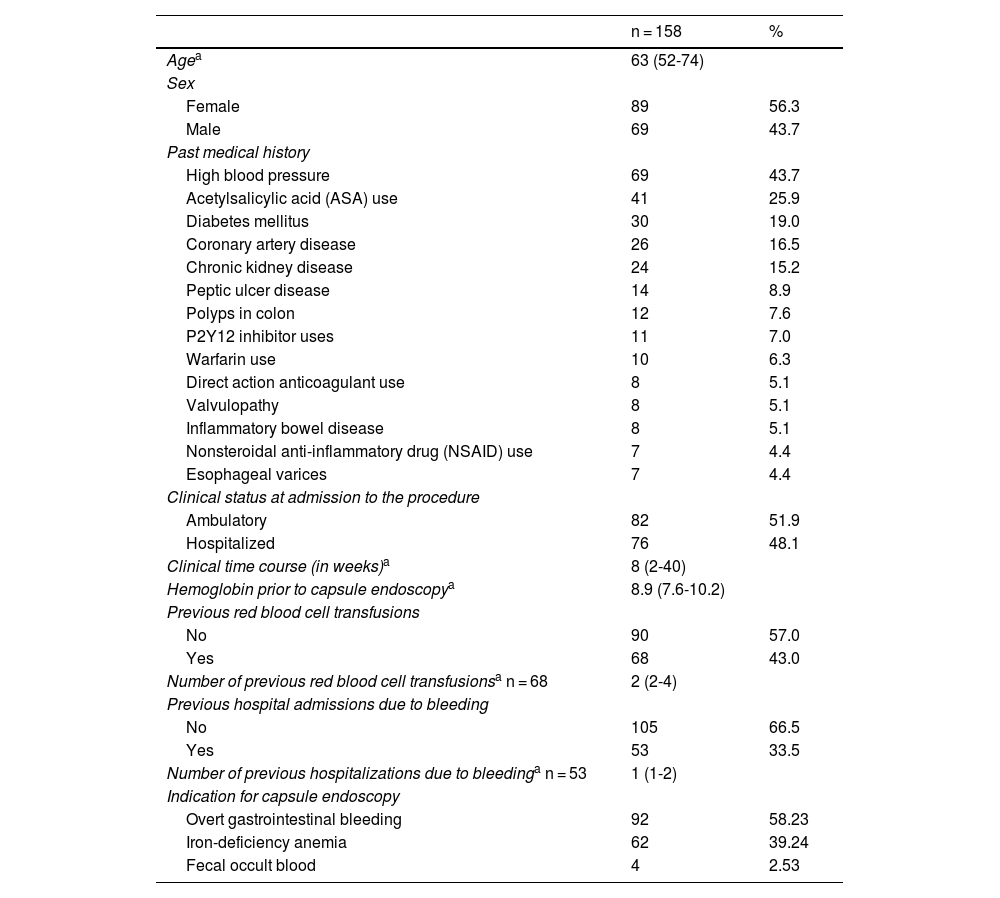
ResultsA total of 158 patients met the inclusion criteria. Mean patient age was 63 years (IQR 52-74) and 53.6% of the patients were women. The most prevalent comorbidities were high blood pressure (43.7%) and diabetes mellitus (19%), and 27.8% of patients had previous abdominal surgery. Acetylsalicylic acid (ASA) was used in 35.9% of the patients (Table 1).
Demographic and clinical characteristics of patients with capsule use.
| n = 158 | % | |
|---|---|---|
| Agea | 63 (52-74) | |
| Sex | ||
| Female | 89 | 56.3 |
| Male | 69 | 43.7 |
| Past medical history | ||
| High blood pressure | 69 | 43.7 |
| Acetylsalicylic acid (ASA) use | 41 | 25.9 |
| Diabetes mellitus | 30 | 19.0 |
| Coronary artery disease | 26 | 16.5 |
| Chronic kidney disease | 24 | 15.2 |
| Peptic ulcer disease | 14 | 8.9 |
| Polyps in colon | 12 | 7.6 |
| P2Y12 inhibitor uses | 11 | 7.0 |
| Warfarin use | 10 | 6.3 |
| Direct action anticoagulant use | 8 | 5.1 |
| Valvulopathy | 8 | 5.1 |
| Inflammatory bowel disease | 8 | 5.1 |
| Nonsteroidal anti-inflammatory drug (NSAID) use | 7 | 4.4 |
| Esophageal varices | 7 | 4.4 |
| Clinical status at admission to the procedure | ||
| Ambulatory | 82 | 51.9 |
| Hospitalized | 76 | 48.1 |
| Clinical time course (in weeks)a | 8 (2-40) | |
| Hemoglobin prior to capsule endoscopya | 8.9 (7.6-10.2) | |
| Previous red blood cell transfusions | ||
| No | 90 | 57.0 |
| Yes | 68 | 43.0 |
| Number of previous red blood cell transfusionsa n = 68 | 2 (2-4) | |
| Previous hospital admissions due to bleeding | ||
| No | 105 | 66.5 |
| Yes | 53 | 33.5 |
| Number of previous hospitalizations due to bleedinga n = 53 | 1 (1-2) | |
| Indication for capsule endoscopy | ||
| Overt gastrointestinal bleeding | 92 | 58.23 |
| Iron-deficiency anemia | 62 | 39.24 |
| Fecal occult blood | 4 | 2.53 |
The majority of the CEs were outpatient procedures (51.9%). Overt bleeding was the primary indication for the procedure, at 58.2%, followed by treatment-refractory iron-deficiency anemia, at 39.2%, and persistent fecal occult blood, for the remaining 2.5%. The time presenting with clinical manifestations of bleeding or the detection of iron-deficiency anemia, before CE was performed, was 8 weeks (IQR 2-40) and the median hemoglobin value available before the procedure was 8.9 mg/dl (Table 1). Fifty-three patients had a history of hospital admission due to previous bleeding episodes and 43% of patients had red blood cell transfusions during the time of symptom manifestation.
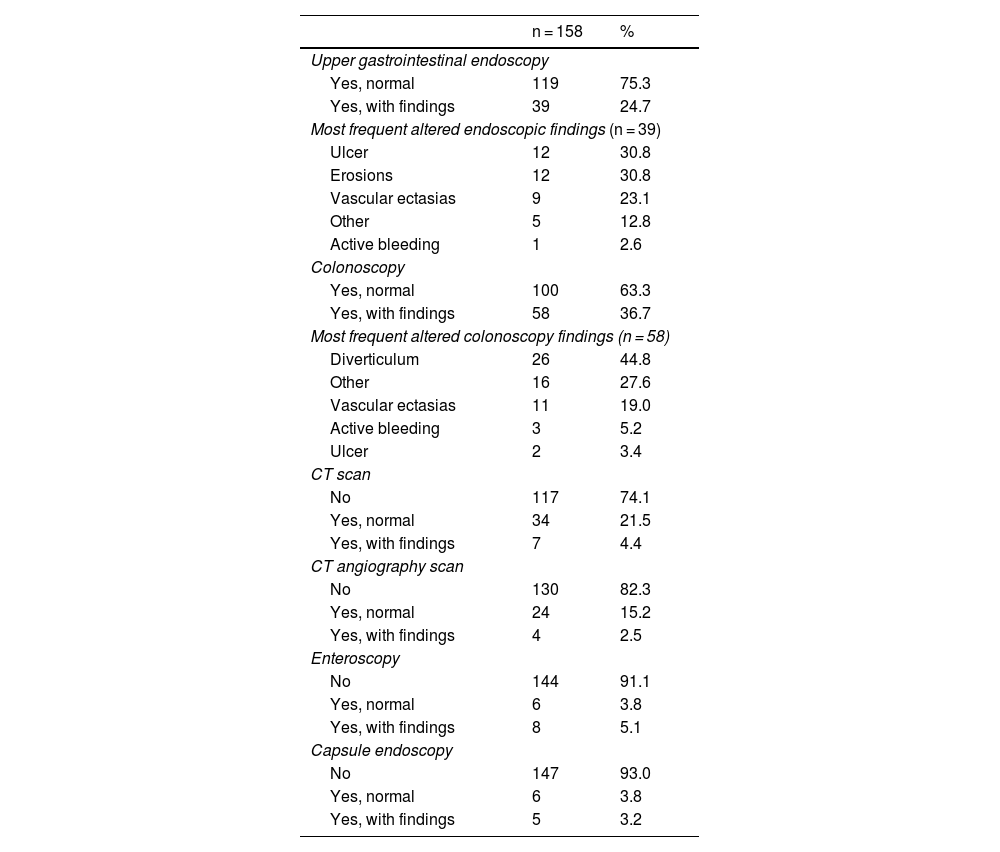
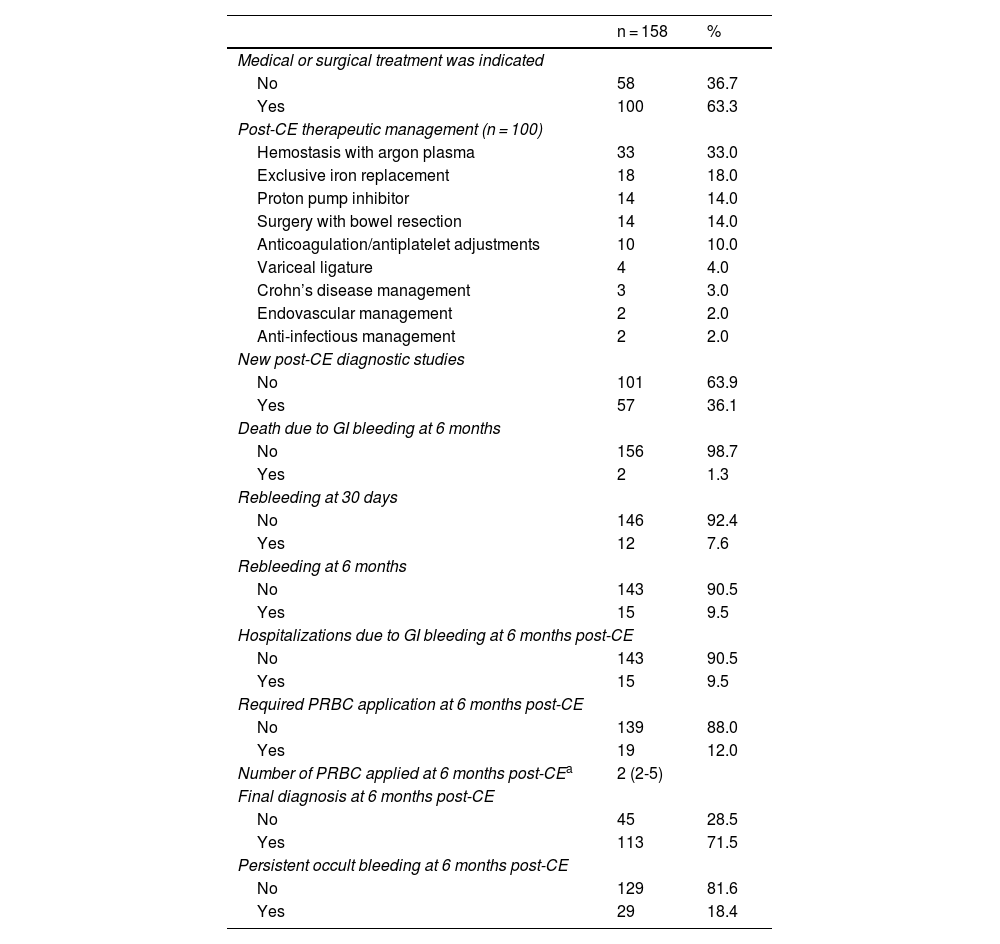
All the patients underwent upper gastrointestinal endoscopy (with abnormalities in 24.7%, the main ones of which were ulcers and erosions) and colonoscopy (with abnormalities in 36.7%, the most frequent of which was diverticulosis), before the performance of CE. Eleven patients had previously undergone CE during symptom manifestation and relevant abnormalities were reported in 5 of those patients (Table 2). Of the CEs carried out, alterations were detected in 144; of those patients, 101 had findings of alterations potentially responsible for the clinical symptoms. Lesions were located in multiple zones (47.2%), followed by lesions exclusively in the jejunum (29.8%) (Table 3). There were no deaths due to gastrointestinal bleeding at 30 days and two deaths at 6 months (one 78-year-old male patient with comorbidities, in whom no lesions were detected, and one 78-year-old female patient with comorbidities and multiple findings of vascular ectasia, managed with serial hemostasis treatment with argon plasma). Fifteen patients presented with bleeding at 6 months; in 8 of those patients no clear origin of the bleeding was found in that period of time, in an outpatient setting. No CEs were repeated in any of the patients. Of the patient total, 18.4% had persistent gastrointestinal bleeding or iron-deficiency anemia. Medical or surgical management was indicated in 63.3% of all the patients. Thirty-three patients had hemostatic management through specific endoscopy, 18 had iron replacement as exclusive treatment, and 18 underwent intestinal resection. Ten patients required adjustments to their anticoagulation or antiplatelet regimens. There were 15 cases of rebleeding at 6 months. There were no cases of death due to gastrointestinal bleeding at 30 days and two deaths at 6 months. A definitive diagnosis was made in 71.5% of the cases (Table 4).
Previous studies.
| n = 158 | % | |
|---|---|---|
| Upper gastrointestinal endoscopy | ||
| Yes, normal | 119 | 75.3 |
| Yes, with findings | 39 | 24.7 |
| Most frequent altered endoscopic findings (n = 39) | ||
| Ulcer | 12 | 30.8 |
| Erosions | 12 | 30.8 |
| Vascular ectasias | 9 | 23.1 |
| Other | 5 | 12.8 |
| Active bleeding | 1 | 2.6 |
| Colonoscopy | ||
| Yes, normal | 100 | 63.3 |
| Yes, with findings | 58 | 36.7 |
| Most frequent altered colonoscopy findings (n = 58) | ||
| Diverticulum | 26 | 44.8 |
| Other | 16 | 27.6 |
| Vascular ectasias | 11 | 19.0 |
| Active bleeding | 3 | 5.2 |
| Ulcer | 2 | 3.4 |
| CT scan | ||
| No | 117 | 74.1 |
| Yes, normal | 34 | 21.5 |
| Yes, with findings | 7 | 4.4 |
| CT angiography scan | ||
| No | 130 | 82.3 |
| Yes, normal | 24 | 15.2 |
| Yes, with findings | 4 | 2.5 |
| Enteroscopy | ||
| No | 144 | 91.1 |
| Yes, normal | 6 | 3.8 |
| Yes, with findings | 8 | 5.1 |
| Capsule endoscopy | ||
| No | 147 | 93.0 |
| Yes, normal | 6 | 3.8 |
| Yes, with findings | 5 | 3.2 |
Capsule findings.
| n = 158 | % | |
|---|---|---|
| Gastric transit time (minutes)a | 19.5 (11-51) | |
| Small bowel transit time (minutes)a | 244 (165-318) | |
| Colon transit time (minutes)a | 263 (224-327) | |
| A lesion was detected | ||
| No | 14 | 8.9 |
| Yes | 144 | 91.1 |
| A lesion potentially responsible for bleeding was detected (n = 144) | ||
| No | 57 | 39.6 |
| Yes | 101 | 70.1 |
| Main location of lesion (n = 144) | ||
| Multiple locations | 68 | 47.2 |
| Jejunum | 43 | 29.9 |
| Duodenum | 16 | 11.1 |
| Stomach | 8 | 5.6 |
| Ileum | 7 | 4.9 |
| Cecum | 2 | 1.4 |
| Stomach (n = 39) | ||
| Erosive gastritis | 23 | 59.0 |
| Ulcer | 8 | 20.5 |
| Vascular ectasia Yamamoto IA | 3 | 7.7 |
| Active bleeding | 2 | 5.1 |
| Venous dilation | 2 | 5.1 |
| Vascular ectasia Yamamoto IB | 1 | 2.6 |
| Duodenum (n = 58) | ||
| Erosions | 29 | 50.0 |
| Vascular ectasia Yamamoto IA | 11 | 19.0 |
| Vascular ectasia Yamamoto IB | 5 | 8.6 |
| Ulcer | 5 | 8.6 |
| Active bleeding | 4 | 6.9 |
| Polyp | 3 | 5.2 |
| Vascular dilations | 1 | 1.7 |
| Jejunum (n = 97) | ||
| Erosions | 25 | 25.8 |
| Vascular ectasia Yamamoto IA | 23 | 23.7 |
| Vascular ectasia Yamamoto IB | 15 | 15.5 |
| Ulcer | 11 | 11.3 |
| Lymphangiectasia | 9 | 9.3 |
| Chylous cysts | 4 | 4.1 |
| Active bleeding | 4 | 4.1 |
| Polyp | 3 | 3.1 |
| Other | 3 | 3.1 |
| Ileum (n = 27) | ||
| Ulcer | 9 | 33.3 |
| Vascular ectasia Yamamoto IA | 6 | 22.2 |
| Vascular ectasia Yamamoto IB | 4 | 14.8 |
| Polyp | 4 | 14.8 |
| Active bleeding | 3 | 11.1 |
| Mass | 1 | 3.7 |
| Cecum (n = 16) | ||
| Vascular ectasia Yamamoto IA | 6 | 37.5 |
| Vascular ectasia Yamamoto IB | 5 | 31.3 |
| Polyp | 2 | 12.5 |
| Active bleeding | 2 | 12.5 |
| Mass | 1 | 6.3 |
Outcomes.
| n = 158 | % | |
|---|---|---|
| Medical or surgical treatment was indicated | ||
| No | 58 | 36.7 |
| Yes | 100 | 63.3 |
| Post-CE therapeutic management (n = 100) | ||
| Hemostasis with argon plasma | 33 | 33.0 |
| Exclusive iron replacement | 18 | 18.0 |
| Proton pump inhibitor | 14 | 14.0 |
| Surgery with bowel resection | 14 | 14.0 |
| Anticoagulation/antiplatelet adjustments | 10 | 10.0 |
| Variceal ligature | 4 | 4.0 |
| Crohn’s disease management | 3 | 3.0 |
| Endovascular management | 2 | 2.0 |
| Anti-infectious management | 2 | 2.0 |
| New post-CE diagnostic studies | ||
| No | 101 | 63.9 |
| Yes | 57 | 36.1 |
| Death due to GI bleeding at 6 months | ||
| No | 156 | 98.7 |
| Yes | 2 | 1.3 |
| Rebleeding at 30 days | ||
| No | 146 | 92.4 |
| Yes | 12 | 7.6 |
| Rebleeding at 6 months | ||
| No | 143 | 90.5 |
| Yes | 15 | 9.5 |
| Hospitalizations due to GI bleeding at 6 months post-CE | ||
| No | 143 | 90.5 |
| Yes | 15 | 9.5 |
| Required PRBC application at 6 months post-CE | ||
| No | 139 | 88.0 |
| Yes | 19 | 12.0 |
| Number of PRBC applied at 6 months post-CEa | 2 (2-5) | |
| Final diagnosis at 6 months post-CE | ||
| No | 45 | 28.5 |
| Yes | 113 | 71.5 |
| Persistent occult bleeding at 6 months post-CE | ||
| No | 129 | 81.6 |
| Yes | 29 | 18.4 |
The detection rate of lesions potentially responsible for bleeding was 63.9%, very close to that reported in international meta-analyses, including the systematic review by Liao et al.,9 with 227 articles involving 22,840 procedures, and the Latin American literature (between 60 and 70%).10–13 With respect to iron-deficiency anemia, Koulaouzidis et al.14 reported a diagnostic yield of 47%, and in our study, it was 53%. Based on our findings in all the CEs, medical or surgical management was indicated in 63.3% of the patients. A total of 101 patients had abnormal CE results, showing findings of lesions potentially responsible for bleeding, among which were Yamamoto grade I (IA and IB) in 45 cases, ulcers in 19 cases, active bleeding in 13 cases, erosions in 10 cases, and other lesions in 14 cases. Of those 101 patients, specific medical or surgical treatment was indicated in 78%. Thirty-three of the 78% underwent endoscopic study for initial hemostatic treatment and one patient had endovascular management. The remaining 22% had no specific therapeutic management, whether due to the comorbidities of the patient, the risk for bleeding, fragility, or self-limitation of symptoms at follow-up; none of the patients had died at 6 months. Those data reinforce the importance of the premorbid condition and clinical situation of the patient, with respect to decision-making.
Even though there is evidence that CE identifies patients that could benefit from subsequent interventions, the data in the literature are heterogeneous. In South Korea the intervention was carried out in 22.9% patients15 but did not influence the rebleeding rate, whereas in the retrospective study by Redondo-Cerezo et al.,16 50.7% received treatment, with clinical improvement in the majority of them. In our study, CE led to treatments that resolved bleeding in 70.1% of the cases, close to that described by Estévez et al. (71.6%)17 but less than that reported by Penazzio et al. (86.9%).18
The standards of quality in the European Society of Gastrointestinal Endoscopy (ESGE) guidelines emphasize the use of enteroscopy in management after CE, with a minimum standard of use of 75%, with low quality of evidence.19 Upon reviewing the current literature, medical and conservative management appear to be predominant over interventionist management, especially with enteroscopy. The percentage of enteroscopies performed varies, and in the case of positive and negative CEs, encompasses from 2.6 to 28.9%,15,20,21 and in the case of positive CEs, increases only to 31.3-56.5%.22–24 In our study, enteroscopic treatment was carried out in only 33% of the patients indicated for management, with subsequent low rebleeding and mortality rates at 6 months. In that sense, and as reported in the literature, small bowel bleeding is very complex and requires highly individualized courses of action.
Management of the cases in which CE does not detect a lesion significant in size remains a clinical challenge. Iron (oral and parenteral) was utilized as single or adjunct management in 59 patients; even in cases of negative CE, support therapy tends to be used.25 In our study, half of the patients with alterations potentially responsible for bleeding had vascular etiologies (angiodysplasia/ectasia), similar to that reported by Hadithi et al.26 Ulcers were in second place (30%), lower than that described by Xin et al.,27 and are controversial, regarding bleeding causality, given that neither their characteristics of location, multiplicity, or histopathologic findings are prognostic factors for symptom persistence at follow-up; up to 84% of ulcers are nonspecific and tend to be part of benign processes.28,29 Erosions were found in 77 cases (32% of lesions) and less than 2% had a final diagnosis of malignancy. Three patients (18, 54, and 60 years of age) had a de novo diagnosis of Crohn’s disease, all in the outpatient setting, that was due to refractory iron-deficiency anemia.
In the present study, all patients underwent endoscopy and colonoscopy, as indicated by the current guidelines. Lesions were detected in up to 24.7% of the upper gastrointestinal endoscopies carried out before CE. The indication for CE in those cases was based on the low probability of those lesions causing the clinical symptoms of the patient or the persistence of bleeding despite treatment. There are strong recommendations, with low quality of evidence, for a “second-look” procedure (with additional diagnostic yields of 29% and 6% for upper gastrointestinal endoscopy and colonoscopy, respectively).30 In our study, said course of action was highly individualized.
Increasingly more patients present with cardiovascular risk comorbidities, such as high blood pressure, diabetes, and chronic kidney disease (CKD), all of which favor the appearance of cardiovascular events and arrhythmias, with indications for antiplatelets and anticoagulants at earlier ages, leading to an increased risk for gastrointestinal bleeding. In our study, lesions causing bleeding presented in 46% of patients that used acetylsalicylic acid (ASA) only, in 90% of those with a double antiplatelet regimen (ASA + P2Y12 inhibitor), in 80% of patients on warfarin, and in 62.8% of direct anticoagulant users. Only one patient was on triple therapy (presenting with ectasia and active bleeding). The risk between anticoagulants and antiplatelets does not appear to differ, according to that reported in the meta-analysis conducted by Tiatzios et al.,31 with an OR of 2.2 and 2.5, respectively. Seventy-five percent of the patients with CKD presented with lesions, although it should be noted that a large number of them also had the diseases and medications of risk mentioned above, making it difficult to determine whether the lesions were the actual cause of bleeding. The use of nonsteroidal anti-inflammatory drugs (NSAIDs) was only described in 7 cases and none of them had a clear timeline as to their use prior to clinical symptoms; 20 to 68% of patients using NSAID for more than 3 months, presented with lesions of the small bowel mucosa.32 Given that there is a greater frequency of clinical events related to the length of time using NSAIDs, that datum should always be noted in the clinical history and the CE protocols.
The timing of CE has an important impact on the diagnostic yield, which is highest, within the first 48 hours of bleeding onset, as reported by Goenka et al.,33 at 87%. It was not carried out within the first 48 hours in any of our study patients, greatly influencing the diagnostic yield. In addition, it is a challenge in the outpatient setting due to potential symptom intermittence and lack of resource availability. The presentation times of overt bleeding before undergoing CE were highly heterogeneous (from 1 to 108 weeks in the outpatient setting and from 1 to 4 weeks in the hospitalized patients). The average time interval from clinical symptoms until CE was 73 weeks, in patients with refractory iron-deficiency anemia. There was only one case (0.6%) of capsule retention, similar to a register in South Korea,34 and in line with the goal of less than 2% promoted by the ESGE.19
There is current concern about the costs associated with the numerous procedures (including CE) and hospital stay. In a recent cost-effectiveness analysis by Jawaid et al.,35 even though it is a non-inferior strategy, with respect to direct costs ($7,362 vs $7,148), a higher detection rate was shown in the first attempt with CE vs standard treatment, enabling shorter hospital stay, when studies were negative. The American College of Gastroenterology (ACG) guidelines endorse the use of CE after negative endoscopic studies, with a strong recommendation and moderate quality of evidence. However, it is important to keep the pre-test probability of detection of clinically diagnostic findings in mind. In that regard, Marya et al.36 found three independent factors for identifying the probable source of bleeding in CE: age above 54 years, hemoglobin lower than 6.4 mg/dl, and inpatient status with overt bleeding. An algorithm was created with those variables to establish risk and potentially limit the use of CE in low-risk patients. Prediction scores could be a valuable tool for the adequate use of the resource, especially in developing countries. In our study, only three patients that had none of those criteria were evaluated through CE, signifying that its indication encompasses more than that of an initial negative study. The risk for rebleeding (in our study it was 7.6% at 30 days and 9.5% at 6 months) is higher when there is ectasia, age above 70 years, hemoglobin below 8 mg/dl, occult bleeding lasting more than 3 months, and continued anticoagulation.37
Our study has several limitations. First, the lack of ambulatory or post-hospitalization clinical follow-up did not allow us to cover a larger number of patients. Second, the retrospective design is a limitation in itself. Third, all the CE studies were evaluated by the same professional expert, but the courses of action before and after the procedure were individually indicated by different clinical specialists, according to each patient’s requirements. Last, the fact that CE could not be performed within 48 hours of symptom onset most likely had a great influence on diagnostic yield, which might have been higher. Even though there are national registers related to the diagnostic yield of CE, it is important to study the clinical impact in other institutions in developing countries.
ConclusionsCE use played an essential role in the clinical, diagnostic, and therapeutic decision-making in patients with occult gastrointestinal bleeding. Its diagnostic yield for detecting lesions with sufficient bleeding potential was comparable to that reported in industrialized countries and in Latin America. Additionally, the rebleeding and hospitalization rates at 6 months were under 10%.
Financial disclosureNo specific grants were received from public sector agencies, the business sector, or non-profit organizations in relation to this study.
Conflict of interestThe authors declare that there is no conflict of interest.