Clostridium difficile infection is the main cause of hospital-acquired diarrhea, and the clinical and endoscopic findings in those patients have been studied very little in Mexico. The aim of the present study was to describe those findings.
Materials and methodsA prospective cohort study was conducted that included patients with hospital-acquired diarrhea associated with Clostridium difficile diagnosed through polymerase chain reaction. The hypervirulent NAP027 strain was also determined. The clinical and endoscopic findings in the study patients, as well as the variables associated with severity, were analyzed.
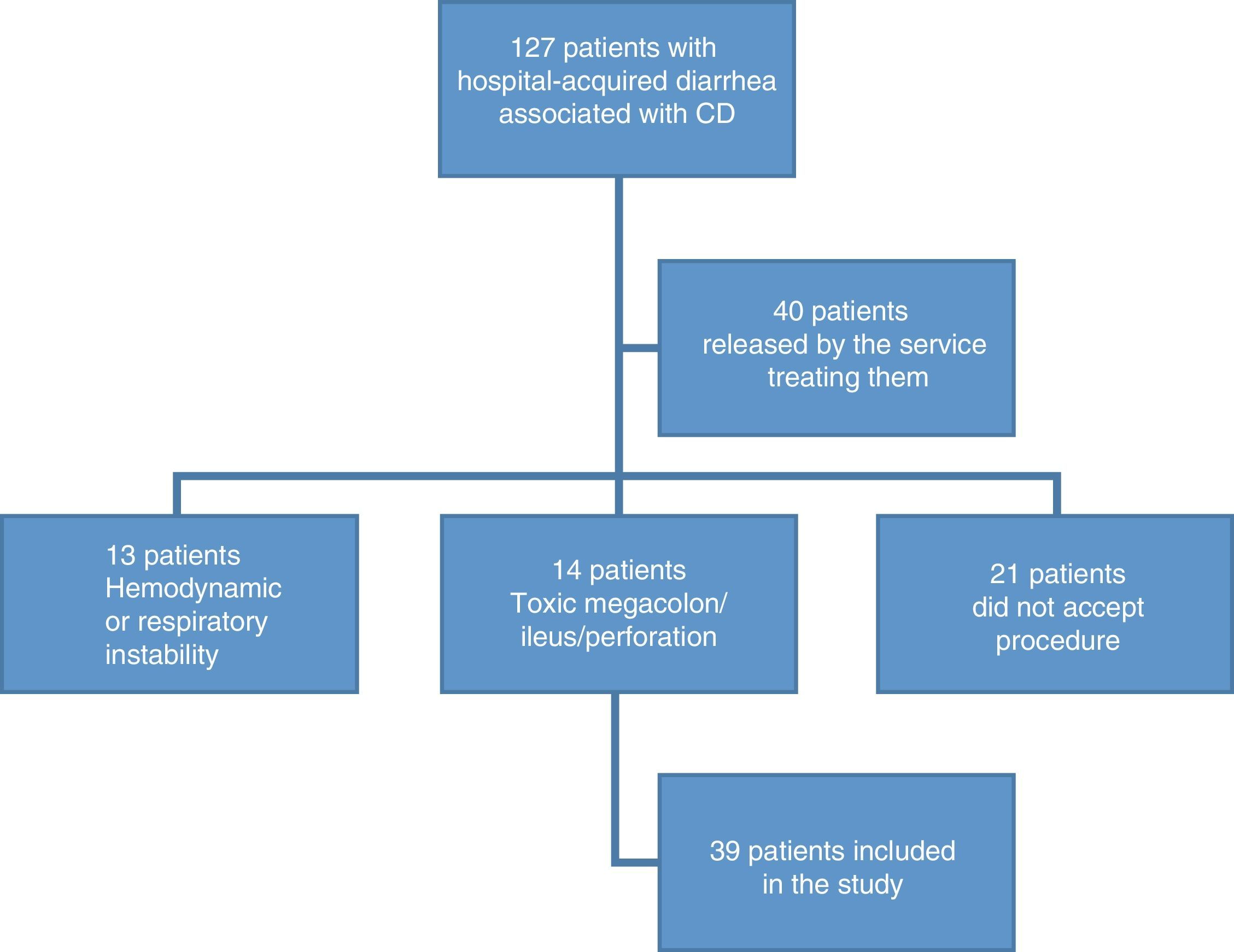
ResultsOf the 127 patients with hospital-acquired diarrhea, 97 were excluded from the study due to lack of colonoscopy. The remaining 39 study patients had a mean age of 48 years, and their most common signs/symptoms were abdominal pain (49%), mucus in stool (41%), and blood in stool (10%). The most common alterations in the laboratory results were leukocytosis in 49%, fecal leukocytes (61%), and hypoalbuminemia (67%). The main risk factor was antibiotic use in 62%, and ceftriaxone was the most widely used. The hypervirulent strain was present in 54% of the cases. Endoscopic abnormalities were found in 87% of the patients. Thirty-eight percent presented with pseudomembranous colitis, with lesions in the left colon in 53%, and in the right colon in 13%. No association was found between proton-pump inhibitor use and Clostridium difficile-associated diarrhea. There was a significant association between hypoalbuminemia (< 3.3g/dL) and a greater risk for severe colitis, with a RR of 8.2 (p=0.008).
ConclusionsPseudomembranous colitis lesions associated with the hypervirulent Clostridium difficile strain were predominant in the left colon. Hypoalbuminemia was a significant severity predictor.
La infección por Clostridium difficile (CD) es la causa principal de diarrea en hospitalizados. Los hallazgos clínicos y endoscópicos han sido poco estudiados en nuestro país. El objetivo de este estudio es describir estos hallazgos.
Material y métodosEstudio de cohorte prospectivo, se incluyeron pacientes con diarrea hospitalaria asociada a CD, diagnosticada mediante PCR y determinación de cepa hipervirulenta NAP027. Se analizaron los hallazgos clínicos y endoscópicos, así como las variables asociadas a severidad.
ResultadosDe 127 pacientes con diarrea hospitalaria, se excluyeron 97 por falta de colonoscopia. De los 39 pacientes incluidos, con edad promedio de 48 años, los signos/síntomas más comunes fueron dolor abdominal (49%), moco en heces (41%) y sangre en heces (10%); las alteraciones de laboratorio más comunes fueron leucocitosis en el 49%, leucocitos en heces (61%) e hipoalbuminemia (67%). El factor de riesgo principal fue el uso de antibiótico en un 62%, más comúnmente la ceftriaxona. La cepa hipervirulenta se presentó en el 54%. Se encontraron anormalidades endoscópicas en el 87%, con colitis seudomembranosa en un 38%, presentándose en colon izquierdo (53%) y en derecho (13%). No se encontró asociación entre uso de IBP y diarrea por CD. Se identificó una asociación significativa entre la hipoalbuminemia (<3.3g/dL) y un mayor riesgo de colitis severa, con un RR de 8.2 (p=0.008).
ConclusionesLas lesiones de colitis seudomembranosa asociada a CD de cepa hipervirulenta predominan en colon izquierdo. La hipoalbuminemia es un predictor significativo de severidad.
Clostridium difficile (CD) infection is the main cause of diarrhea in hospitalized patients in the developed world and the main cause of diarrhea due to antibiotic use (10-35% of all cases), having reached epidemic levels in the last decade.1,2
CD is a Gram-positive bacterium that grows under strict anaerobic conditions. The strains of clinical interest are those that produce toxin A (TcdA) and toxin B (TcdB), and there are strains that produce a binary toxin.3–5
In 2002, there was an outbreak of a hypervirulent strain, NAP1/BI/027, that is a hyperproducer of toxins, capable of producing 16 to 20 times more toxins than the other strains. It is resistant to fluoroquinolones, and its global extension was reported in 2008.1,6
Community-origin CD infection is considered when symptoms begin in the community or in the first 48h after hospital admission. The main risk par excellence for CD infection is the previous use of antibiotics, but said use is nonexistent in up to 65% of community cases.7,8
The diagnosis of diarrhea due to CD is based on diarrheic stool studies, by detection of toxin A and toxin B through enzyme immunoassays (EIAs), with a sensitivity of 63-94% and a specificity of 75-100%, or through nucleic acid amplification methods (PCR) with a sensitivity of 86-93% and a specificity of 98%. The American College of Gastroenterology recommends the latter as the standard diagnostic test.1,9,10 Endoscopy is not recommended when infection is suspected, due to its low sensitivity at disease onset. The characteristic lesion of CD infection is pseudomembranous colitis, which is present in 40-60% of the cases.11 However, CD infection can have a normal endoscopic appearance and mild cases can present with minimal erythema, edema, or nonspecific erosions. Pseudomembranes have also been described in other bacterial infections, in viruses, and even in amoebiasis.12–18
The aim of this study was to describe the clinical and endoscopic characteristics of patients with diarrhea associated with the CD strain, NAP027, as well as the associated risk factors, at a tertiary care hospital.
Materials and methodsA prospective cohort study was conducted that included patients diagnosed with hospital-acquired diarrhea associated with CD infection that were seen at the Hospital Civil Fray Antonio Alcalde, within the time frame of March to December 2015.
The PCR test for CD in diarrheic stools (Bristol 6-7) from the patients with hospital-acquired diarrhea was carried out at the nosocomial infection division of the Infectious Disease Service of the Hospital Civil Fray Antonio Alcalde. The patients that had positive results and met the inclusion criteria underwent an endoscopic study with an Olympus Exera 150 colonoscope. All the procedures were performed with intravenous sedation, administered by an anesthesiologist. The patients prepared for the study with polyethylene glycol electrolyte solution.
The “Xpert C. Difficile/Epi Assay” PCR test was used for rapid in vitro detection of the sequences of the toxin B gene regulator and the presumptive identification of the hypervirulent 027/NAP1/BI strain, through the detection of the binary toxin (CDT) gene regulator sequences and the elimination of a single pair of bases in nucleotide 117 of the tcd gene.
The study patients were above the age of 18 years, diagnosed with hospital-acquired diarrhea from CD, and hospitalized at the Hospital Civil Fray Antonio Alcalde. Exclusion criteria were: pregnant patients, patients under 18 years of age, and those that refused to sign statements of informed consent for the endoscopic study. Patients with toxic megacolon, prolonged ileus, suspicion of colonic perforation, hemodynamic and/or respiratory instability, patients that abandoned the study, and those released by the service treating them before undergoing colonoscopy, were eliminated from the study. The registered variables were age, sex, history of proton pump inhibitor (PPI) use, abdominal surgery, comorbidities such as diabetes and chronic kidney disease, hospital stay, signs and symptomatology, fecal characteristics (mucus, blood, etc.), laboratory results such as leukocytosis, fecal leukocytes, hypervirulent NAP 027 strain identification, serum albumin, procalcitonin, and acute phase reactants.
Statistical analysisDescriptive statistics were carried out on the study population. The categorical variables were reported as total number and percentage and the numeric variables as median and interquartile range (IQR), based on abnormal distribution identified by the Shapiro-Wilk test. The association of the categorical variables was carried out through the Pearson's chi-square test or the Fisher's exact test, as corresponded, reporting the measure of association with relative risk (RR). The Mann-Whitney U test was used to identify differences between the continuous variables distributed between the 2 groups. An ROC curve of the serum albumin levels was constructed to predict severity, determining significance of its area under the curve with the Hanley-McNeil test. The optimum cutoff point that balanced the greatest sensitivity and specificity was calculated through the Youden's index. A linear regression analysis was done with the Spearman rank correlation test to corroborate the relation between the continuous variables. The two-tailed p value was calculated for all the tests and a value under 0.05 was considered statistically significant. The statistical analysis and graphs were carried out using the SPSS version 20 software program. The institutional ethics committee approved the study and statements of informed consent were obtained, following the guidelines of the Declaration of Helsinki in relation to research on humans.
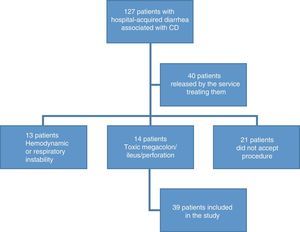
ResultsDemographic findingsOne hundred and twenty-seven patients were recruited that presented with hospital-acquired diarrhea associated with CD. Ninety-seven patients were excluded, given that 16% did not accept the procedure; 11% presented with toxic megacolon, metabolic ileus, or bowel perforation; 10% presented with hemodynamic or respiratory instability; and 31% were released by physicians from the services treating them, without undergoing endoscopy (fig. 1). A remainder of 39 patients fit the inclusion criteria. The mean age of the patients was 48.9 years (SD±17) and 67% of the patients were men. A total of 20.5% of the patients were above 64 years of age.
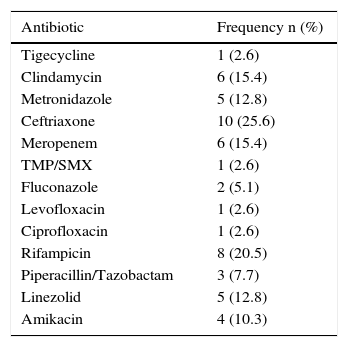
Risk factors and comorbiditiesRegarding the important past medical histories, 36% of the patients had previous surgery, 7% abdominal surgery, 31% chronic kidney disease, 43% diabetes mellitus, 33% high blood pressure, and 5% pneumonia. Only one patient had pancreatitis, 2 patients had chronic hepatopathy, 2 patients had ulcerative colitis, one patient had leukemia, 2 patients had lymphoma, and there was one case of solid tumor. Three patients were immunocompromised, two of whom were receiving chemotherapy. Sixty-two percent of the patients reported previous antibiotic use. Ceftriaxone was the most common antibiotic at 26%, followed by rifampicin and clindamycin-meropenem (Table 1). PPI use was reported in 80% of the patients.
Frequency of antibiotics used.
| Antibiotic | Frequency n (%) |
|---|---|
| Tigecycline | 1 (2.6) |
| Clindamycin | 6 (15.4) |
| Metronidazole | 5 (12.8) |
| Ceftriaxone | 10 (25.6) |
| Meropenem | 6 (15.4) |
| TMP/SMX | 1 (2.6) |
| Fluconazole | 2 (5.1) |
| Levofloxacin | 1 (2.6) |
| Ciprofloxacin | 1 (2.6) |
| Rifampicin | 8 (20.5) |
| Piperacillin/Tazobactam | 3 (7.7) |
| Linezolid | 5 (12.8) |
| Amikacin | 4 (10.3) |
Forty-nine percent of the patients presented with abdominal pain, 41% with mucus in stool, 10% with blood in stool, 54% presented with the hypervirulent NAP027 strain, 49% had leukocytosis, and 61% had fecal leukocytes. Sixty-seven percent presented with hypoalbuminemia, with a mean albumin level of 2.3g/dL (SD±0.85). Procalcitonin was reported with a median of 1.2 ng/mL (IQR: 0.47-3.0), the mean erythrocyte sedimentation rate (ESR) was 52mm/h (SD±45), and C-reactive protein was 51mg/L (IQR: 2-9). It should be pointed out that the ESR and C-reactive protein measures were only obtained in 38% of the patients.
Median hospital stay was 4 days (IQR: 2-9) and 36% of the patients had prolonged hospital stay. There was only one death in the study population.
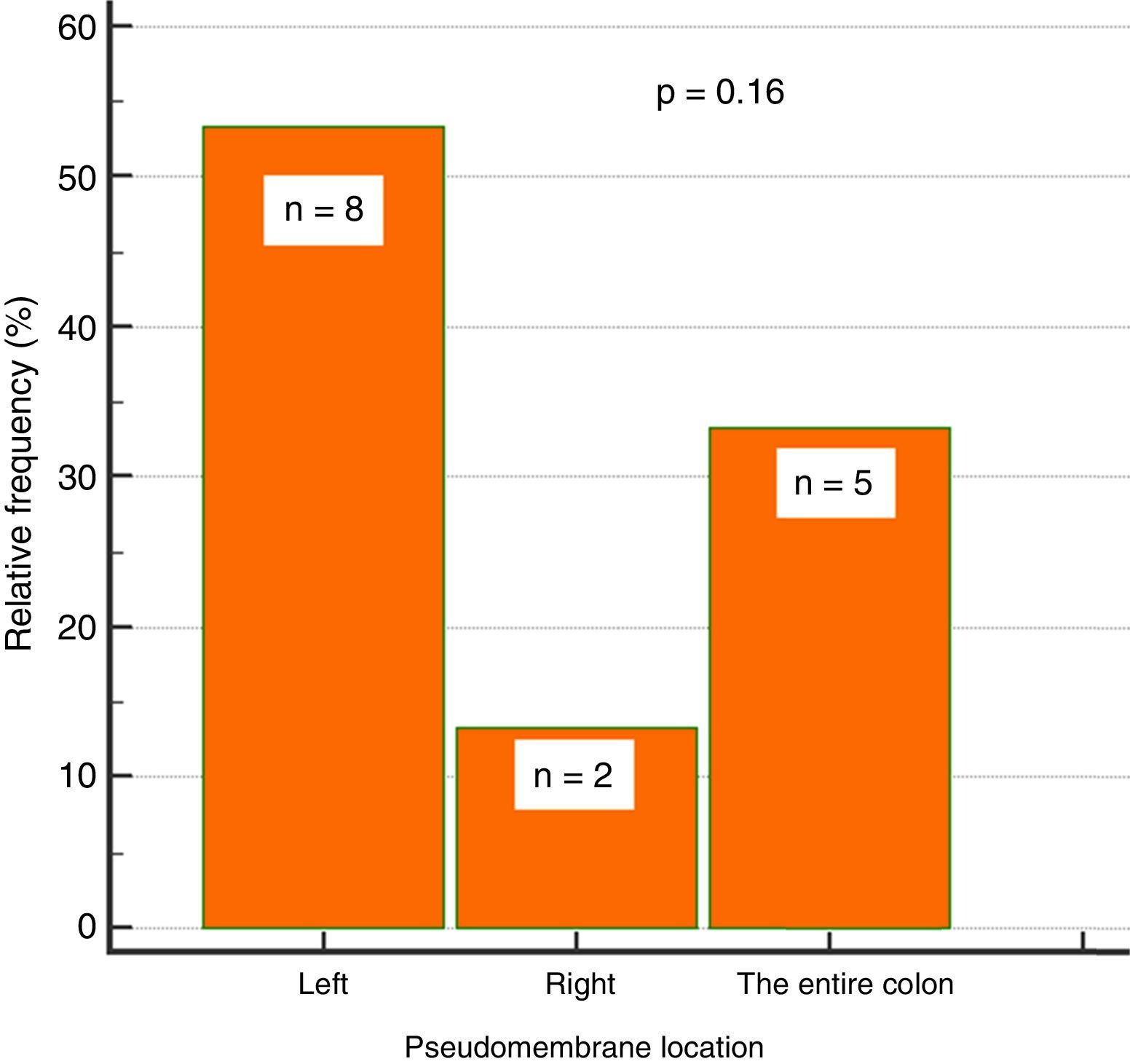
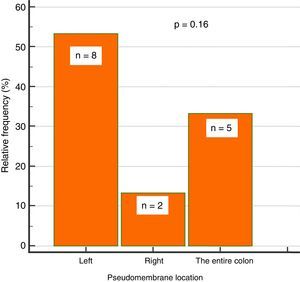
Endoscopic findingsIn regard to colonoscopy, they were performed within a median of 7 days (IQR: 5-11.5). A total of 87% of the patients had abnormal colonoscopy (colitis), 38% of whom presented with pseudomembranous colitis. Of those patients 53% (n=8) had pseudomembranes in the left colon, 33% (n=5) in the entire colon, and 13% (n=2) in the right colon, only. That involvement trend did not reach statistical significance (p=0.16) (fig. 2).
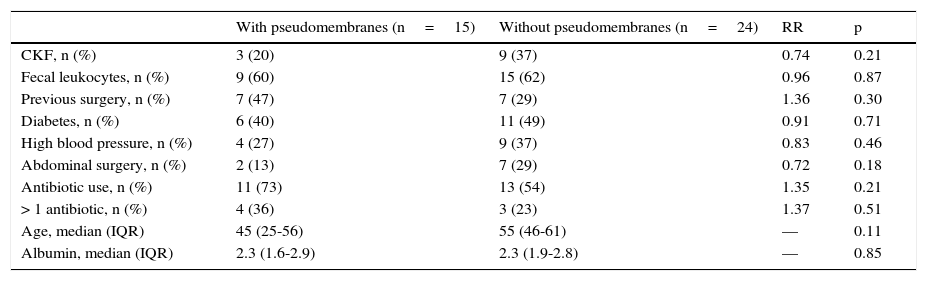
Likewise, the presence of pseudomembranes was not significantly associated with disease severity, and no other independent risk factor for their presence was found (Table 2).
Association between pseudomembranous colitis and risk factors.
| With pseudomembranes (n=15) | Without pseudomembranes (n=24) | RR | p | |
|---|---|---|---|---|
| CKF, n (%) | 3 (20) | 9 (37) | 0.74 | 0.21 |
| Fecal leukocytes, n (%) | 9 (60) | 15 (62) | 0.96 | 0.87 |
| Previous surgery, n (%) | 7 (47) | 7 (29) | 1.36 | 0.30 |
| Diabetes, n (%) | 6 (40) | 11 (49) | 0.91 | 0.71 |
| High blood pressure, n (%) | 4 (27) | 9 (37) | 0.83 | 0.46 |
| Abdominal surgery, n (%) | 2 (13) | 7 (29) | 0.72 | 0.18 |
| Antibiotic use, n (%) | 11 (73) | 13 (54) | 1.35 | 0.21 |
| > 1 antibiotic, n (%) | 4 (36) | 3 (23) | 1.37 | 0.51 |
| Age, median (IQR) | 45 (25-56) | 55 (46-61) | — | 0.11 |
| Albumin, median (IQR) | 2.3 (1.6-2.9) | 2.3 (1.9-2.8) | — | 0.85 |
There was no significant association between PPI use and the presence of CD-associated diarrhea, nor was there any significant association between their use and the presence of pseudomembranous colitis at endoscopy (p=0.94). The only variable that was significantly associated with the use of PPIs was the number of bowel movements per day, with 6/day vs 4/day in patients with and without PPI use, respectively (p=0.04).
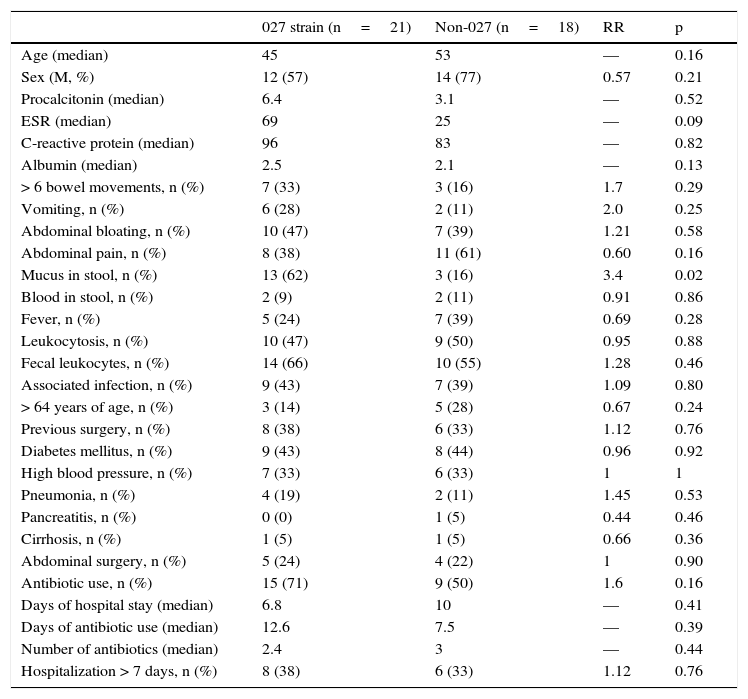
In relation to the hypervirulent NAP027 strain (Table 3), the only variable significantly associated with that strain was the presence of mucus, with a RR of 3.4 (62 vs 16%, p=0.02). The presence of fecal leukocytes showed a trend toward an increased relation to the NAP027strain, but it was not significant (66 vs 55%) (RR:1.28, p=0.46). Even though the patients with the NAP027 strain had double the amount of procalcitonin, it was abnormal in both groups, and the difference was not significant (6.4 ng/mL vs 3.1 ng/mL, p=0.52). With respect to antibiotics, there was only a certain trend towards greater risk with rifampicin (28 vs 11%), but it was not significant (p=0.25). Colonoscopy was abnormal in the majority of the patients in both groups, and there was a certain trend for pseudomembranous colitis to present more frequently with the hypervirulent strain (47 vs 28%), but with no statistical significance (RR: 1.62, p=0.23). Severity was similar in both groups, as was the time within which colonoscopy was performed (median 7 days).
Bivariate analysis of the hypervirulent NAP027 strain and the non-027 strains.
| 027 strain (n=21) | Non-027 (n=18) | RR | p | |
|---|---|---|---|---|
| Age (median) | 45 | 53 | — | 0.16 |
| Sex (M, %) | 12 (57) | 14 (77) | 0.57 | 0.21 |
| Procalcitonin (median) | 6.4 | 3.1 | — | 0.52 |
| ESR (median) | 69 | 25 | — | 0.09 |
| C-reactive protein (median) | 96 | 83 | — | 0.82 |
| Albumin (median) | 2.5 | 2.1 | — | 0.13 |
| > 6 bowel movements, n (%) | 7 (33) | 3 (16) | 1.7 | 0.29 |
| Vomiting, n (%) | 6 (28) | 2 (11) | 2.0 | 0.25 |
| Abdominal bloating, n (%) | 10 (47) | 7 (39) | 1.21 | 0.58 |
| Abdominal pain, n (%) | 8 (38) | 11 (61) | 0.60 | 0.16 |
| Mucus in stool, n (%) | 13 (62) | 3 (16) | 3.4 | 0.02 |
| Blood in stool, n (%) | 2 (9) | 2 (11) | 0.91 | 0.86 |
| Fever, n (%) | 5 (24) | 7 (39) | 0.69 | 0.28 |
| Leukocytosis, n (%) | 10 (47) | 9 (50) | 0.95 | 0.88 |
| Fecal leukocytes, n (%) | 14 (66) | 10 (55) | 1.28 | 0.46 |
| Associated infection, n (%) | 9 (43) | 7 (39) | 1.09 | 0.80 |
| > 64 years of age, n (%) | 3 (14) | 5 (28) | 0.67 | 0.24 |
| Previous surgery, n (%) | 8 (38) | 6 (33) | 1.12 | 0.76 |
| Diabetes mellitus, n (%) | 9 (43) | 8 (44) | 0.96 | 0.92 |
| High blood pressure, n (%) | 7 (33) | 6 (33) | 1 | 1 |
| Pneumonia, n (%) | 4 (19) | 2 (11) | 1.45 | 0.53 |
| Pancreatitis, n (%) | 0 (0) | 1 (5) | 0.44 | 0.46 |
| Cirrhosis, n (%) | 1 (5) | 1 (5) | 0.66 | 0.36 |
| Abdominal surgery, n (%) | 5 (24) | 4 (22) | 1 | 0.90 |
| Antibiotic use, n (%) | 15 (71) | 9 (50) | 1.6 | 0.16 |
| Days of hospital stay (median) | 6.8 | 10 | — | 0.41 |
| Days of antibiotic use (median) | 12.6 | 7.5 | — | 0.39 |
| Number of antibiotics (median) | 2.4 | 3 | — | 0.44 |
| Hospitalization > 7 days, n (%) | 8 (38) | 6 (33) | 1.12 | 0.76 |
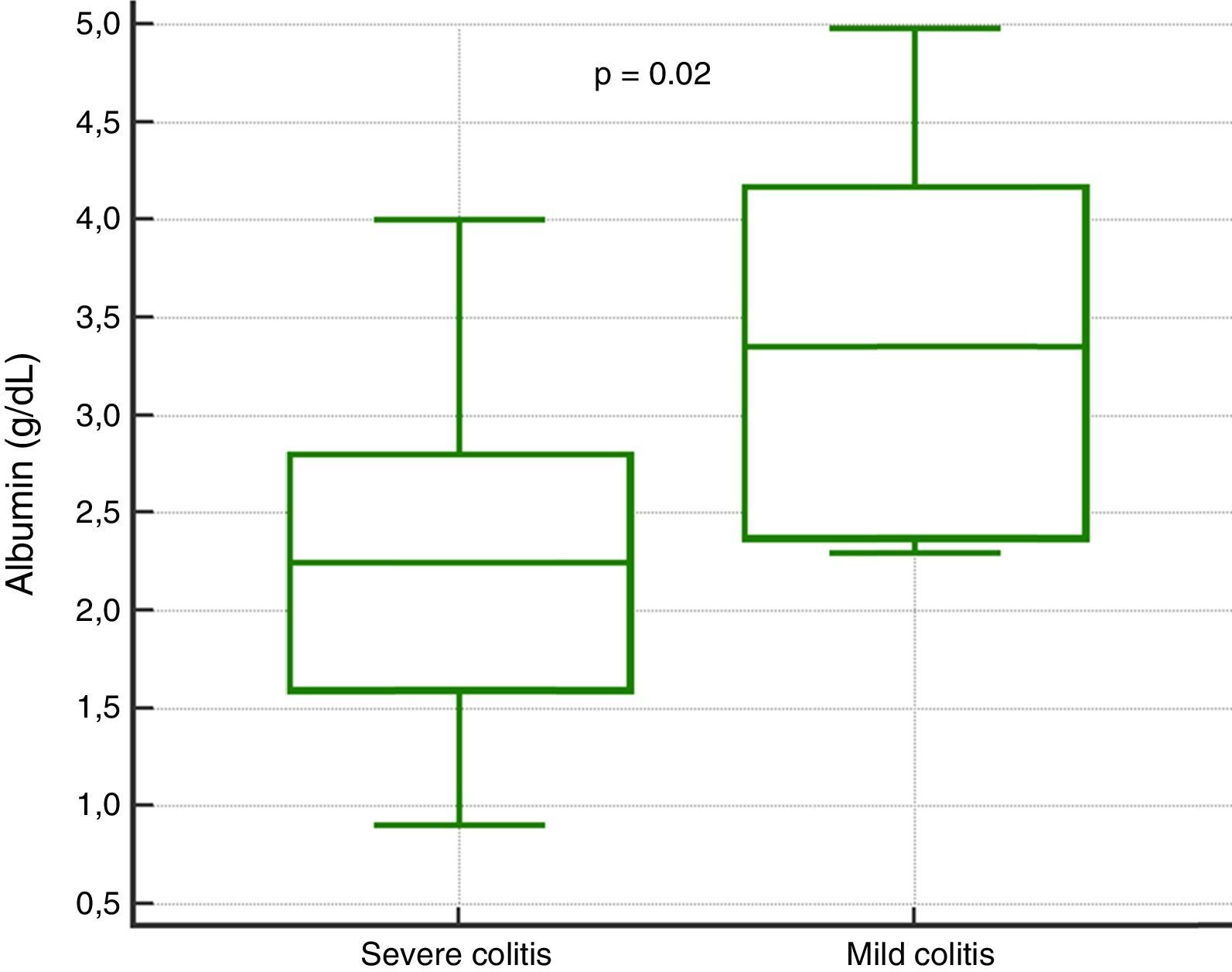
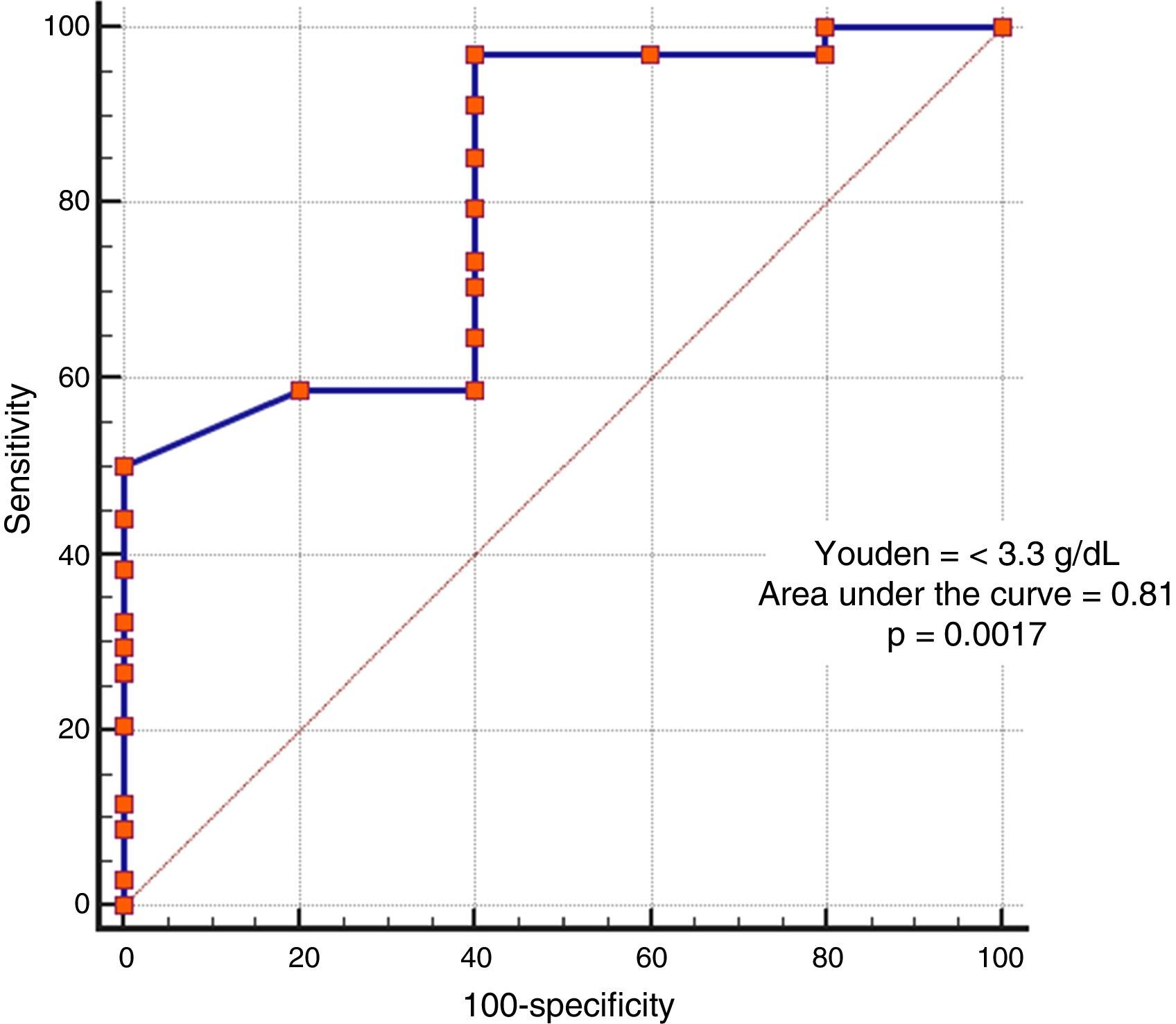
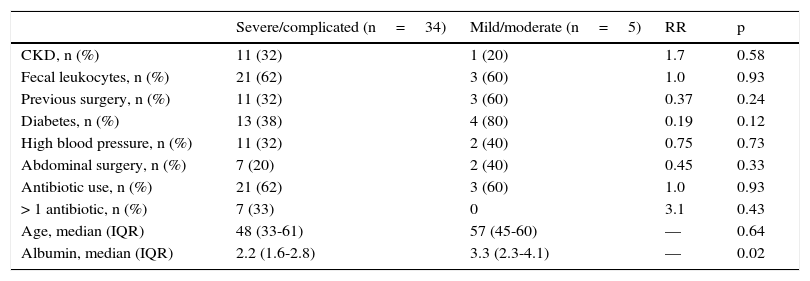
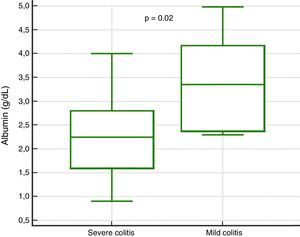
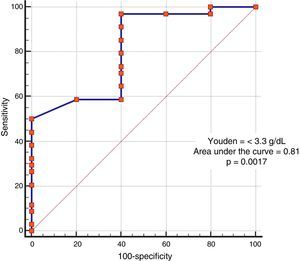
In the analysis of clinical presentation severity, the only statistically significant variable was the level of serum albumin (p=0.02) (Table 4, fig. 3). When the ROC curve was constructed and the Youden's index calculated, there was a significant area under the curve of 0.81 (p=0.0017) and a cutoff point of ≤ 3.3g/dL for predicting severe/complicated symptoms (fig. 4). A RR of 8.2 was calculated (p=0.008), with 97% sensitivity and 60% specificity, as well as a 52% positive predictive value and an 82% negative predictive value.
Severity of diarrhea.
| Severe/complicated (n=34) | Mild/moderate (n=5) | RR | p | |
|---|---|---|---|---|
| CKD, n (%) | 11 (32) | 1 (20) | 1.7 | 0.58 |
| Fecal leukocytes, n (%) | 21 (62) | 3 (60) | 1.0 | 0.93 |
| Previous surgery, n (%) | 11 (32) | 3 (60) | 0.37 | 0.24 |
| Diabetes, n (%) | 13 (38) | 4 (80) | 0.19 | 0.12 |
| High blood pressure, n (%) | 11 (32) | 2 (40) | 0.75 | 0.73 |
| Abdominal surgery, n (%) | 7 (20) | 2 (40) | 0.45 | 0.33 |
| Antibiotic use, n (%) | 21 (62) | 3 (60) | 1.0 | 0.93 |
| > 1 antibiotic, n (%) | 7 (33) | 0 | 3.1 | 0.43 |
| Age, median (IQR) | 48 (33-61) | 57 (45-60) | — | 0.64 |
| Albumin, median (IQR) | 2.2 (1.6-2.8) | 3.3 (2.3-4.1) | — | 0.02 |
The clinical characteristics of our patients differed somewhat from those reported internationally.1,7,9 Reports state that greater risk is seen in patients above 65 years of age, but only 20% of the patients in our study were in that age group. The mean presentation age of our patients was 49 years. Only 36% had prolonged hospital stay (greater than 15 days). Thirty to forty percent of our population had a past medical history of chronic diseases (diabetes, high blood pressure, chronic kidney disease).
Symptom frequency, as well as systemic leukocytosis frequency (49%) and fecal leukocytes (61%), coincided with that reported in the international literature.2–4 In regard to inflammation markers, procalcitonin was elevated in the majority of the patients (90%) with a median of 1.2 ng/mL, mean ESR was 52mm/h, and median C-reactive protein was 51mg/L. However, the last two were reported in a low percentage of our sample.
Prior antibiotic use was reported in 62% of the patients, concurring with the figures in the current literature.4,9,10 One third of the patients did not have a history of antibiotic use. Two possible explanations for that are: first, the use of other drugs that increase the risk for CD infection, such as PPIs; and second, contact with another patient, in the room or ward, that had received broad spectrum antibiotics.19 It is worth noting that fluoroquinolones were used in only 6% of the patients. This reflects the fact that our hospital center is hyper-endemic for CD, and one of the measures taken 3 years ago by the nosocomial infection service was to restrict fluoroquinolone use, especially because of their association with the hypervirulent strain.
Previous PPI use and the risk for CD infection is currently a subject of debate. We reported PPI use in approximately 80% of the patients, but an association risk was not demonstrated. The most feasible explanation for that result is our study's relatively small sample size and its very specific inclusion criteria (positive PCR). At any rate, recent evidence suggests that continuing the use of a PPI after the first symptoms of CD-associated diarrhea increases the risk for relapse, and the interruption of its use is recommended if there is no precise indication for it.20–22
There was only one death in our study population. That number is clearly biased, given that patients in a critical state were excluded from the study. Nevertheless, the mortality rate from this entity at our hospital, recently published by the nosocomial infection service, is 5%.23
In relation to endoscopic findings, colonoscopy was abnormal in the large majority of patients, with normal findings in only 13%. This concurs with studies that have reported normal results in 6-31% of patients.11,12
Of the patients with pseudomembranous colitis, left colon involvement predominated in more than half of the patients. Isolation in the right colon was less common. Given that the frequency of location was not statistically significant, our study does not support performing rectosigmoidoscopy, alone, as has been done in some studies.12 Lesions can present solely in the right colon, even though frequency is low, and they have a reported incidence of 16%.11,24 In addition, previous studies reported 91% sensitivity with flexible sigmoidoscopy for diagnosing pseudomembranous colitis and 100% sensitivity with colonoscopy.25
Colonoscopy is not exempt from risks, due to the possibility of perforation. Therefore, performing the procedure with a minimum of air insufflation, avoiding endoscopic loop formation, and suspending the examination if there is suspicion of producing a complication are recommended.11,18 Our suggestion is to perform the complete endoscopic study, reviewing the right colon as much as possible, and if classic lesions (pseudomembranes) present in the left colon, to take samples and conclude the study.
It should be mentioned that because of the primary aim of the study, colonoscopy was performed in all of the patients for research purposes only. It is well known that the procedure is not indispensable in patients with a positive PCR result, unless there is no treatment response.12,18
In the extended risk analysis for pseudomembranes, there was no association with severity, but we observed a nonsignificant trend toward pseudomembrane presentation in patients that used more than one antibiotic (RR 1.37, p=0.51) and in patients with previous surgery (RR 1.36, p=0.30).
The only variable that had statistical significance for severity was a serum albumin level ≤ 3.3g/dl, suggesting 2 hypotheses. One, that patients with hypoalbuminemia associated with other pathologic states (malnutrition, severe colitis with protein-losing enteropathy) will present with severe disease symptoms if they acquire CD infection; and the other, that albumin levels are reduced as a response to inflammatory symptoms, because albumin is a negative acute phase reactant. Based on our results, albumin levels could be useful in predicting severity, but further randomized trials with follow-up of the trends of the levels throughout the disease are required to reliably establish this fact.
One of the main strengths of the present study was that it analyzed the endoscopic characteristics resulting from CD infection, a variable not previously reported on in Mexico. Other study strengths were the fact that all the patients were diagnosed through the PCR method and that the hypervirulent NAP027 strain was identified, providing a more reliable association between the lesions with the pathogen studied. The limitations of our study include its small sample size, which does not allow solid conclusions to be made, with respect to the comparisons between groups. However, the majority of studies conducted on this specific theme also have a limited number of study patients.12,25
ConclusionsColonoscopy is not indicated in all patients with hospital-acquired CD infection, but when an endoscopic procedure is required, colonoscopy should be preferred over the performance of rectosigmoidoscopy, alone. The present study showed that a serum albumin level < 3.3g/dL was a factor that could predict severity symptoms in our specific group of patients.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Financial disclosureNo financial support was received in relation to this study.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Velarde Ruiz-Velasco JA, Aldana-Ledesma JM, Ibarra-Estrada MA, et al. Características clínicas y endoscópicas en diarrea hospitalaria asociada a infección por Clostridium difficile. Revista de Gastroenterología de México. 2017;82:301–308.