The chronic use of nonsteroidal anti-inflammatory drugs (NSAIDs) can cause complications in the gastrointestinal tract. The use of proton pump inhibitors (PPIs) is recommended in high-risk patients to prevent them.
ObjectiveThe aim of this article was to evaluate the gastroprotection measures taken in persons with chronic NSAID use.
Materials and methodsA descriptive cross-sectional study was conducted. The clinical records were reviewed of patients seen as outpatients at the Rheumatology Department over a 4-month period, choosing those with chronic NSAID use, and intentionally looking for gastroprotection measures according to the recommendations published by the American College of Gastroenterology.
ResultsA total of 417 patients (347 women; mean age: 48.12±14.2 years) were included. The most frequent diagnosis was rheumatoid arthritis (65%). Nine patients (2.1%) had a history of peptic ulcer, 48 (11.5%) patients were 65 years of age or older, 26 (6.2%) patients took NSAIDs and aspirin, and 130 (31.2%) took NSAIDs with steroids. Tests for Helicobacter pylori infection were done in just 53 cases, and there were positive results in only 9 (16%). Some risk for gastrointestinal toxicity was established in 211 cases and only 65 (30.8%) received gastroprotection. In contrast, 31 (15%) patients received gastroprotection when there was no indication for it.
ConclusionProphylaxis with PPIs in chronic NSAID users was inadequately employed. It was not prescribed in the majority of patients (69.2%) and it was used with no justification in others (15%).
El uso crónico de antiinflamatorios no esteroideos (AINE) puede provocar complicaciones en el tracto gastrointestinal. Para prevenirlas, se recomienda el uso de inhibidores de la bomba de protones (IBP) en los enfermos de alto riesgo.
ObjetivoEvaluar las medidas de gastroprotección en personas que usan AINE en forma crónica.
Material y métodosEstudio descriptivo y transversal. Se revisaron los expedientes clínicos de los enfermos que acudían a la consulta externa de reumatología durante 4 meses y se eligieron a los que utilizaban AINE de forma crónica. Se buscaron intencionadamente las medidas de gastroprotección de acuerdo con las recomendaciones publicadas por el Colegio Americano de Gastroenterología.
ResultadosSe incluyó a 417 pacientes (347 mujeres; edad promedio= 48.12±14.2 años). El diagnóstico más frecuente fue artritis reumatoide (65%). Nueve pacientes (2.1%) tenían historia de úlcera péptica. Cuarenta y ocho (11.5%) enfermos tenían 65 años o más. Veintiséis (6.2%) tomaban AINE y aspirina, y 130 (31.2%) AINE con esteroides. En 53 casos (12.7%) se conocía el estatus de infección por Helicobacter pylori que fue positivo en solo 9 (16%). En 211 casos se estableció algún riesgo para toxicidad gastrointestinal y solo 65 (30.8%) recibía gastroprotección. En cambio, 31 (15%) lo recibieron sin ninguna indicación.
ConclusiónLa profilaxis con IBP en usuarios crónicos de AINE se emplea de manera inadecuada. En su mayoría no se indica (69.2%) y en otras se utiliza sin justificación (15%).
Nonsteroidal anti-inflammatory drugs (NSAIDs) are the most widely prescribed drugs worldwide and their use has substantially decreased the morbidity and mortality of cardiovascular disease and improved the quality of life in persons that suffer from chronic pain.1 Low doses of platelet antiaggregants have been shown to be efficacious in primary as well as secondary prevention of cardiovascular events in high-risk persons, mainly those of advanced age and that present with comorbidities.2,3 In addition, they are efficacious as analgesics and anti-inflammatory agents and are used as part of treatment for rheumatologic diseases, post-trauma, and neoplasias.4 Unfortunately their chronic use can cause adverse effect in the digestive tract that vary from dyspeptic symptoms to severe complications such as bleeding and perforation.5,6 Thus an effort has been made to identify the risk factors associated with these complications in order to provide preventive measures. Several studies have shown that age above 65 years, a previous history of peptic ulcer disease, high doses of NSAIDs, concomitant use of anticoagulants, steroids and/or aspirin, and H. pylori infection are determining factors of NSAID damage.7,8 Clinical guidelines have described various strategies for the prevention of harmful NSAID effects.9,10 The latest ones published in 2009 by the American College of Gastroenterology suggest gastroprotection measures in accordance with the risk for gastrointestinal involvement classified as low, moderate, and high, and they include cardiovascular risk, given the known cardiotoxicity of NSAIDs and aspirin.11 The primary prophylactic measure is to use the standard dose of proton pump inhibitor (PPI), as well as the least ulcerogenic NSAID and the lowest effective dose.12,13 But despite the guidelines, there is evidence of inadequate use of the primary prophylactic measures.14,15 There are no studies in Mexico on gastroprotection measures in chronic NSAID users, and therefore in this work we decided to evaluate whether: 1) gastroprotection was carried out, and 2) if the clinical guideline criteria were applied.
Materials and methodsA descriptive, cross-sectional study was conducted that reviewed the case records of the patients seen as outpatients at the Rheumatology Department of the Instituto Nacional de Ciencias Médicas y Nutrición “Salvador Zubirán”, Mexico City, within the time frame of February 1, 2015 to May 30, 2015. Patients that were chronic NSAID users were chosen for the analysis. Those patients that had already presented with NSAID-associated complications and those in whom it was not possible to complete the required data were excluded from the study. The demographic data, comorbidities, risk factors for adverse effects related to NSAID use, and the gastroprotection measures employed were recorded.
Chronic NSAID use was defined as the ingestion of the medication for 3 or more days per week for more than a month. The prescribed NSAID dose above that which is recommended was considered high dose. The guideline recommendations published by the American College of Gastroenterology (2009) and ratified in the Clinical Guidelines for the Diagnosis and Treatment of Peptic Ulcer Disease published by the Asociación Mexicana de Gastroenterología11–13 were used to evaluate the implementation of gastroprotection measures. The primary outcome measure was to evaluate the prevalence rate of the use of gastroprotection measures according to the stratification of gastrointestinal and cardiovascular risk.
The results were expressed in percentages (%) and means.
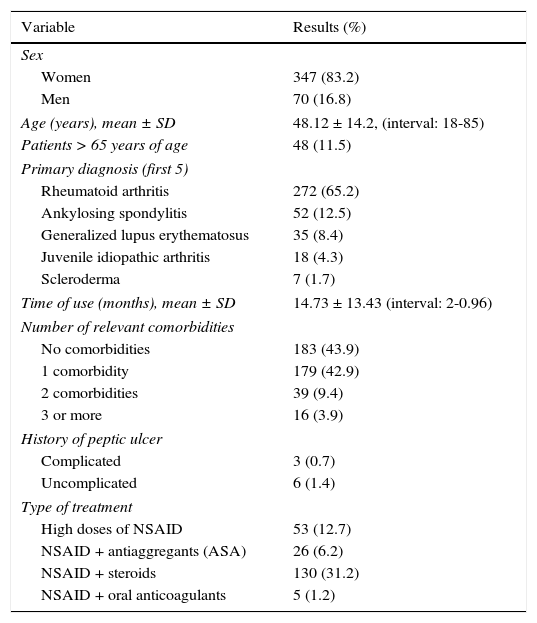
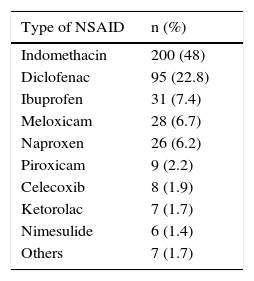
ResultsA total of 4,500 case records were reviewed during the study period and 417 patients that were chronic NSAID users were identified. Three hundred forty-seven (83.2%) of those patients were women and 70 (16.8%) were men. The mean age of the total group was 48.12 ± 14.2 years. Table 1 shows the patient characteristics. Indomethacin was the most widely used NSAID (Table 2). Tables 3 and 4 show the stratification of the patients according to the number of risk factors and the group to which they belonged.
General characteristics of the patients using NSAIDs (n = 417).
| Variable | Results (%) |
|---|---|
| Sex | |
| Women | 347 (83.2) |
| Men | 70 (16.8) |
| Age (years), mean ± SD | 48.12 ± 14.2, (interval: 18-85) |
| Patients > 65 years of age | 48 (11.5) |
| Primary diagnosis (first 5) | |
| Rheumatoid arthritis | 272 (65.2) |
| Ankylosing spondylitis | 52 (12.5) |
| Generalized lupus erythematosus | 35 (8.4) |
| Juvenile idiopathic arthritis | 18 (4.3) |
| Scleroderma | 7 (1.7) |
| Time of use (months), mean ± SD | 14.73 ± 13.43 (interval: 2-0.96) |
| Number of relevant comorbidities | |
| No comorbidities | 183 (43.9) |
| 1 comorbidity | 179 (42.9) |
| 2 comorbidities | 39 (9.4) |
| 3 or more | 16 (3.9) |
| History of peptic ulcer | |
| Complicated | 3 (0.7) |
| Uncomplicated | 6 (1.4) |
| Type of treatment | |
| High doses of NSAID | 53 (12.7) |
| NSAID + antiaggregants (ASA) | 26 (6.2) |
| NSAID + steroids | 130 (31.2) |
| NSAID + oral anticoagulants | 5 (1.2) |
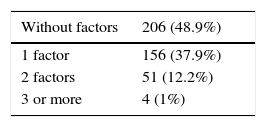
Presence of gastrointestinal risk factorsa (n = 417 [%]).
| Without factors | 206 (48.9%) |
|---|---|
| 1 factor | 156 (37.9%) |
| 2 factors | 51 (12.2%) |
| 3 or more | 4 (1%) |
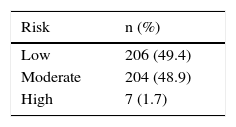
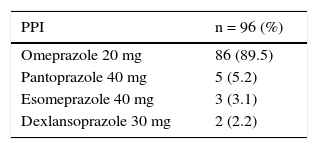
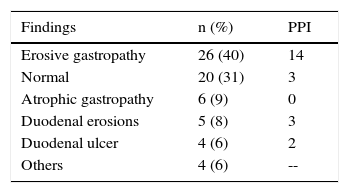
PPIs were prescribed in 65 (31%) of the 211 patients that had one or 2 risk factors (moderate) and in 31 (15%) that had none. Two of the 7 patients classified as high-risk received PPIs. Gastrointestinal prophylaxis was indicated in 18 (38%) of the 48 patients 65 years of age or older, in 9 (35%) of the 26 patients with concomitant aspirin use, and in 44 (34%) of the 130 patients that also received steroids. Table 5 shows the medication used for gastroprotection. Proximal gastrointestinal endoscopy was performed in 65 cases (15.3%) that was indicated for anemia or a history of gastrointestinal bleeding and was reported as normal in 20 of the patients. Twenty-six had erosive gastropathy and the rest of the findings are summarized in Table 6.
Chronic NSAID use in high-risk patients has been related to severe complications. Around 25% of users may develop peptic ulcer disease and 2 to 4%, bleeding and perforation.1 Evidence shows that the application of prophylactic measures considerably reduces these eventualities that cause over 100,000 hospitalizations per year in the United States and 7,000 to 10,000 annual deaths, mainly in high-risk patients.2,3,11,12
The group of patients included in the present study showed a high prevalence of risk factors for gastrointestinal adverse effects. All were chronic anti-inflammatory drug consumers, a significant percentage were older than 65 years of age, and at least one third had concomitant use of steroids or aspirin, thus representing a population requiring gastroprotection. In addition, indomethacin, one of the most ulcerogenic NSAIDS, was the most widely used.
It is not surprising that half of these patients required gastroprotection, but only 65 of the 211 (31%) received it. Unfortunately, the discouraging findings of this study are not exclusive to our environment. Morini et al. analyzed 869 patients that were chronic NSAID users of which 68.2% (593) had gastroprotection, but only 34.4% in an adequate manner. Underuse was observed in 30.6% of the patients above 65 years of age.14 Sturkenboom et al., with a greater number of evaluated patients (n = 69,648), reported that of the 2,811 patients with 2 or more risk factors, no measure of gastrointestinal prophylaxis was taken in 81.2% of them.15 On the other hand, PPI overuse as a prophylactic measure has been shown in several studies. We found such overuse in 15% of the cases, a lower figure than is reported in other analyses.15
Different studies have analyzed the influence that H. pylori infection has on persons with chronic NSAID use.16,17 Its importance is such that all the clinical guidelines, including those in Mexico, recommend searching for it and eliminating it in patients that require chronic NSAID or PPI use.18 Patients with rheumatoid diseases, such as those in the present study, require both medications and therefore are patients in whom H. pylori must be ruled out. This was done in only 53 of the cases, corresponding to a mere 13% of the study population. It was more striking to find that only 16%, that is to say, 9 of the 53 subjects, had a positive test, which was a gastric biopsy analyzed by a pathologist. This is a relevant fact in a country where H. pylori infection affects a large percentage of adults, and even more so, considering the high number of patients 65 years of age or older. We have no satisfactory explanation for this finding.
As can be seen from the present analysis, primary prophylaxis for lesions attributable to chronic NSAID use was sub-optimal. It was not indicated in the majority of cases and was overused in others. The intentional search for an additive damage factor, such as H. pylori infection, was low, but the infection detected in a small group of patients was surprisingly lower than that reported in the general population. The factors that were most associated with PPI subuse in this group of patients were the high doses of NSAIDs, the concomitant use of steroids or aspirin, and age above 65 years. Even though there were no severe complications in any of the cases, endoscopic study that had to be performed on 65 patients showed ulcerative lesions in an important number of them (70%). It is possible that the lesions attributable to NSAID-induced gastropathy go unperceived, in addition to the fact that many patients present with asymptomatic peptic ulcer due to the analgesic effect of the NSAIDs and then have the complication of bleeding with no prior symptoms.
In conclusion, prophylaxis with PPIs in chronic NSAID users was inadequately employed. It was not prescribed in the majority of cases (69.2%) and it was used unjustifiably in others (15%). There were very few intentional searches for H. pylori infection, a factor with a synergic capacity for gastric involvement. While the confirmation of these results at other sites is pending, the different medical groups and healthcare providers should establish measures that guarantee the application of the clinical guidelines that exist to improve the quality of medical attention standards.
Ethical responsibilitiesProtection of persons and animalsThe authors declare that no experiments were performed on humans or animals for this study.
Data confidentialityThe authors declare that they have followed the protocols of their work center in relation to the publication of patient data.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects referred to in the article. This document is in the possession of the corresponding author.
Financial disclosureNo financial support was received in relation to this study.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Velasco-Zamora JA, Gómez-Reyes E, Uscanga L. ¿Qué tanto se siguen las recomendaciones de las guías clínicas sobre gastroprotección? Una revisión en enfermos que consumen antiinflamatorios no esteroideos. Revista de Gastroenterología de México. 2016;82:121–125.
See related content at DOI: http://dx.doi.org/10.1016/j.rgmxen.2016.06.016, Abdo-Francis JM. Clinical practice guidelines: What is their actual usefulness? Rev Gastroenterol Méx. 2016;81(3):119–20.