Enteral Nutrition Therapy (ENT) is considered an important tool for the appropriate maintenance of nutritional conditions. ENT tolerance may be limited due to gastrointestinal (GI) events resulting from formula composition and/or simultaneously administered drug therapies.
AimsTo verify the possible association between GI events and drug therapies being administered to patients receiving ENT at a university hospital.
MethodsA prospective observational cohort study was conducted. Medical records from 95 patients requiring ENT at the Hospital de Clínicas de Porto Alegre (HCPA) were randomly evaluated until discharge, death, or initiation of oral or parenteral diet occurred. Details of the administered medications and enteral formula, together with the presenting patient disease and digestive manifestations, were recorded by the medical team. Three experienced gastroenterologists evaluated the possible association between the digestive symptoms and the medications employed. The study protocol was approved by the HCPA Research Ethics Committee and patient consent forms were signed.
ResultsMean patient age: 65±17 (24-95) years; 94.70% presented with GI events: constipation 70.50%, diarrhea 38.90%, abdominal distension 18.90%, vomiting 16.80%, and pulmonary aspiration 1.10%. ENT was most indicated in neurologic (50.50%) and neoplastic (25.30%) disease. Medications given to the patients showed a positive relation: 63.20% to 86.70% of GI symptoms could be attributed to the drugs being administered.
ConclusionsGI complications during ENT are common; they are frequently linked to administered drug therapy. Health care teams should consider all risk factors present, specifically those related to prescribed medication, before modifying/suspending ENT.
La terapia de apoyo con nutrición enteral (TNE) se considera una herramienta importante para el mantenimiento adecuado de la condición nutricional. La tolerancia de la TNE puede estar limitada por los efectos gastrointestinales (GI) que resultan de la composición de la fórmula y/o de tratamientos farmacológicos administrados simultáneamente.
ObjetivoDeterminar la posible asociación entre efectos GI y tratamientos farmacológicos administrados simultáneamente, a pacientes que reciben TNE en un Hospital Universitario.
MétodosSe llevó a cabo un estudio prospectivo observacional de cohorte. Los expedientes médicos de 95 pacientes que requirieron TNE en el Hospital de Clínicas de Porto Alegre (HCPA) fueron aleatoriamente evaluados hasta que ocurriera su egreso, muerte o iniciación de vía oral o dieta parenteral. El equipo médico registró los detalles de los medicamentos administrados y la fórmula enteral en conjunto con la enfermedad presentada por los pacientes, así como las manifestaciones digestivas. Tres gastroenterólogos experimentados evaluaron la posible asociación entre los síntomas digestivos y los medicamentos utilizados. El protocolo de estudio fue aprobado por el Comité de Investigación y Ética del HCPA; y los pacientes firmaron un consentimiento informado.
ResultadosLa edad media de los pacientes fue: 65±17 (24-95) años; 9470% presentó efectos GI: estreñimiento 7050%, diarrea 38,90%, distensión abdominal 18,90%, vómito 16,80%, y aspiración pulmonar 1,10%. La TNE fue indicada con mayor frecuencia en enfermedades neurológicas (50,50%) y neoplásicas (25,30%). Los medicamentos administrados a los pacientes mostraron una relación positiva: 63,20% a 86,70% de los efectos GI fueron atribuidos a los tratamientos farmacológicos.
ConclusionesLas complicaciones GI durante TNE son comunes. Se asocian con frecuencia a los tratamientos farmacológicos administrados. Los equipos encargados del cuidado de la salud de estos pacientes deben considerar todos los factores de riesgo presentes, específicamente aquellos relacionados con los medicamentos ordenados antes de modificar/suspender la TNE.
Gastrointestinal (GI) events in Enteral Nutrition Therapy (ENT) may be a result of the nutritional formula that is infused, the drug therapy being taken by these patients, or the background illness justifying its use.1 Adverse events occurring from medications can vary depending on the drug prescribed, as well as on the clinical condition of the patient. Medications to be administered should be carefully evaluated in order to prevent such problems, primarily in the hospital setting where patients are undergoing multiple drug therapies.2 Few studies have attempted to investigate this association between medication use and patients in such a scenario.
The aims of this study were to verify the frequency of GI complications (diarrhea, abdominal distension, nausea/vomiting, and constipation) associated with the use of ENT in adult inpatients at the Hospital de Clínicas de Porto Alegre (HCPA), and to identify the possible association with medications simultaneously administered during treatment.
MethodsDesign and patientsA prospective observational cohort study was conducted using data collected from medical records of the HCPA inpatients requiring enteral nutrition support. The sample included patients with a minimum age of 18 years that required ENT for more than five days. Patients receiving parenteral nutrition, enteral nutrition for less than five days, oral intake concomitant with enteral nutrition, pregnant women, and women who had recently given birth were excluded from the study.
The study was approved by the Research Ethics Committee of the HCPA and consent forms were signed by all participants.
Data collectionThe prospective selection of study participants was carried out from a list of ENT users at the HCPA, using the existing institutional database that is part of its electronic medical prescription system. Patients were enumerated consecutively by placing numbered cards in a container and drawing them out at random. Data from electronic medical records were collected from day five of ENT up to therapy interruption due to patient discharge or death or to the beginning of an oral or parenteral diet. Medical records were analyzed three times a week by the same researcher on alternating days. The evaluations of the medical team and nursing staff were noted, along with medication prescriptions and occurrences of GI complications during the previous 48h to 72h. These data were entered in the electronic chart system of the HCPA, where all patient information is consolidated. The most recent electronic record was used for those patients that were discharged or that died on days when data were not collected.
The nursing team responsible for the daily hospital routine was not aware of the study objective, in order to avoid the Hawthorne effect.3
A data collection instrument was developed to evaluate the clinical GI manifestations of patients, the medications administered, and the possible association between these two variables. The patients’ observed GI symptoms were described using the following terminology:
- a)
Defined relationship: events with a high probability of being caused by medications used during the period of drug therapy; a cause-effect relationship stated as complications described on the patient information leaflet that could not be explained by known characteristics of the illness.
- b)
Probable relationship: events with a low probability of being caused by medications used during the period of drug therapy; complications described on the patient information leaflet that could not be explained by known characteristics of the illness.
- c)
Possible relationship: events with a very low probability of being caused by medications used during the period of drug therapy and that could more likely be explained by known characteristics of the patient's clinical condition.
- d)
Doubtful relationship: events with a greater probability of being linked to factors other than the medication under scrutiny.
Three gastroenterologists familiar with the terminology used in the data collection instrument independently evaluated the medications taken by the patients and any subsequent GI events that were registered. In cases in which there was a difference of opinion, the evaluators met together to work out a consensus. For data analysis purposes, the terms “probable” and “possible” were considered to be a positive association, whereas the term “doubtful” implied a negative association.
Outcomes measurementDiarrhea was defined as the occurrence of three or more episodes of liquid or semi-liquid evacuations within a 24-hour timespan.4 Constipation was defined as less than three evacuations occurring per week.4,5 Pulmonary aspiration was confirmed by clinical criteria/symptoms reported and/or by radiologic method (data present in medical records). Abdominal distension, nausea, and vomiting were included when reported by the medical team in daily patient evaluations. Medical records were used to obtain information regarding the medical prescription for body positioning of the patient in bed.
Sample calculationsA total of 95 patients using ENT were evaluated, resulting in an approximate 42.00% frequency value of adverse gastroenterologic and pulmonary events, with a maximum acceptable difference of 10.00%. These adverse events included diarrhea, abdominal distension, nausea/vomiting, constipation, and clinical evidence of pulmonary aspiration. The margin of error calculation, which varied from 8.00% to 10.00%, was used for the grouped outcomes when they were evaluated separately.
Data analysisCalculations of frequency, percentage, mean±standard deviation (SD), and median (including the 25th and 75th percentiles) were performed for the descriptive data analysis. Pearson's chi-squared test and Fisher's exact test were used to compare categorical variables between groups that did or did not present adverse gastroenterological events. The relationship between GI complications and osmolality was calculated using a Student's t test. The Mann-Whitney test was used to evaluate the relationship of adverse events with the duration of enteral nutrition. Concordance among gastroenterologists (in relation to medications and adverse events) was evaluated using weighted Kappa values.6
ResultsThe study involved 95 patients with no loss of follow-up.
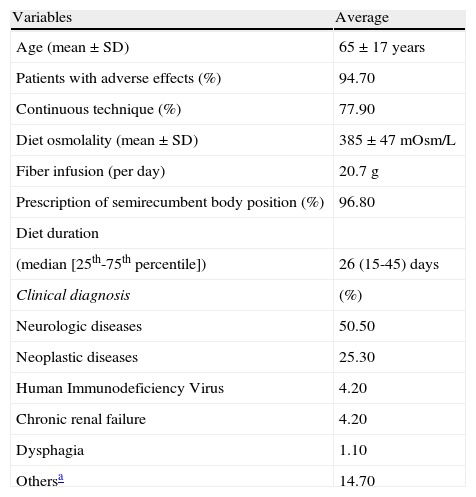
Demographic data and population characteristics are presented in Table 1.
Sample characteristics.
| Variables | Average |
| Age (mean±SD) | 65±17 years |
| Patients with adverse effects (%) | 94.70 |
| Continuous technique (%) | 77.90 |
| Diet osmolality (mean±SD) | 385±47 mOsm/L |
| Fiber infusion (per day) | 20.7 g |
| Prescription of semirecumbent body position (%) | 96.80 |
| Diet duration | |
| (median [25th-75th percentile]) | 26 (15-45) days |
| Clinical diagnosis | (%) |
| Neurologic diseases | 50.50 |
| Neoplastic diseases | 25.30 |
| Human Immunodeficiency Virus | 4.20 |
| Chronic renal failure | 4.20 |
| Dysphagia | 1.10 |
| Othersa | 14.70 |
SD: standard deviation.
The medical prescription for the body positioning of the patient in bed was not related to the occurrence of clinically relevant pulmonary aspiration, with only one patient presenting this condition. Patients with diarrhea used enteral diet for a longer period of time than those who did not present with this complication (median: 32 days) (p=0.001).
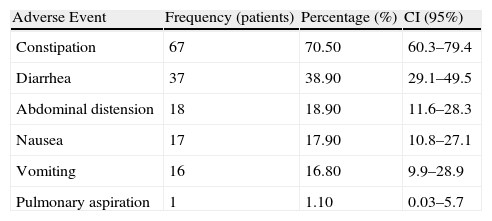
Observed GI events that occurred in this population are shown in Table 2. All prescribed medications presented in the medical records were considered for the purpose of this study.
Frequency of Adverse Events (n=95 patients).
| Adverse Event | Frequency (patients) | Percentage (%) | CI (95%) |
| Constipation | 67 | 70.50 | 60.3–79.4 |
| Diarrhea | 37 | 38.90 | 29.1–49.5 |
| Abdominal distension | 18 | 18.90 | 11.6–28.3 |
| Nausea | 17 | 17.90 | 10.8–27.1 |
| Vomiting | 16 | 16.80 | 9.9–28.9 |
| Pulmonary aspiration | 1 | 1.10 | 0.03–5.7 |
CI: confidence interval.
Note: The sum of cases was greater than 100% because some patients presented with more than one adverse event.
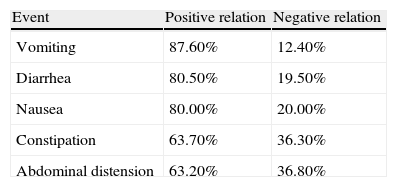
The weighted Kappa values, which analyzed the concurrence between gastroenterologists in relation to the evaluated variables, showed a variation of 0.45-0.62, an interval considered moderate to good.6,7 The frequency of adverse events in relation to the negativity and positivity of association with medications used by patients during their stay at the HCPA is shown in Table 3.
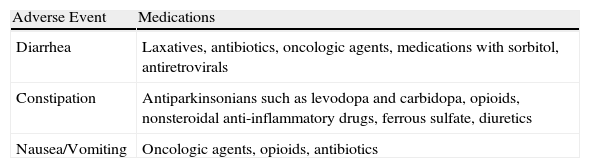
Table 4 shows the medications frequently associated with adverse gastroenterological events in this sample.
Medications frequently associated with Adverse Gastroenterologic Events.
| Adverse Event | Medications |
| Diarrhea | Laxatives, antibiotics, oncologic agents, medications with sorbitol, antiretrovirals |
| Constipation | Antiparkinsonians such as levodopa and carbidopa, opioids, nonsteroidal anti-inflammatory drugs, ferrous sulfate, diuretics |
| Nausea/Vomiting | Oncologic agents, opioids, antibiotics |
We believe that this is the first study attempting to evaluate the relationship between GI events and simultaneously administered pharmacologic agents in hospitalized patients being treated with ENT.
Our results confirm a high incidence of digestive symptoms in this population, with constipation and diarrhea being the most important manifestations.
The occurrence of diarrhea was higher in the group of patients that received ENT for a longer period of time. This fact can be explained by the multifactorial causes of diarrhea. The aggravation of diarrhea can occur in patients with longer periods of hospitalization, extensive use of medications (laxatives, medications that contain sorbitol, antibiotics, and oncologic agents), or as a result of underlying illnesses or medical conditions (such as diabetes mellitus or contamination with the Human Immunodeficiency Virus-HIV).1,8 It is worth noting that in our sample, patients who were using antibiotic therapy presented higher frequencies of diarrhea, which agrees with findings from other studies.8,9
When comparing our results to a study conducted by Luft et al.10 involving 302 patients receiving or not receiving ENT and that represented a population with similar characteristics to ours in terms of patient age, number of days of hospitalization, applied definition of diarrhea, and type of diet used, the prevalence of diarrhea was found to be 18.20% as opposed to the 38.90% obtained in our study. Methodological data collection differences were probably responsible for this discrepancy.
The medications most frequently associated with diarrhea involved stimulants or osmotic laxatives for the treatment of constipation, such as mineral oil, lactulose, bisacodyl, and also the use of antibiotics.11,12
Approximately two-thirds of our sample (70.50%) presented with constipation. This result represented a higher percentage than that found in studies conducted by Montejo13 (15.70%) and Pancorbo-Hidalgo et al.14 (29.80%). However, it was comparable to the findings of Nassar5 (69.90%), whose study design was similar to ours. This result can be explained by our sample characteristic in which 50.50% of patients suffered from neurological diseases which can predispose to constipation in a hospital setting. These patients often spend longer periods of time in bed, as well as being subject to certain constipating medications (levodopa and carbidopa, for example) that inhibit both colonic motility and perception of evacuative impulses.15,16 A lack of fiber in the ENT formula does not appear to be related to this symptom, as the average daily fiber infusion was 20.7g, an amount within the recommended daily consumption.17,18
Few clinical studies have evaluated the risk associated with medication use and the occurrence of constipation in patients receiving ENT; this association presented a positive relationship in 63.70% of our patients.1 The occurrence of this clinical problem can be caused by many medications regularly used in the hospital setting, such as opioid analgesics (morphine, codeine) and nonsteroidal anti-inflammatory drugs (naproxen, ibuprofen).12,16,19,20
The underlying disease itself that necessitates the use of ENT is also an important factor in the occurrence of GI complications.21,22 This should be taken into consideration when modifications to infused medications and/or changes in the enteral feeding formula are contemplated, with the aim of improving newly arising symptoms in the clinical setting.
Caution should be exercised when making any generalization of the results of this study, given that our sample was obtained from a university hospital context and we had a team of experienced health professionals with well-established routines for the care of patients requiring ENT; the HCPA is a regional reference center for health services in Southern Brazil.
A limitation of this study with respect to the quality of information could involve the method of data collection (patient medical records), as both under-reporting and over-reporting of signs and symptoms may occur. A further drawback to consider is the lack of a validated tool for evaluating the relationship between medication use and GI complications developed by patients. It was necessary to create such an instrument based on the content of the National Agency for Sanitary Vigilance (ANVISA),23 as well as the tool of Naranjo and Busto24 that is widely used in clinical trials. This latter instrument could not be used in clinical practice due to methodological issues, because it attempts to study one variable (pharmacologic intervention) at a time and uses information related to placebo intervention in order to define the level of association. For the purposes of our study, we used only the terminology and operational definitions of Naranjo and Busto, without using the other characteristics they applied in their clinical research.8,10,13,14
Although the instrument used had not previously been validated, the Kappa test applied to evaluate the agreement relationship between the gastroenterologists considered it to be moderate to good,6,7 which suggests that it may be used in a larger sample in order to evaluate sensitivity, specificity, and predictive values.
The strength of this research lies in the fact that it is an observational study set in the real-life context of the operational realities of enteral nutrition application within a tertiary reference hospital in Southern Brazil. Care was taken to avoid the Hawthorne effect whereby behavior or performance can be modified simply because a person is aware that they are being evaluated.4 To this end, medical, nursing, and nutrition teams were never aware that a researcher was collecting study data from patient medical records. It was equally important for the data to be collected in a randomized manner, thus enabling a representative sample of the hospital as a whole to be obtained. Additionally, all data collection was conducted by the same researcher and no sample loss occurred, resulting in the matching of the sample calculation with the number of patients studied.
ConclusionsGI symptoms are frequent in patients using ENT. However, the causes associated with them should be carefully examined in the clinical context of each patient, as they are often connected to the prescribed medications that are jointly administered with ENT. Consequently, enteral nutrition per se should not be seen as a principal cause of GI complications. Healthcare teams should consider all risk factors present for each patient when evaluating the occurrence of these adverse effects, paying particular attention to the prescribed medications in use before modifying, reducing, or suspending ENT administration.
Conflict of interestThe authors declare that there is no conflict of interest.
Financial disclosureNo financial support was received in relation to this article.