Hereditary hemorrhagic telangiectasia (HHT) is an autosomal dominant disease mainly associated with mutations in the genes encoding endoglin (ENG) and the ALK1 receptor (ACVRL1), which play different roles in the transforming growth factor-beta (TGF-beta) and bone morphogenetic protein (BMP) signaling pathways. Loss of function of those proteins results in an imbalance in angiogenesis, leading to excessive neoangiogenesis and/or failure of vascular stabilization during its course.1 Clinically, it is characterized by the presence of vascular malformations (VMs) in the skin, the nasal and digestive tract mucosa, the brain, lung, and liver.1 Gastrointestinal bleeding is a frequent complication of HHT, which can occur in up to 30% of cases and occasionally is considerably severe, with a very high number of transfusions required, high morbidity and mortality, and deterioration of quality of life.2
A 61-year-old man was diagnosed with HHT due to epistaxis, family history, and the heterozygous deletion of exon 2 of ENG in the molecular study. Along the course of the disease, contrast echocardiography, abdominal angiotomography, and magnetic resonance angiography of the brain were performed, revealing no VMs. Spanning a 20-year period, the patient had episodes of anemia associated with digestive bleeding that had required frequent parenteral iron therapy and regular transfusions over the last 8 years, with transfusion dependence in the last 2 years. Successive gastroscopies and capsule endoscopy studies showed gastric and duodenal telangiectasias, occasionally with active bleeding, treated with argon electrocoagulation.
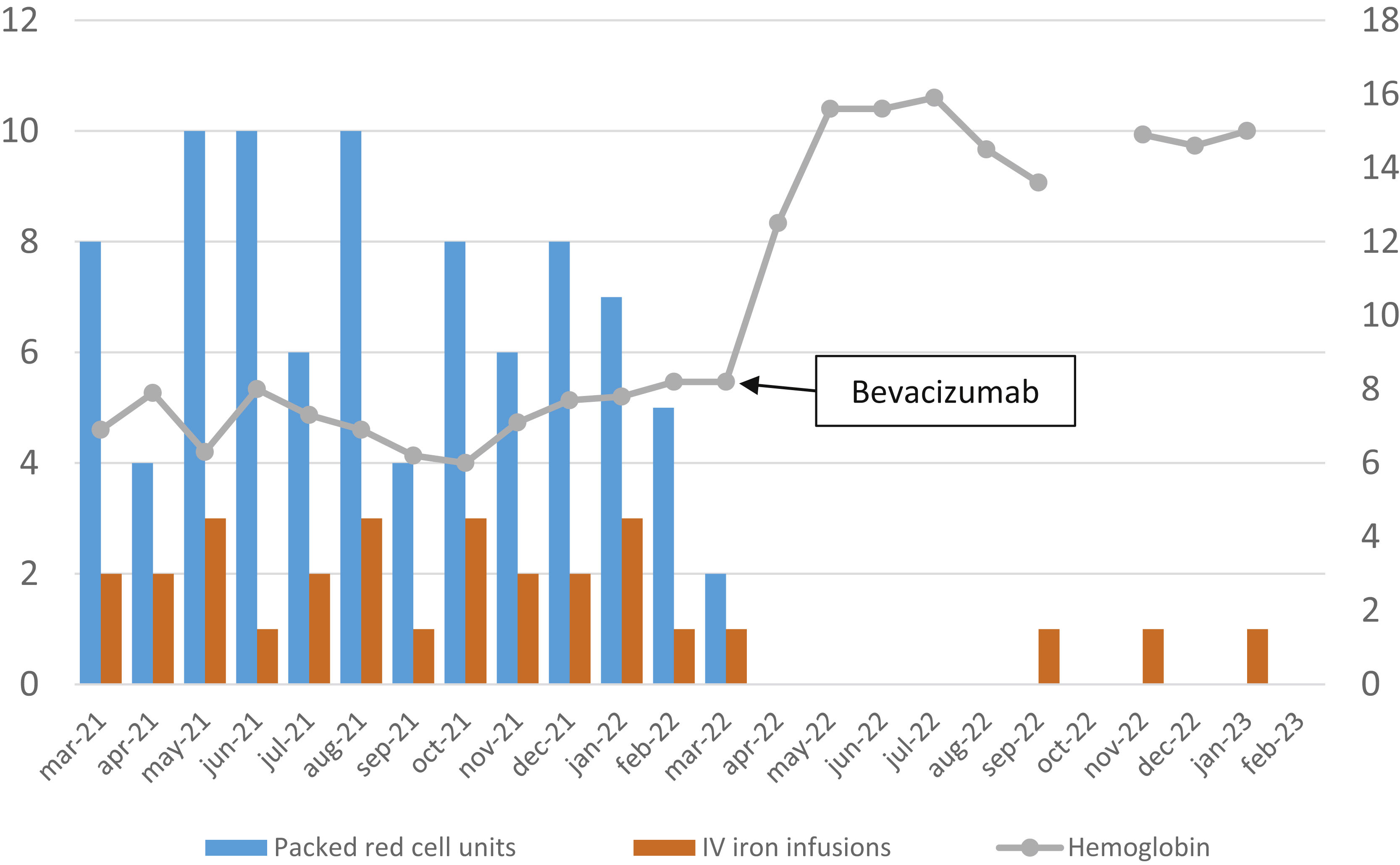
Treatment was started with bevacizumab 5 mg/kg/2 weeks for a total of 6 doses and then switched to 5 mg/kg/6 weeks. Fig. 1 shows the required transfusions and parenteral iron therapy in the 12 months before and after starting bevacizumab. Tolerance to treatment has been good, although headache, arthromyalgia, and elevated plasma creatinine have been observed as adverse effects. All those symptoms have improved, with the spacing of bevacizumab doses. No arterial hypertension or proteinuria was observed in the controls that were carried out.
Evolution of packed red cell units required, parenteral iron infusions, and hemoglobin levels before and after treatment with bevacizumab. The ordinate axis on the left reflects the number of units of packed red cells and the doses of intravenous iron administered, and the right axis reflects the hemoglobin levels expressed in g/dl.
In recent years, recognition of the role of angiogenesis in the pathogenesis of HHT has supported the use of different antiangiogenic treatments; among them, the most extensively studied is bevacizumab, a humanized monoclonal antibody that selectively binds to vascular endothelial growth factor (VEGF) and neutralizes its biological activity, thus inhibiting angiogenesis. The evidence for its use derives from observational studies showing a reduction in the severity of epistaxis or digestive bleeding, improvement of anemia and reduction of transfusions required, and progression of liver disease, so its use is indicated in patients with severe epistaxis, chronic digestive bleeding, and heart failure with high cardiac output related to hepatic VM.3,4
Bevacizumab is contraindicated in patients with severe arteriopathy or arterial hypertension and recent venous thromboembolic disease, infection, or surgery.5 It should be used with caution in patients with high thromboembolic risk and attention should be paid to wound healing. Its administration should be interrupted in the event of surgery.1,5 The most widely accepted dosing regimen consists of an induction phase of 6 doses of 5 mg/kg every 2 weeks, followed by a maintenance phase, in which bevacizumab is administered, either on a fixed schedule with programmed intervals or on demand, depending on bleeding episodes and hemoglobin levels.1 The decision between those options should be made on an individual basis, according to the severity of previous bleeding and the response and tolerance to treatment. The most frequent adverse effects are arterial hypertension, asthenia, proteinuria, arthromyalgia, headache, and venous thromboembolic disease, which in general, do not require discontinuation of the drug.3 Other antiangiogenic drugs used in HHT are pazopanib, a tyrosine kinase inhibitor that blocks the VEGF receptor, and thalidomide, albeit there is less evidence on their use.5
In conclusion, bevacizumab was extraordinarily effective in controlling our patient’s gastrointestinal bleeding, in line with results reported in the literature. Pending the completion of controlled clinical trials to confirm said data, bevacizumab should be considered a useful alternative in patients with HHT and epistaxis or difficult-to-control gastrointestinal bleeding.
Ethical considerationsThe authors have obtained informed consent from the patient referred to in the article, this document is in the possession of the corresponding author. The authors declare that they have followed their center’s protocols for the publication of patient data, that this article does not contain personal information that would allow identification of the patient.
Financial disclosureNo specific grants were received in relation to this article.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Gutiérrez-Macías A, Barroso-Benayos I, Burzako-Sánchez A. Utilidad del bevacizumab en sangrado gastrointestinal crónico en telangiectasia hemorrágica hereditaria. Rev Gastroenterol Mex. 2023;88:433–434.