Lactose intolerance is a condition with an elevated prevalence worldwide, especially in Latin American, Asian, and African countries. The aim of the present narrative review was to highlight the importance of accurately diagnosing lactose intolerance to prevent self-diagnosis that results in the unnecessary elimination of milk and dairy products from the diet and the consequent deprivation of nutrients that could be essential at certain stages of life. The pathophysiologic mechanism of deficient lactose absorption in the intestine can be primary, secondary to other enteropathies, or coexistent with other intestinal diseases with similar symptoms, such as irritable bowel syndrome, bacterial overgrowth syndrome, or celiac disease, causing confusion in relation to diagnosis and treatment. Lactose intolerance consists of a set of symptoms attributed to the consumption of milk and dairy products that are assumed to be due to deficient digestion of that disaccharide. A wide range of tests have been validated to detect deficient digestion that include blood tests, genetic mutation analyses, breath tests, and recently, a urine test, all of which are described in the present article. Nevertheless, there are few validated questionnaires for symptom evaluation and measurement, partly due to the heterogeneity of concepts and the subjectivity of each of the symptoms.
La intolerancia a la lactosa es un padecimiento con elevada prevalencia a nivel mundial principalmente en países hispanoamericanos, asiáticos y africanos. El objetivo de esta revisión narrativa es destacar la importancia del diagnóstico correcto de intolerancia a la lactosa, que evite el autodiagnóstico que conlleva a la eliminación innecesaria de lácteos de la dieta con la privación de nutrimentos que podrían ser esenciales en algunas etapas de la vida. El mecanismo fisiopatogénico de la absorción deficiente de lactosa en el intestino puede ser primario, secundario a otras enteropatías o coexistir con otras enfermedades intestinales cuyos síntomas son parecidos como en el caso del síndrome de intestino irritable, síndrome de sobre crecimiento bacteriano o enfermedad celiaca entre otros; provocando confusión en el diagnóstico y tratamiento. La intolerancia a la lactosa constituye un conjunto de síntomas atribuidos al consumo de leche y sus derivados y se asume que se debe a la digestión deficiente de este disacárido. Se han validado una amplia gama de pruebas para detectar digestión deficiente como: exámenes en sangre, análisis de mutaciones genéticas, pruebas en aire espirado y recientemente, una en orina, mismas que se describen en este artículo. Sin embargo, para la evaluación y medición de los síntomas, existen pocos cuestionarios validados, en parte, debido a la heterogeneidad de conceptos y subjetividad de cada uno de los síntomas.
Lactose intolerance (LI) is a condition with a high prevalence worldwide, whose clinical manifestations are varied and similar to those of other entities, such as celiac disease, bacterial overgrowth syndrome, or irritable bowel syndrome. The gastrointestinal functional disorders are a group of conditions that are defined based on gastrointestinal symptoms, in the absence of structural alterations and their overall prevalence is elevated (10-20%). They are varied, given that they depend on different factors, such as age, geographic region, manner in which they are registered, tests and criteria used for their diagnosis, genetic burden, dietary, environmental, psychosocial, cultural, and economic factors, nutritional status, and exposure to infectious agents.1–3 Velasco-Benítez described the behavior of those disorders in adolescents in Argentina and other Latin American countries. The most frequent in Argentina were abdominal migraine, irritable bowel syndrome, functional constipation, and aerophagia, whereas functional constipation, irritable bowel syndrome, and functional abdominal pain were more frequent in the other countries.4 In Mexico, Remes-Troche et al. calculated that 7.6% (±6.8-8.5%) of the general population suffers from some type of gastrointestinal functional disorder, and irritable bowel syndrome is one of the most frequent.5 Similar to results from other publications, the measured frequency in Mexico in women is higher than in men, in persons with professional careers, and those with middle and high socioeconomic levels. That same article describes the overlap with other digestive disorders, which could include LI. However, physicians often view said intolerance as a consequence of other gastrointestinal diseases, and instead of confirming it, assume it is part of another syndrome and simply eliminate dairy products from the patient’s diet.
The aim of the present narrative review was to highlight the importance of an accurate diagnosis of LI, in the context of patients that chronically suffer from gastrointestinal symptoms, to prevent self-diagnosis and misdiagnosis that leads to the elimination of dairy products in the diet and the deprivation of nutrients that could be essential at different stages of life.
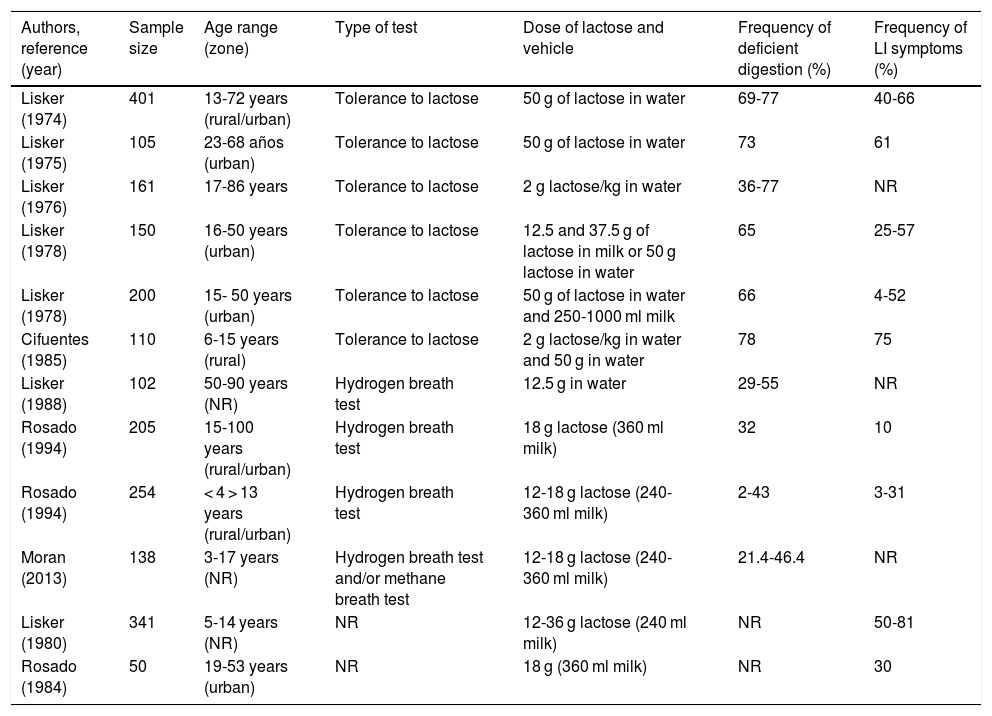
Prevalence of lactose intolerance and deficient lactose digestionLI is a frequent condition worldwide. There are European, American, Asian, and African case series that describe a varied prevalence of 15%, 50%, 70%, and 100%, respectively.6–8 Studies in Mexico found that almost half the adult population complained of gastrointestinal discomfort related to the consumption of dairy products, and of those, 70% had a positive test, suggesting deficient lactose digestion.8 Nevertheless, one of the main issues with those studies was the lack of standardization in the quantity of lactose administered for performing the tests, the conditions in which they were carried out, and their interpretation, as well as the genetic variability of the study populations. Table 1 shows the results of studies conducted in Mexico.
The prevalence of deficient digestion and symptoms related to lactose intolerance in different studies in Mexico.
| Authors, reference (year) | Sample size | Age range (zone) | Type of test | Dose of lactose and vehicle | Frequency of deficient digestion (%) | Frequency of LI symptoms (%) |
|---|---|---|---|---|---|---|
| Lisker (1974) | 401 | 13-72 years (rural/urban) | Tolerance to lactose | 50 g of lactose in water | 69-77 | 40-66 |
| Lisker (1975) | 105 | 23-68 años (urban) | Tolerance to lactose | 50 g of lactose in water | 73 | 61 |
| Lisker (1976) | 161 | 17-86 years | Tolerance to lactose | 2 g lactose/kg in water | 36-77 | NR |
| Lisker (1978) | 150 | 16-50 years (urban) | Tolerance to lactose | 12.5 and 37.5 g of lactose in milk or 50 g lactose in water | 65 | 25-57 |
| Lisker (1978) | 200 | 15- 50 years (urban) | Tolerance to lactose | 50 g of lactose in water and 250-1000 ml milk | 66 | 4-52 |
| Cifuentes (1985) | 110 | 6-15 years (rural) | Tolerance to lactose | 2 g lactose/kg in water and 50 g in water | 78 | 75 |
| Lisker (1988) | 102 | 50-90 years (NR) | Hydrogen breath test | 12.5 g in water | 29-55 | NR |
| Rosado (1994) | 205 | 15-100 years (rural/urban) | Hydrogen breath test | 18 g lactose (360 ml milk) | 32 | 10 |
| Rosado (1994) | 254 | < 4 > 13 years (rural/urban) | Hydrogen breath test | 12-18 g lactose (240- 360 ml milk) | 2-43 | 3-31 |
| Moran (2013) | 138 | 3-17 years (NR) | Hydrogen breath test and/or methane breath test | 12-18 g lactose (240- 360 ml milk) | 21.4-46.4 | NR |
| Lisker (1980) | 341 | 5-14 years (NR) | NR | 12-36 g lactose (240 ml milk) | NR | 50-81 |
| Rosado (1984) | 50 | 19-53 years (urban) | NR | 18 g (360 ml milk) | NR | 30 |
NR: not reported.
Reproduced with permission from the author (Rosado8).
LI is a syndrome characterized by bowel sounds, meteorism, abdominal pain, flatulence, nausea, vomiting, and occasional loose stools caused by the consumption of milk and dairy products.9 Symptoms are nonspecific, and intensity is variable, depending on the quantity of lactose ingested and individual susceptibility. They generally present within 30 min to 2 h after dairy product intake. In some cases, constipation can present instead of diarrhea, as a consequence of exaggerated methane production. Intolerance much less frequently causes extraintestinal symptoms, such as headache, dizziness, loss of concentration, short-term memory difficulty, intense fatigue, muscle pain, and arthralgias. The majority of persons, despite having deficient lactose digestion, have been shown to tolerate up to 240 ml of milk or dairy products at wide intervals with no problem, whereas one-third of patients complain of intolerance to small quantities.6
Presenting symptoms upon consuming dairy products is not synonymous with deficient lactose digestion, given that there are other components in milk. Principally proteins and peptides derived from their digestion (caseins-casomorphins) can cause similar discomfort. Thus, deficient digestion should only be considered when there is evidence of a functional incapacity of lactase to hydrolyze lactose and convert it into glucose and galactose.
Lactose is digested by lactase, an enzyme found in the brush border of enterocytes. Its maximum expression is in the middle jejunum, where it is subject to little fermentation because the bacterial burden at that level is low. Lactose hydrolysis produces glucose and galactose, conferring beneficial effects. On the one hand, glucose is a source of energy for different enterocyte functions, and on the other, galactose forms part of the glycoproteins and glycolipids. When lactose is not completely digested, it arrives intact at the distal parts of the small bowel and colon, where it is fermented by bacteria, causing the symptoms previously described.10
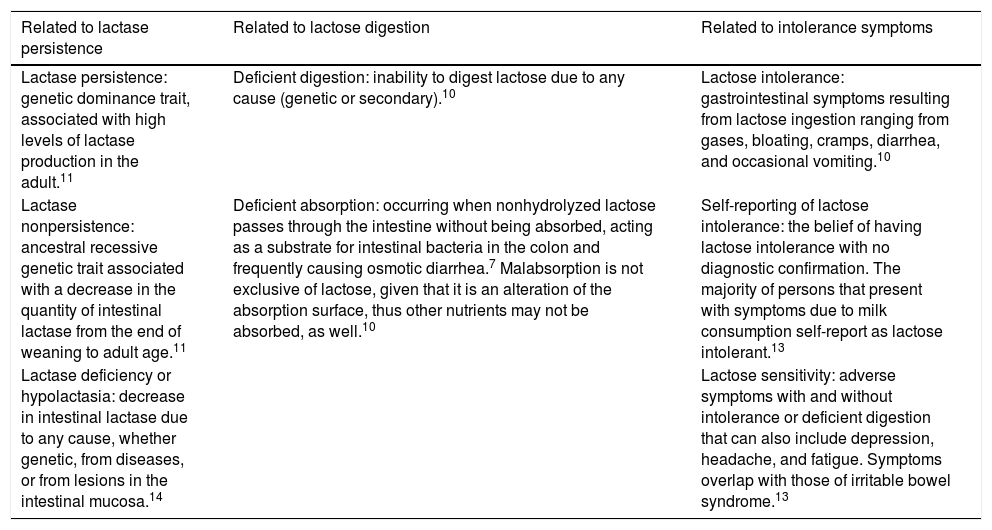
Lactase is encoded and synthetized in the enterocyte by the MCM6 gene, located on the chromosome 2q21 region. At least 3 polymorphic variants of the single nucleotide polymorphism (SNP) are known to regulate its gene expression. The substitution of a thymine for cytosine at position 13910 (SNP C/T-13910) has been found in persons with lactase persistence (LP), whereas SNP C/C-13910 has been associated with lactase nonpersistence (LNP).11,12 That finding has been observed in different regions of the world and explains the varying frequency of LI and deficient lactose digestion reported in different articles.7 There is little clinical usefulness in determining the mutation responsible for LNP. On the other hand, LP cannot be determined with absolute certainty, even in samples of small bowel mucosa, due to the irregular distribution of the enzyme throughout the digestive tract. Therefore, LNP is indirectly evaluated through breath tests, in which deficient digestion is inferred by hydrogen production after an oral load of lactose (Table 2).10
Concepts of deficient digestion and lactose intolerance.
| Related to lactase persistence | Related to lactose digestion | Related to intolerance symptoms |
|---|---|---|
| Lactase persistence: genetic dominance trait, associated with high levels of lactase production in the adult.11 | Deficient digestion: inability to digest lactose due to any cause (genetic or secondary).10 | Lactose intolerance: gastrointestinal symptoms resulting from lactose ingestion ranging from gases, bloating, cramps, diarrhea, and occasional vomiting.10 |
| Lactase nonpersistence: ancestral recessive genetic trait associated with a decrease in the quantity of intestinal lactase from the end of weaning to adult age.11 | Deficient absorption: occurring when nonhydrolyzed lactose passes through the intestine without being absorbed, acting as a substrate for intestinal bacteria in the colon and frequently causing osmotic diarrhea.7 Malabsorption is not exclusive of lactose, given that it is an alteration of the absorption surface, thus other nutrients may not be absorbed, as well.10 | Self-reporting of lactose intolerance: the belief of having lactose intolerance with no diagnostic confirmation. The majority of persons that present with symptoms due to milk consumption self-report as lactose intolerant.13 |
| Lactase deficiency or hypolactasia: decrease in intestinal lactase due to any cause, whether genetic, from diseases, or from lesions in the intestinal mucosa.14 | Lactose sensitivity: adverse symptoms with and without intolerance or deficient digestion that can also include depression, headache, and fatigue. Symptoms overlap with those of irritable bowel syndrome.13 |
Deficient lactose digestion can be understood as a normal and expected situation in the life cycle of certain ethnicities or as a secondary defect in lactase production or expression.15 In general, three types of LNP are accepted:
The first is due to genetic mutations that present in the neonatal stage, in which neonates do not have the capacity to digest lactose. They present with diarrhea that is sometimes associated with acidosis and hypercalcemia upon consuming breastmilk. Primary hypolactasia is rare, related to a certain geographic localization, and can be total or partial.7,13
The second, the most common across the globe, is characterized by a gradual decrease in lactase activity. It also arises from a genetic regulation, although its distribution depends on the merging of native populations. The migration of “old world” inhabitants has been documented as creating a different distribution between LP and LNP. The nonpersistent phenotype is the more recent in the “new world” and can begin between 2 and 5 years of age, but does not usually present before 8 years of age. In populations with a LP/LNP mixture, the phenotype is expressed at later ages, as occurs in Mexico (Table 1). From the functional perspective, persons with both homozygous and heterozygous LP have been reported to express lactase levels at the brush border that are 10-times higher than those in persons with LNP.15
The third, known as secondary hypolactasia, is due to gastrointestinal diseases that cause villous atrophy. In other words, any damage to the mucosa causes temporary lactase deficiency that improves when the initial problem is resolved, but in the case of genetic predisposition, could permanently cause LNP (Table 3).11,16
Agents identified as lactose modifiers in the small bowel.
| Type of agent | Identified agents |
|---|---|
| Bacterial and viral | Rotavirus, single-celled parasites (Giardia)16 |
| Diseases | Gastroenteritis, celiac disease, Crohn’s disease, ulcerative colitis, irritable bowel syndrome, immunologic deficiencies, bacterial overgrowth, malnutrition11,16 |
| Mechanical, physical, pharmacologic | Radiation exposure, gastrointestinal surgeries16 |
| Olmesartan, antibiotics11 |
The symptoms attributed to LI are due to the osmotic property of lactose that attracts water from the intravascular space to the intestinal lumen and partially depends on the speed at which the disaccharide arrives at the intestine, in turn, reflecting the quantity of lactose that was not hydrolyzed. In addition, the bacterial fermentation of lactose produces hydrogen, methane, nitrogen, and short-chain fatty acids that also contribute to symptoms.13 With respect to intolerance, symptom intensity depends on the quantity of the nutrient or food substance consumed, a fact that should not be forgotten. Thanks to hydrogen breath tests, even persons with deficient lactose digestion, hence with LNP, can tolerate the quantity of lactose contained in a glass of whole milk (12.5 g in 250 ml).13
LNP does not appear to be associated with other digestive diseases, even though individuals with irritable bowel syndrome have been shown to have a greater probability of presenting with LI than the general population.2,3,17 Curiously, in persons with inflammatory bowel disease, in whom the frequency of LI would be thought to be high, prevalence similar to that in the general population has been described. Thus, there is no reason to eliminate dairy products from the diet in that group of patients.18,19
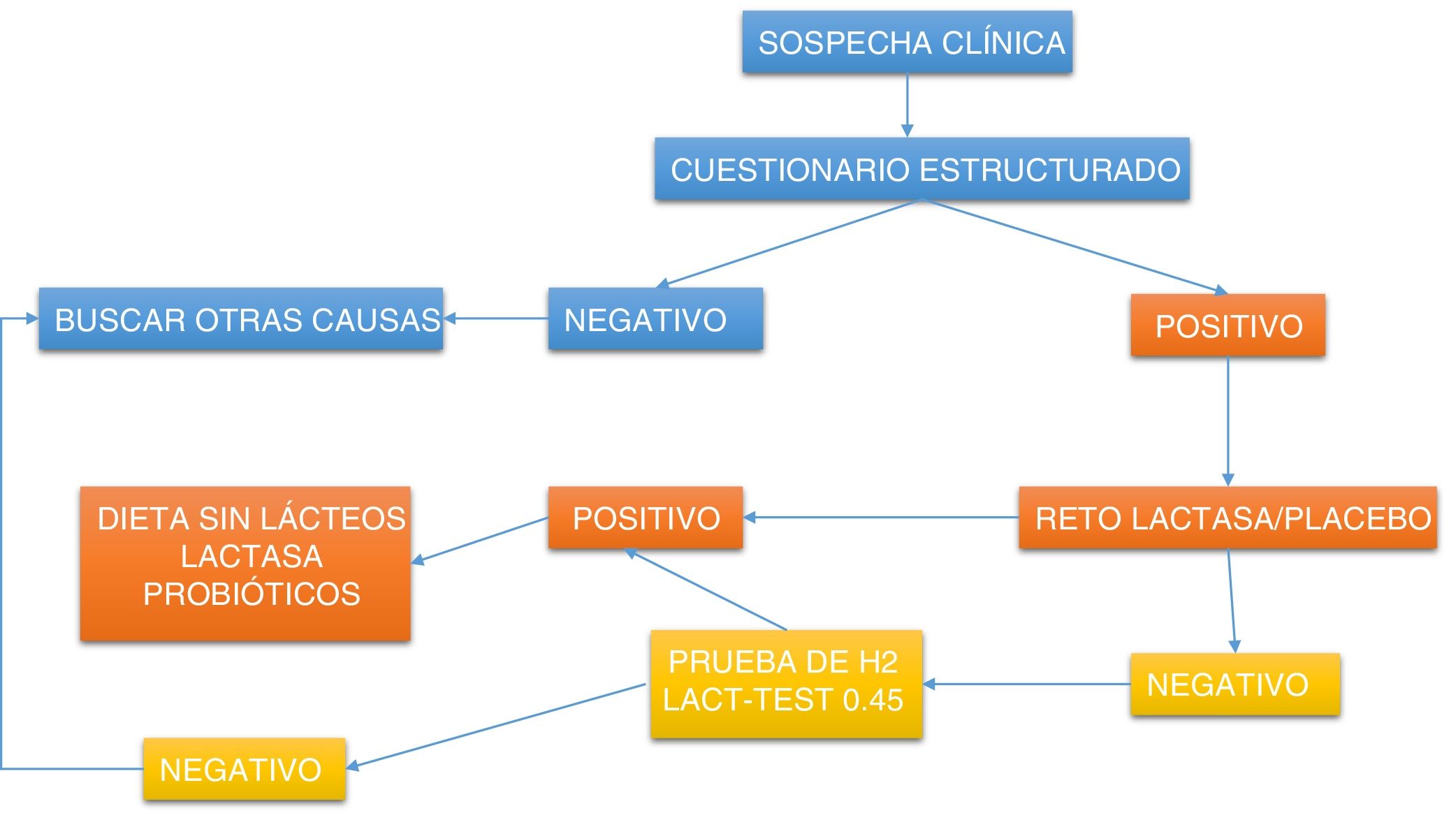
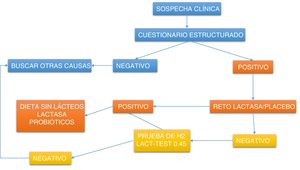
Diagnosis of lactose intoleranceDirected questioning should be carried out to relate symptoms with milk and dairy products, or foods made with them, to then evaluate the coexistence of deficient digestion. Fig. 1 shows a practical clinical approach.
Diagnostic approach algorithm for detecting lactose intolerance.
LacTEST: urine xylose test that indirectly measures lactase intestinal activity, dividing a sugar composed of galactose and xylose; Lactase challenge: 5-day supplementation with 10,000 IU of lactase per meal to evaluate symptoms.
There are few questionnaires that evaluate gastrointestinal symptoms related to dairy product consumption. In fact, there is only one validated questionnaire that measures symptom intensity, utilizing a visual analogue scale to grade the intensity of diarrhea, vomiting, cramping, bowel sounds, and flatulence or gases. It was applied before and after the breath test (reference standard) to be validated in an adult Spanish population, in which LI prevalence varied from 36% to 51%. Deficient digestion was documented in that group at 45%, showing 0.75 sensitivity and 0.67 specificity, for a cutoff point of 7.20
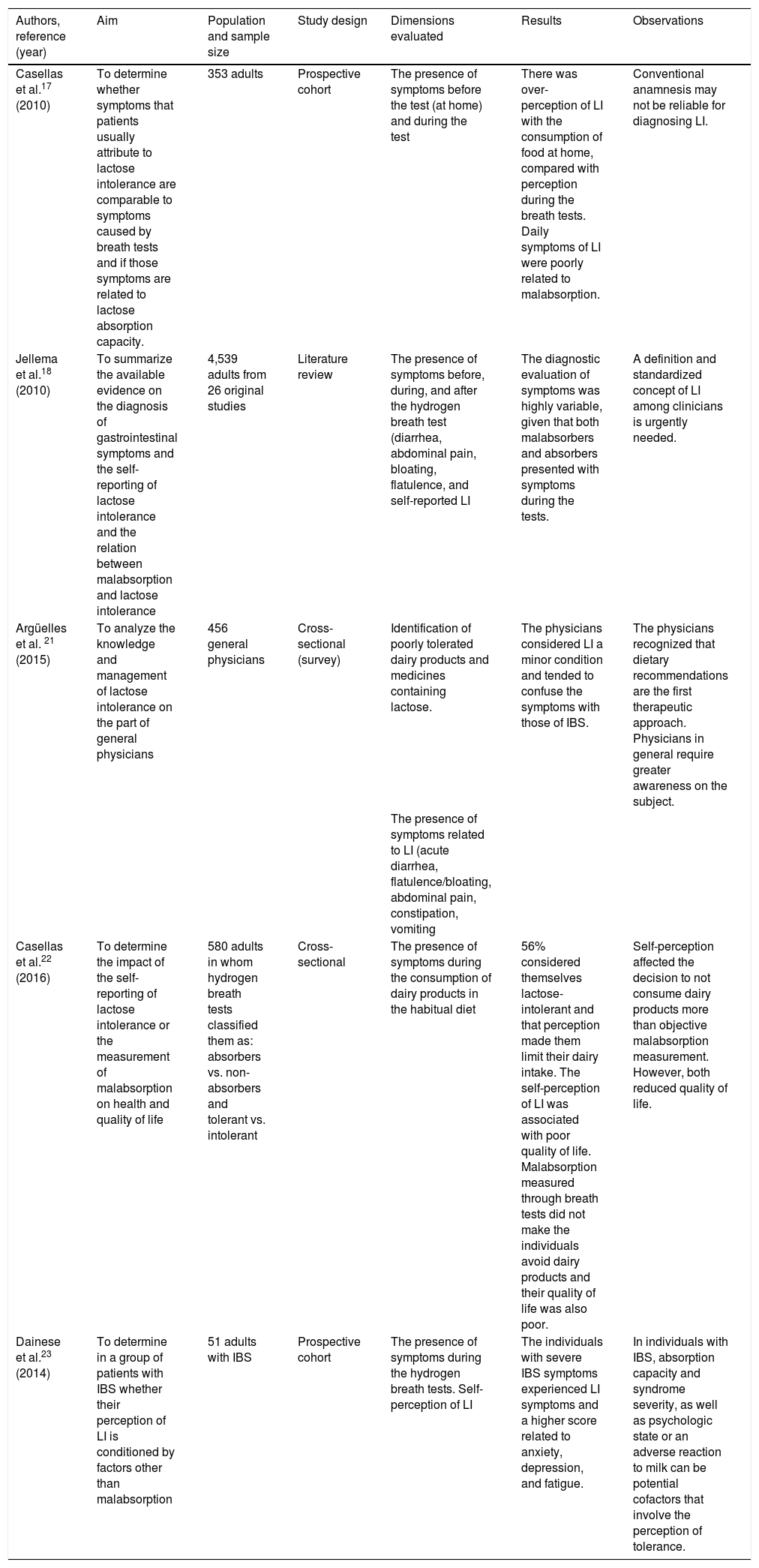
Other studies have examined symptoms with questions that were non-validated but directly described the related symptoms, either simultaneously with or before and after the breath tests (Table 4).
Studies that have evaluated and compared lactose intolerance symptoms presenting around the hydrogen breath tests.
| Authors, reference (year) | Aim | Population and sample size | Study design | Dimensions evaluated | Results | Observations |
|---|---|---|---|---|---|---|
| Casellas et al.17 (2010) | To determine whether symptoms that patients usually attribute to lactose intolerance are comparable to symptoms caused by breath tests and if those symptoms are related to lactose absorption capacity. | 353 adults | Prospective cohort | The presence of symptoms before the test (at home) and during the test | There was over-perception of LI with the consumption of food at home, compared with perception during the breath tests. Daily symptoms of LI were poorly related to malabsorption. | Conventional anamnesis may not be reliable for diagnosing LI. |
| Jellema et al.18 (2010) | To summarize the available evidence on the diagnosis of gastrointestinal symptoms and the self-reporting of lactose intolerance and the relation between malabsorption and lactose intolerance | 4,539 adults from 26 original studies | Literature review | The presence of symptoms before, during, and after the hydrogen breath test (diarrhea, abdominal pain, bloating, flatulence, and self-reported LI | The diagnostic evaluation of symptoms was highly variable, given that both malabsorbers and absorbers presented with symptoms during the tests. | A definition and standardized concept of LI among clinicians is urgently needed. |
| Argüelles et al. 21 (2015) | To analyze the knowledge and management of lactose intolerance on the part of general physicians | 456 general physicians | Cross-sectional (survey) | Identification of poorly tolerated dairy products and medicines containing lactose. | The physicians considered LI a minor condition and tended to confuse the symptoms with those of IBS. | The physicians recognized that dietary recommendations are the first therapeutic approach. Physicians in general require greater awareness on the subject. |
| The presence of symptoms related to LI (acute diarrhea, flatulence/bloating, abdominal pain, constipation, vomiting | ||||||
| Casellas et al.22 (2016) | To determine the impact of the self-reporting of lactose intolerance or the measurement of malabsorption on health and quality of life | 580 adults in whom hydrogen breath tests classified them as: absorbers vs. non-absorbers and tolerant vs. intolerant | Cross-sectional | The presence of symptoms during the consumption of dairy products in the habitual diet | 56% considered themselves lactose-intolerant and that perception made them limit their dairy intake. The self-perception of LI was associated with poor quality of life. Malabsorption measured through breath tests did not make the individuals avoid dairy products and their quality of life was also poor. | Self-perception affected the decision to not consume dairy products more than objective malabsorption measurement. However, both reduced quality of life. |
| Dainese et al.23 (2014) | To determine in a group of patients with IBS whether their perception of LI is conditioned by factors other than malabsorption | 51 adults with IBS | Prospective cohort | The presence of symptoms during the hydrogen breath tests. Self-perception of LI | The individuals with severe IBS symptoms experienced LI symptoms and a higher score related to anxiety, depression, and fatigue. | In individuals with IBS, absorption capacity and syndrome severity, as well as psychologic state or an adverse reaction to milk can be potential cofactors that involve the perception of tolerance. |
IBS: irritable bowel syndrome; LI: lactose intolerance.
Methods are divided into invasive and noninvasive.24 The noninvasive methods include genetic testing, the measurement of hydrogen in exhaled breath (currently considered the test of choice),25,26 the glycemia curve,27 and the 4-galactosyl-xylose curve. Lactase activity determination in intestinal mucosa fragments obtained through biopsy (the gold standard for hypolactasia diagnosis) is the invasive method. There is currently no standardization of noninvasive tests to determine deficient absorption, leading to false negatives (10%) and false positives (20%),28–31 making it necessary to establish a lactose dose that resembles physiologic conditions to adequately evaluate its hydrolysis.
Due to its easy application, hydrogen titration in exhaled breath is the most widely accepted method worldwide. However, it takes time (about 5 h) and exposes the recipients to a lactose load that can trigger discomfort. The test has 77.5% sensitivity and 97.6% specificity.32 Its principle is simple: the lactose that is not hydrolyzed remains in the intestinal lumen until reaching the colon, where its anaerobic fermentation by the gut microbiota occurs. The fermentation products are short-chain fatty acids (methane, acetic acid, propionic acid, and butyric acid) and hydrogen. Both methane and hydrogen are absorbed and reach the lungs by way of the bloodstream, where they are eliminated through exhalation and can be titrated.29
LacTEST® is a noninvasive method that utilizes 4-galactosyl-xylose, whose structure resembles that of the lactose molecule, as a substrate, and evaluates lactase activity throughout the intestine. In other words, it is an in vivo assessment of total lactase activity that has no limitations or risk for the patient.33–37 Unlike the breath test, it has none of the limitations associated with intestinal transit, dysbiosis, previous antibiotic use, or chronic lung diseases. It has the advantage of easier application for both physician and patient, given that it requires no sequential measurements and can be determined in a single measurement. It is also economically accessible and uncomplicated because it requires only a colorimeter or a simple spectrophotometer. Values obtained from a sample of 205 individuals self-defined as lactose intolerant were 93.5% sensitivity, 91.8% specificity, and predictive values of 92.7% and 92.7%, with an area under the curve of 0.93.33 Its main disadvantage is that it does not measure symptoms and therefore is useless for evaluating intolerance.
The most accurate diagnostic method for corroborating LI is the challenge, i.e., the secondary presence of symptoms immediately after dairy product consumption. Nevertheless, the quantity consumed and the best method for identifying lactase deficiency, as well as the associated symptoms, are still subjects of debate, because the lack of lactase does not necessarily produce symptoms, nor is the presence of symptoms synonymous with lactose intolerance.
The importance of an accurate diagnosisThe elimination of dairy products from the diet does not appear to have detrimental effects on health and they have even been proposed as a nonessential food group for adult life. However, several analyses have associated their elimination with cancer and autoimmune diseases. A meta-analysis consisting of 29 cohort studies, with 938,465 participants, whose aim was to evaluate milk and dairy product consumption and the risk for mortality, coronary heart disease, and cardiovascular disease, concluded that there was no association between those variables. Nevertheless, there were inverse associations between fermented dairy product consumption (20 g/day) and mortality (RR = 0.98, 95% CI: 0.97-0.99, I2 = 94.4%) and the risk for cardiovascular disease (RR = 0.98, 95% CI: 0.97-0.99, I2 = 87.5%). A subsequent analysis of the same study indicated that for every intake of 10 g of cheese, there was a 2% lower risk for cardiovascular disease (RR = 0.98, 95% CI: 0.95-1.00, I2 = 82.6%).38 In another meta-analysis that included 22 studies, an inverse association was found between dairy product consumption and the total risk for cardiovascular disease (RR = 0.88, 95% CI: 0.81- 0.96) and infarction (RR = 0.87, 95% CI: 9.77-0.99). That study stated that the risk for infarction decreased with the consumption of low-fat dairy products (RR = 0.93, 95% CI: 0.88-0.99) and cheese (RR = 0.91, 95% CI: 0.84-0.98). Likewise, those authors found that the risk for coronary heart disease decreased with cheese consumption (RR = 0.84, 95% CI: 0.71-1.00). They concluded that dairy products provided a beneficial effect with respect to cardiovascular diseases, finding that both fat-free dairy products and cheese protected against the incidence of coronary heart disease and infarction.39 A systematic review showed that habitual dairy product consumption was inversely associated with the incidence (RR = 0.85, 95% CI: 0.73-0.98) and prevalence (RR = 0.88, 95% CI: 0.82-0.95) of metabolic syndrome, with an increased portion of any of them daily.40 Finally, a prospective study on a cohort of 1,573 post-menopausal Korean women that evaluated milk, yogurt, and other dairy product consumption and the incidence of osteoporosis, found that women with high consumption of those dairy products had a lower risk for osteoporosis in radial bone (HR = 0.52, 95% CI: 0.33-0.80, p = 0.0027) compared with women that did not consume them. Similarly, high milk and yogurt consumption (> 5 times a week) had a protective effect on radial osteoporosis, but that relation was not consistent with measurements of the tibia.41
Little is known about the modifications in the gut microbiota resulting from dairy product consumption. The most recent studies indicate that there are modifications in its composition and alpha diversity, if dairy consumption is added or suppressed. Studies on healthy individuals that consumed fermented milk or yogurt, with or without added lactobacilli, had no association with pathogenic mechanisms, such as bloating due to exaggerated gas production, even though symptoms were observed in persons exposed to psychologic stress. The promotion of balance in the microbiota was also observed in patients whose diets included high meat consumption.42–45
Li et al. assessed the effect of the supplementation with milk on the gut microbiota and cardiovascular risk markers in individuals, with and without deficient lactose digestion. They found no differences in short-chain fatty acid production or the cardiovascular risk markers between groups, but did find modifications in the microbiota of the individuals with deficient digestion (p < 0.01).46 An elegant randomized clinical trial by Odamari et al.47 showed that supplementation of a fermented dairy product with a probiotic (yogurt with Bifidobacterium longum) in a red meat-based diet resulted in the maintenance of a normal microbiota composition in healthy individuals.
Poor concordance between symptoms suggestive of LI and deficient digestion, as well as the fact that many uncomfortable symptoms are shared with other gastrointestinal diseases (bacterial overgrowth, celiac disease, gluten-sensitive enteropathy, irritable bowel syndrome), the prebiotic effect of lactose, possible implications for the microbiota, the role of dairy products on bone health, gastrointestinal health and health in general are a good reason for promoting and confirming the diagnosis of deficient lactose digestion.
But beyond the biologic effects of LI, accurate diagnosis enables patient empowerment, providing him or her with the information to make dietary decisions that affect quality of life. It also enables dietary modification, taking into account patient likes and preferences, but especially preventing the unnecessary elimination of foods. Foods whose lactose content is modified are available in Mexico, which is highly advantageous for persons that tolerate fewer than 12 g of lactose per day. Supplements with lactase that are administered at a dose of 10,000 IU for each ration of dairy products are also available. Studies utilizing lactobacilli and other probiotics, whose aim is to improve the digestion of molecules, such as lactase, have recently been carried out. Even though they appear promising, results are still inconclusive as to strain, dose, safety, and efficacy.14
ConclusionsLI and deficient lactose digestion are frequent in Mexico. It is an over-diagnosed condition, with symptoms that are easily confused with other digestive tract problems, often leading to a poorly substantiated recommendation to stop milk and dairy product consumption. Currently there are diagnostic methods that are noninvasive, easy to apply, and inexpensive, as well as products that are lactose-free or dairy-free. Lactase-based enzyme supplements are also available that can make life easier for persons that enjoy milk and dairy product consumption but have occasionally seen a specialist for digestive problems.
Ethical considerationsThe present document did not require authorization from research and ethics committees because it is a literature review that collects evidence from observational and experimental studies, systematic reviews, meta-analyses, and consensuses to make up a narrative review. All valid national and international scientific norms were observed.
Financial disclosureNo financial support was received in relation to this review of the literature.
Conflict of interestSophia E Martínez-Vázquez, MS, has been a speaker for Ferrer, Carnot, Asofarma, and Takeda and a scientific consultant for Asofarma and Liomont.
Dr. José Ramón Noguiera-de Rojas has been a speaker for Asofarma and Astra Zeneca.
Dr. José María Remes-Troche is a member of the advisory board of Asofarma and Takeda. He has received research funding from Sanfer and Senosian and has been a speaker for Asofarma, Alfasigma, Takeda, Sanfer, Johnson & Johnson, and Endomédica.
Dr. Enrique Coss-Adame is a member of the advisory board of Asofarma, Takeda, Ferrer, and Abbott and a speaker for Laboratorios Takeda, Asofarma, Ferrer, Carnot, Grunenthal, Alfa-Sigma, and Endomédica. He has received research funding from Alfa-Sigma and Bustard S.A. de C.V.
Dr. Rodolfo Rivas-Ruíz has been a speaker for Abbott, Sanofi, and Roche.
Dr. Luis F. Uscanga-Domínguez is a member of the advisory board of Asofarma and a speaker for Asofarma and Astra Zeneca.
Please cite this article as: Martínez Vázquez SE, Nogueira de Rojas JR, Remes Troche JM, Coss Adame E, Rivas Ruíz R, Uscanga Domínguez LF. Importancia de la intolerancia a la lactosa en individuos con síntomas gastrointestinales. Revista de Gastroenterología de México. 2020;85:321–331.