Important advances have been made since the last Mexican consensus on the diagnosis and treatment of Helicobacter pylori (H. pylori) infection was published in 2007. Therefore, the Asociación Mexicana de Gastroenterología summoned 20 experts to produce “The Fourth Mexican Consensus on Helicobacter pylori”. From February to June 2017, 4 working groups were organized, a literature review was performed, and 3 voting rounds were carried out, resulting in the formulation of 32 statements for discussion and consensus. From the ensuing recommendations, it was striking that Mexico is a country with an intermediate-to-low risk for gastric cancer, despite having a high prevalence of H. pylori infection. It was also corroborated that peptic ulcer disease, premalignant lesions, and histories of gastric cancer and mucosa-associated lymphoid tissue lymphoma should be considered clear indications for eradication. The relation of H. pylori to dyspeptic symptoms continues to be controversial. Eradication triple therapy with amoxicillin, clarithromycin, and a proton pump inhibitor (PPI) should no longer be considered first-line treatment, with the following 2 options proposed to take its place: quadruple therapy with bismuth (PPI, bismuth subcitrate, tetracycline, and metronidazole) and quadruple therapy without bismuth (PPI, amoxicillin, clarithromycin, and metronidazole). The need for antimicrobial sensitivity testing when 2 eradication treatments have failed was also established. Finally, the promotion of educational campaigns on the diagnosis and treatment of H. pylori for both primary care physicians and the general population were proposed.
Desde el último consenso mexicano para el diagnóstico y tratamiento de la infección por Helicobacter pylori (H. pylori) en el 2007, han existido avances importantes al respecto. Por tal motivo, la Asociación Mexicana de Gastroenterología convocó a 20 expertos para la realización del «IV consenso mexicano sobre H. pylori». Durante febrero y junio del 2017 se organizaron 4 mesas de trabajo, una revisión de la literatura y 3 rondas de votaciones donde se establecieron 32 enunciados para discusión y consenso. Dentro de las recomendaciones se destaca el reconocer a México como un país con riesgo de cáncer gástrico bajo a intermedio a pesar de la alta prevalencia de infección por H. pylori. Se corrobora que enfermedad ulcerosa péptica, presencia de lesiones premalignas, antecedentes de cáncer gástrico y linfoma asociado a la mucosa deben considerarse indicaciones claras para erradicación. La relación del H. pylori con los síntomas dispépticos sigue siendo controversial. La triple terapia de erradicación con amoxicilina, claritromicina y un inhibidor de la bomba de protones ya no debe ser considerada la primera línea de tratamiento. En su lugar, se proponen 2 opciones: la terapia cuádruple con bismuto (inhibidor de la bomba de protones, subcitrato de bismuto, tetraciclina y metronidazol) y la terapia cuádruple sin bismuto (inhibidor de la bomba de protones, amoxicilina, claritromicina y metronidazol). Se establece la necesidad de la realización de sensibilidad antimicrobiana ante la falla a 2 tratamientos de erradicación. Finalmente, se proponen campañas de educación respecto al diagnóstico y tratamiento del H. pylori para médicos de primer contacto y población general.
In 2007, the Asociación Mexicana de Gastroenterología (AMG) produced “The Third Mexican Consensus on Helicobacter pylori”.1 Since then, national and international articles have provided relevant information with respect to Helicobacter pylori (H. pylori) in terms of epidemiology, pathophysiology, diagnosis, resistance to antibiotics, treatment, and public health measures.
Therefore, in February of 2017, the AMG summoned a group of experts to formulate “The Fourth Mexican Consensus on Helicobacter pylori” and they established useful recommendations for the medical community.
MethodsThe consensus was developed utilizing the Delphi process as previously described.2 Five general coordinators were designated (FJBP, JMRT, MSGH, GPP, and JTL) and 15 experts on the subject matter were invited to participate. The coordinators performed a thorough review using the following databases: CENTRAL (The Cochrane Central Register of Controlled Trials), MEDLINE (PubMed), EMBASE (Ovid), LILACS, CINAHL, BioMed Central and the World Health Organization International Clinical Trials Registry Platform (ICTRP). The search covered the time frame of January 1, 2010 to February 28, 2017 and the search criteria included: “H. pylori” combined with the following terms: “epidemiology”, “incidence”, “prevalence”, “Mexico”, “pathophysiology”, “inflammation”, “microbiota”, “diagnosis”, “differential diagnosis”, “treatment”, “antibiotics”, “therapy”, “management”, “review”, “guidelines”, and “meta-analysis” and their Spanish equivalents. All consensus members had access to the complete bibliography.
The coordinators then formulated 45 statements that were put to a first anonymous electronic vote (March 28 to April 9, 2017) to evaluate the content and wording of the statements. The consensus members issued their votes with the following responses: a) in complete agreement, b) in partial agreement, c) uncertain, d) in partial disagreement, and e) in complete disagreement.
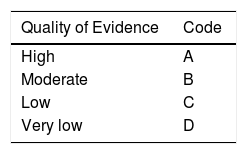
After the first voting round, the coordinators made the corresponding modifications. The statements in which the “complete agreement” total was > 75% were kept and those in which the “complete disagreement” total was > 75% were eliminated. The statements in which the “complete agreement” total was ≤ 75% and the “complete disagreement” total was ≤ 75% were reviewed and restructured. Strength of recommendation and quality of evidence were established for each of the statements using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system.3 Quality of evidence in the GRADE system is not only based on study design or methodology, but also on a clearly formulated question related to a clearly stated outcome measure,4 and thus can be high, moderate, low, or very low. The GRADE system also establishes the strength of recommendation as strong or weak, and in favor of or against an intervention or statement. As shown in Table 1, the GRADE system employs a code written in an upper-case letter to express the quality of evidence, followed by a number to indicate the strength of recommendation in favor of or against an intervention or statement.
Classification of the quality of evidence and strength of recommendation (GRADE).
| Quality of Evidence | Code |
|---|---|
| High | A |
| Moderate | B |
| Low | C |
| Very low | D |
| Strength of Recommendation | Code |
|---|---|
| Strong in favor of the intervention | 1 |
| Weak in favor of the intervention | 2 |
| Weak against the intervention | 2 |
| Strong against the intervention | 1 |
The statements revised and graded using the GRADE system were then put to a second anonymous electronic vote (April 25 to May 7, 2017) and the results were presented at the face-to-face meeting on June 1, 2017, in Guadalajara, Jalisco. At that meeting, the statements in which agreement was > 75% were ratified. Those statements in which agreement did not reach 75% in the previous voting rounds were discussed in an attempt to reach a consensus, and if that was not possible, they were eliminated. A third voting round was then carried out.
Once all the consensus statements were established, the coordinators formulated the present manuscript, which was then reviewed and approved by all the members of the consensus.
ResultsThe coordinators initially proposed 45 statements. In the first voting round, 5 statements (11%) were eliminated because they did not reach consensus. The second voting round included 40 statements. In accordance with the results of the second vote, 35 statements (87%) were presented at the face-to-face meeting for ratification and 5 (13%) were to be re-voted upon. At the end of the face-to-face meeting after the revision, fusion, and elimination of several statements, a total of 32 were decided upon to make up the consensus.
The final statements and voting results are presented below.
Associations/Indications1.H. pyloricolonization in humans induces an inflammatory process in the gastric mucosa.
H. pylori is a flagellated Gram-negative bacillus that is a potential pathogen for humans and is capable of producing different degrees of inflammation in all colonized subjects. [5] The gastric inflammatory process (gastritis) is variable and independent of the presence of symptoms.6H. pylori is currently considered the main cause of chronic gastritis.7,8 That inflammatory process is associated with the development of ulcers, atrophy, intestinal metaplasia, dysplasia, gastric adenocarcinoma (GAC), and gastric mucosa-associated lymphoid tissue (MALT) lymphoma.7,8
The degree of inflammation and the predominant site of colonization by H. pylori are determining factors for establishing the risk for gastroduodenal complications.9 The predominant inflammation in the gastric antrum without atrophy is associated with a state of acid hypersecretion and its complications, such as the development of gastroduodenal ulcers.10 On the other hand, atrophic gastritis in the corpus and antrum are associated with hyposecretion and achlorhydria and condition greater risk for the development of premalignant lesions and GAC.11
It should be mentioned that in an important number of patients, H. pylori infection can remain asymptomatic and not progress to the complications described above, and at present that behavior is unpredictable. It is probable that factors such as type of diet, genetic susceptibility, alcohol and tobacco consumption, among others, act synergically with the bacterium to promote the development of complications.7,8
2.H. pyloriinfection has a causal association with peptic ulcer disease (PUD).
H. pylori infection is the main cause of PUD.7,8,12 Up to 95% of duodenal ulcers and 80% of gastric ulcers are associated with that infection. However, the large majority of patients with chronic H. pylori infection do not develop PUD, suggesting that there is a varying susceptibility in each individual. Urease is the enzyme responsible for the survival of the bacterium, whereas adhesins (BabA and OipA) facilitate adhesion to the epithelium.5 Fifty percent of the H. pylori strains produce the vacuole-forming cytotoxin that is encoded by the vacA gene. Allelic variations associated with a greater inflammatory response have been described in that gene. The strains also have the gene associated with the cagA cytotoxin, which encodes for a high molecular weight antigenic protein (CagA) present in up to 60% of the isolations.13 Between 80% and 100% of patients with duodenal ulcer produce anti-CagA antibodies, whereas they are detected in only 63% of persons with gastritis. In addition, genetic polymorphisms of different cytokines, such as interleukin 1 beta have been associated with the development of PUD.14
3.H. pyloriinfection is one of the main risk factors for developing gastric adenocarcinoma (GAC) and low-grade gastric mucosa-associated lymphoid tissue (MALT) lymphoma.
In 1994, the World Health Organization recognized H. pylori as a type 1 carcinogen based on a solid epidemiologic association.15,16 It is currently considered the main risk factor for GAC and it is estimated that up to 90% of distal GAC cases are attributable to that infection.17 Although GAC is multifactorial, it is clear that the chronic inflammation associated with H. pylori and subsequent progression to premalignant lesions (atrophy, intestinal metaplasia, and dysplasia) is fundamental.18 Inflammation produced by H. pylori, in addition to producing hypochlorhydria, induces genetic instability, proto-oncogene activation, and alterations in the gastric microbiome.19
In a recent meta-analysis conducted by Lee et al.,20 they combined the results of 24 studies on eradication therapy and showed that asymptomatic subjects that received treatment for H. pylori had a lower incidence of GAC than subjects that did not receive the therapy (cumulative incidence of 0.53, 95% confidence interval (95% CI) of 0.44-0.64, p = 0.037). Eradication therapy has also been reported to reduce mortality (hazard ratio of 0.26, 95% CI of 0.09-0.79) and the incidence of intestinal metaplasia or dysplasia (odds ratio of 0.56, 95% CI of 0.34 to 0.91).21 Likewise, eradication has been shown to prevent the appearance of metachronous GAC (incidence rate-ratio of 0.54, 95% CI of 0.46-0.65).21,22
With respect to MALT, also known as gastric marginal zone B-cell lymphoma, 92% of the cases are reported to be infected with H. pylori.23 That neoplasia is the result of an aberrant adaptive immune response when confronted with chronic “immunoinflammatory” stimuli that favor lymphoid hyperplasia and then produce apoptotic alterations, proliferation, and cellular resistance that condition the development of malignant cellular clones.24 From those observations, in 1993 Wotherspoon et al.25 reported that H. pylori eradication was associated with regression of that type of lymphoma (see statement 8).
4. Mexico has an intermediate-to-low risk profile forH. pylori-associated GAC.
GAC represents a public healthcare problem worldwide, despite the decrease in its incidence and mortality in the last decades.26,27 There are currently areas where incidence and mortality of GAC continue to be high, such as Eastern Asia, mainly China, Central Africa, Eastern Europe, and Latin American countries, such as Chile, Costa Rica, and Colombia.28
In 2012, cancer held third place as cause of death after heart disease and diabetes mellitus in Mexico.29 According to a recent study by Sánchez-Barriga et al. on trends in mortality from GAC in Mexico, 69,107 individuals died from gastric cancer within the time frame of 2000 to 2012.30 The mortality rate per 100,000 inhabitants adjusted to the world population decreased from 7.5 to 5.6. The man:woman ratio was 1.15:1.0 and Southeast Mexico, specifically Chiapas, had the highest mortality rate due to gastric cancer (9.2, 95% CI: 8.2-10.3 in the year 2000 and 8.2, 95% CI: 7.3-9 in 2012).
Therefore, Mexico is considered to have an intermediate-to-low risk for GAC associated with H. pylori.28 Nevertheless, in Mexico, the relation between H. pylori infection and the development of GAC is complex and it is difficult to calculate the true risk level, given that there is no national incidence register and studies describing regional differences are lacking. In addition, there is variability in the strains reported in the different studies, health conditions and climate conditions are not the same, and resistance to antibiotics varies.31
5. The association between functional dyspepsia (FD) andH. pyloriinfection is controversial.
In general, the term “dyspepsia” is utilized to refer to symptoms that originate in the upper abdominal region and they include epigastric pain and/or burning sensation, early satiety, and postprandial fullness.32 The term “uninvestigated dyspepsia” is used in patients with symptoms in whom a diagnostic approach has not been carried out, especially through endoscopy. In the absence of identifiable structural alterations (PUD, neoplasias, chronic use of nonsteroidal anti-inflammatory drugs), symptoms are considered functional and can be the consequence of emptying alterations, impaired gastric accommodation, hypersensitivity, gastric dysrhythmias, and immunocellular alterations mediated by an infectious agent such as H. pylori.33
The relation between FD and H. pylori is considered controversial because it is known that the prevalence of FD in population studies can reach 30%, whereas the prevalence of H. pylori infection can reach 90% in some regions.34 According to 2 meta-analyses, the therapeutic gain of H. pylori eradication in patients with FD is only 10% (a figure similar to that of other therapies, such as proton pump inhibitor [PPI] use) and the number needed to treat is 14 (95% CI: 10-20).35,36 It was even concluded that PPI use is more cost-effective in the treatment of dyspepsia than the search for H. pylori.35,36
There are other positions with respect to the relation between FD and H. pylori infection that are worth considering. The Kyoto global consensus report7 stated that a subgroup of patients with dyspepsia can have symptoms associated with the infection, and that even though the current number needed to treat is 14, there are currently no criteria that can predict which patient will respond to eradication therapy. Therefore, the recommendation is to give patients eradication therapy and wait 6 months to see if there was significant symptomatic gain, which is the required time for histologic recovery. In fact, that consensus proposes that the term “H. pylori-associated dyspepsia” be used for those patients.
The Rome IV group of experts32 emitted recommendations similar to those of the Kyoto consensus, regarding H. pylori infection as a “possible” cause of FD and that in a subgroup of patients, its eradication produces symptom resolution that is maintained for a period of 6 to 12 months. Patients in whom dyspeptic symptoms do not improve with eradication therapy are considered the patients with true FD by the Rome IV group.
The Maastricht V Consensus is the most categorical and considers gastritis associated with H. pylori an infectious disease that causes active chronic gastritis and that eradication is the only option for curing the histologic lesion.8 In that context, that group of experts believes that the true diagnosis of FD should be made in the absence of H. pylori infection.
6. H. pylori determination is recommended in individuals that have first-degree relatives with a history of GAC, and if positive, treatment should be offered.
There is evidence that patients with first-degree relatives that have GAC have a greater probability of presenting with chronic gastritis and premalignant lesions associated with H. pylori.37,38 In relative terms, those subjects have 7 times more risk of presenting with atrophic gastritis. Thus, genetic susceptibility influences the expression of the type of gastritis and subsequently the risk for premalignant and malignant lesions associated with H. pylori. Patients with H. pylori infection that have relatives with GAC are considered a high-risk group and should be offered eradication treatment.
7. The “search and treat” strategy in relation toH. pyloriis recommended as a first option, before empiric antisecretory therapy or endoscopy, in patients with uninvestigated dyspepsia under 55 years of age and with no alarm symptoms.
In other parts of the world, statement 7 is a recommendation with a GRADE level of evidence and strength of recommendation A2, but in Mexico there are no studies that clinically evaluate the “search and treat” strategy or that establish whether the cutoff point of 55 years of age is a low-risk or high-risk organicity threshold for the country.
In fact, that recommendation resulted in discrepancy among the participants of the consensus, given that the evidence and cost-benefit studies have reported contradictory and extremely varied results. Numerous management guides in countries with a low prevalence of H. pylori suggest the “search and treat” strategy as the strategy of choice in young patients with no alarm symptoms.7,8,39 A predominant factor in favor of that strategy is the apparent association described between H. pylori and GAC, in which case, eradication could significantly reduce the risk for GAC.7,8,39
On the other hand, it is estimated that only 5% of dyspepsia cases are due to H. pylori.40 In Mexico, where the prevalence of H. pylori is above 70%, the strategy is controversial, and its cost-effectiveness is unknown.39,41
Finally, one of the positions of the Maastricht V consensus8 on massive eradication utilizing the “search and treat” strategy is that it can select and create antimicrobial resistance.
8.H. pylorieradication is first-line treatment for localized low-grade MALT.
Eradication has been considered first-line treatment for that type of neoplasia, especially in cases of early-stage disease, in which the cure rate can reach 80%.42,43 In cases that have achieved cure, the patients should remain in follow-up. In patients that have no response or present with disease recurrence, other therapies, such as radiotherapy or chemotherapy, should be considered.44 It is important to emphasize that in patients with MALT that are carriers of translocation t (11,18), H. pylori eradication therapy is not effective.42 Even though the evidence supports that recommendation, it is necessary to know that there are no controlled clinical trials or meta-analyses on the subject, which is the reason the quality of evidence for that statement is B in the present consensus.
9. Long-term treatment with PPIs alters the topography ofH. pylori-associated gastritis.
It is known that in situations of reduced acid secretion, such as in chronic PPI use, H. pylori can affect the entire topography of the stomach, thus producing pangastritis.45,46 That can also occur in other situations that predispose to hyposecretion, such as in the case of vagotomy. On the other hand, in situations of normal or high acid secretion, H. pylori predominantly affects the gastric antrum.47
It is important to know the topography of H. pylori infections, to have a more appropriate sampling, as well as to predict the risk for the development of premalignant lesions. For example, severe atrophic gastritis in the corpus (with or without intestinal metaplasia) confers a greater risk for developing intestinal GAC.48
10. The role ofH. pylorias a causal agent of diseases or specific symptoms has not been confirmed in the pediatric population.
In the majority of cases, H. pylori is acquired before 12 years of age through an intrafamilial transmission route.49,50 Other associated factors are: over-crowded living conditions, low socioeconomic level, and poor hygiene.51,52 The diagnosis in children is difficult to make, given that symptoms such as abdominal pain, nausea, vomiting, and occasionally diarrhea, are not very specific.
Even though some studies have established a relation between recurrent chronic abdominal pain and H. pylori, a recent meta-analysis found no such association.53 Similar to what occurs in adults, children whose digestive symptoms improve after eradication are those that have signs of PUD.54 In general, routine treatment for children with H. pylori infection and abdominal pain is not recommended.55
Some studies have shown that children with refractory iron-deficiency anemia improve after eradication treatment.56 However, the most solid evidence in that respect is in the adult population (see statement 11). Evidence is very controversial regarding the relation between H. pylori infection and short stature, difficulty to gain weight, type 1 diabetes mellitus, and celiac disease.55
11. There is evidence of an association betweenH. pyloriinfection and unexplained iron deficiency anemia and idiopathic thrombocytopenic purpura.H. pylorisearch and treat strategy is recommended in those cases.
The mechanism by which H. pylori induces the alterations in the iron reserves is not completely understood, but it appears to involve several routes, including gastrointestinal blood loss, reduced dietary iron absorption, and increased iron uptake by the bacterium.57 Antibodies have been identified that are directed against epitopes of gastric mucosal cells in atrophic gastritis that involve an autoimmune mechanism triggered by H. pylori and directed against the parietal cells through a phenomenon of molecular mimicry.57
In a meta-analysis published in 2008, Muhsen and Cohen58 reported that iron-deficiency anemia was 2.6 times more frequent in patients with H. pylori than in subjects with no infection. A group of authors59 recently confirmed in a meta-analysis of 14 studies that patients with H. pylori had a 1.72 risk of presenting with iron-deficiency anemia. Moreover, eradication produced an increase in serum ferritin levels.
With respect to idiopathic thrombocytopenic purpura, there is evidence in adults that eradication treatment produces an increase in platelets. In a 2009 systematic review by Stasi et al.,60 they showed that up to 90% of patients had a favorable response (43% with complete resolution and 50% with an increase of at least double the number of platelets). Based on that, the American Society of Hematology61 considers that H. pylori infection should be searched for in adult patients with ITP (quality of evidence and grade of recommendation C2) and eradication therapy offered to the patients that result positive (quality of evidence and grade of recommendation B1). Even though the pathophysiologic mechanism is unknown, it has been proposed that some H. pylori peptides can mimic platelet antigens, triggering an autoimmune response.62
Diagnosis12. The most sensitive and specific noninvasive test for diagnosingH. pyloriinfection and confirming treatment response is the labeled urea breath test. Another alternative is the monoclonal antibody-based stool antigen test.
The labeled urea breath test is a simple, relatively economic, and widely available test. It has 96% sensitivity and 93% specificity.63 The test utilizes a 13C or14C isotope. The former is the most widely used, given that the latter is a radioactive isotope and therefore is not recommended in children or pregnant women. The labeled urea breath test is used for both diagnosing H. pylori and confirming its eradication.8 The determination of monoclonal antibodies directed against H. pylori antigens in stool through ELISA is an excellent diagnostic alternative that is comparable to the breath test in relation to sensitivity and specificity. In the meta-analysis and systematic review by Gisbert et al.,64 sensitivity and specificity for the diagnosis of infection prior to treatment was 94 and 97%, respectively. In Mexico, the cost is slightly lower than that of the labeled urea breath test, but it appears that adherence to the test in stool is lower. The availability of the monoclonal antibody-based stool antigen test is also lower than that of the labeled urea breath test.
13.- The detection of serum anti-H. pyloriantibodies is useful for epidemiologic studies.
Serum antibody detection tests are widely available and low-cost, with an approximate sensitivity of 95% and a specificity of 90%.65 Those values can vary greatly (57 to 100%), depending on the commercial test utilized.66 The tests should be locally validated.67 In addition, the anti-H. pylori antibodies do not distinguish active infection from exposure.
Antibody detection is extremely useful in epidemiologic studies68 and can also be useful in special situations in which the results of the breath test and/or stool antigen can be affected, as can occur in cases of gastrointestinal bleeding, atrophic gastritis, and MALT.66
14.-When performing tests forH. pyloridiagnosis, the use of antibiotics and/or bismuth should be suspended four weeks prior to the test. PPIs should be suspended at least two weeks before testing.
Antibiotics and compounds with bismuth have antibacterial activity and must be suspended 4 weeks before the breath test or stool antigen test, so that if there is a bacterial load, it can be detected by the tests. During its use, bismuth has a reported frequency of false negatives of up to 55% in the breath test and 15% in the stool antigen test.69
PPI use reduces the sensitivity of the breath test and the stool antigen test because the increased intragastric pH consequently reduces the bacterial load, mainly in the gastric antrum, leading to false negatives of up to 30% in the two types of tests.70 Parente et al. showed that a 7-day removal of those drugs was sufficient for normalizing the test results, but 14 days was considered the safe interval.71
It is important to comment that the use of antacids does not modify the results of those tests, but the prolonged use of H2 receptor antagonists has a minimal effect on them, possibly related to the development of tolerance, which is characteristic of those drugs.72
15. The decision to perform endoscopy should be based on the clinical context of the patient, and ifH. pyloriis suspected, gastric biopsies should be taken.
The decision to perform endoscopy depends on the comprehensive evaluation of each patient.73 Indications include: patients above 50 years of age with symptoms of dyspepsia with alarm symptoms (anemia, weight loss, dysphagia, gastrointestinal bleeding) or patients that do not respond to empiric treatment.74 The search for H. pylori alone is not a valid indication for endoscopy.64 When endoscopy is carried out for a different indication and H. pylori infection is suspected, samples of gastric tissue should be taken for a rapid urease test (RUT) and histopathologic analysis according to the updated Sydney protocol (see further ahead).75
16. When performing endoscopy, the RUT is the first-line diagnostic test for investigatingH. pyloriinfection.
The RUT is a direct test for active H. pylori infection. It depends on the presence of the enzyme, urease, which is produced by the bacterium. Urease catalyzes the hydrolysis of urea into ammonia and CO2. RUT has a sensitivity of 90-100% and a specificity of 97-99%.64,76 A sample from the gastric antrum and one from the corpus are sufficient for maximizing the diagnostic yield. The rapidness of the result depends on the bacterial density and the test may require 24h. The main causes of false negatives are PPI and antibiotic use, atrophic gastritis, intestinal metaplasia, and recent gastrointestinal bleeding. Less frequently, false positives can be due to the presence of other urease-producing bacteria, such as Proteus mirabilis, Citrobacter freundii, Klebsiella pneumoniae, Enterobacter cloacae, and Staphylococcus aureus.7,39 The RUT has the advantage of fast results for immediate treatment commencement. It should be noted that the RUT identifies the presence of H. pylori, but it does not evaluate the grade of gastritis or the presence of premalignant lesions.64,75
17. To adequately evaluate gastritis, atrophy and/or intestinal metaplasia due toH. pylori, samples should be obtained through endoscopy, in accordance with the updated Sydney protocol.
H. pylori infection is a continuous process that progresses to chronic inflammation and can evolve to atrophic gastritis, intestinal metaplasia, and finally cancer, according to the sequence proposed by Correa et al.77 Therefore the histologic characterization of gastritis, as well as the presence of premalignant lesions, have prognostic value in relation to both gastroduodenal complications and the development of GAC.78
Following the updated Sydney protocol is recommended for efficient sampling, which includes the taking of 5 biopsy samples: 2 from the greater curvature and 2 from the lesser curvature at the level of the gastric corpus and antrum at 3cm from the pylorus and 1 sample from the incisura to maximize the identification of premalignant lesions.75 Which patients need endoscopic surveillance of the lesions is determined by their severity and extension. That is based on a staging proposal for stratifying GAC risk that considers the gravity and topographic extensions of atrophic gastritis and intestinal metaplasia, called the Operative Link for Gastritis Assessment (OLGA) and Operative Link on Gastric Intestinal Metaplasia (OLGIM), respectively.79–81 The OLGA and OLGIM systems are endorsed by the European Society of Gastrointestinal Endoscopy (ESGE) and the European Helicobacter Study Group (EHSG).82 Atrophy is classified on a scale of 4 levels (0-3) in accordance with the Sydney system scale.79 The OLGIM scale tends to replace the OLGA system for detecting patients with gastric alterations that have premalignant potential and basically replace the atrophy score with only an evaluation of intestinal metaplasia.79–81 Gastritis staging organizes the histologic phenotypes of gastritis along a progressively increasing scale of risk for gastric cancer from the lowest (stage 0) to the highest (stage IV).79–81
18. The eradication ofH. pyloriinfection should be confirmed through a breath test or a monoclonal antibody-based stool antigen test at least 4 weeks after the completion of the antimicrobial regimen.
It is of the utmost importance to confirm infection eradication after the antimicrobial regimen because infection persistence can have important clinical consequences, such as the recurrence of duodenal ulcer, MALT lymphoma, and/or the risk for developing premalignant lesions or GAC. Noninvasive tests (breath test or stool antigen test) are recommended at least 4 weeks after having completed the antibiotic regimen and at least 2 weeks after having suspended PPI use, to confirm H. pylori eradication.83,84 Endoscopy is not indicated to confirm eradication.85
19.H. pylorieradication and the absence of recurrence after treatment should be corroborated through a breath test or monoclonal antibody-based stool antigen test.
Even though eradication with a first antibiotic regimen is confirmed, the risk for recurrence should always be contemplated. Recurrence is greater in regions with a high prevalence of H. pylori infection, such as Mexico, where recurrence the first year varies from 11.7 to 18.8%.86,87 That can be attributed to one of the following causes: a) recrudescence of the infection (temporary disappearance of evidence of the infection with later reappearance of the same strain) or b) reinfection (new infection caused by another strain of H. pylori). The differentiation between the two requires molecular analysis of the strain.86,87 Corroborating that the patient has no recurrence after the first treatment, carried out through noninvasive tests, is recommended. The time at which that is done varies (6 to 12 months) and there is limited evidence with respect to that in Mexico.86,87
20.- In Mexico, antimicrobial susceptibility evaluations should be carried out to validate the efficacy of the treatment regimens recommended in the international guidelines.
There is worldwide evidence of growing antimicrobial resistance by H. pylori to the currently recommended eradication regimens. In fact, in February of 2017 the WHO issued a list of 16 bacteria with important antimicrobial resistance that threaten the health of humanity, and one of them is H. pylori.88 That resistance varies in different geographic regions, even within the same country.88 In Mexico, in a systematic review by Camargo et al.,85 they reported resistance to clarithromycin of 13% (IC 7-20), to metronidazole of 60% (IC 47-72), to amoxicillin of 4% (IC 0-13), to tetracyclines of 2% (IC 0-9), and to dual metronidazole/clarithromycin of 13% (IC 6-21).89 Undoubtedly those figures vary geographically, regionally, and temporally, and are related to local uses of antibiotic therapy, including that for other indications.90,91 Regional studies are required in Mexico that evaluate sensitivity to antibiotics utilizing correct methodologies and including the antimicrobials used in the eradication regimens, mainly macrolides, metronidazole, and quinolones.
Treatment21. In regions with rates of resistance to clarithromycin equal to or greater than 15%, triple therapy with PPI, amoxicillin, and clarithromycin should not be used.
H. pylori infection is commonly treated with the combination of 2 or 3 antibiotics and a PPI, taken concomitantly or sequentially, for 7 to 14 days. In clinical practice, the course of initial eradication therapy is called “first-line therapy”. The 2007 AMG consensus guidelines recommended treatment with a PPI, clarithromycin, and amoxicillin for 14 days, or substituting amoxicillin with metronidazole in patients allergic to penicillin. At that time, the reported eradication rate varied from 70 to 85%.1 We should emphasize that no treatment regimen guarantees H. pylori infection cure in 100% of the patients. In fact, today there are few regimens that reach eradication rates above 90%.92
In addition, there is growing concern with respect to triple therapy with clarithromycin. In Mexico City, susceptibility to 3 antibiotics in 195 strains of H. pylori isolated from the same number of patients was studied. Eighty percent of the strains were resistant to metronidazole, 24% were resistant to clarithromycin, and 18% showed transitory resistance to amoxicillin. In addition, resistance to 2 or more antibiotics increased significantly from 1995 to 1997 and was more notable for clarithromycin, which increased from 10% in 1995 to 27% in 1997.93 In a 2010 meta-analysis, eradication rates of only 22% were reported in cases of H. pylori strains that were resistant to clarithromycin, contrasting noticeably with the success rate of 90% in cases with strains that were sensitive to clarithromycin.94
Therefore, if resistance to clarithromycin is currently > 15%, first-line treatment with amoxicillin and clarithromycin should no longer be used. Even though in some areas the eradication rate with the triple therapy could be above 80%, considering the information stated above, the recommendation to use that regimen is up to the judgement of the treating physician.
22. In regions with rates of resistance to clarithromycin greater than 15%, beginning treatment with quadruple regimens with or without bismuth is recommended.
Today, the eradication rate with triple therapy with clarithromycin is below 80%.91,95 Based on the experience in other parts of the world, using quadruple therapies, with or without bismuth, as described in the following section, is recommended.96–98
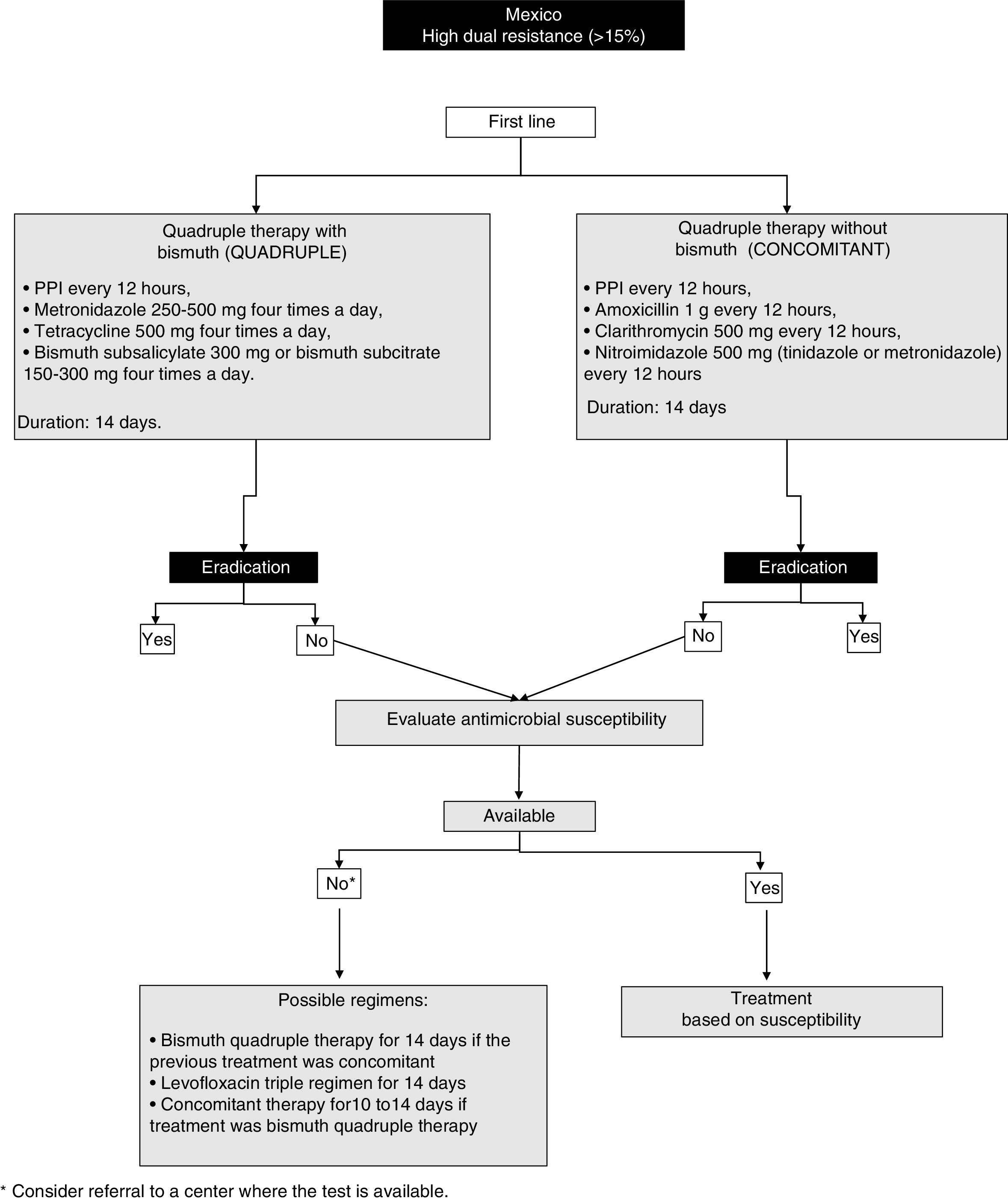
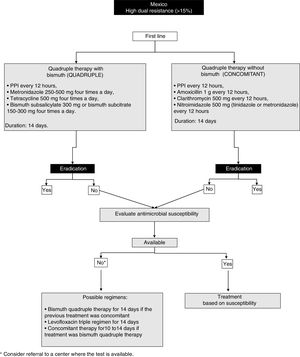
23. In regions with elevated dual resistance rates (clarithromycin and metronidazole), such as Mexico, quadruple therapy for 14 days is recommended as first-line treatment, considering the following 2 regimens as possible options:
- a)
Quadruple therapy with bismuth: PPI, bismuth subcitrate, tetracycline, and metronidazole
- b)
Quadruple therapy without bismuth (concomitant therapy): PPI, amoxicillin, clarithromycin, and metronidazole
The efficacy of quadruple therapy with bismuth is not affected by resistance to clarithromycin. Resistance to metronidazole has a minor impact on the efficacy of quadruple therapy with bismuth, and in many cases the high resistance to metronidazole has been reported “in vitro”.96 In addition, resistance can be partially overcome by increasing the dose, duration, and frequency of administration.97,98 Thus, in regions where resistance to clarithromycin is high, such as in Mexico, or in patients that have been treated with macrolides for any reason, quadruple therapy with bismuth for 14 days should be strongly considered first-line therapy.99
The quadruple therapy recommended herein consists of a PPI every 12h with metronidazole 500mg four times a day, tetracycline 500mg four times a day, and bismuth subsalicylate 300mg or bismuth subcitrate 150-300mg four times a day for 14 days (fig. 1). In the third AMG consensus guidelines, quadruple therapy with PPI, bismuth, metronidazole, and tetracycline was also recommended.1 A recent meta-analysis that included 12 clinical controlled trials (CCTs) and 2,753 patients, reported eradication rates in the intention-to-treat analysis of 77% with the quadruple therapy with bismuth vs. 69% with the triple therapy with clarithromycin (difference of risk = 0.06, 95% CI: -0.01 to 0.13).92 A recent more robust and complex analysis model, called “network meta-analysis” that provides a global estimate of the comparative treatment effectiveness of a condition, combining direct and indirect evidence, included different treatment regimens for H. pylori, and found that 10-14 days of quadruple therapy with bismuth was superior to a 7-day regimen of triple therapy with clarithromycin (85 vs. 73%, RR = 1.17; 95% CI 95%:1.12-1.21).92
Another option is the so-called concomitant therapy that consists of a PPI every 12h, amoxicillin 1g every 12h, clarithromycin 500mg every 12h, and nitroimidazole 500mg (tinidazole or metronidazole) every 12h for 14 days. That recommendation is supported by data from a meta-analysis that included 19 clinical trials on concomitant therapy with 2,070 patients with H. pylori infection and showed a cure rate of 88% (95% CI: 85-91%).100 In addition, in the CCTs that compared concomitant therapy (481 patients) with triple therapy with clarithromycin (503 patients), the cure rate in the intention-to-treat analysis was 90 and 78%, respectively (OR of 2.36; 95% CI: 1.67-3.34).98 The majority of those studies were conducted in Europe or Asia and only one was conducted in Latin America. Finally, the consensus group recommends that, when employing concomitant therapy, the most appropriate duration is at least 10-14 days.
It is important to comment that some current limitations to those regimens are the scant availability of subcitrate of bismuth and tetracycline in Mexico, the adverse effects of metronidazole, the need for a higher dose of medications, and a greater probability of poor treatment adherence.
24. If first-line eradication treatment fails, the antimicrobial susceptibility of theH. pyloristrain should be studied, isolating it through endoscopy with sampling and tissue culture, to adjust treatment and improve the eradication rate.
Ideally, H. pylori eradication therapy should be based on local resistance patterns and each patient's history of exposure to antibiotics. Unfortunately, such “targeted” therapy is not possible and empiric regimens are employed, which facilitates resistance to antibiotics. In some regions of Mexico, there is information about patterns of resistance to antibiotics during treatment of H. pylori infection.93,101–105 Evidence shows that therapy guided by culture and sensitivity tests is better in the intention-to-treat analysis (RR, 0.84; 95% CI: from 0.77 to 0.90; p<0.00001), at a lower overall cost.106 However, tests to determine resistance to antimicrobials are not available in all the geographic regions of Mexico, and their accessibility requires the support of governmental healthcare institutions and policies.8,91
25. If it is not possible to evaluate the resistance to antimicrobials, a second-line regimen should be considered that includes high doses of PPI, amoxicillin, bismuth subcitrate, and a different antibiotic from the one utilized in the first regimen.
The selection of a salvage regimen in cases of persistent H. pylori infection after one or more eradication attempts is an increasingly frequent scenario. The most important determining factor for eradication treatment success is the sensitivity or resistance of H. pylori to the antibiotics used.107 If it is not possible to determine resistance, empiric decisions must be made. In that sense, clarithromycin and fluoroquinolones should not be reused empirically, because resistance is not resolved through an increase in the dose, treatment duration, or the frequency of administration.97,98,108 However, amoxicillin and tetracycline can be reused, given that resistance is rare even after their previous use.105 In general, the reuse of metronidazole should be avoided, but as mentioned above, resistance decreases with an increase in the dose.97,98 Thus metronidazole can be reused as a component of a bismuth-containing quadruple therapy course for 14 days, especially if previous use was brief or low-dose.
The available information about salvage regimens has been obtained from studies outside of Mexico, but the consensus group has based its recommendations on analyses conducted in North America from the year 2000. The use of clarithromycin or levofloxacin as a salvage regimen is considered an option if the patient received previous first-line treatment with a bismuth-containing quadruple therapy. Quadruple therapy with bismuth or a salvage regimen with levofloxacin are preferred options if the patient received a first-line treatment that contained clarithromycin. The following three regimens can be considered for use with salvage regimens:
- •
Bismuth-containing quadruple therapy for 14 days
- •
Levofloxacin-containing triple therapy for 14 days
- •
Concomitant therapy for 10 to 14 days
The efficacy of sequential therapy guided by genotype determination of bacterial resistance in third-line treatment has been reported. After second-line treatment failure, treatment should be guided by susceptibility to antibiotic testing, whenever possible.109
The consensus group recommends that in the case of treatment failure with a second regimen, the patient should be sent to a referral center to evaluate H. pylori antibiotic susceptibility.
26. In cases of penicillin allergy, the quadruple regimen with bismuth is recommended.
Bismuth-containing quadruple therapy is one of the regimens today that does not contain amoxicillin that can be used in patients that are truly allergic to the drug. If treatment with 1 or 2 eradication regimens has failed in a patient that is allergic to penicillin, verifying that the patient truly is allergic to the drug is recommended to determine if regimens with amoxicillin can be used. Numerous studies have shown that, even though 5-10% of the population is reported to be allergic to penicillin, ∼90% of those patients have negative skin tests and can tolerate penicillin with no hypersensitivity.110
Public health27. The prevalence ofH. pyloriinfection in Mexico is declining in the younger generations.
In Mexico, a decrease in the prevalence of H. pylori infection has been reported, especially in children and young adults.111–115 For example, in 1998, Torres et al.51 found that after 10 years of age, 50% of the children were infected with H. pylori and that figure rose to 70% when they reached the age of 20 years. Almost 10 years later, the same group of researchers found that prevalence descended close to 10% in young adults and to 15% in children between 11 and 14 years of age.116 Even though the descent could be attributed to multiple factors, it is assumed that there could have been improvement in the socioeconomic conditions of Mexico within that time frame. Nevertheless, it is important to recognize that those results can vary in the different geographic regions of the country.
28.H. pylorieradication reduces the inflammatory response, and when treatment is opportune, it reduces the risk for progression to premalignant lesions (atrophic gastritis and intestinal metaplasia).
H. pylori eradication has been shown to detain the progression of premalignant lesions in patients that receive treatment, especially in those with early and non-severe lesions.117 Thus, H. pylori eradication is a basic aspect of primary prevention strategy in the development of GAC, especially if it is eradicated when premalignant lesions are at an early stage.118,119
29.H. pylorieradication to prevent the development of GAC is cost-effective in geographic areas with a high risk for that disease.
Even though GAC incidence is decreasing in some populations, the absolute number of cases is increasing, due to both the growth and aging of the populations.120H. pylori eradication in populations with a high risk for GAC has been shown to be associated with a reduced GAC incidence rate. Economic analyses show that it is a cost-effective measure.121
30. The eradication ofH. pyloriin the general population is NOT recommended as a strategy for GAC prevention in Mexico.
In areas with a low incidence of H. pylori and in those in which important risk factors or the development of GAC have not been described, the generalized eradication of the bacteria is not recommended. As is expressed in statement 4, eradication in the general population is not recommended, given that Mexico is a country with an intermediate-to-low risk for the development of GAC.28 Indiscriminate eradication can lead to the development of resistance to drugs, increase community Clostridium difficile infection, and elevate the prevalence of diseases negatively associated with H. pylori colonization, such as GERD, Barrett's esophagus, asthma, and obesity.
31. The presence of premalignant lesions (atrophy/intestinal metaplasia) requires adequate histologic stratification and periodic endoscopic surveillance.
Based on the current knowledge about the biology of gastritis and incorporating the experience acquired worldwide through the application of the updated Sydney System, utilizing the OLGA and OLGIM staging systems is recommended, as expressed in statement 17. With respect to the periodicity of endoscopic surveillance, even though it is debatable and might not be applicable in all populations, the European Group for the Surveillance of Premalignant Lesions of the Stomach recommends that endoscopic surveillance be offered every 3 years to patients with atrophy or extensive intestinal metaplasia in the antrum and corpus with no displasia.82 On the other hand, in the case of low-grade dysplasia, endoscopic surveillance is recommended every 12 months, and in the case of high-grade disease, every 6 months.82 When there are visible or evident endoscopic lesions, resection for detailed histologic study is recommended.
In the Mexican population there are no studies on that subject and the cost-benefit of those surveillance regimens must be evaluated.
32. Educational campaigns should be promoted to provide information about the influence ofH. pylorion health and its relation to gastrointestinal diseases.
It is very important for the general population to be aware of the significance of H. pylori and the diseases it causes, to facilitate diagnosis and early treatment. The participation of the population is essential in recognizing alarm symptoms and the clinical manifestations that require the medical attention of a specialist.
Likewise, educational campaigns are recommended for healthcare professionals that provide primary care to patients in Mexico. For example, in a recent study by Cano-Contreras et al.,122 430 general practitioners were surveyed with respect to the diagnosis and treatment of H. pylori in Mexico, and the results showed there is a great lack of knowledge about the theme.
ConclusionsThe present consensus provides updated recommendations adapted to the Mexican population with respect to the epidemiology, diagnosis, and treatment of H. pylori infection. It identifies many areas in which evidence is scarce or null Mexico, highlighting the need for studies to be conducted that are areas of opportunity for research.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Financial disclosureThe present consensus received the support of the Asociación Mexicana de Gastroenterología during the face-to-face meeting in relation to participation, transportation, and lodging. No fees were received.
Conflict of interestDr. Francisco J. Bosques-Padilla has been a Speaker for Takeda, Abbvie, and MSD.
Dr. José María Remes-Troche is a Member of the Advisory Board of Takeda Pharmaceuticals, Alfa-Wassermann, and Almirall. He has received research funds from Sanfer. He is a Speaker for Takeda, Asofarma, Alfa-Wassermann, Carnot, Almirall, and Astra-Zeneca.
Dr. Maria Sarai Gonzalez-Huezo is or has been on the Advisory Board of Bayer, Bristol, Falk, Merck, and Roche. She is a Speaker for Bayer, Janssen, Abbvie, Roche, Falk, Asofarma, Ferring, and Gilead.
Dr. Juan Miguel Abdo-Francis is or has been a Speaker for Takeda, Abbot, and Grünenthal.
Dr. Francisco Esquivel-Ayanegui is or has been a Speaker for Asofarma, Astra-Zeneca, and Menarini.
Dr. Guillermo Pérez-Pérez declares he has no conflict of interest.
Dr. Javier Torres-López declares he has no conflict of interest.
Dr. María Victoria Bielsa-Fernández declares she has no conflict of interest.
Dr. M. Constanza Camargo declares she has no conflict of interest.
Dr. Elvira Garza-González declares she has no conflict of interest.
Dr. Angélica I Hernández-Guerrero declares she has no conflict of interest.
Dr. Roberto Herrera-Goepfert declares he has no conflict of interest.
Dr. Francisco M Huerta-Iga is or has been a Speaker for Takeda, Astra-Zeneca, and Asofarma.
Dr. Yelda Leal-Herrera declares she has no conflict of interest.
Dr. Aurelio Lopéz-Colombo declares he has no conflict of interest.
Dr. Nayelli X. Ortíz-Olvera declares she has no conflict of interest.
Dr. Arnoldo Riquelme-Pérez declares he has no conflict of interest.
Dr. Clara Luz Sampieri-Ramírez declares she has no conflict of interest.
Dr. Luis F. Uscanga-Domínguez declares he has no conflict of interest.
Dr. José Antonio Velarde Ruiz Velasco is or has been a Speaker for Takeda, Asofarma, Alfa-Wassermann, Abbvie, Abbott, Gilead, and MSD.
Please cite this article as: Bosques-Padilla FJ, Remes-Troche JM, González-Huezo MS, Pérez-Pérez G, Torres-López J, Abdo-Francis JM, et al. IV consenso mexicano sobre Helicobacter pylori. Revista de Gastroenterología de México. 2018;83:325–341.