Rumination syndrome is a functional gastrointestinal disorder characterized by effortless postprandial regurgitation of ingested food into the mouth. An unperceived postprandial contraction of the abdominal wall could be a key mechanism. In those patients, retrograde flow of the ingested gastric content into the mouth is produced due to a simultaneous combination of elevated intra-abdominal pressure and negative intrathoracic pressure. The estimated prevalence is around 2% in the general adult population. The main clinical characteristics include: a) early postprandial regurgitation, b) the effortlessly regurgitated material is similar to the ingested food, c) the regurgitated material is spit out or swallowed again. The clinical diagnosis of rumination syndrome relies on the clinical criteria. High resolution esophageal manometry, ideally including impedance monitoring, can be an important adjunct for making the clinical diagnosis. Its management is based on instruction as to the nature of the pathology, education in postprandial diaphragmatic breathing, and the assessment of possible psychiatric comorbidity. Baclofen use is reserved for second-line treatment in patients with refractory symptoms.
El síndrome de rumiación es un trastorno funcional gastrointestinal que se caracteriza por la regurgitación posprandial sin esfuerzo de los alimentos ingeridos hasta la boca, en el que la contracción posprandial no percibida de la pared abdominal sería un mecanismo clave. En estos pacientes, el flujo retrógrado del contenido gástrico ingerido hasta la boca se produce debido a la combinación de una presión intraabdominal elevada con una presión intratorácica negativa. La prevalencia estimada es de alrededor del 2% en la población general adulta. Las características clínicas principales incluyen: a) regurgitación posprandial precoz; b) el material regurgitado sin esfuerzo es similar al alimento ingerido; c) el material regurgitado se escupe o se vuelve a tragar. El diagnóstico clínico del síndrome de rumiación requiere el cumplimiento de los criterios clínicos. Las pruebas de manometría esofágica de alta resolución idealmente con impedancia pueden representar un complemento importante para el diagnóstico clínico. Su manejo se basa en la instrucción acerca de la naturaleza del trastorno, la educación en respiración diafragmática posprandial y la evaluación de posible comorbilidad psiquiátrica. El uso del baclofeno se reserva como segunda línea para pacientes con síntomas refractarios.
The term rumination, which is applied to both humans and animals, is derived from the Latin ruminor and means “to bring from the throat” or “chew that which is regurgitated”.1 It is a normal process in animals known as ruminants, such as cows, sheep, and goats.2
Rumination syndrome is a functional gastrointestinal disorder characterized by repetitive effortless regurgitation of recently ingested food into the mouth, followed by a new episode of chewing, and then swallowing or expelling the food bolus, which typically does not taste acidic or bitter because it occurs a few minutes after eating. Diagnosis entails key and common characteristics: a) the episode tends to be repeated until the regurgitated material becomes acidic, and b) the taste of the regurgitated material is usually pleasant to the patient.2
Rumination syndrome affects both the pediatric and adult populations.2–4 It is frequently misdiagnosed as refractory gastroesophageal reflux disease or recurrent vomiting, often resulting in a prolonged diagnostic delay.5
The syndrome is described and characterized as a functional gastroduodenal disorder, with established criteria included in the Rome IV classification,6 and is described in Table 1.
Diagnostic criteria for rumination syndrome.
| They should include the following: |
| Persistent or recurrent regurgitation of recently ingested food, with oral expulsion or re-chewing and subsequent re-swallowing. |
| Regurgitation is not preceded by retching. |
| The criteria are met during the past 3 months, with symptom onset at least 6 months prior to diagnosis. |
| Observations that support the diagnosis: |
| The effortless regurgitation events are not preceded by nausea. |
| The regurgitated material contains recognizable food, whose taste may be agreeable. |
| The process tends to cease when the regurgitated content becomes acidic. |
Taken from Stanghellini et al.6
There is scant data on incidence and prevalence in adults. Even though it is considered a rare disorder, its true prevalence is most likely underestimated, given the lack of recognition of said pathology by both clinicians and patients. Utilizing the Rome II criteria, the authors of a Canadian study reported a prevalence of 0.8% (95% CI 0.3%-1.3%) in the general population, through the application of questionnaires.7 The global prevalence of functional gastrointestinal pathologies, according to the Rome IV criteria, was established in a recently published multicenter study, describing a prevalence of rumination syndrome of 2.8% (3.1% women, 2.5% men) through an online survey and a prevalence of 1.1% (1.4% women, 0.8% men) through personal interviews.8 The prevalence of rumination syndrome is higher in patients with fibromyalgia or eating disorders and can reach 7-8%.9,10
In a study on a pediatric population from 4 provinces of Sri Lanka, 2,163 children and adolescents, between the ages of 10 and 16 years, were surveyed. Utilizing the Rome III criteria, prevalence obtained by questionnaire was 5.1%, and was similar in boys and girls (5.1% and 5%, respectively). Of the patients affected, 12% had symptoms that were sufficiently severe to cause school absenteeism.11
PathophysiologyThe pathophysiology of rumination syndrome is not yet completely understood, but unperceived activation of the abdominal wall in the postprandial period appears to be a cardinal pathogenic characteristic within the pathophysiologic process. Gastroduodenal manometry, and more recently the high-resolution esophageal impedance manometry technique, have shown that retrograde flow of the gastric content that reaches the buccal cavity in patients presenting with rumination is produced due to the simultaneous combination of elevated intra-abdominal pressure and negative intrathoracic pressure, resulting in an esophagogastric gradient in the direction of the oral cavity. In postprandial high-resolution esophageal impedance manometry, the ruminative phenomena follow gastric pressurizations, with pressures typically above 30mmHg in the majority of proximal reflux episodes,12 which is associated with upper and lower esophageal sphincter relaxation at the time of the gastric pressurization. Those observations reveal that the increase of intra-abdominal pressure, alone, cannot explain the rumination phenomenon, and that upper and lower esophageal sphincter relaxation most likely plays a central role in that process.13,14 In manometric studies, the increase in pressure of the abdominal musculature is simultaneously observed in all sensors, which is recognized in the “R wave” trace. Likewise, upon comparing patients with rumination syndrome with patients that have gastroesophageal reflux disease, the former reached an abdominal pressure peak of a median of 43mmHg, which was significantly higher than the gastroesophageal reflux group, whose abdominal pressure peak was a median of 6mmHg.12
In the comparison of rumination syndrome patients with controls, utilizing the gastric barostat test, the patients with rumination syndrome presented with more nausea and abdominal bloating sensation than the controls. At 4 and 8mmHg of gastric distension pressure, the lower esophageal sphincter tone was more reduced in those patients than in the controls, suggesting greater gastric sensitivity and lower esophageal sphincter relaxation during the gastric distension maneuver. Nevertheless, accommodation and gastric emptying were within normal ranges in the majority of patients with rumination syndrome.15
Electromyography studies of the abdominal musculature have shown that the regurgitation of gastric content is produced by the coordinated maneuver of a sudden contraction of the intercostal muscles, together with a contraction of the anterior abdominal muscles.16
In a group of 5 patients with rumination syndrome, diagnosed through the Rome III criteria, and monitored with high-resolution esophageal impedance manometry and videofluoroscopic recording to evaluate the barrier of the gastroesophageal junction in rumination episodes, ascension of the gastroesophageal junction was observed from the abdominal cavity to the thoracic cavity, generating a “pseudo-hernia” with gastric content and air. However, the importance of that observation is still being disputed, especially because it has also been described in healthy persons during physical activity.17 Prevalence of sliding hiatal hernia was observed in 50% of the cohort of both a rumination syndrome group and a gastroesophageal reflux disease group.12
According to patterns observed in high-resolution esophageal impedance manometry, 3 rumination variants have been described:12
Primary rumination: characterized by an increase in abdominal pressure that precedes retrograde gastric flow into the esophagus.
Secondary rumination: similar to primary rumination but the increase in abdominal pressure follows an event of gastroesophageal reflux.
Rumination associated with supragastric belching: produced by a mechanism similar to that of a supragastric belch, in which the aboral movement of the diaphragm creates subatmospheric pressure in the esophageal body and the upper esophageal sphincter relaxes. Air then enters the esophageal body, which can be seen as an increase in impedance toward the oral cavity, after which the air is rapidly (in less than a second) expelled from the esophageal body and is manifested as a return to baseline impedance. The increase in abdominal pressure is produced during the expulsion of air and precedes the retrograde flow of the gastric content.
In addition to the pathophysiologic mechanisms described, patients with rumination syndrome have been thought to present with a premonitory impulse, similar to that which occurs in patients with motor or vocal tics. It is an averse and disagreeable somatosensory experience the person has beforehand that is relieved after the rumination event, reinforcing the abdominal contraction. That recently described multiple mechanism model, or “maintenance model”, proposes that some patients could present with secondary psychologic and/or pathophysiologic mechanisms that contribute to the reinforcement process produced in response to the primary mechanism that maintains the usual contraction of the abdominal wall:18
- -
The primary maintenance route: rumination phenomena can be habits or reflexes that develop through a conditioned response to stimuli, in this case, food. There is a hypothesis that when the material is regurgitated, the premonitory impulse is temporarily resolved, reinforcing the contraction of the abdominal wall.
- -
Possible secondary psychologic mechanisms: rumination syndrome can be maintained secondarily by environmental, cognitive, and behavioral mechanisms that reinforce continuous regurgitation. Firstly, some individuals state they have a high probability of regurgitation after eating certain foods. Secondly, some individuals describe difficulty in tolerating the premonitory impulse and allow the production of regurgitation to temporarily alleviate their discomfort. Thirdly, some individuals are preoccupied with the body shape/weight that could partially maintain symptoms, but that is not attributable to an eating disorder. In fourth place, repetitive regurgitation could be a positive function for alleviating anxiety or providing a sensation of relief or pleasure, the latter of which has been described in children and adults with developmental disorders.
- -
Possible secondary pathophysiologic mechanisms: certain comorbidities could contribute to maintaining rumination. Rumination syndrome can arise in the context of other conditions that present with regurgitation and vomiting, such as gastroparesis and gastroesophageal reflux disease.18
A group of researchers studied the vagal tone in patients with rumination syndrome and healthy volunteers through the variability of heart rate that was measured at the baseline and during and after meals. Baseline vagal tone was similar in the two groups, and even though there was a trend toward greater vagal tone in postprandial episodes in the rumination group, it was not statistically significant.19
From the histopathologic perspective, Halland et al.20 analyzed duodenal biopsies from 22 patients diagnosed with rumination syndrome and compared them with 10 controls. They found that the number of intraepithelial lymphocytes was significantly higher in the rumination group (mean 15, range 8-29, and 2 cases with ≥ 25 intraepithelial lymphocytes/100 enterocytes), compared with the control group (mean 11, range 11-18). In addition, the patients with rumination syndrome had a significantly higher mean eosinophilic count than the controls, with 26 per mm2 versus 18 per mm2 (p=0.006). No pathogens were observed in the histology of either group, nor was there villous atrophy or other characteristics of celiac disease.20 The authors speculate that the muscle mechanisms leading to rumination could be in a context of low-grade inflammation, but further research is needed to confirm said hypothesis.20
Clinical characteristicsWhen we suspect rumination in a patient, the predominant symptom is the rapid appearance of postprandial and effortless regurgitation, typically within 10minutes from finishing a meal. 6,21 It can follow each meal or the majority of meals and generally occurs while the patient is eating or a few minutes after finishing the meal, thus the flavor of the regurgitated food is similar to that of the recently ingested food.21 Regurgitation can be produced, even when only liquids are ingested, which favors the regurgitation of previously ingested solid foods.22 Episodes tend to persist for one or 2hours after the meal, and even when they occur within that period of time, the regurgitated material consists of partially digested foods that are recognizable by their flavor. Regurgitation is often triggered by a sensation of abdominal discomfort (pressure, pain, burning).
The condition is difficult to recognize, given that both the patient and the clinician unfamiliar with the pathology confuse rumination with vomiting or refractory gastroesophageal reflux, delaying the diagnosis,21 with an increase in additional studies and associated costs. To differentiate rumination from vomiting, the regurgitation episodes experienced in rumination syndrome are generally not preceded by nausea. In addition, when a patient vomits, it is difficult to keep the content in the mouth due to the intensity of the impulse, rapidly expelling it. Nevertheless, there can also be rumination phenomena that present several hours after a meal, have a bad taste, and contain acid or bile.21 Patients with rumination can rechew the content, spit it out, or swallow it again, depending on the circumstances. That habit can lead patients to limit their social activities, especially eating in the presence of others at restaurants or in the workplace.23
Some individuals with rumination syndrome have described postprandial dyspeptic symptoms of fullness or epigastric burning sensations,24 and the presence of early satiety has been reported in 3/4 of patients.20 Other symptoms, such as heartburn, abdominal pain, and diarrhea and/or constipation, are less frequent but do not rule out the diagnosis. On the other hand, nocturnal regurgitation, dysphagia, or symptoms that are not close to meals make the diagnosis less likely.22 Patients have been reported to have symptoms of gastroesophageal reflux disease, such as heartburn or regurgitation, that do not respond to proton pump inhibitors, reaching figures close to 50%, with a higher risk in females, Latinos, and younger individuals.25 In that respect, some of those patients categorized as having refractory gastroesophageal reflux disease, with regurgitation as the predominant symptom, could actually correspond to patients with rumination syndrome. In fact, in some case series, up to 100% of patients with rumination syndrome take proton pump inhibitors.20
Likewise, certain patients with other pathologies, such as achalasia and accommodation or gastric emptying disorders, that present with similar symptoms, should be evaluated for rumination syndrome with the pertinent studies, according to the degree of suspicion.23
The presence of an inciting event prior to the diagnosis of rumination syndrome, such as illness, or the psychosocial stress of divorce, a death in the family, or problems at school or work, has been described in some patients.26
Among the complications associated with rumination syndrome, dental erosions have been detected in up to 77% of children27 and halitosis has been described as a reason for referral.28 Weight loss has also been reported as an associated symptom in adolescents and adults, in up to 40% of cases.2 In severe cases of nutritional compromise, jejunostomy has been performed to stabilize those patients.26
ComorbiditiesRumination syndrome has been associated with the presence of mental health disorders, including episodes of anxiety, depression, somatoform disorders, and adaptation disorders. Psychiatric comorbidity is a quite frequent finding (more than 90% in one case series). Thirteen percent of patients diagnosed with rectal evacuation disorder have concomitant rumination syndrome.2,5,26 Dietary restrictions due to the fear of regurgitation or to a previous history of eating disorders (7-17%), as well as a history of abuse (33%), have stood out in some case series.5,29,30
Even though some studies have suggested that rumination syndrome is predominantly produced in children and adults with developmental delay, that has not been corroborated by other studies, given that there are case series conducted on populations with normal intelligence.13,16,24
Diagnostic processRumination syndrome is a clinical diagnosis. It should be suspected in patients that present with postprandial effortless regurgitation, with no retching. The presence of “refractory reflux”, with regurgitation as the predominant symptom, suggests underlying rumination, which is more frequent in the subgroup of young women.31 Patients see a mean of up to 5 physicians and have symptoms for nearly 3 years before the diagnosis.2 That delay can also play a deleterious role in the patient’s emotional state, often making it difficult in that context for him/her to accept the diagnosis of a functional gastrointestinal disorder. The support of an objective diagnostic test is often helpful. Confidence in the diagnosis and a solid doctor-patient relationship is of the utmost importance for achieving good treatment adherence and the patient’s acceptance of the disease.
The diagnostic keys of the initial clinical evaluation should be centered on establishing whether or not the postprandial symptoms are vomiting (as often reported by the patient) or regurgitation. As previously explained, regurgitation, unlike vomiting, requires no effort, may not be associated with nausea, and is not preceded by retching or spasms. Some patients may also refer to regurgitation as a belch, making a detailed anamnesis important for correctly interpreting the symptom. Patients with rumination syndrome often state there is an event that triggers the appearance of rumination symptoms.32
In customary clinical practice, mechanical obstruction in patients suspected of having rumination syndrome is usually ruled out through upper endoscopy, with biopsies taken to rule out other disorders (e.g., eosinophilic gastroenteritis, celiac disease, or Helicobacter pylori infection, among others), and enterography techniques (enteroclysis by computed tomography or magnetic resonance imaging). Upper gastrointestinal endoscopy tends to be normal in patients with rumination syndrome, with the exception of the finding of esophagitis, which is reported in some case series at around 10%.12
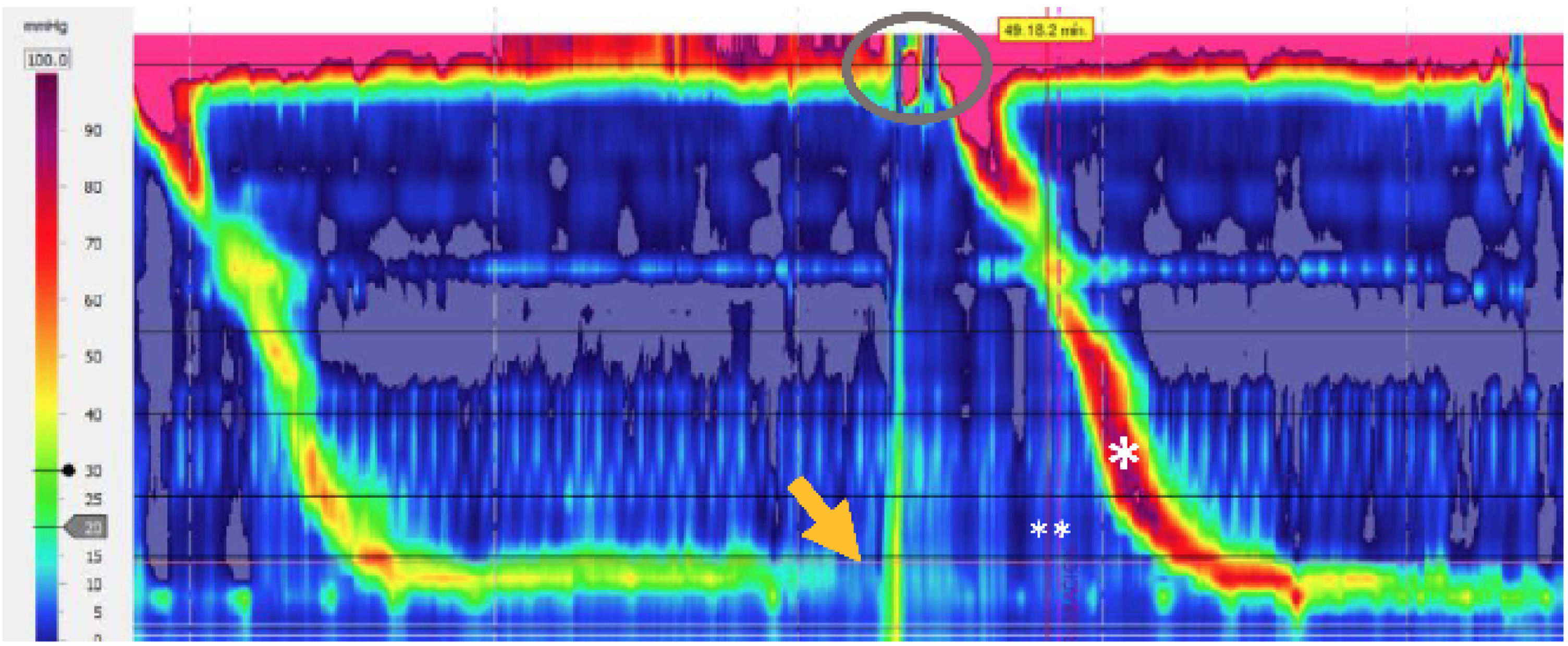
In selected cases, antroduodenal manometry or high-resolution esophageal manometry, ideally with impedance monitoring in the postprandial period, can be employed.6 The latter is considered the gold standard by some authors, albeit normative values have not yet been established.31 Neither of the two diagnostic techniques is widely available in our environment, and are mainly limited to specialized centers. The diagnostic criteria of high-resolution esophageal impedance manometry for rumination syndrome are based on the presence of reflux into the proximal esophagus, associated with gastric pressurization above 30mmHg.12 The analysis in the postprandial period, following a standardized meal,23 enables the presence of gastroesophageal reflux to be characterized through transitory relaxations of the lower esophageal sphincter, supragastric belches, or rumination phenomena. Upon evaluating the performance of that technique, up to 20% of the patients with symptoms of gastroesophageal reflux disease catalogued as refractory to proton pump inhibitors, present with rumination criteria.33Fig. 1 shows high-resolution esophageal manometry with postprandial motor findings characteristic of rumination syndrome.
Postprandial trace of the high-resolution esophageal manometry with no impedance monitoring. Gastric contraction at 30mmHg (R wave) (arrow), opening of the upper sphincter (oval), and secondary swallow (*) in conjunction with the symptom (**) referred to by the patient are observed.
Classically, esophageal body motility is preserved but there is a case report of rumination syndrome associated with ineffective esophageal motility.34
In addition to high-resolution esophageal manometry, the utility of 24-h pH/impedance monitoring has been tested for the diagnosis of rumination. A recent study by Nakagawa et al.31 evaluated patient characteristics through that technique, compared with high-resolution esophageal impedance manometry, considered the gold standard. Patients with rumination rarely presented with episodes of reflux in the recumbent position and had significantly more postprandial early nonacid reflux episodes with high proximal extent. Unlike the patients with gastroesophageal reflux disease, the patients with rumination had a higher pH nadir shortly after eating, that became acidic over time. The time interval from the detection of reflux through impedance monitoring and the appearance of symptoms was significantly shorter in the patients with rumination syndrome than in those with gastroesophageal reflux disease.31
The same study proposed a score for rumination diagnosis of 0 to 2 points by means of 24-h pH/impedance monitoring as follows:
The number of episodes of postprandial nonacidic reflux (up to one hour after the meal), with a cutoff value of 3/h: 1 point
Postprandial symptom index with a cutoff value of 60%: 1 point
Rumination syndrome is diagnosed with 1 or 2 points, with 91.7% and 58% sensitivity and 78.6% and 93% specificity, respectively.
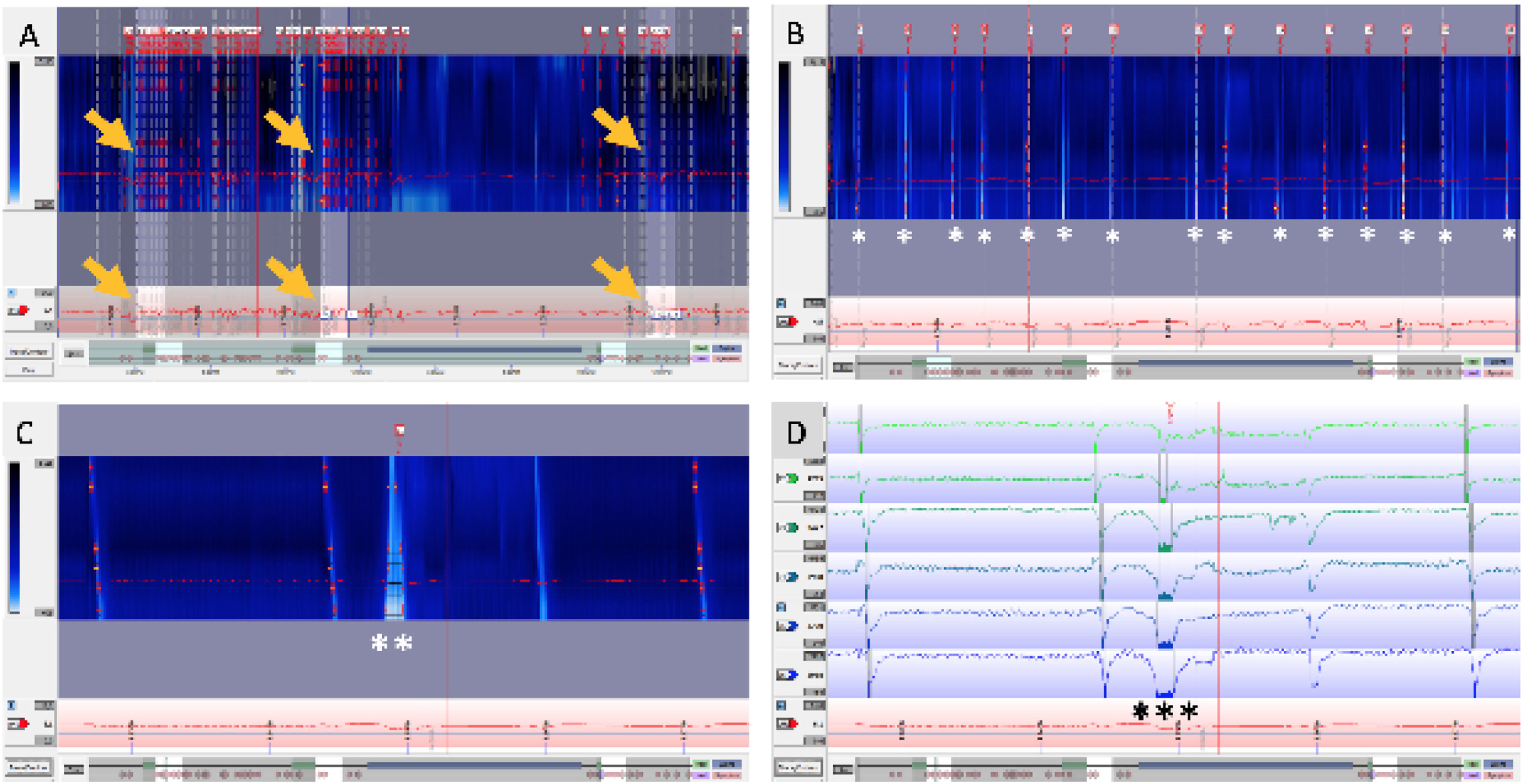
Fig. 2 shows an example of a 24-h pH/impedance trace with the previously described analysis protocol, consistent with rumination syndrome.
In the same patient, 24-h pH-impedance trace with the postprandial analysis protocol in 3 meals. A) 24-h time window: 3 postprandial periods (arrows) analyzed for obtaining the criteria proposed by Nakagawa et al.31 are observed. B) 60-min time window: postprandial period with 15 nonacid reflux events (*). C) 5-min time window in colorimetry, with nonacid reflux event (**). D) Impedance trace showing the nonacid reflux event in detail (***) for the same period analyzed in C.
There are few gastrointestinal disorders that present with frequent postprandial effortless regurgitation other than rumination syndrome:
Achalasia: Some patients with achalasia and gastric accommodation alteration have postprandial regurgitation. Nevertheless, the predominant symptom in patients with achalasia is dysphagia,35 which is not a classic characteristic of rumination syndrome. Achalasia can be distinguished from rumination syndrome by its characteristic patterns in high-resolution esophageal manometry, as well as by the presence of late postprandial regurgitation and/or vomiting.
Gastroesophageal reflux disease: The symptom of regurgitation in rumination does not have the sour, bitter, or acidic taste reported by patients with gastroesophageal reflux disease. Unlike pathologic reflux, rumination syndrome episodes are not nocturnal. Medications that suppress gastric acid production, such as proton pump inhibitors, do not tend to improve symptoms in patients with rumination syndrome.
TreatmentThe therapeutic strategy begins with an adequate explanation to the patient as to the nature of the pathology, commenting to him/her that it corresponds to a benign functional disorder, describing the underlying mechanisms that produce it, so that the patient takes an active role in the management of the condition. A multidisciplinary approach with the participation of gastroenterologists and mental health teams is recommended, especially in patients with underlying depression, anxiety disorders, or refractory symptoms.
The available therapies focus on increasing the pressure of the lower esophageal sphincter or reducing the gastric pressure produced by the contraction of the striated musculature of the abdominal wall. The cornerstone of therapy for rumination syndrome is the technique of diaphragmatic breathing or abdominal breathing. Diaphragmatic breathing reduces the postprandial intragastric pressure and increases the pressure of the gastroesophageal juncture zone, restoring the pressure gradient to that level.13 With respect to the technical concepts, patients are instructed to place one hand on the abdomen and the other on the chest. They are then told to slowly inhale through the nose, with only the hand on the abdomen rising during the inhalation and the hand on the chest remaining still. The patients then exhale slowly through the mouth. Each inhalation and exhalation should be slow and complete, with the goal of carrying out six to eight respirations per minute. The technique can be taught with the patients in the decubitus supine position, as well as in a seated position (which is how patients generally eat).36 Some patients require several sessions to adequately learn the technique. The patients are typically instructed to do the diaphragmatic breathing exercises immediately after a meal and continue them for 10 to 15min or more until the regurgitative sensation is resolved. The aim of diaphragmatic breathing is that, with time, it occurs unconsciously during the events that precipitate regurgitation. Patients should be advised that diaphragmatic breathing can feel uncomfortable at the beginning of the therapy. To reduce that sensation and prevent limited adherence, patients should be told to loosen belts and not wear tight clothes. Gastroenterologists or other clinicians familiar with the technique could initially demonstrate how it is done during the medical consultation.36 Physiotherapists with knowledge of the technique can also be part of the treatment process.
That technique has been taught through biofeedback of the thoraco-abdominal musculature by means of electromyographic control. Electromyography-guided biofeedback, consisting of 3 sessions over a 10-day period, was conducted on 12 patients in a randomized clinical trial. Before a standardized meal, the patients were trained to control the muscles of the abdomen and chest under electromyographic vision, connected to a monitor. The patients were instructed to reduce the activity of the intercostal and anterior abdominal muscles and to increase the activity of the diaphragm. After eating, they were taught how to prevent the rumination episodes by controlling those muscles. After the session, the patients were told to perform the exercises every day at home, 5min before and after the 3 daily meals (breakfast, lunch, and dinner). The biofeedback patients showed reduced activity of the intercostal and anterior abdominal muscles, compared with the control group of 11 patients that received simethicone. Biofeedback also reduced the episodes of regurgitation, compared with the placebo group (74±6% vs 1±14%, respectively) and the difference was statistically significant. No adverse effects were reported.29
Diaphragmatic breathing associated with behavioral therapy and assisted by esophageal impedance manometry has been well tolerated by patients, resulting in reduced intragastric pressure, increasing the pressure of the gastroesophageal junction and re-establishing a negative gastroesophageal pressure.13 However, that combined diagnostic technique is more expensive and not widely available.
Diaphragmatic breathing has been shown to increase vagal tone, but that mechanism does not explain the reduction of the rumination episodes.19 Most likely, diaphragmatic breathing more specifically affects the increase in both abdominal wall muscle contractibility and the pressure of the gastroesophageal junction, as well as reducing anxiety.19
In the context of refractory symptoms, pharmacologic therapy has been proposed, especially the use of baclofen. Said drug is a gamma-aminobutyric acid type B (GABAB) that is capable of reducing the transitory relaxations of the lower esophageal sphincter and increasing the pressure of the lower esophageal sphincter in healthy individuals and patients with gastroesophageal reflux disease.37,38 Only 2 trials have evaluated the impact of baclofen on regurgitation in rumination syndrome. In a double-blind, placebo-controlled, cross-over study that assessed the effectiveness of baclofen in patients with rumination syndrome, reduced regurgitation episodes were reported by the patients that received baclofen, registering symptoms through high-resolution esophageal manometry study.39 The effectiveness of baclofen was also shown in another study on patients with rumination episodes, achieving a reduction in the number of events and an increase in lower esophageal sphincter pressure. Nevertheless, there are no long-term data on its efficacy and tolerance profile.40 Baclofen crosses the blood-brain barrier and has a variety of secondary effects related to the central nervous system that include confusion, dizziness, vertigo, somnolence, weakness, and trembling. In general, a 5 to 10mg initial dose at bedtime is recommended and can be increased to 10mg, 3 times a day, with careful monitoring in relation to secondary effects.
Tricyclic antidepressants, together with diaphragmatic breathing and audio relaxation techniques for at least 3 months, have also been used in patients with rumination syndrome. At the mean follow-up of almost 9 months, 90% of the patients had symptom improvement, with a mean subjective improvement close to 70%, as well as weight gain or weight stability in 80% of the patients that initially reported weight loss.26 The most widely used tricyclic antidepressant in that study was nortriptyline, starting at a dose of 10mg at night, with a weekly increase of 10mg, until achieving symptom improvement, with a minimum of adverse effects.26 The mean time until improvement was 6.3 weeks. The study was not blinded, and improvement was determined through a non-validated questionnaire, reflecting the need to create and validate scales that would enable the comparison of treatment strategies.
In case reports, the use of chewing gum has been associated with a reduced number of rumination episodes but there are no randomized trials that demonstrate the effectiveness of that intervention.41,42
Finally, regarding surgery, there is one report on 5 patients diagnosed with rumination syndrome. Four of them had low resting lower esophageal sphincter pressure and reported complete cessation of ruminating behavior after undergoing Nissen fundoplication. The post-Nissen adverse event of dysphagia was described in one of the 5 patients. Notably, the employment of biofeedback therapy before the surgery or the use of baclofen were not reported.43
There is also a case report in the literature of a female patient with a history of regurgitation of recently ingested food, who had been diagnosed with a number of diseases (bile duct pathology, gastroparesis, among others). She underwent cholecystectomy, medical treatment, and pyloroplasty, until rumination syndrome was diagnosed through antroduodenal manometry. A gastric histologic study revealed a normal number of nerve fibers and ganglia (myenteric plexus) and an adequate number of Cajal cells within the muscular propria. The patient presented with important malnutrition and received enteral nutrition through jejunostomy. Despite carrying out treatment with breathing exercises, she continued to have nausea and vomiting, and so a subtotal gastrectomy (80%), with Roux-en-Y reconstruction, was performed. At the postoperative follow-up at 4 months, the patient had reduced symptomatology above 70%, with improved nutritional status and quality of life.44,45
PrognosisFew studies provide information on the long-term results in patients with rumination syndrome, but the limited data available suggest that symptoms can recur after successful treatment. Forty-seven adolescents that underwent an intensive inpatient program were surveyed around one year after hospital discharge. Twenty percent of them had symptom resolution but 73% had rumination recurrence.46 That datum is very important, showing that patient follow-up should be carried out, given that rumination can be associated with significant weight loss with its consequent malnutrition, as described above.
ConclusionRumination syndrome is a clinically recognized entity, with clinical criteria included in the Rome IV criteria. There are also well-established diagnostic support tests for the condition. It should be suspected in patients with a history of postprandial regurgitation, in those described as having proton pump inhibitor-refractory gastroesophageal reflux disease, and in patients with numerous consultations for “vomiting”. Within the complementary evaluation available in our environment, beginning the study of suspected cases with 24-h pH-impedance with the rumination protocol (three meals in 24h and an additional analysis of the postprandial period) could have a favorable impact on the diagnostic process of patients with a long history of “refractory gastroesophageal reflux”, enabling a better selection of patients referred for the costlier and less available procedures, such as high-resolution esophageal manometry, ideally with impedance monitoring, and the postprandial analysis protocol. Treatment is based on a detailed explanation of the condition to the patient and a solid doctor-patient relationship, to promote adherence to the diaphragmatic breathing exercises, with or without biofeedback at referral centers, and on the use of baclofen as second-line drug therapy.
Conflict of interestThe authors declare that they have no conflict of interest.
Please cite this article as: Chahuan J, Rey P, Monrroy H. Síndrome de rumiación. Artículo de revisión. Revista de Gastroenterología de México. 2021;86:163–171.