Primary intestinal lymphangiectasia is a rare congenital disease described by Waldmann in 1961 that is a consequence of obstruction of the lymphatic drainage of the small bowel with secondary lymph vessel dilation. This distorts the architecture of the villi and causes a leakage of lymph into the intestinal lumen, resulting in protein-losing enteropathy and malabsorption.
AimTo describe the clinical, biochemical, radiologic, endoscopic, and histologic characteristics in children with primary intestinal lymphangiectasia.
MethodA retrospective observational, descriptive, cross-sectional study was conducted that reviewed the case records of children diagnosed with primary intestinal lymphangiectasia that were seen at the Department of Gastroenterology and Nutrition of the Instituto Nacional de Pediatría within the time frame of January 1, 1992 to September 30, 2012.
ResultsFour patients were found that presented with primary intestinal lymphangiectasia. Three of them had been diagnosed before 3 years of age. All the patients presented with chronic diarrhea, edema, lymphopenia, hypocalcemia, and hypogammaglobulinemia, and 3 patients presented with hypocholesterolemia. Bowel transit time, endoscopy, and intestinal biopsies were characteristic of this pathology.
ConclusionsIntestinal lymphangiectasia should be suspected when there is a clinical picture of chronic diarrhea and protein-losing enteropathy accompanied with edema at any level, as well as hypoalbuminemia, hypocalcemia, lymphopenia, hypogammaglobulinemia, and hypocholesterolemia, which are the main biochemical findings of this pathology. All children presenting with intestinal lymphangiectasia should undergo an upper gastrointestinal series with bowel transit time and endoscopy with biopsies taken at the level of the duodenum. Treatment includes diet and the periodic administration of albumin and gamma globulin.
La linfangiectasia intestinal primaria es una enfermedad congénita rara descrita por Waldmann en 1961, y que es consecuencia de una obstrucción del drenaje linfático del intestino delgado con dilatación secundaria de los vasos linfáticos, que distorsionan la arquitectura de las vellosidades ocasionando pérdida de linfa hacia la luz intestinal,dando como resultado una enteropatía perdedora de proteínas y malabsorción de nutrimentos.
ObjetivoDescribir las características clínicas, bioquímicas, radiológicas, endoscópicas e histológicas en niños con linfangiectasia intestinal primaria.
MétodoEstudio observacional, descriptivo, transversal y retrospectivo de niños con linfangiectasia intestinal primaria que fueron vistos en el Servicio de Gastroenterología y Nutrición del Instituto Nacional de Pediatría desde el 1 de enero de 1992 al 30 de septiembre de 2012, en donde se revisaron los expedientes clínicos de los niños con este diagnóstico.
ResultadosSe encontraron 4 pacientes con linfangiectasia intestinal primaria, 3 de ellos diagnosticados antes de los 3 años; todos se presentaron con diarrea crónica, edema, linfopenia, hipocalcemia, hipoalbuminemia e hipogammaglobulinemia, y en 3 pacientes, con hipocolesterolemia, y con tránsito intestinal, endoscopia y biopsias intestinales características de esta enfermedad.
ConclusionesLa linfangiectasia intestinal debe sospecharse ante un cuadro de diarrea crónica, enteropatía perdedora de proteínas, que se acompaña de edema a cualquier nivel, así como hipoalbuminemia, hipocalcemia, linfopenia, hipogammaglobulinemia e hipocolesterolemia como los principales hallazgos bioquímicos de esta entidad. Ante esta enfermedad debe realizarse estudio de serie esofagogastroduodenal con tránsito intestinal y endoscopia con toma de biopsias a nivel de duodeno. El tratamiento incluye dieta y administración periódica de albúmina y gammaglobulina.
Intestinal lymphangiectasia (IL) is a cause of chronic diarrhea. It is a protein-losing enteropathy that can have a primary (PIL) or secondary presentation.1,2
PIL is a rare congenital disease that was first described by Waldmann in 1961 and is caused by obstruction of the lymphatic draining of the small bowel with a secondary dilation of the mucosal, submucosal, and/or serous lymphatic vessels that distorts the architecture of the villi,3 causing a leakage of lymph into the intestinal lumen that results in nutrient malabsorption. The loss of lymph produces hypoproteinemia, hypoalbuminemia, and lymphopenia,4,5 which in turn can lead to peripheral edema (inferior members), ascites, pleural effusion, weight loss, and delayed growth as typical clinical manifestations.6 PIL is a congenital disorder of the lymphatic system that is usually diagnosed before 3 years of age4,7,8 and is a typical example of secondary immunodeficiency. Laboratory findings include hypoproteinemia, hypoalbuminemia, lymphocytopenia, hypogammaglobulinemia, and reduced levels of transferrin and fibrinogen.6,9,10 Diagnosis is confirmed by radiologic, endoscopic, and histologic findings of the dilated lymph ducts and the deformity of the intestinal villi.11 In general, intestinal lymphangiectasia treatment can be divided into: a) nutritional support, b) medication use, and in some cases, c) surgery.1
The aim of this study was to know the clinical, biochemical, radiologic, and endoscopic characteristics of children with PIL.
MethodsAn observational, descriptive, cross-sectional, and retrolective study was conducted on children diagnosed with PIL that were seen at the Gastroenterology and Nutrition Service of the Instituto Nacional de Pediatría of the Department of Health within the time frame of January 1, 1992 to September 30, 2012. A complete medical history was taken for each patient, but for the purposes of this study only the following variables were included: age at the time of diagnosis, sex, and clinical manifestations. The laboratory tests reviewed were total lymphocyte count, albumin, cholesterol, serum calcium, and immunoglobulins; the specialized studies taken into account were the esophagogastroduodenal transit series and endoscopic and histologic findings. The treatment review included a) type of diet, and b) whether or not albumin and/or gamma globulin were used, and c) clinical progression.
The variables were analyzed using the SPSS® version 16.0 program and the result database was registered therein.
Descriptive statistics were employed. Frequencies were used for the categorical variables and measures of central tendency and dispersion were used for the quantitative variables.
ResultsFour patients were found to have PIL, and 3 of them were female. The mean age at the time of diagnosis was 22 months (range 9-39 months). The clinical manifestations are shown in Table 1, the biochemical findings in Table 2, and the radiologic, endoscopic and histologic findings in Tables 3 and 4, respectively.
Clinical characteristics of the patients with primary intestinal lymphangiectasia.
| Patient | Sex | Age at time of diagnosis | Length of time of progression | Extraintestinal lymphatic malformation | Edemas/pleural effusion/ascites | Height at time of diagnosis, cm | Present height, cm |
| 1 | Female | 2 years | 14 years | Yes | Yes | 81 | 147.4 |
| 2 | Female | 9 months | 16 years and 6 months | Yes | Yes | 68 | 145 |
| 3 | Female | 3 years and 3 months | 6 years and 6 months | No | Yes | 87 | 121 |
| 4 | Male | 1 year and 3 months | 5 months | No | Yes | 75 | 75 |
Biochemical characteristics of the patients with primary intestinal lymphangiectasia.
| Patient | Albumin, g/dl | Calcium, mg/dl | IgA, mg/dl | IgG, mg/dl | IgM, mg/dl | Lymphocytes, uL | Cholesterol, mg/dl | Triglycerides, mg/dl |
| 1 | 1.6 | 7.3 | 23.6 | 156 | 24.2 | 600 | 55 | 56 |
| 2 | 1.8 | 7.9 | 24 | 132 | 67.1 | 1,800 | 105 | 52 |
| 3 | 1.4 | 5.7 | 74.8 | 205 | 42.5 | 150 | 94 | 53 |
| 4 | 1.8 | 5.6 | 27 | 71.3 | 21.9 | 728 | 110 | 88 |
Radiologic characteristics of the patients with primary intestinal lymphangiectasia.
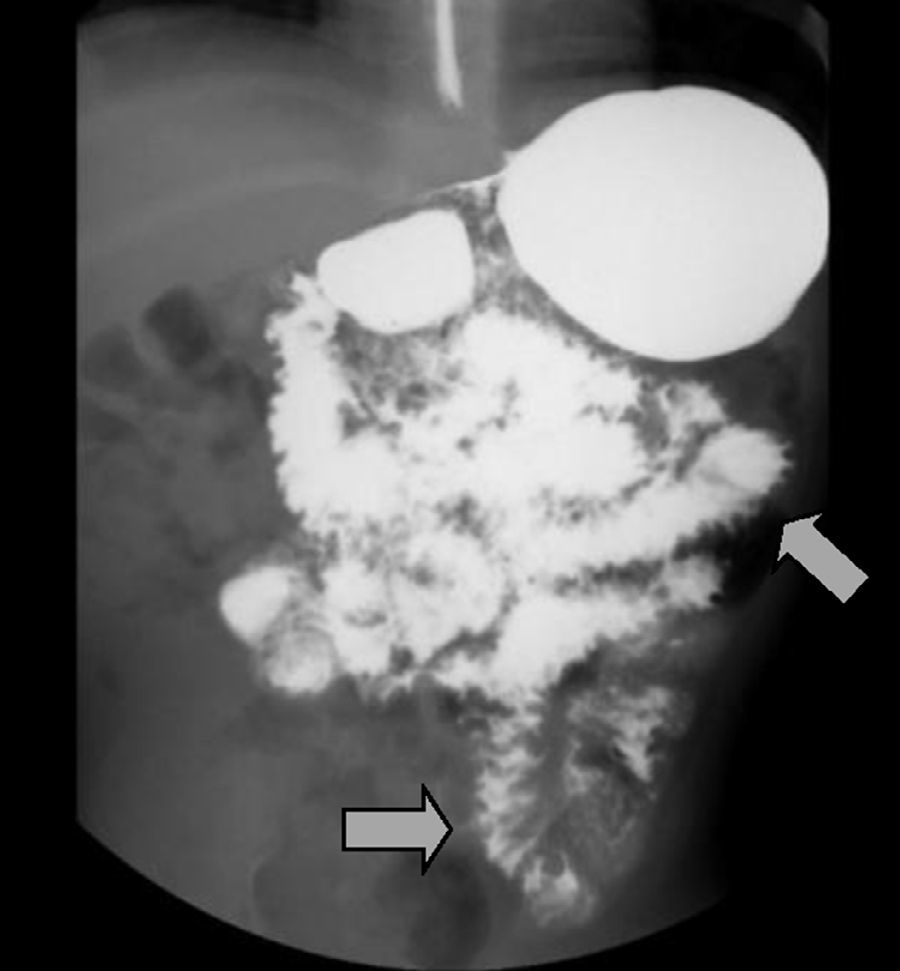
| 1 | Spiculated pattern, data suggestive of inflammatory process in the duodenal mucosa and ileal jejunum |
| 2 | Thick connivent folds in the entire small bowel, thickening of the duodenal mucosa. Double-rail images |
| 3 | Thickening of the folds at the jejunal level and spiculated edges, «stack of coins» image |
| 4 | Thickened folds in the small bowel with spiculated edges and some «stack of coins» images |
Endoscopic and histologic findings in the patients with primary intestinal lymphangiectasia.
| Patient | Endoscopy | Histology |
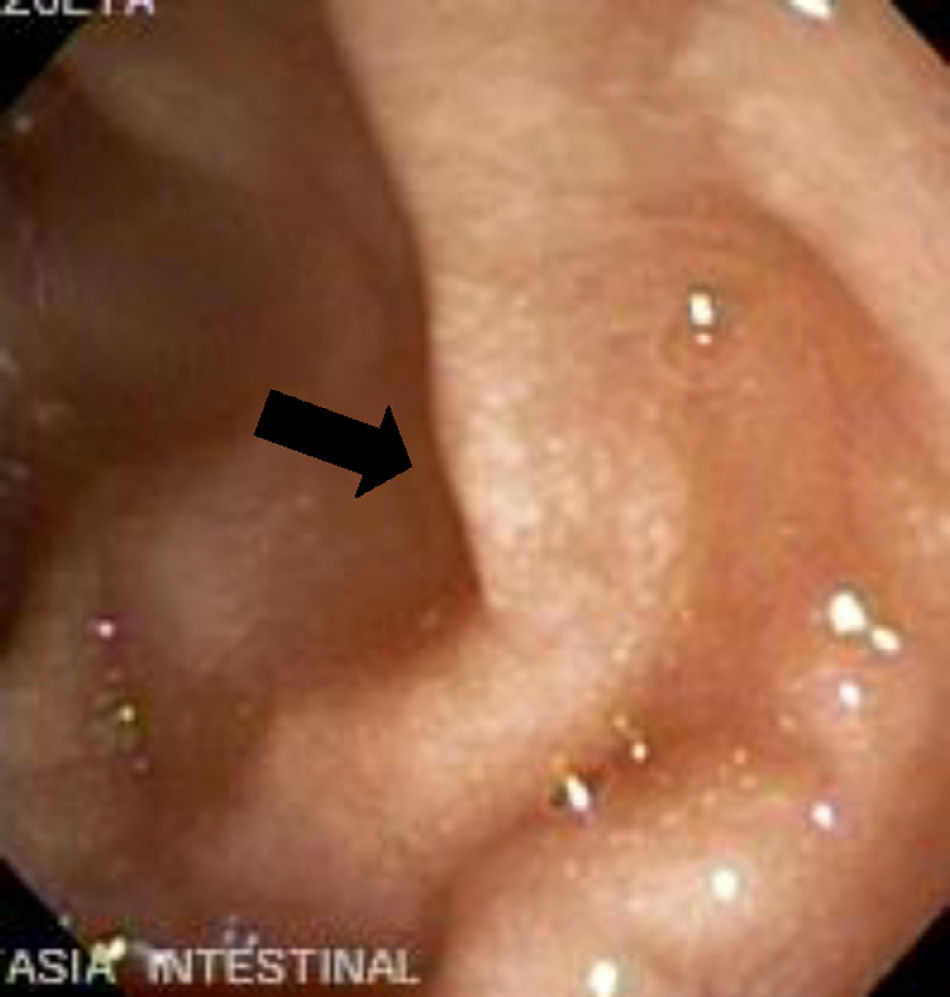
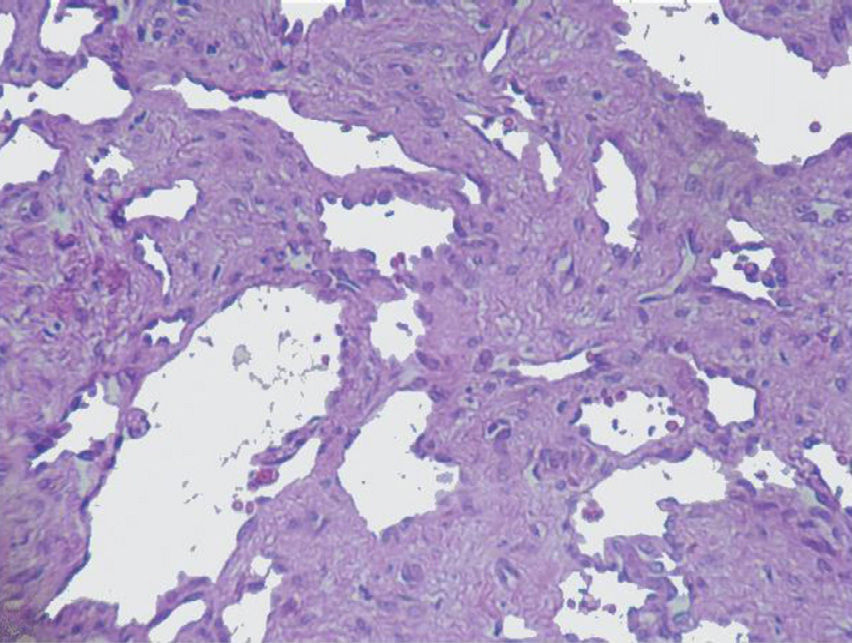
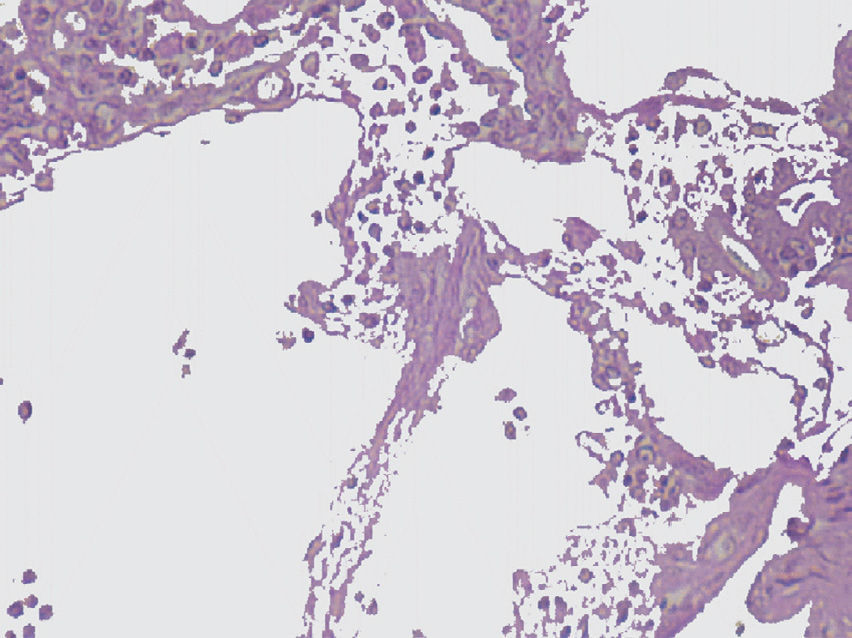
| 1 | Increase in the duodenal folds and whitish matter that could be lymph | Lymph vessel dilation. Left inguinal lymph node with fusiform-cell lymphangioma |
| 2 | Second portion of the duodenum with abundant whitish spots along its entire course | Vascular dilations corresponding to lymphangiectasia |
| 3 | White frost images | Lymphangiectasia. Moderate chronic duodenitis with moderate atrophy of the villi |
| 4 | Snowflake images in the duodenum | Lymph vessel dilation corresponding to intestinal lymphangiectasia |
Dietary and pharmacologic treatment was used in all of the patients; all of the patients received a protein-rich and low-fat diet with a liposoluble vitamin supplement, calcium, and half-chain triglycerides.4,12,13 Drug treatment was based on diuretics such as furosemide and/or spironolactone at the recommended doses. Albumin was administered intravenously at 1g/kg/day for 5 days and gamma globulin at 600mg/kg/day. These last 2 drugs were administered every 21-28 days in a continuous manner.
DiscussionThe incidence of PIL is low. At the Instituto Nacional de Pediatría, a national hospital referral center, we found only 4 cases over a 20-year period (1992-2012). Three of them were females, in contrast to the male predominance reported in the international literature in relation to sex. However, our case series was small, and most likely a larger number of cases is needed in order to establish prevalence in relation to sex. According to different reports in the literature, PIL is diagnosed before the age of 3 years.2,4,7,8 In our case series, the mean age at the time of diagnosis was 22 months, coinciding with that published by other authors in which the majority of PIL cases were diagnosed in patients under the age of 3 years. Only one of our patients was diagnosed after 3 years of age, being a late referral. Nevertheless, there are also reports in the literature of diagnoses after the age of 3, such as the study published by Suresh et al. in 2008.14
In all the cases, the clinical manifestations were characterized by diarrhea, edema of the extremities, and ascites; 2 of the patients presented with severe sepsis. As is known, edema and ascites present in PIL due to its being a protein-losing enteropathy,4,15 and it can often be associated with other extraintestinal lymphatic anomalies such as lymphangiomas,16,17 present in the same 2 cases of ours that had asymmetric lymphedema of the extremities with no other clinical manifestations consistent with syndromic diseases. The increased pressure in the lymph vessels causes lymph extravasation with an accumulation of chylomicron-rich fluid in the peritoneal cavity;18 this explains the ascites that all the patients presented with. Severe malnutrition is a consequence of nutrient malabsorption, chronic diarrhea, and protein-losing enteropathy. An important characteristic is that the height of all of our patients was below normal parameters, a situation that continues up to the present, making under-height a constant finding in PIL1 that persists despite medical treatment.
Hypoalbuminemia, hypocalcemia, lymphocytopenia, and hypogammaglobulinemia were findings in all of our patients, whereas hypocholesterolemia was only observed in 3 of them (Table 2).
Immunoglobulin concentrations are reduced in PIL and cellular immunity is also affected by the lymphocytopenia, resulting in secondary immunodeficiency. Nevertheless, the children in our case series presented with few severe infections, which is probably explained by the fact that our treatment included periodic gamma globulin administration.12,19
In an article published in 2010, Wen et al. reviewed reports in the international literature spanning the time frame of 1995 to 2009, and found 80 cases, adding 4 diagnosed at their hospital center, in which the female sex, together with edema as a clinical characteristic, predominated in 78% of the patients; this concurs with the results of our study. They reported a mean age at the time of diagnosis of 13.3 years, including children and adults. In that study is was striking that there was a constant elevation of IgE in the 4 patients described and lymphopenia in only 2 of them, in contrast with our study, and the presence of lymphoma in 5% of the cases, manifested as abdominal pain of rapid onset.20
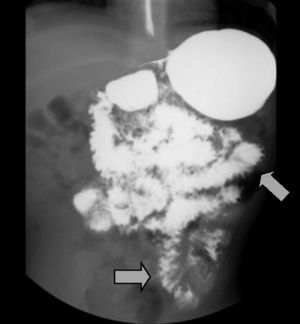
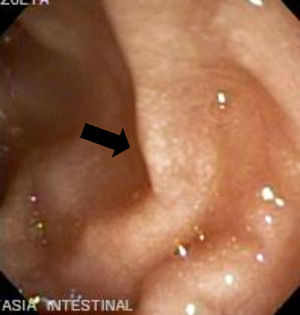
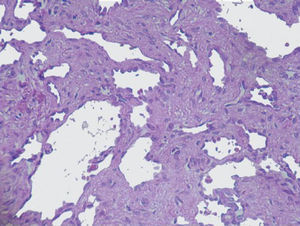
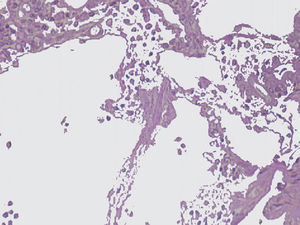
The imaging studies revealed a thickening of the mucosa in 3 of the 4 patients, a «stack of coins» image (2/4), a spiculated pattern (4/4), and double-rail images (1/4). These present in children with this pathology (fig. 1) and our findings were concordant with reports in the literature (see Table 3).21 The endoscopic image in our cases showed the typical aspect of white frost (4/4) or snowflakes in the intestinal villi (fig. 2). This contrasts with other published case series in which the aspect is nodular (Table 4).7 Histologically, lymph vessel dilation was observed (figs. 3 and 4), corroborating the intestinal lymphangiectasia diagnosis (Table 4).22
The dietary treatment was based on a high-protein, low-fat diet with medium-chain triglycerides and liposoluble vitamins. In regard to the medications used, in addition to albumin and gamma globulin, diuretics like furosemide and/or spironolactone were necessary. Considering all of the above, the clinical progress of the children was favorable.18,23
Compared with other case series, the study by Xinias et al. published in March 2013 presents 2 male patients with clinical characteristics very similar to those of our patients. However, management with albumin was used only in the periods of exacerbation. They do not mention the use of diuretics or gamma globulin and their patients progressed well.24
Other medical treatments have been proposed for PIL such as the use of octreotide, tranexamic acid, and some cytotoxic drugs, but they are expensive and their results are controversial; they have yet to be used in our hospital center. In reference to octreotide, its use has been suggested in treatment-refractory patients. However, there is little experience with this drug in children and apparently good results have only been reported in adults.25–28 Surgical treatment such as enterectomy of the affected intestinal segment is only indicated when the lesion is local or segmentary, with initially good results. When the part of the intestine that is affected does not have clear margins defining the lesion, the risk/benefit of surgery must be evaluated, given that wide resections can cause short bowel syndrome.4,9,29 There are reports in the literature in which labeled albumin scintigraphy and abdominal tomography were used with apparently good results to delimit the affected segment.30,31 In our case series these studies were not done. In relation to follow-up it is suggested to continue with surveillance and periodic check-ups of these children.19
ConclusionsIntestinal lymphangiectasia in children should be suspected whenever there are symptoms of chronic diarrhea and protein-losing enteropathy accompanied with edema of the inferior extremities, even though this can be present at any level. Hypoalbuminemia, hypogammaglobulinemia, hypocalcemia, lymphopenia, and hypocholesterolemia are the main biochemical findings in this disease. An esophagogastroduodenal transit study and endoscopy with biopsy at the duodenal level should be done on every child presenting with this pathology.
These children require opportune diagnosis and multidisciplinary treatment by the gastroenterologist, nutritionist, and immunologist, given the fact that they can present with severe life-threatening intercurrent infections and should be considered as children with secondary immunodeficiencies and managed as such. Their frequent hospital visits predispose them to school absenteeism and increased family expense.
Financial disclosureNo financial support was received in relation to this article.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Valdovinos-Oregón D, Ramírez-Mayans J, Cervantes-Bustamante R, Toro-Monjaraz E, Cázares-Méndez M, Cadena-León J, et al. Linfangiectasia intestinal primaria: 20 años de experiencia en el Instituto Nacional de Pediatría. Revista de Gastroenterología de México. 2014;79:7–12.