Endoscopy is the most effective method for identifying gastric adenocarcinoma (GAC). Interval gastric cancer (IGC) is GAC that is diagnosed 2–3 years after a normal endoscopy. Its characteristics are unknown in the Colombian environment. The clinical, histopathologic, and endoscopic characteristics were evaluated, along with the presentation rate, proton pump inhibitor (PPI) use, and IGC survival rate, and compared with other types of GAC.
MethodsA retrospective, analytic study was conducted on a prospective cohort. It evaluated 513 patients with GAC treated at our institution, within the time frame of January 2012 and June 2018. The patients had endoscopic diagnosis of GAC and endoscopy within the past three years that was negative for tumor.
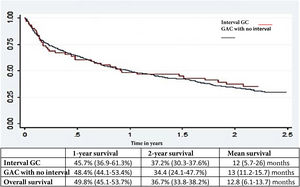
ResultsA total of 513 patients diagnosed with GAC were evaluated. Forty-two of the patients had IGC (8.2%): 9 early lesions and 33 advanced lesions (79%). The IGCs were smaller (31 vs. 41 mm; P < .01), as well as flatter and more depressed (P < .01). There was no association with PPI use, but there was an association with a history of gastrectomy and anastomosis (P = .02), as well as the absence of red flags (P < .003). The most frequent locations were the gastric body (52%) and the antrum (26%). Overall two-year survival was similar between IGC and GAC (37.1 vs. 39.3%, P = .72).
ConclusionA total of 8.2% of recently diagnosed GAC were cases of IGC. The presence of anastomosis and the absence of red flags were related to IGC. Overall survival was poor and there were no differences from the other types of GAC detected.
La endoscopia es el método más efectivo para identificar el adenocarcinoma gástrico (ACG). El cáncer gástrico de intervalo (CGI) es aquel ACG diagnosticado de 2 a 3 años posteriores a una endoscopia normal. Las características de esta entidad son desconocidas en nuestro medio. Se evaluaron características clínicas, histopatológicas, endoscópicas, tasa de presentación, consumo de inhibidores de bomba de protones (IBP) y sobrevida del CGI, y se comparó con los otros ACG.
MétodosEstudio retrospectivo analítico de cohorte prospectiva, realizado entre enero de 2012 y junio de 2018; evaluó 513 pacientes con ACG manejados en esta institución que tenían la endoscopia del diagnóstico de ACG y una endoscopia negativa para tumor en los últimos 3 años.
ResultadosSe evaluaron 513 pacientes con diagnóstico de ACG, 42 eran CGI (8.2%): 9 lesiones tempranas y 33 avanzados (79%). Los CGI fueron más pequeños (31 vs. 41 mm; p < 0.01), más planos y deprimidos (p < 0.01), no tuvo asociación con el uso de IBP, pero sí con el antecedente de gastrectomía y anastomosis (p = 0.02) y con la ausencia de signos de alarma (p < 0.003). La localización más frecuente fue el cuerpo gástrico (52%) y el antro (26%). La supervivencia global a 2 años fue similar entre CGI y ACG (37.1 vs. 39.3%; p = 0.72).
ConclusiónEl CGI representó el 8.2% de los ACG recién diagnosticados. La presencia de anastomosis y la ausencia de signos de alarma se relacionaron con CGI. La supervivencia global fue pobre y no tuvo diferencia con los otros ACG detectados.
According to GLOBOCAN 2020 figures, gastric adenocarcinoma (GAC) is the sixth most common cancer and the third cause of cancer death worldwide.1 In Colombia, despite great advances in diagnostic techniques and treatment, the disease is frequently diagnosed at advanced stages and only 30% of cases are candidates for curative treatment.2,3 Inversely, 5-year survival rates have been reported at above 70% for localized or early disease in Asian countries, primarily due to early detection programs in the population,4 emphasizing the importance of early detection, which nevertheless, is dependent on the local risk.
Esophagogastroduodenoscopy (EGD) with biopsy is the primary tool for detecting GAC,5 but it does not always detect existing cancers and its failure rate has been examined in several studies.6 In a 2014 meta-analysis, Menon et al. found that upper gastrointestinal tract cancer went undetected by EGD in 11.3% of the cases.7
Different authors define previously missed GAC, or interval gastric cancer (IGC), as disease undiagnosed by EGD, within 3 years prior to diagnosis. That is based on the hypothesis that GAC has a doubling time of 2 to 3 years.8 However more recent Japanese studies suggest that those times vary, according to disease stage, going from 16.6 months, for early lesions, to 7.6 months, for more advanced disease.9–10 Centered on that evidence, different studies have investigated the IGC rate from 2 to 5 years prior to diagnosis, finding the widely ranging rates of 4.6% to 25.8%.11–14
Strictly speaking, the term interval cancer is applied to undetected neoplastic lesions during the course of a screening program in at-risk populations, such as those implemented in the West for colorectal cancer (CRC) or those for gastric cancer conducted in Korea or Japan. Thus, the term would not fit lesions outside of those program, nor undetected lesions during endoscopy that is usually performed for diagnostic purposes. A more pertinent description of those lesions would be undetected or post-endoscopy gastric cancer.
The term “interval gastric cancer” was coined in Korea and Japan, countries that have nationally established gastric cancer detection programs. For Western physicians, interval CRC may be a more familiar corresponding term.15 Currently, low quality colonoscopy is recognized as the most important factor for the appearance of interval CRC,16 and is inclined to be extrapolated to ICG and EGD. The exact rate of undiagnosed or undetected GAC during endoscopy has not been established. Japanese studies describe high figures of 25.8%,12 studies from the United Kingdom report 2.3 to 14%,17 Australia 0.41%,18 the United States 5.5 to 11.5%,19 and Korea 0.2%,20 but the factors that induce the diagnostic error of GAC have not been specified and there are few and fairly non-standardized articles describing the correct techniques for performing high quality endoscopy and preventing said error.
IGC is more common in tumors located in the middle part of the stomach and in lesions of undifferentiated cancer.20 Previous reports revealed that IGC was more frequent in upper gastrointestinal tract radiography, compared with endoscopic studies.21
IGC includes both unseen and latent lesions. The occurrence of undetected lesions can be reduced through detailed examination with chromoendoscopy and/or a repeat EGD with improved images and good biopsy sampling, whereas the development of latent lesions may be inevitable. Treatment with proteolytic enzymes to prevent mucus, before the procedure, is another option for improving endoscopic visibility,22 although its use has recently been questioned,23 and it is not routinely implemented at endoscopy services. Another problem is controlling the quality of the endoscopy. The experience of the endoscopist can influence the development of IGC and endoscopists should take care to avoid blind spots. Currently, the Korean Society of Gastrointestinal Endoscopy recommends 8 EGD images as the standard, which includes only 4 images of the stomach. That was initially suggested by the European guidelines.24
The Korean screening program found that background atrophy and intestinal metaplasia (IM) of the stomach were related to the development of IGC. The unevenness of the gastric mucosa in IM is a plausible hindrance for the endoscopic detection of early gastric cancer,25 making the use of techniques, such as digital or vital chromoendoscopy, magnification, and closer follow-up, more valuable.
The aims of the present study were: 1) to determine the rate of IGC, 2) to establish the demographic characteristics of those patients, 3) to evaluate their endoscopic and histologic characteristics, and 4) to determine the IGC survival rate and compare it with that of other types of GAC, with no previous negative endoscopies for neoplasia.
Materials and methodsStudy of the population and proceduresAll the patients with gastric cancer seen in consultation by one of the authors, within the time frame of January 2012 and July 2018, were evaluated. The inclusion criteria were patients with a histopathologic diagnosis of GAC made during the study period and having a one-year follow-up.
The exclusion criteria were an incomplete EGD or an EGD with an abnormal finding, suggesting neoplasia, but uncorroborated by biopsy.
The physical and electronic clinical histories of all the patients with GAC were reviewed, including previous negative EGDs performed at other institutions.
The following aspects were evaluated: demographic (age and sex) and clinical data; EGD indication (dysphagia, hematemesis, melena, vomiting, and constitutional syndrome were considered red flags); and a history of proton pump inhibitor (PPI) therapy within the past three months, for patients with IGC, as well as for patients with GAC and no previous negative endoscopy. The endoscopic characteristics obtained from the endoscopy report were lesion size (in millimeters); the presence of ulceration; location (gastroesophageal junction, fundus, body [with the incisura], and antrum); and tumor morphology (depressed, flat, sessile, or mass-like). When there was more than one negative EGD, the most recent one was selected for the analysis, discarding previous endoscopies, to adjust to the time intervals stipulated in the inclusion criteria.
The histologic subtype (intestinal or diffuse adenocarcinoma) and differentiation grade (undifferentiated, poorly differentiated, moderately differentiated, or well-differentiated) were also obtained from the pathology reports.
Tumor stage was determined, according to the tenth edition of the TNM system of the American Joint Committee on Cancer. Overall survival was established from the date of GAC diagnosis to the date of death. Survivors were recorded, utilizing the date of their last medical visit.
Study pointsIGC was defined as the number of patients with GAC that had a negative endoscopy within 36 months prior to diagnosis. The primary aim was to evaluate the proportion of IGC and its endoscopic and histologic characteristics. The secondary aim was to evaluate the differences in survival between IGC and GAC with no prior negative endoscopy.
Statistical analysisThe mean, standard deviation, median, and range were calculated for the continuous variables and frequency and percentage were calculated for the categorical variables. Utilizing the Wilson method, the 95% confidence intervals for the proportions were calculated. The data were analyzed using parametric methods for normally distributed continuous data (Student’s t test) and nonparametric methods (Mann-Whitney U test) for continuous data that were not normally distributed.
The chi-square test and Fisher’s exact test were employed for the categorical data. To reduce the risk for type I error, only the variables previously reported as risk factors for IGC, or with a plausible pathophysiologic relation to IGC, were included.
One and 2-year survival probabilities were calculated for IGC and GAC with no interval, through the Kaplan-Meier method. The log-rank test was utilized to evaluate overall survival. All the analyses were bilateral (two-tailed) and p values below 0.05 were considered significant. All the statistical calculations were performed at the research institution, utilizing IBM SPSS® version 24 software.
Ethical considerationsThe present study conducted at the institution of cancerology was classified as research with no biologic, physiologic, psychologic, or social risks, according to the international Declaration of Helsinki, the Belmont report, and the 1993 Resolution 8430 of the Colombian Department of Health, Title II, Article 11. The 1999 Colombian Resolution 1995, which establishes the norms for clinical history management was also taken into account.
ResultsWithin the 6.5 years of the study period, 631 patients with gastric tumors, of which 513 were GAC (81%), were evaluated at their medical consultations. The majority of the patients were men (68%). Forty-two patients presented with IGC for an overall rate of 8.2%. The mean age of the patients with detected GAC was 72 ± 14.2 years, whereas the mean age for the patients with IGC was 66 ± 16.6 years. Table 1 shows the characteristics of the two groups of patients.
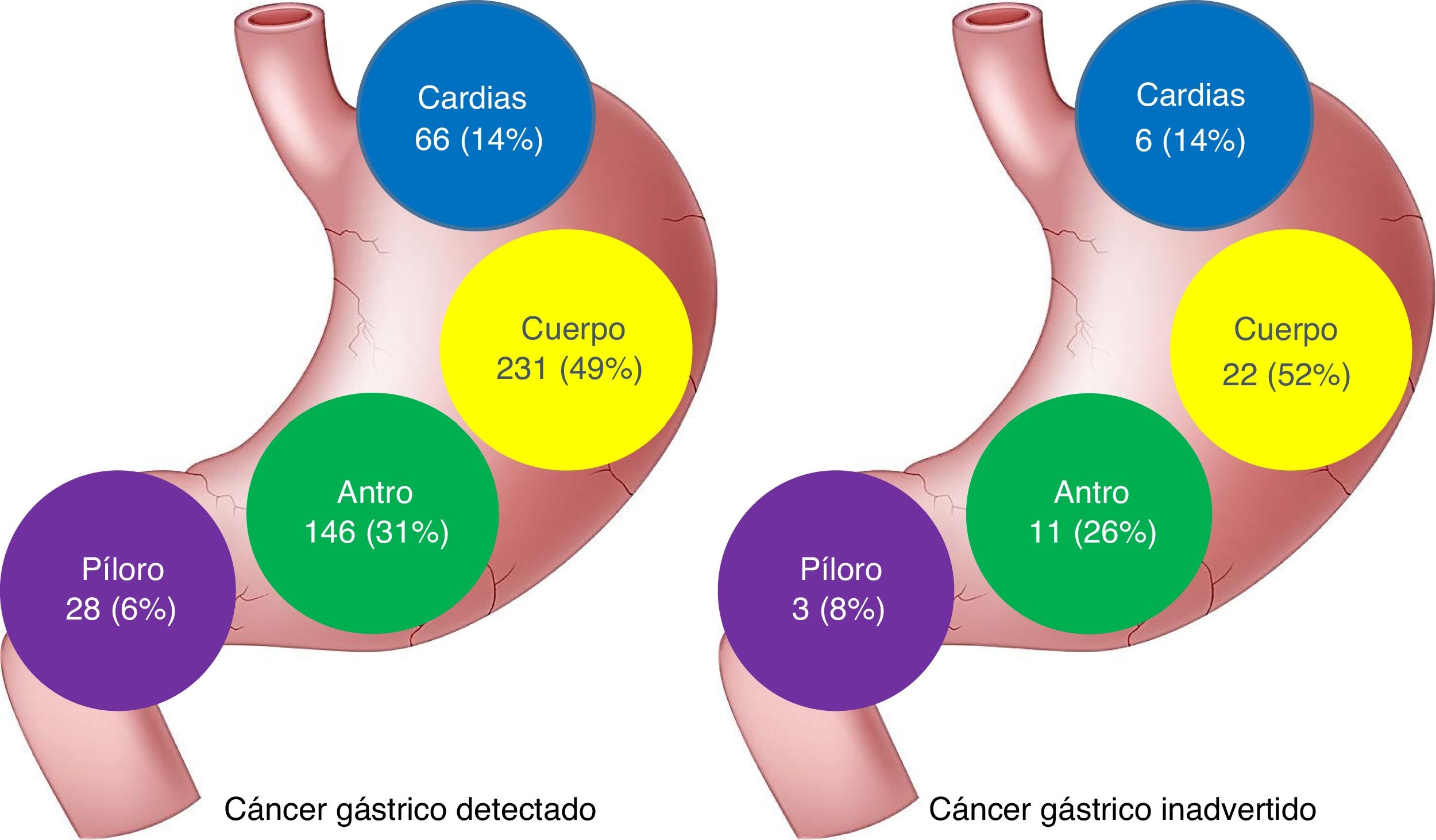
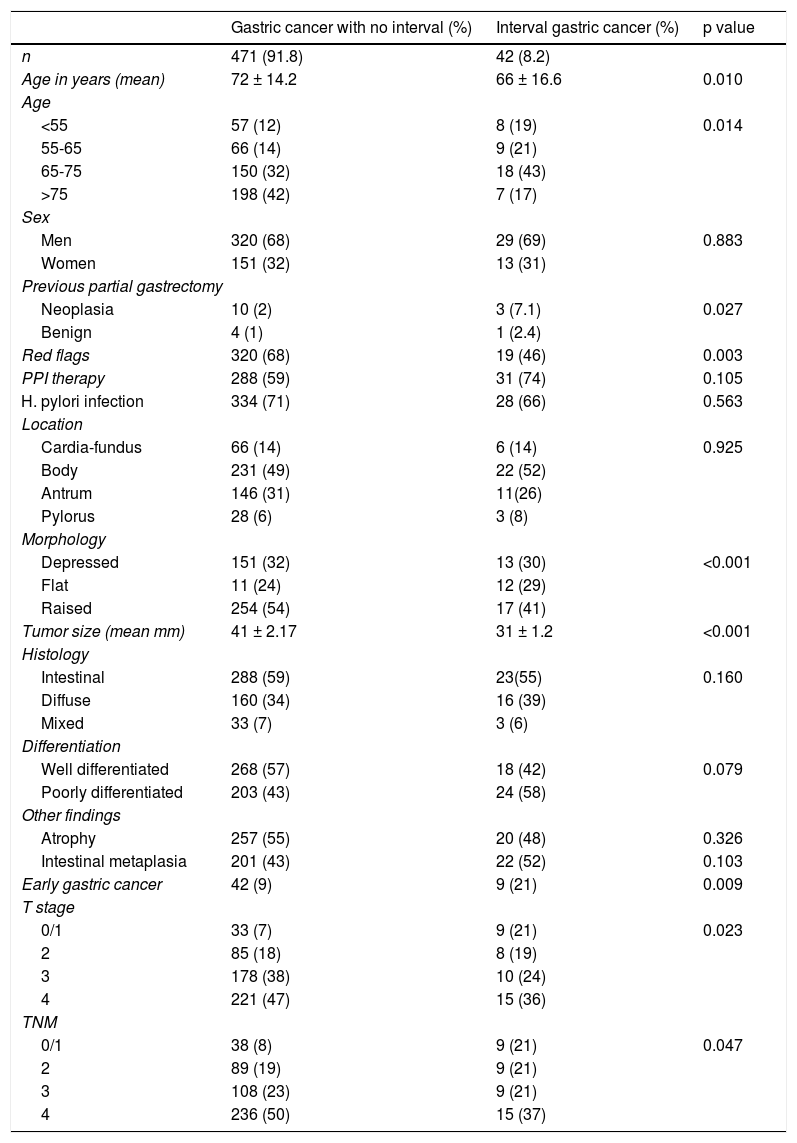
Number and characteristics of the patients that had undergone an endoscopy within 3 years before their gastric cancer diagnosis.
| Gastric cancer with no interval (%) | Interval gastric cancer (%) | p value | |
|---|---|---|---|
| n | 471 (91.8) | 42 (8.2) | |
| Age in years (mean) | 72 ± 14.2 | 66 ± 16.6 | 0.010 |
| Age | |||
| <55 | 57 (12) | 8 (19) | 0.014 |
| 55-65 | 66 (14) | 9 (21) | |
| 65-75 | 150 (32) | 18 (43) | |
| >75 | 198 (42) | 7 (17) | |
| Sex | |||
| Men | 320 (68) | 29 (69) | 0.883 |
| Women | 151 (32) | 13 (31) | |
| Previous partial gastrectomy | |||
| Neoplasia | 10 (2) | 3 (7.1) | 0.027 |
| Benign | 4 (1) | 1 (2.4) | |
| Red flags | 320 (68) | 19 (46) | 0.003 |
| PPI therapy | 288 (59) | 31 (74) | 0.105 |
| H. pylori infection | 334 (71) | 28 (66) | 0.563 |
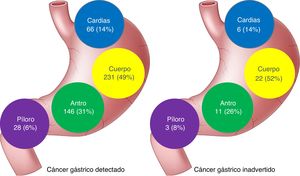
| Location | |||
| Cardia-fundus | 66 (14) | 6 (14) | 0.925 |
| Body | 231 (49) | 22 (52) | |
| Antrum | 146 (31) | 11(26) | |
| Pylorus | 28 (6) | 3 (8) | |
| Morphology | |||
| Depressed | 151 (32) | 13 (30) | <0.001 |
| Flat | 11 (24) | 12 (29) | |
| Raised | 254 (54) | 17 (41) | |
| Tumor size (mean mm) | 41 ± 2.17 | 31 ± 1.2 | <0.001 |
| Histology | |||
| Intestinal | 288 (59) | 23(55) | 0.160 |
| Diffuse | 160 (34) | 16 (39) | |
| Mixed | 33 (7) | 3 (6) | |
| Differentiation | |||
| Well differentiated | 268 (57) | 18 (42) | 0.079 |
| Poorly differentiated | 203 (43) | 24 (58) | |
| Other findings | |||
| Atrophy | 257 (55) | 20 (48) | 0.326 |
| Intestinal metaplasia | 201 (43) | 22 (52) | 0.103 |
| Early gastric cancer | 42 (9) | 9 (21) | 0.009 |
| T stage | |||
| 0/1 | 33 (7) | 9 (21) | 0.023 |
| 2 | 85 (18) | 8 (19) | |
| 3 | 178 (38) | 10 (24) | |
| 4 | 221 (47) | 15 (36) | |
| TNM | |||
| 0/1 | 38 (8) | 9 (21) | 0.047 |
| 2 | 89 (19) | 9 (21) | |
| 3 | 108 (23) | 9 (21) | |
| 4 | 236 (50) | 15 (37) | |
PPI: proton pump inhibitor.
There were no differences in relation to sex, albeit there was a predominance of men with the disease. The patients with IGC were younger than the patients with GAC detected at the first endoscopy (p = 0.01).
Clinical dataRed flags (dysphagia, hematemesis, melena, vomiting, and constitutional syndrome) were more frequent in the patients with GAC detected at the first endoscopy (68 vs 46%, OR: 0.28, p = 0.003) and a history of gastrectomy was less frequent in those patients (p = 0.027). Unlike that suggested in the literature, neither PPI use nor H. pylori infection was predominant in either of the groups.
Endoscopic aspectsThe mean time interval between negative EGD and IGC diagnosis was 14.4 months (range: 2-34.6). Of the 42 patients with IGC, 45% (19/42) had a negative EGD at an interval of < 1 year, 22% (9/42) within 1-2 years, and 33% (14/42) within 2-3 years. The median number of negative endoscopies in the IGC group was 1 (range: 1-3). The most common findings in the negative EGDs were gastritis (31/42, 73%), IM (15/42, 36%), gastric atrophy (19/42, 45%), and gastric ulcer (12/42, 29%). Four patients (4/42, 2%) had a negative EGD reported as normal.
No differences in the location of the lesions were found between the 2 groups of patients, but the missed lesions tended to have a more proximal location (Fig. 1).
Regarding morphology, flat and excavated lesions (ulcers) predominated in IGC (p < 0.001). IGC tumors were smaller than the GAC tumors with no negative endoscopy (31 ± 1.2 mm vs 41 ± 2.17 mm, OR: 0.98, p < 0.001)
Histologic findingsThere were no significant differences between the histologic variants (p = 0.160) or in the differentiation grades between the 2 groups. Early gastric cancer was more frequent in the patients with previous negative endoscopies (21 vs 9%, p = 0.009).
Neither gastric atrophy nor IM were risk markers for the presentation or not of IGC.
Tumor stagingTumors were deeper in GAC with no negative endoscopy than in IGC, with 75% T3-4 vs 60% T3-4, respectively (p = 0.023). However, 21% of the GAC tumors diagnosed in patients with a prior negative endoscopy were in stage 0 or 1 (curable stages), compared with only 9% of the tumors in patients with no prior negative endoscopy (p = 0.009). Likewise, only 58% of the IGC tumors were in stage 3 or 4, compared with 73% in the patients that had not had a previous endoscopy (p = 0.047).
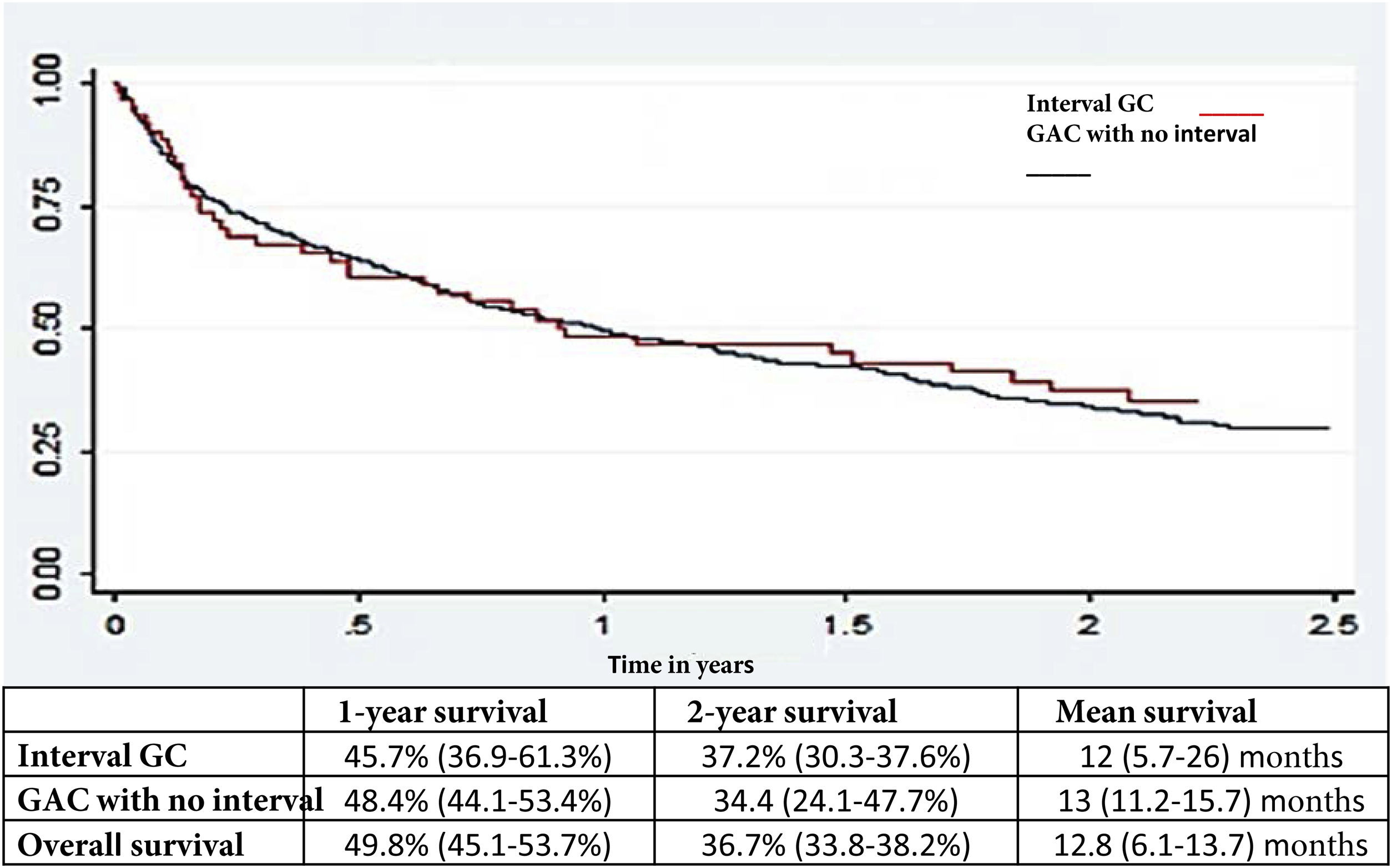
Survival analysisThere was no difference in the general survival between IGC and non-IGC (Fig. 2).
DiscussionThe present study is the first in Colombia to evaluate the IGC rate in a cohort of patients diagnosed with GAC, and found an intermediate rate (8.2%), with respect to that reported in the international medical literature (4.6-14.3%).26 The lack of a unified IGC definition and substantial heterogeneity among authors, mainly regarding the amount of time for determining the time interval of IGC, are important factors to point out.
Based on the historic study by Fujita8 that suggests a doubling time of 2 to 3 years for GAC, the majority of studies have considered a time interval of 6 months to 3.5 years. If the doubling time for cancer of the mucosa is supposed at 2 to 3 years, GAC diagnosed within that interval, after a normal endoscopy, could have gone unnoticed in the initial EGD.
Unlike results reported in other publications,14,27–29 we found that PPI therapy was not an independent predictor of negative endoscopy. Partial cure of the mucosa of the lesions is attributed to PPIs, which would result in less advanced lesions. However, despite the fact that PPI use is massive in our environment, we found no differences between groups. Our study ratified what has previously been reported,14 with respect to the higher incidence of IGC in patients with prior gastric surgery (subtotal gastrectomy) due to cancer or benign lesions. A possible explanation is that the altered gastric anatomy could contribute to not seeing the lesions. Female sex, younger age, an endoscopist that is not a gastroenterologist, studies carried out without sedation, and a patient with more comorbidities have all been postulated to increase the probability of undiagnosed upper gastrointestinal cancer in previous endoscopies.11,30,31
The presence of red flags was significantly lower in IGC, compared with the diagnosis of GAC with no previous negative endoscopies (46 vs 68%, p < 0.003), which is consistent with the fact that IGC tumors were smaller (mean size: 31 ± 1.2 mm vs 41 ± 2.17 mm, OR: 0.98, p < 0.001) and were diagnosed at a less advanced stage (early gastric cancer in 21 vs 9%, p = 0.009).
We found that almost one out of every 3 patients with a negative EGD had a gastric ulcer, some of which could be GAC that was not correctly diagnosed. Those ulcers should be biopsied and reevaluated after treatment, including H. pylori eradication, when indicated, within 6-8 weeks.32 Sensitivity in the diagnosis of GAC increases with the number of biopsy samples, and if malignancy is suspected, at least seven samples of the heaped up edges of the ulcer and base should be taken.33
In our cohort, the IGC tumors were more frequently found to be flat or depressed lesions and were smaller than the GAC tumors with no negative endoscopies, which could have contributed to their not being observed in the EGD. In accordance with the available literature,11,13,14,17,19,30,32,34,35 no differences in the histologic subtype or differentiation grade were found. Stage 1 disease was identified in only 21% of the IGC tumors and 76% of the patients were diagnosed within 2 years from the negative EGD, making it probable that the majority of the IGC tumors were “true” lesions, explained by an unrecognized lesion, albeit the possibility of new fast-growing lesions continues to be plausible.
The most frequent location of IGC was the gastric body and no significant differences were found with the primarily detected GAC tumors. Unlike CRC, in which the right colon has been shown to be a risk factor for interval cancer,36,37 there appears to be no relation between location and IGC.7,26
Another crucial factor, despite the fact that IGC is increasingly being diagnosed in stages 1-2, was that one and 2-year survival rates continue to be discouraging, emphasizing the importance of early diagnosis, as well as the consequences of missing a malignant lesion.
There are several possible explanations for IGC, and they include: limitations regarding the endoscopic technique and lesion recognition; inadequate supervision of students; sampling error (very few or inexact biopsies); lack of tolerance of the patient toward the procedure or inadequate sedation, resulting in a poor or incomplete evaluation of the mucosa; inadequate follow-up; and errors in the histopathologic interpretation. The Japanese experience with gastric cancer emphasizes the importance of meticulous EGD. That involves the preparation of the patient with an antifoaming agent combined with a mucolytic agent, for better visibility; careful and systemic inspection of the stomach, with adequate air insufflation to flatten the gastric folds; and extensive photographic documentation (> 20 images), to guarantee adequate visualization of all the areas of the stomach.38 Clinical trials on antifoaming and mucolytic agents have shown that their administration improves the visualization of the mucosa.39
A call to action could be made based on the IGC rate found in the present study, using it as a possible indicator of upper gastrointestinal endoscopy quality.11,30,31,40 Quality indicators have been studied in relation to colonoscopy, whereas such indicators have not been standardized for EGD, creating a space for greater investigation. In future studies on quality indicators for EGD, rates of failure in the detection of upper gastrointestinal cancers could be evaluated as indicators of quality, after unifying the definition of interval gastric cancer.
Key factors for said improvement include better training for endoscopists and greater advancement in endoscopes. Establishing an improved training system for endoscopists and ensuring the quality of endoscopy are essential for more successful endoscopic detection of gastric cancer.41,42 With respect to advances in endoscopes in the detection and diagnosis of upper gastrointestinal cancers, better endoscopic images, with or without magnification, would be a contributing factor to improvement.32 The rate of IGC has been suggested to range from 4.6 to 14.3% in Western countries.26
From the perspective of endoscopy as a limited resource, the stratification of individual risk is required, instead of performing surveillance endoscopy within the 3 years after the previous endoscopy on all persons. Thus, how to incorporate the risk factors for gastric cancer, such as the status of H. pylori infection43 and atrophic gastritis with IM,44 should be studied in detection programs, as well as surveillance programs. A large prospective study on those factors is justified.
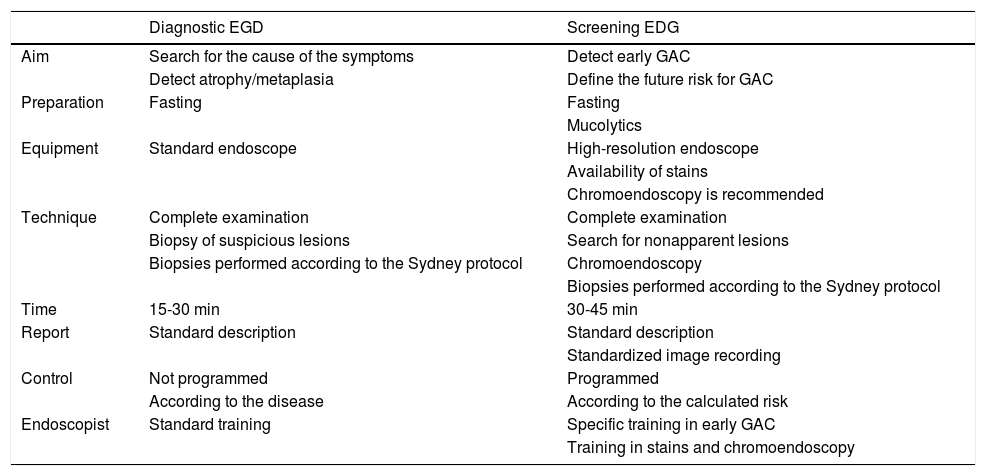
The endoscopist must differentiate the type of endoscopy to be performed for improving the possibility of detecting incipient, or even premalignant, gastric lesions. It requires a different posture from that of habitual diagnostic endoscopy or screening endoscopy. Table 2 underlines those points.
Differences between diagnostic endoscopy and screening endoscopy for the detection of preneoplastic lesions or early GAC.
| Diagnostic EGD | Screening EDG | |
|---|---|---|
| Aim | Search for the cause of the symptoms | Detect early GAC |
| Detect atrophy/metaplasia | Define the future risk for GAC | |
| Preparation | Fasting | Fasting |
| Mucolytics | ||
| Equipment | Standard endoscope | High-resolution endoscope |
| Availability of stains | ||
| Chromoendoscopy is recommended | ||
| Technique | Complete examination | Complete examination |
| Biopsy of suspicious lesions | Search for nonapparent lesions | |
| Biopsies performed according to the Sydney protocol | Chromoendoscopy | |
| Biopsies performed according to the Sydney protocol | ||
| Time | 15-30 min | 30-45 min |
| Report | Standard description | Standard description |
| Standardized image recording | ||
| Control | Not programmed | Programmed |
| According to the disease | According to the calculated risk | |
| Endoscopist | Standard training | Specific training in early GAC |
| Training in stains and chromoendoscopy |
EGD: esophagogastroduodenoscopy; GAC: gastric adenocarcinoma.
The present study has certain limitations. First, the retrospective, observational design did not allow us to collect relevant information, such as the time of the gastric examination, sedation and/or tolerance to the study, or family history and genetic data, among others. Second, the lack of a national cancer register database did not enable us to determine whether a patient with a negative EGD at our institution was later diagnosed with GAC at other hospitals. Lastly, the present study may have little power for detecting a small difference in survival due to the reduced sample size.
In conclusion, IGC accounted for 8.2% of all the GAC treated over a 6.5-year period and most likely arose from an early undetected cancer in the majority of cases. Anastomoses can contribute to missing malignant lesions and pump blockers do not increase the incidence of omission. Adequate biopsy sampling and follow-up of the healing of ulcers are easily available strategies for daily clinical practice that could reduce the IGC rate and improve the prognosis of GAC in Western countries. To impact that IGC rate, we make a call for better training for endoscopists, further study and development of endoscopy in relation to detection and diagnosis, and the establishment of appropriate surveillance programs in places that require them. It is also essential for each endoscopist to always have in mind the risk for missing important lesions and make an effort to achieve the best performance in every upper gastrointestinal endoscopy procedure.
Conflict of interestThe authors declare that there is no conflict of interest.
Financial disclosureNo financial support was received from any public or private institution in relation to this study/article.
Please cite this article as: Castaño-Llano R, Piñeres A, Jaramillo R, Molina S, Aristizábal F, Puerta JE. Cáncer gástrico de intervalo: un llamado a la atención y a la acción. Rev Gastroenterol Méx. 2023;88:91–99.
The present study was carried out with the support of the Sustainability Project of the Research Vice Chancellery of the Universidad de Antioquia, Medellín-Colombia.