An anastomotic leak is one of the most dreaded complications in colorectal surgery because it increases postoperative morbidity and mortality. The aim of the present study was to identify whether indocyanine green fluorescence angiography (ICGFA) reduced the anastomotic dehiscence rate in colorectal surgery.
Material and methodsA retrospective study on patients that underwent colorectal surgery with colonic resection or low anterior resection and primary anastomosis, within the time frame of January 2019 and September 2021, was conducted. The patients were divided into the case group, in which ICGFA was performed for the intraoperative evaluation of blood perfusion at the anastomosis site, and the control group, in which ICGFA was not utilized.
ResultsA total of 168 medical records were reviewed, resulting in 83 cases and 85 controls. Inadequate perfusion that required changing the surgical site of the anastomosis was identified in 4.8% of the case group (n = 4). A trend toward reducing the leak rate with ICGFA was identified (6% [n = 5] in the cases vs 7.1% in the controls [n = 6] [p = 0.999]). The patients that underwent anastomosis site change due to inadequate perfusion had a 0% leak rate.
ConclusionsICGFA as a method to evaluate intraoperative blood perfusion showed a trend toward reducing the incidence of anastomotic leak in colorectal surgery.
La fuga de anastomosis es una de las complicaciones más temidas de la cirugía colorrectal ya que aumenta la morbimortalidad postoperatoria. El objetivo de este manuscrito fue identificar si la angiografía por fluorescencia con verde de indocianina (AFVI) disminuía la tasa de dehiscencia de anastomosis en cirugía colorrectal.
Material y métodosSe realizó un estudio retrospectivo de enero 2019 a septiembre 2021 de los pacientes que fueron sometidos a cirugía colorrectal con resección colónica o resección anterior baja y anastomosis primaria. Se dividió a los pacientes en dos grupos, un grupo de estudio en los que se realizó la AFVI para valoración transoperatoria de la perfusión sanguínea en el sitio de anastomosis y un grupo control en los que no se utilizó.
ResultadosSe revisaron un total de 168 expedientes obteniendo un grupo control de 85 pacientes y un grupo de estudio de 83 pacientes. En el grupo de estudio se identificó una inadecuada perfusión que requirió un cambio del sitio de anastomosis en el 4.8% (n = 4). Se identificó una tendencia a la disminución de la tasa de fuga con la AFVI en el grupo de estudio 6% (n = 5) vs grupo control 7.1% (n = 6) (p .999). Se encontró una tasa de 0% de fugas en los pacientes con cambio de sitio de anastomosis por perfusión inadecuada.
ConclusionesEl uso de AFVI como método de valoración de la perfusión sanguínea de manera transoperatoria muestra una tendencia a disminuir la incidencia de fuga de anastomosis en cirugía colorrectal.
Anastomotic leak is one of the most dreaded complications in colorectal surgery because it increases postoperative morbidity and mortality1,2. It is defined as “the communication between the intraluminal and extraluminal compartments due to a defect in intestinal wall integrity at the site of the anastomosis” 1. The rate of anastomotic leak in colorectal surgery varies widely, with an average incidence of 1–19%2–4. Multiple factors are related to anastomotic leaks and one of the most important is ischemia at the anastomosis site5–8. During surgery, the surgeon makes a clinical evaluation of the perfusion at the anastomosis site, to decide whether or not it is viable. However, the sensitivity and specificity for the prediction of anastomotic leaks by surgeons is 38% and 46%, respectively, for anastomoses located more than 15 cm from the anal verge, and 62% and 52%, respectively, for anastomoses located at fewer than 15 cm from the anal verge9.
Indocyanine green is a sterile, anionic, water-soluble, and relatively hydrophobic molecule of tricarbocyanine; once it is injected intravenously into the vascular system, it binds to plasma proteins10,11. As stated by Reinhart et al.12, the dye has been used as a contrast agent since the mid 1950s to evaluate heart and liver function, and at the beginning of 2000, its fluorescent reactivity was discovered, when stimulated by infrared light. Thus, it enables the real-time visualization of the blood flow through angiography, resulting in multiple clinical applications13. In colorectal surgery, it was first described by Kudszus et al.14 as a way to evaluate perfusion at the anastomosis site. The aim of our study was to determine if the incidence of anastomotic leaks was reduced by the use of indocyanine green for intraoperatively evaluating blood perfusion at the anastomosis site.
Materials and methodsA retrospective case-control study was conducted on patients that underwent colorectal surgery with procedures of colectomy or low anterior resection (LAR) and primary anastomosis, within the time frame of January 2019 and September 2021. A total of 178 patients were identified, 10 of whom were excluded due to a history of prior laparotomy or noncompliance with the postoperative follow-up in the first 30 days after surgery. Eighty-three patients underwent indocyanine green fluorescence angiography (ICGFA) (cases) and 85 patients had the standard procedure (controls). All the surgeries were performed by the same group of colorectal surgeons (Coloncare, Monterrey, Mexico), with extensive experience in both the conventional and laparoscopic approaches. According to the surgical procedure, the patients were categorized into 5 groups: right colectomy, left colectomy, sigmoid colectomy, LAR, and ultralow anterior resection (ULAR). Patient comorbidities, diagnoses, and preoperative study results were registered.
Surgical techniqueAll the surgical procedures were performed by the same group of colorectal surgeons through the laparoscopic approach. All the patients were placed in the Lloyd-Davis position and received balanced general anesthesia. Those undergoing right colectomy were placed in the left-sided partial Trendelenburg position and the patients that underwent left colectomy, sigmoid colectomy, LAR, or ULAR were placed in the right-sided Trendelenburg position. Anastomotic donut examination, the air leak test, and direct visualization through colonoscopy or rectosigmoidoscopy were evaluated in the patients that underwent left colectomy/sigmoid colectomy or LAR/ULAR; ICGFA was also evaluated in the group of patients in which the imaging technique was used (cases).
Right colectomyThe surgeon was at the left side of the patient and placed 4 trocars. First, the cecum and the ileocolic pedicle were identified. Medial to lateral dissection was carried out. Once the right colon was freed, the proximal and distal resection sites were identified. ICGFA was performed in the case group at the levels of the terminal ileum and the transverse colon, or the resection was directly performed in the control group, utilizing a 60 mm linear stapler with a purple cartridge at the level of the terminal ileum and a purple cartridge at the level of the transverse colon. Lastly, a side-to-side isoperistaltic intracorporeal anastomosis was performed with a purple cartridge stapler through enterotomy (in the case group, once the stapler was placed, the perfusion test was repeated before the shot) and the anastomosis was closed with 2–0 barbed continuous sutures. The specimen was put in a 12–15 mm laparoscopic bag and removed by means of a Pfannenstiel incision, placing the Alexis® O™ (Applied Medical, California, USA) wound protector.
Left colectomy/sigmoid colectomyThe surgeon was at the right side of the patient and 4 trocars were used. First, the lesion was identified. The medial to lateral approach was utilized and the splenic angle was freed, if required, to have a tension-free anastomosis. If the pathology was benign, the superior hemorrhoidal artery was spared. Once the colon was freed, the resection sites were marked with clips at the proximal level and sectioning at the distal level was performed with a 60 mm linear stapler. A Pfannenstiel incision was made, the Alexis® O™ (Applied Medical, California, USA) wound protector was placed, and the specimen was extracted. ICGFA was then performed in the case group patients, or the resection was directly performed in the control group. The resection was carried out with a scalpel at the site identified with adequate perfusion, either clinically or utilizing ICGFA. The anvil of a circular stapler was inserted, and the wound was closed using 0 monofilament purse-string sutures. The cavity was closed, turning the Alexis protector, and pneumoperitoneum was restarted to perform the colorectal anastomosis with a circular stapler. ICGFA was then repeated in the case group patients, confirming adequate perfusion at the anastomosis site. The specimen was removed through a Pfannenstiel incision.
Low anterior resection/ultralow anterior resectionThe surgeon was at the right side of the patient and used 4 trocars. First, the lesion was identified. The medial to lateral approach was utilized and the splenic angle was freed. Once the left colon and sigmoid colon were freed, the peritoneal reflexion was incised and the mesorectum dissected, respecting its integrity inside the surgical specimen. Dissection was carried out, depending on the lesion site, to obtain an adequate distal margin. The resection sites were marked with hemoclips at the proximal level and sectioning was performed at the distal level with a 60 mm linear stapler. A Pfannenstiel incision was made, the Alexis® O™ (Applied Medical, California, USA) wound protector was placed, and the specimen was extracted. ICGFA was performed in the case group, or resection was directly performed in the control group. The resection was carried out with a scalpel at the site identified with adequate perfusion, either clinically or utilizing ICGFA. The anvil was inserted, and the wound was closed using 0 monofilament purse-string sutures. The cavity was closed, turning the Alexis protector, and pneumoperitoneum was restarted to perform the colorectal anastomosis with a 28 mm circular stapler. ICGFA was then repeated in the case group patients, confirming adequate perfusion at the anastomosis site. The specimen was removed through a Pfannenstiel incision. If the surgeon deemed it necessary, a diverting loop ileostomy was done.
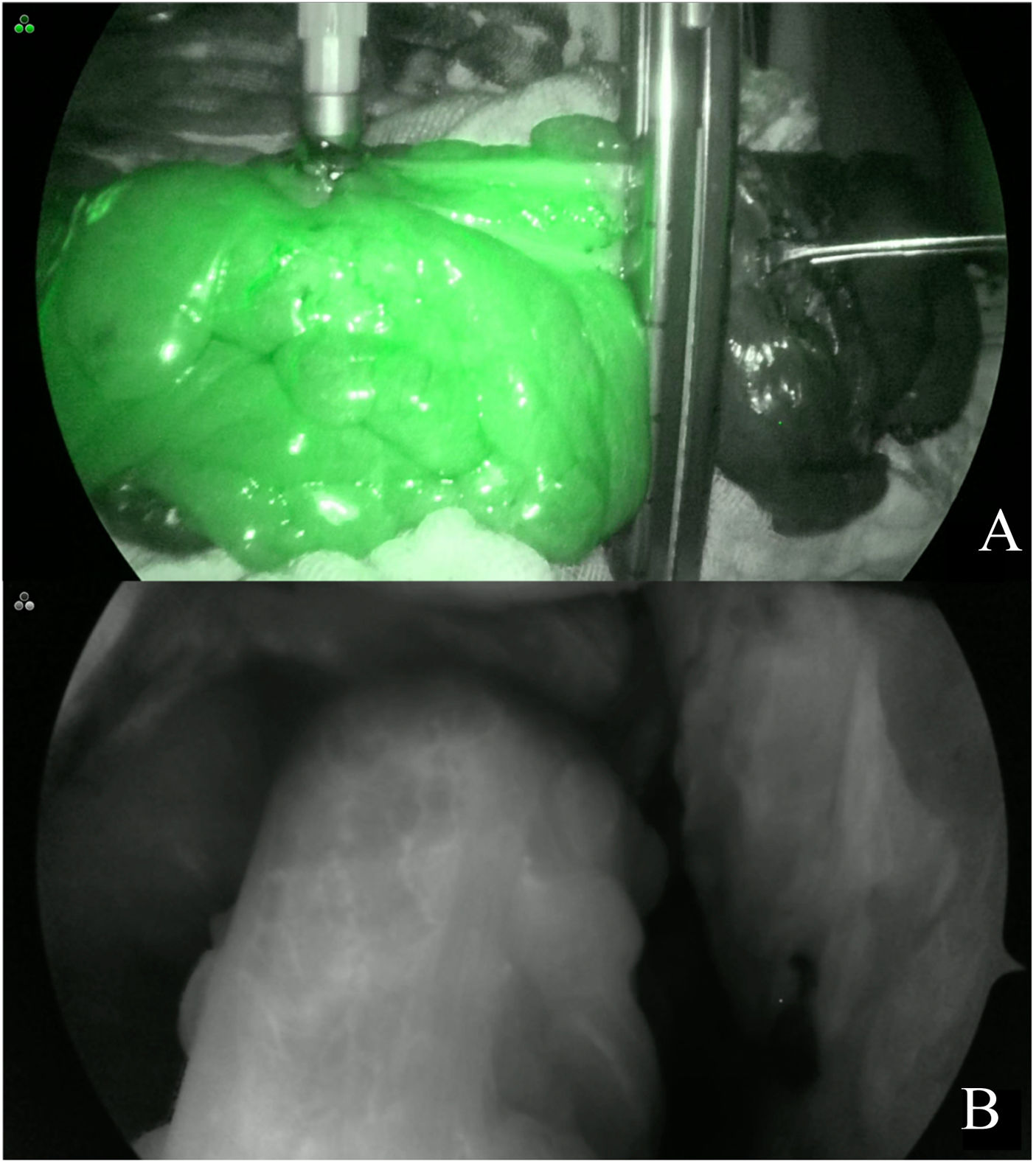
Indocyanine green fluorescence angiographyA Stryker 1688 platform was utilized for the ICGFA. Once the specimen was dissected and the proximal resection site was selected according to its macroscopic characteristics, a bolus of indocyanine green at a dose of 7.5 mg in 3 ml of physiologic solution was intravenously administered by the peripheral route. Near-infrared light was then utilized at the resection site to perform ICGFA and visually evaluate whether there was adequate perfusion (Fig. 1A, B). When ICGFA revealed a proximal resection site with inadequate perfusion because the contrast could not be visualized, the site was changed to perform the anastomosis at a location where the contrast could be seen; said changes were considered therapeutic decision modifications. Once the resection and anastomosis were carried out, the imaging test was repeated at the same dose.
Patients were considered to have “adequate perfusion” when, after the intravenous administration of indocyanine green and the use of near-infrared light, the contrast could be seen in the tissue in fewer than 60 s in the SPY ENV and SPY Contrast imaging modalities.
Patients were considered to have “inadequate perfusion” when, after the intravenous administration of indocyanine green and the use of near-infrared light, the contrast could not be seen in the SPY ENV and SPY Contrast imaging modalities in the tissue, or visualization time was longer than 60 s.
Study designThe registered patient data were sex, age, body mass index (BMI), comorbidities, preoperative test results (hemoglobin, albumin, leukocytes, percentage of lymphocytes, platelets), and indication for surgery, as well as the operation variables, such as the surgical procedure performed, conversions, blood loss, surgery duration, complications, ICGFA, change in the previously decided anastomosis site, the use of drainage and diverting ileostomy, and the findings of obstruction, perforation, local sepsis, generalized sepsis, focal peritonitis, or purulent peritonitis.
Statistical analysisFor the statistical analysis, the quantitative variables were reported as median and interquartile range, with prior proof of their distribution through the Kolmogorov-Smirnov test, and the qualitative variables were reported as frequency and percentage. For the comparison of the two groups (the case group [with indocyanine green use] and the control group [without indocyanine green use]), the chi-square test was used to compare the qualitative variables and the Mann-Whitney U test for the quantitative variables. Statistical significance was set at a p < 0.05. The SPSS Statistics version 24 (Armonk, NY) was used for the statistical analysis.
Ethical considerationsThe authors declare that no experiments on humans or animals were conducted for the present study, that they followed the protocols of each work center of the participating hospitals on the publication of patient data. The collaborating physicians applied the standards, ethical requisites, informed consent for patient diagnosis, management. Written statements of informed consent were not requested for this study, given that it was a retrospective multicenter analysis, included no personal data that could identify the patients.
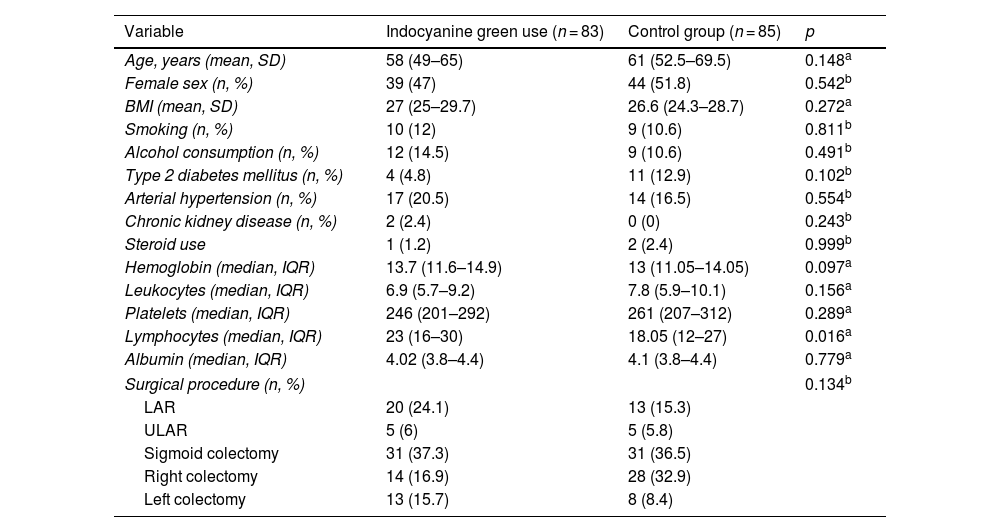
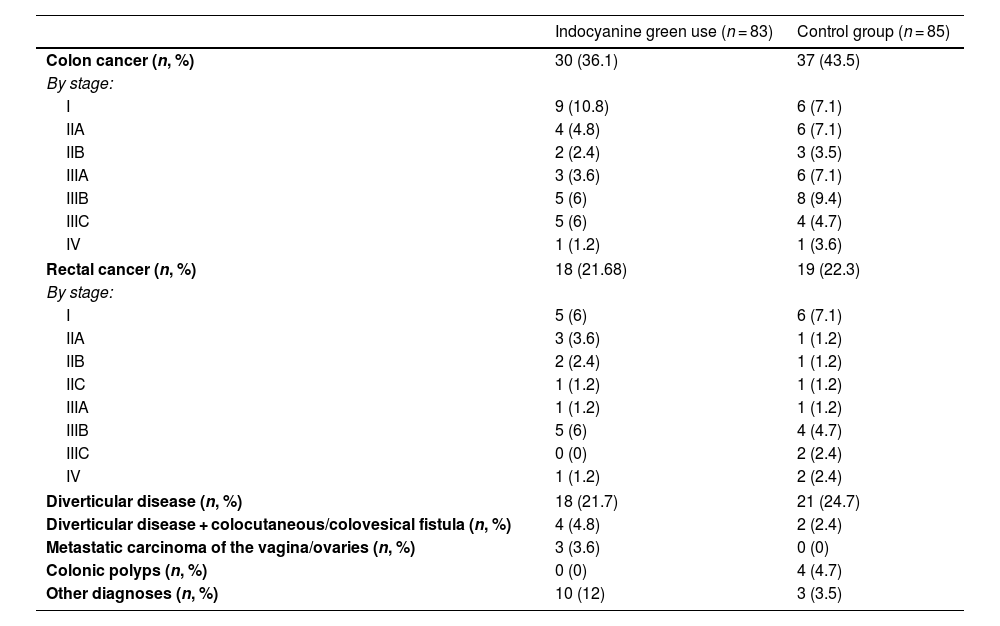
ResultsOf the 168 participants included in the study, 83 underwent ICGFA to evaluate the perfusion in the anastomosis and 85 underwent the standard procedure. The mean patient age was 60 (51.2–68) years, the mean BMI was 26.8 (24.4–29.2) kg/m2, and 49.4% of the patients were women (Table 1). There were no statistically significant differences in the comparison of the clinical and demographic characteristics between the cases and controls, except in the percentage of lymphocytes, in which there was a slight trend toward being higher in the case group. Table 2 shows the different diagnoses made.
Sociodemographic characteristics of the patients.
| Variable | Indocyanine green use (n = 83) | Control group (n = 85) | p |
|---|---|---|---|
| Age, years (mean, SD) | 58 (49–65) | 61 (52.5–69.5) | 0.148a |
| Female sex (n, %) | 39 (47) | 44 (51.8) | 0.542b |
| BMI (mean, SD) | 27 (25–29.7) | 26.6 (24.3–28.7) | 0.272a |
| Smoking (n, %) | 10 (12) | 9 (10.6) | 0.811b |
| Alcohol consumption (n, %) | 12 (14.5) | 9 (10.6) | 0.491b |
| Type 2 diabetes mellitus (n, %) | 4 (4.8) | 11 (12.9) | 0.102b |
| Arterial hypertension (n, %) | 17 (20.5) | 14 (16.5) | 0.554b |
| Chronic kidney disease (n, %) | 2 (2.4) | 0 (0) | 0.243b |
| Steroid use | 1 (1.2) | 2 (2.4) | 0.999b |
| Hemoglobin (median, IQR) | 13.7 (11.6–14.9) | 13 (11.05–14.05) | 0.097a |
| Leukocytes (median, IQR) | 6.9 (5.7–9.2) | 7.8 (5.9–10.1) | 0.156a |
| Platelets (median, IQR) | 246 (201–292) | 261 (207–312) | 0.289a |
| Lymphocytes (median, IQR) | 23 (16–30) | 18.05 (12–27) | 0.016a |
| Albumin (median, IQR) | 4.02 (3.8–4.4) | 4.1 (3.8–4.4) | 0.779a |
| Surgical procedure (n, %) | 0.134b | ||
| LAR | 20 (24.1) | 13 (15.3) | |
| ULAR | 5 (6) | 5 (5.8) | |
| Sigmoid colectomy | 31 (37.3) | 31 (36.5) | |
| Right colectomy | 14 (16.9) | 28 (32.9) | |
| Left colectomy | 13 (15.7) | 8 (8.4) | |
IQR: interquartile range; LAR: low anterior resection; SD: standard deviation; ULAR: ultralow anterior resection.
List of diagnoses per study group.
| Indocyanine green use (n = 83) | Control group (n = 85) | |
|---|---|---|
| Colon cancer (n, %) | 30 (36.1) | 37 (43.5) |
| By stage: | ||
| I | 9 (10.8) | 6 (7.1) |
| IIA | 4 (4.8) | 6 (7.1) |
| IIB | 2 (2.4) | 3 (3.5) |
| IIIA | 3 (3.6) | 6 (7.1) |
| IIIB | 5 (6) | 8 (9.4) |
| IIIC | 5 (6) | 4 (4.7) |
| IV | 1 (1.2) | 1 (3.6) |
| Rectal cancer (n, %) | 18 (21.68) | 19 (22.3) |
| By stage: | ||
| I | 5 (6) | 6 (7.1) |
| IIA | 3 (3.6) | 1 (1.2) |
| IIB | 2 (2.4) | 1 (1.2) |
| IIC | 1 (1.2) | 1 (1.2) |
| IIIA | 1 (1.2) | 1 (1.2) |
| IIIB | 5 (6) | 4 (4.7) |
| IIIC | 0 (0) | 2 (2.4) |
| IV | 1 (1.2) | 2 (2.4) |
| Diverticular disease (n, %) | 18 (21.7) | 21 (24.7) |
| Diverticular disease + colocutaneous/colovesical fistula (n, %) | 4 (4.8) | 2 (2.4) |
| Metastatic carcinoma of the vagina/ovaries (n, %) | 3 (3.6) | 0 (0) |
| Colonic polyps (n, %) | 0 (0) | 4 (4.7) |
| Other diagnoses (n, %) | 10 (12) | 3 (3.5) |
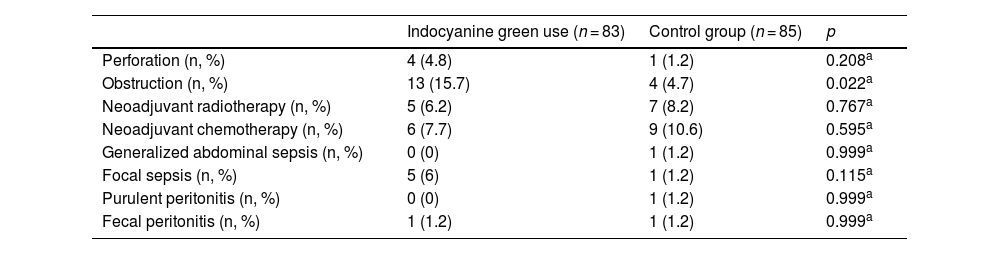
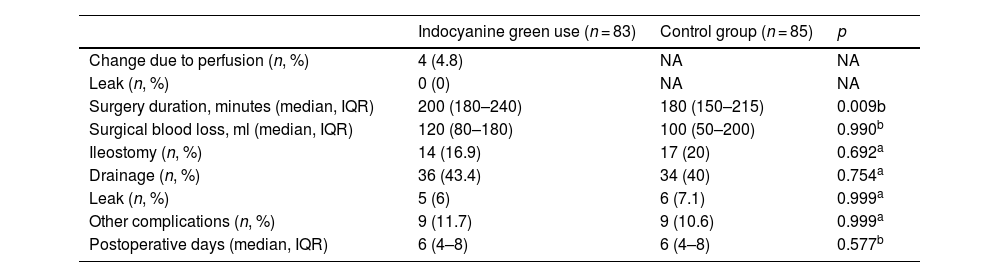
In the evaluation by the surgeon of the resection site in the case group patients, only 4.8% had signs of inadequate perfusion (poor visualization of the indocyanine green dye after ICGFA or a perfusion time greater than 60 s) that merited a change in the anastomosis site. There were no statistically significant differences in the comparison between the cases and controls, regarding the preoperative variables of perforation rate, focal sepsis, generalized sepsis, purulent peritonitis, fecal peritonitis, neoadjuvant radiotherapy, or neoadjuvant chemotherapy. Only a higher number of patients with preoperative obstruction was seen in the case group, compared with the controls (15.7% vs 4.7%; p = 0.022) (Table 3). Surgery duration was statistically longer in the case group (p = 0.009), by a difference of 20 min. There were no differences between the cases and controls, with respect to perioperative blood loss or postoperative days of hospital stay (Table 4).
Preoperative conditions.
| Indocyanine green use (n = 83) | Control group (n = 85) | p | |
|---|---|---|---|
| Perforation (n, %) | 4 (4.8) | 1 (1.2) | 0.208a |
| Obstruction (n, %) | 13 (15.7) | 4 (4.7) | 0.022a |
| Neoadjuvant radiotherapy (n, %) | 5 (6.2) | 7 (8.2) | 0.767a |
| Neoadjuvant chemotherapy (n, %) | 6 (7.7) | 9 (10.6) | 0.595a |
| Generalized abdominal sepsis (n, %) | 0 (0) | 1 (1.2) | 0.999a |
| Focal sepsis (n, %) | 5 (6) | 1 (1.2) | 0.115a |
| Purulent peritonitis (n, %) | 0 (0) | 1 (1.2) | 0.999a |
| Fecal peritonitis (n, %) | 1 (1.2) | 1 (1.2) | 0.999a |
Clinical and surgical outcome comparison.
| Indocyanine green use (n = 83) | Control group (n = 85) | p | |
|---|---|---|---|
| Change due to perfusion (n, %) | 4 (4.8) | NA | NA |
| Leak (n, %) | 0 (0) | NA | NA |
| Surgery duration, minutes (median, IQR) | 200 (180–240) | 180 (150–215) | 0.009b |
| Surgical blood loss, ml (median, IQR) | 120 (80–180) | 100 (50–200) | 0.990b |
| Ileostomy (n, %) | 14 (16.9) | 17 (20) | 0.692a |
| Drainage (n, %) | 36 (43.4) | 34 (40) | 0.754a |
| Leak (n, %) | 5 (6) | 6 (7.1) | 0.999a |
| Other complications (n, %) | 9 (11.7) | 9 (10.6) | 0.999a |
| Postoperative days (median, IQR) | 6 (4–8) | 6 (4–8) | 0.577b |
IQR: interquartile range.
In the leak analysis, the trend toward their reduction was similar in the case group and the controls (6% vs 7.1%; p = 0.999). Of the patients that had ICGFA and presented with a leak, 60% (n = 3) underwent LAR, 20% (n = 1) had sigmoid colectomy, and 20% (n = 1) had right colectomy. In the case group patients that needed an anastomosis site change due to inadequate perfusion (4.8%), the leak rate was 0%. Lastly, there were no differences in the rate of ileostomy and drainage use or in the appearance of other types of complications (Table 3).
DiscussionAs stated above, anastomotic leak is one of the complications with greater morbidity and mortality in colorrectal surgical procedures. With respect to cost-benefit, in Mexico there are no studies evaluating the estimated cost of performing ICGFA versus the benefit it could have in reducing anastomotic leaks, but numerous studies on the subject have been conducted in Europe and the United States. In a retrospective study conducted in the United States by Hammond et al.15, with a total of 101,929 patients that underwent colorrectal surgery and an anastomotic leak rate of 6.18% (6174 patients), those authors estimated that anastomotic dehiscence added $24,129 USD to hospital costs and increased hospital stay by a mean of 7.3 days. Likewise, Liu et al.16 carried out a cost analysis to determine whether ICGFA routinely reduced hospital costs. Using the base case assumptions of a mean cost of laparoscopic colon surgery of $8652.92 CAD, an incremental cost due to anastomotic leak of $9934.50 CAD, and a cost of $250 CAD for ICGFA, they concluded that routine indocyanine green use for colorectal surgery reduced cost per case by 2.06%. In the United Kingdom, anastomotic leak represents an annual expense of 3.5 to 5 million euros for the healthcare system, whereas the mean cost of an ampoule of indocyanine green is 13 2.
Determining the value of ICGFA is currently one of the main study objectives in colorectal surgery because results regarding its usefulness for reducing the anastomotic leak rate have been contradictory2,11,17–19. In their meta-analysis, Blanco-Colino and Espin-Basany2 reported a significant reduction in the anastomotic leak rate with ICGFA in patients that underwent surgery due to colon cancer and rectal cancer. Likewise, the meta-analysis by Lin et al.3 revealed a significant reduction in leak rates with the use of indocyanine green (OR = 0.31, p < 0.0001). On the other hand, the PILLAR III randomized clinical trial carried out by Jafari et al.20 demonstrated no significant reduction in leak rate in the group that underwent ICGFA versus the control group that did not have it (9% vs 9.6%, p = 0.37), but the study did not reach the predetermined sample size. According to the published editorial by Keller and Hompes21, the clinical trial was prematurely suspended, partially due to the fact that the surgeons involved were not willing to continue performing procedures in the control group, without utilizing the indocyanine green imaging technique.
It is important to take the type of surgery to be performed into account. According to the study by Morales-Conde et al.4 that evaluated the percentage of resection site changes based on ICGFA, the surgery in which that imaging tool had the greatest usefulness was left hemicolectomy (25.9%), followed by LAR (25.7%); its utility decreased in the cases of right hemicolectomy (6%). In our study, the incidence of anastomotic leak was low in both groups. Pertinently, inadequate perfusion that required resection site change occurred in 4 patients (4.8%), none of whom presented with a leak. Nevertheless, due to the low incidence of that complication in the study, further studies with a larger number of patients are needed, to reach conclusions regarding the usefulness of ICGFA.
Currently, one of the main limitations in relation to ICGFA is the lack of consensus on the definition of inadequate perfusion, especially in cases in which the perfusion is visualized, but at a low intensity. Aiba et al.22 described such a scenario and also suggested the use of arterial perfusion time, defined as the time from indocyanine green application to visualizing the angiography, with the aim of standardizing and establishing a defined protocol for ICGFA. A perfusion time above 60 s, particularly for evaluating the perfusion at the level of the rectum, is conducive to inadequate perfusion and favors the risk for leaks. However, no objective manners to measure the visualization or intensity of the perfusion with indocyanine green have been established.22 Serra-Aracil et al.23 recently designed a computer program for the objective and quantitative evaluation of perfusion through indocyanine green use called SERGREEN, in an effort to establish an objective protocol for the interpretation of ICGFA.
ConclusionAccording to the data collected in the present study, a slight, statistically non-significant, reduction in the anastomotic leak rate was identified in the group of patients that underwent ICGFA, compared with the control group. The fact that an anastomosis site change due to inadequate perfusion was carried out in only 4.8% of the patients that underwent ICGFA, none of whom presented with a leak, is a limitation of the study. ICGFA is a low-risk intervention that enables a rapid and simple evaluation of blood perfusion at the anastomosis site. As with other tests for evaluating anastomosis, such as the air leak test, direct visualization through colonoscopy or rectosigmoidoscopy, and anastomotic “donut” examination, among others, the aim of ICGFA is to improve safety in patients. Even though none of the tests is perfect, together they enable us to improve surgical practices, as well as surgical outcomes. The data presented in the literature reveal a trend toward a decrease in the anastomotic leak rate through ICGFA. Nevertheless, no significant difference in the leak rate was found in the PILLAR III trial or in our study. Due to the low incidence of leaks, further studies on larger patient cohorts need to be conducted. The establishment of a usage protocol for ICGFA and its objective evaluation are also necessary to make it possible to reach conclusions about the usefulness of this imaging test.
Financial disclosureNo specific grants were received from public sector agencies, the business sector, or non-profit organizations in relation to this study.
Conflict of interestDr. Luis E. Salgado Cruz is a consultant for Stryker regarding the use of indocyanine green. The rest of the authors declare they have no conflict of interest.