Fibrosis staging in patients with nonalcoholic fatty liver disease (NAFLD) is carried out through the application of stepwise algorithms but there is little real-world data on their use. Our aim was to calculate the number of patients with NAFLD and indeterminate or high risk for fibrosis, assessed through noninvasive scores, that consequently underwent further staging evaluation.
Materials and methodsA cross-sectional multicenter cohort study was conducted on patients with NAFLD evaluated by hepatologists within the time frame of June 1 and July 31, 2018. The FIB-4 and NAFLD fibrosis scores were calculated in all the patients, and if at least one of the scores suggested indeterminate or high risk for fibrosis, we believed the patient should have undergone additional fibrosis staging assessment.
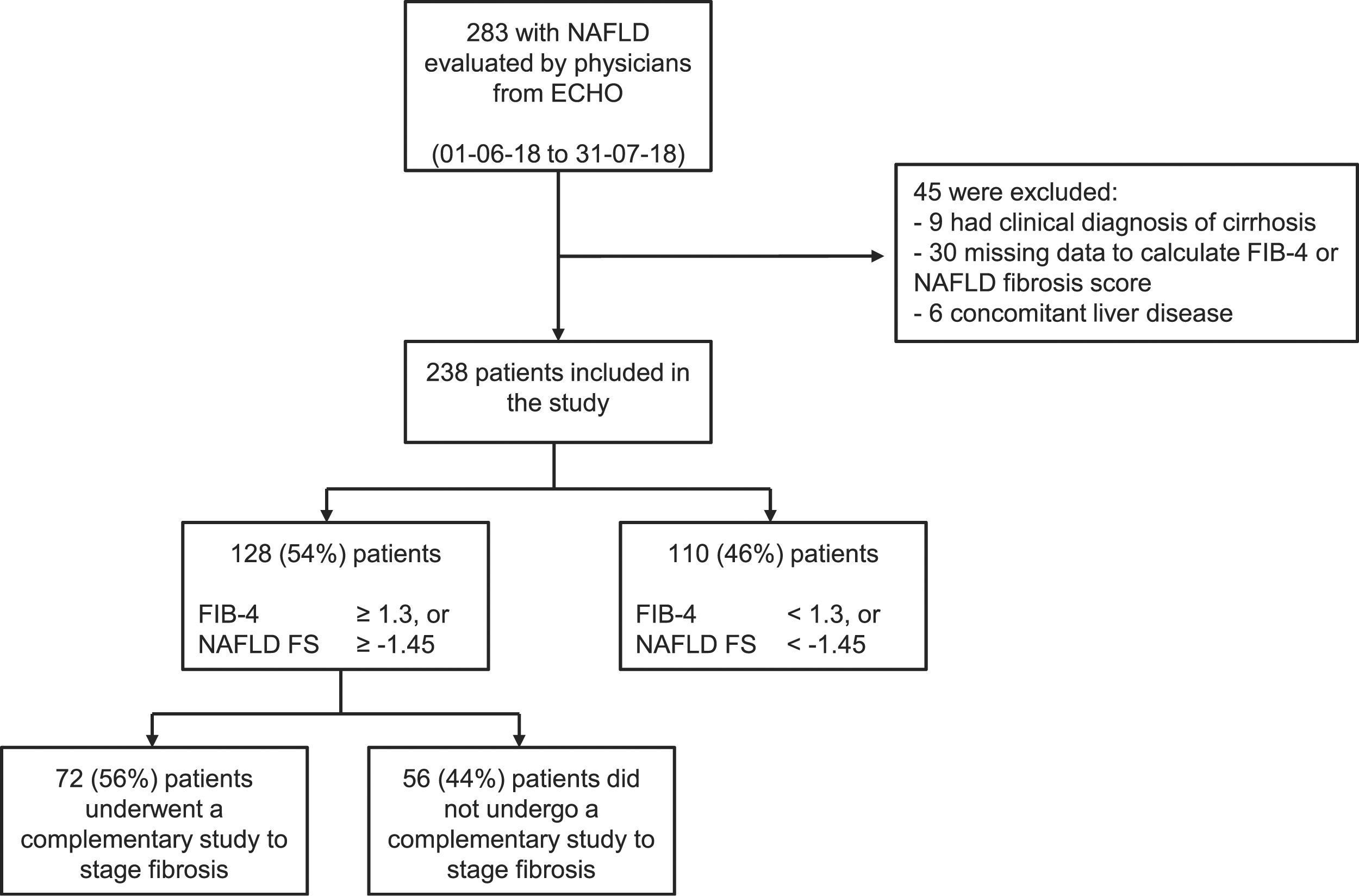
ResultsThe study included 238 patients. The median time interval from NAFLD diagnosis and inclusion in the analysis was 12.2 months (IQR 3.0–36.5). A total of 128 (54%) patients had at least one noninvasive score that suggested indeterminate or high risk for fibrosis but studies to confirm the fibrosis grade (elastography, biopsy, etc.) were performed on only 72 (56%). The main barriers encountered by the physicians for applying the staging algorithms were related to health insurance coverage and imaging study costs.
ConclusionsA high percentage of patients with NAFLD were at indeterminate or high risk for fibrosis, according to noninvasive scores, but additional studies were carried out on only half of them, showing low adherence to current recommendations.
La estadificación de la fibrosis en pacientes con enfermedad por hígado graso no alcohólico (EHGNA) se realiza aplicando distintos algoritmos. Sin embargo, hay pocos reportes de cómo se utilizan en la vida real. Nuestro objetivo fue estimar la proporción de pacientes con EHGNA que teniendo riesgo indeterminado o alto de fibrosis según scores no-invasivos se someten a estudios adicionales de estadificación.
Materiales y métodosEstudio de corte transversal multicéntrico de pacientes con EHGNA evaluados por hepatólogos del 1 de junio del 2018 al 31 de julio del 2018. Se calcularon FIB-4 y NAFLD fibrosis score en todos los pacientes y se consideró que, si al menos uno de los scores sugería riesgo indeterminado o alto de fibrosis, el paciente debería haberse sometido a estudios adicionales de estadificación.
ResultadosSe incluyeron 238 pacientes. La mediana de tiempo entre el diagnóstico de EHGNA y la inclusión en el estudio fue de 12.2 (IQR 3.0–36.5) meses. En total, 128 (54%) pacientes tuvieron al menos una evaluación con score no-invasivo que sugería riesgo indeterminado o alto de fibrosis. Sin embargo, solamente 72 (56%) se realizaron estudios para confirmar el grado de fibrosis (elastografía, biopsia, etc.). Las principales barreras encontradas por los médicos para aplicar los algoritmos de estadificación fueron relacionadas a cobertura de salud y a costos.
ConclusionesUn alto porcentaje de pacientes con EHGNA tiene riesgo indeterminado o alto de fibrosis, de acuerdo con los resultados obtenidos con scores no invasivos. Sin embargo, solamente la mitad se realizó estudios adicionales, evidenciando una baja adherencia a las recomendaciones actuales.
Nonalcoholic fatty liver disease (NAFLD) is considered the most prevalent cause of liver disease worldwide1, affecting approximately 25% of the world population2. There is an abundance of data regarding the epidemiology of NAFLD in developed countries, indicating a mean prevalence of 24% in Europe and in the United States3,4. The prevalence of NAFLD in South America appears to be even higher, affecting more than 30% of the population3.
The spectrum of NAFLD ranges from nonalcoholic fatty liver, a stage with a more favorable liver-related prognosis, to nonalcoholic steatohepatitis (NASH), which is associated with progressive fibrosis and a higher risk for developing cirrhosis and hepatocellular carcinoma5. Given that NASH and its consequences will occur in only a minority of patients3,5, the identification of that population is paramount, so they can be offered proper follow-up and care.
The gold standard for the diagnosis of NASH is liver biopsy, but due to the epidemic proportions of the disease, offering an invasive procedure that entails the risk of complications and is prone to sampling errors appears impractical. Thus, several guidelines have proposed the use of noninvasive tools for fibrosis evaluation, and a stepwise algorithm has been recommended to simplify the clinical management of patients with NAFLD6. The initial steps include calculating noninvasive scores based on clinical features and serum biomarkers to classify the risk of fibrosis, and only patients with indeterminate or high risk are referred for a second assessment, with other noninvasive imaging techniques, such as elastography. Liver biopsy is offered only to patients in whom noninvasive assessment cannot exclude advanced fibrosis6.
The use of noninvasive tools is recommended and they are widely available7, but there is a well-known gap between the knowledge of guidelines and their implementation in daily practice8. Furthermore, other factors, such as barriers related to healthcare insurance coverage or local availability of technology, can impact the use of certain tools, such as elastography, in developing countries.
The adherence to recommended algorithms for fibrosis assessment in patients with NAFLD in Latin America is unknown. Thus, the primary aim of the present study was to estimate the proportion of NAFLD patients with indeterminate or high risk of fibrosis, as determined by noninvasive scores, that consequently underwent imaging studies to assess fibrosis, in real-world practice. Additionally, we sought to identify barriers to complying with the proposed fibrosis staging algorithms.
Materials and methodsStudy design and variablesWe conducted a cross-sectional multicenter study of patients with NAFLD, utilizing the Extension for Community Healthcare Outcomes (ECHO) NAFLD Clinic. Said model employs videoconferencing technology as a platform to deliver specialty medical care, by training and supporting healthcare providers so they can provide the best medical care in their local communities9.
The ECHO-NAFLD clinic was launched in February 2018 and consists of monthly 90-min teleconferences directed by an expert multidisciplinary team at the Hospital Italiano in Buenos Aires, Argentina. During the teleconference, participants from different regions of Argentina present patients with NAFLD to the multidisciplinary expert panel and the additional participants, who then discuss the case and give recommendations for patient care.
All physicians participating in the ECHO-NAFLD clinic were invited to take part in the present study.
From June 1 to July 31, 2018, all consecutive patients with NAFLD, above 17 years of age, that received medical care from physicians participating in the ECHO-NAFLD clinic were invited to participate in the study. Patients diagnosed with cirrhosis, based on a composite of clinical signs provided by laboratory tests, endoscopy, and radiologic imaging, were excluded from the study. Patients were also excluded if they had concomitant liver diseases, were HIV positive, or if either the NAFLD fibrosis score10 or the FIB-410,11 score could not be calculated with the available data. NAFLD was defined as evidence of hepatic steatosis, through imaging modalities or histologic analyses, and the lack of secondary causes of liver fat accumulation, such as significant alcohol consumption12, long-term use of steatogenic medication, or monogenic hereditary disorders13. The selection criteria were verified using a pre-designed form, in the presence of the patients.
For each patient, the study consisted of a single visit, in which the following data were collected from medical records, complementary studies, and interrogation:
- •
Demographics and social determinants: age, sex, type of medical insurance (public system, private insurance, or social welfare system), educational level (primary, secondary, or post-secondary), employment status (employed vs unemployed), and place of residence in an urban or non-urban area.
- •
Comorbidities: dyslipidemia (patients receiving any lipid-lowering treatment or that presented with any of the following situations: LDL cholesterol >160 mg/dl, HDL cholesterol <40 mg/dl, or triglycerides >200 mg/dl), arterial hypertension (patients receiving antihypertensive drugs and/or that presented with a systolic or diastolic blood pressure at examination >140 or 90 mmHg, respectively), diabetes (patients receiving oral anti-diabetic drugs and/or insulin, or that had glycated hemoglobin >6.5 g/dl or at least 2 fasting glucose levels >126 mg/dl), and a history of major cardiovascular ischemic disease or cerebrovascular disease. Body mass index (BMI) was categorized according to the World Health Organization classification: normal: 15.5–24.9; overweight: 25-29.9; class I obesity: 30–34.9; class II obesity: 35–39.9; class III obesity: 4014.
- •
Noninvasive scores: the FIB-411 score and NAFLD fibrosis score10 were calculated in all patients. Predefined cutoff points were applied to each score, to classify patients into different categories, according to the risk of fibrosis. For the FIB-4 score, indeterminate or high risk of fibrosis was set as follows: ≥1.3 for patients 36–64 years of age and ≥2 for patients above 64 years of age15. Indeterminate or high risk of fibrosis was set at >–1.455 for the NAFLD fibrosis score15. The FIB-4 score was not calculated in patients under 36 years of age15, based on data precluding its use to rule in advanced fibrosis in that population, and as a result, failing to identify which of those patients should undergo further staging studies15–17.
- •
Outcome variable: for the purpose of the present study, and according to recommendations, we accepted the following staging algorithm: patients with at least one noninvasive score that suggested indeterminate or high risk of fibrosis should have undergone imaging studies to stage fibrosis, such as transient elastography, among others6. Therefore, the outcome variable was constructed using the number of patients that had at least one noninvasive score with indeterminate or high risk of fibrosis, as the denominator, and the number of patients that underwent at least one imaging study (imaging techniques ± ultrasound-guided biopsy) to stage fibrosis, as the numerator.
The age and sex of the participating physicians were also collected, and they completed a survey assessing their perception of potential barriers to having access to imaging studies for staging fibrosis in patients with NAFLD. The survey consisted of questions regarding four potential barriers, which they ranked from “most important” to “least important”, according to their opinion: a) insurance and cost-related obstacles, b) a lack of awareness about the potential consequences of NAFLD in patients and the general population, c) insufficient information in the medical community on how to apply algorithms to stage fibrosis in patients with NAFLD, and d) distance from the patient’s place of residence to the closest center performing fibrosis staging studies.
The present article was structured in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.
Statistical analysisThe qualitative variables were presented as absolute numbers and percentages and the quantitative variables were shown as median and interquartile range (IQR: 25th percentile and 75th percentile). The Fisher’s exact test or chi-square test for the categorical variables and the Mann–Whitney U test for numerical variables were employed to compare the variables between patients with indeterminate or high-risk noninvasive scores, according to whether imaging studies to stage fibrosis had been conducted. When an ordinal variable was evaluated, the chi-square for trend function was applied. Tests were two-sided and significance was accepted at a p < 0.05. STATA software (StataCorp LLC, TX; version 14.2) was used for the calculations.
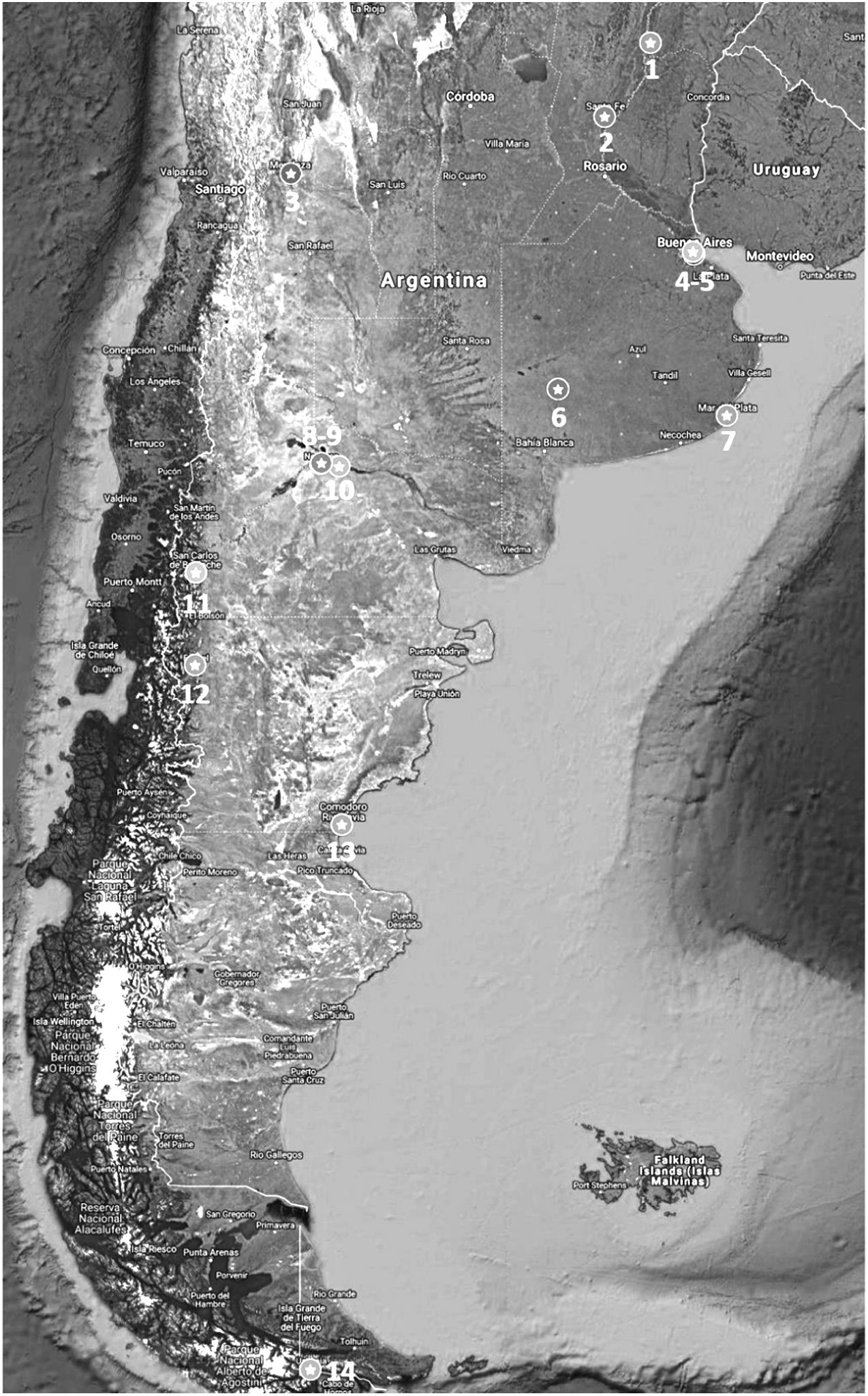
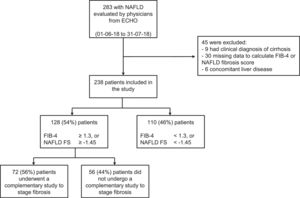
ResultsDuring the study period, a total of 24 physicians from 12 cities in Argentina were actively participating in the ECHO-NAFLD clinic, 14 of whom accepted to participate in the study (Fig. 1). Overall, 283 patients with NAFLD were evaluated within the time frame of the study and included in the analysis (Fig. 2).
Distribution of the Argentinian sites that participated in the study.
The research sites were: 1: Esquina, 2: Santa Fe, 3: Mendoza, 4 and 5: Ciudad de Buenos Aires, 6: Coronel Suárez, 7: Mar del Plata, 8 and 9: Neuquén, 10: General Roca, 11: Bariloche, 12: Esquel, 13: Comodoro Rivadavia, 14: Ushuaia.
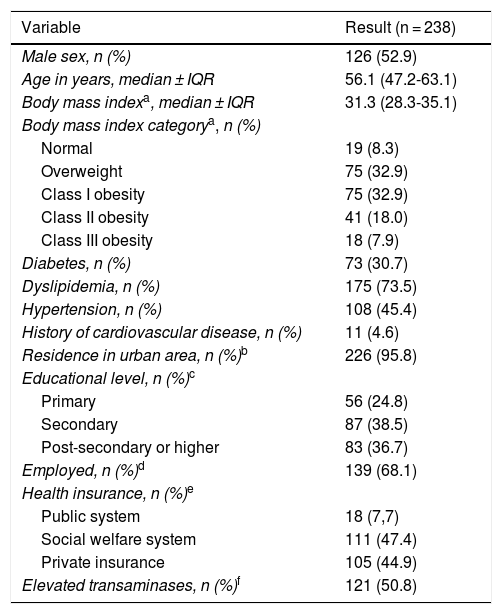
Patient characteristics and comorbidities are detailed in Table 1. The median time interval from NAFLD diagnosis to inclusion in the study was 12.2 (3.0–36.5) months. A total of 126 (53%) patients were men, and the median patient age was 56 years (47–63). All patients had at least one risk factor for NAFLD. Dyslipidemia, obesity, and diabetes were registered in 175 (73%), 134 (59%), and 73 (31%) patients, respectively. Regarding social determinants, most of the patients lived in urban areas, were employed, had at least completed primary school, and had healthcare insurance.
Patient characteristics collected from the ECHO-NAFLD Clinic, from June 1 to July 31, 2018 (n = 238).
| Variable | Result (n = 238) |
|---|---|
| Male sex, n (%) | 126 (52.9) |
| Age in years, median ± IQR | 56.1 (47.2-63.1) |
| Body mass indexa, median ± IQR | 31.3 (28.3-35.1) |
| Body mass index categorya, n (%) | |
| Normal | 19 (8.3) |
| Overweight | 75 (32.9) |
| Class I obesity | 75 (32.9) |
| Class II obesity | 41 (18.0) |
| Class III obesity | 18 (7.9) |
| Diabetes, n (%) | 73 (30.7) |
| Dyslipidemia, n (%) | 175 (73.5) |
| Hypertension, n (%) | 108 (45.4) |
| History of cardiovascular disease, n (%) | 11 (4.6) |
| Residence in urban area, n (%)b | 226 (95.8) |
| Educational level, n (%)c | |
| Primary | 56 (24.8) |
| Secondary | 87 (38.5) |
| Post-secondary or higher | 83 (36.7) |
| Employed, n (%)d | 139 (68.1) |
| Health insurance, n (%)e | |
| Public system | 18 (7,7) |
| Social welfare system | 111 (47.4) |
| Private insurance | 105 (44.9) |
| Elevated transaminases, n (%)f | 121 (50.8) |
The categorical data are expressed as absolute numbers and percentages. The numerical data are expressed as median and interquartile range.
The distribution of the categories of the NAFLD fibrosis score and the FIB-4 score is presented in Table 2. Of the 238 patients included in the study, 128 (54%) had at least one noninvasive score result that suggested indeterminate or high risk of fibrosis but only 72 (56%) of them underwent imaging studies for fibrosis staging. The most frequently performed studies were transitional elastography, acoustic radiation force impulse (ARFI), or shear wave elastography, carried out in 30 (42%) patients, and magnetic resonance elastography, performed in 14 (19%) patients. Liver biopsy and a combination of methods (one or more imaging techniques plus liver biopsy) were carried out to stage fibrosis in 19 (26%) and 9 (13%) patients, respectively. Taking into account all 44 patients that underwent noninvasive imaging studies to assess fibrosis (transitional elastography, acoustic ARFI, shear wave elastography, or magnetic resonance imaging), 32% presented a result that suggested F0 to F1 fibrosis, whereas 68% presented a result that suggested F2 to F4 fibrosis. In the 26 patients that underwent liver biopsy (either as a single approach or in combination with noninvasive imaging studies), 78% presented a METAVIR score of F0 or F1, whereas 22% had scores ranging from F2 to F4.
Distribution of the noninvasive score results (risk of fibrosis), collected through the ECHO-NAFLD Clinic, from June 1 to July 31, 2018.
| Risk of fibrosis | NAFLD fibrosis score (n = 145) | FIB-4 score (n = 220) |
|---|---|---|
| Low risk | 51 (35.2) | 140 (63.6) |
| Indeterminate risk | 61 (42.1) | 55 (25.0) |
| High risk | 33 (22.7) | 25 (11.4) |
Data is presented as absolute numbers and percentages. The NAFLD fibrosis score could be calculated in 145 patients and the FIB-4 score in 220 patients.
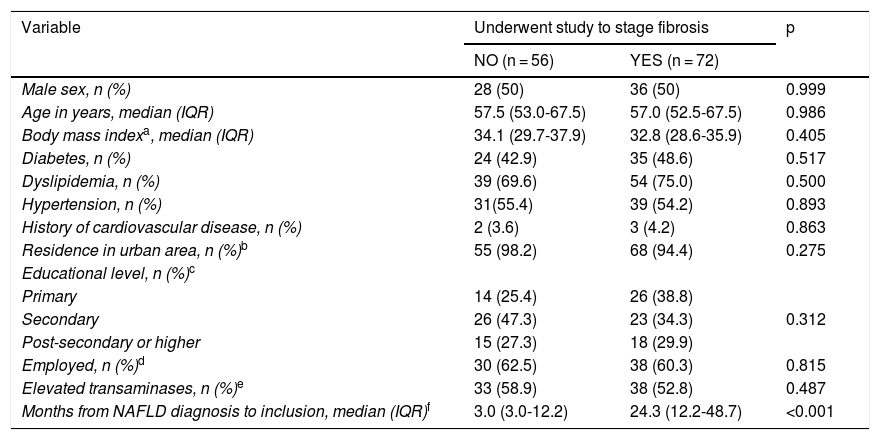
In the 128 patients with noninvasive score results that suggested indeterminate or high risk of fibrosis, the time interval from diagnosis of NAFLD to inclusion in the study was the only variable that was different between the patients that underwent imaging studies to stage fibrosis and those that did not (Table 3). The median time interval from NAFLD diagnosis to inclusion in the study was 24.3 months (12.2–48.7) in the patients that had a complementary study to stage fibrosis vs 3.0 months (3.0–12.2) in the patients that did not (p < 0.001).
Comparison of characteristics of patients with indeterminate or high-risk scores, according to whether or not a complementary study for staging fibrosis was performed, collected from the ECHO-NAFLD Clinic, from June 1 to July 31, 2018 (n = 128).
| Variable | Underwent study to stage fibrosis | p | |
|---|---|---|---|
| NO (n = 56) | YES (n = 72) | ||
| Male sex, n (%) | 28 (50) | 36 (50) | 0.999 |
| Age in years, median (IQR) | 57.5 (53.0-67.5) | 57.0 (52.5-67.5) | 0.986 |
| Body mass indexa, median (IQR) | 34.1 (29.7-37.9) | 32.8 (28.6-35.9) | 0.405 |
| Diabetes, n (%) | 24 (42.9) | 35 (48.6) | 0.517 |
| Dyslipidemia, n (%) | 39 (69.6) | 54 (75.0) | 0.500 |
| Hypertension, n (%) | 31(55.4) | 39 (54.2) | 0.893 |
| History of cardiovascular disease, n (%) | 2 (3.6) | 3 (4.2) | 0.863 |
| Residence in urban area, n (%)b | 55 (98.2) | 68 (94.4) | 0.275 |
| Educational level, n (%)c | |||
| Primary | 14 (25.4) | 26 (38.8) | |
| Secondary | 26 (47.3) | 23 (34.3) | 0.312 |
| Post-secondary or higher | 15 (27.3) | 18 (29.9) | |
| Employed, n (%)d | 30 (62.5) | 38 (60.3) | 0.815 |
| Elevated transaminases, n (%)e | 33 (58.9) | 38 (52.8) | 0.487 |
| Months from NAFLD diagnosis to inclusion, median (IQR)f | 3.0 (3.0-12.2) | 24.3 (12.2-48.7) | <0.001 |
The categorical data are expressed as absolute numbers and percentages. The numerical data are expressed in median and interquartile range.
All participating physicians were hepatologists. Their median age was 45 years (37–59) and 9 (64%) were women. Regarding access to updated guidelines and recommendations for NAFLD assessment, 7 (50%) participants stated that they had attended an international hepatology congress or postgraduate course, within the time frame of 2016–2018.
When assessing their perception of potential barriers to having access to imaging studies for staging fibrosis, 7 (50%) physicians stated that the leading barrier was related to insurance coverage and cost of the study, 4 (29%) described a lack of awareness about the potential consequences of NAFLD in patients and the general population, and 3 (21%) asserted that it was the distance from the patient’s place of residence to the closest center performing studies to stage fibrosis. None of the participating physicians stated that the main barrier was insufficient information in the medical community on how to apply algorithms to stage fibrosis in patients with NAFLD.
DiscussionOur study has several interesting findings. First, its results showed that more than 50% of the patients with NAFLD followed by hepatologists had indeterminate or high risk of liver fibrosis, as assessed by noninvasive scores. Second, imaging studies were performed in only 50% of the patients in whom further studies to stage fibrosis were indicated by algorithms. Third, the primary barrier to staging fibrosis perceived by the hepatologists was related to imaging study cost and insurance coverage.
As stated above, NAFLD has reached epidemic proportions worldwide, and said trend appears to be rapidly escalating, concomitant with the increase in the prevalence of type 2 diabetes and obesity3,18,19. Given that morbidity and mortality in patients with NAFLD are associated with the development of fibrosis, the detection of patients at high risk for that outcome is crucial13,20. The recent recommendations to use noninvasive scores as the first tool for fibrosis assessment in patients with NAFLD6,17 appears to be a reasonable practical approach: those scores are affordable, widely accessible, and have a high negative predictive value for ruling out advanced fibrosis21. In a recent meta-analysis, the NAFLD fibrosis score and FIB-4 score were found to be the noninvasive scores with higher diagnostic accuracy for advanced fibrosis, with an area under the receiver operating characteristic curve of approximately 0.8422.
In our study, we found that a high proportion (54%) of patients had indeterminate or high risk of fibrosis, according to noninvasive scores. That finding was higher than usually expected because our cohort was predominantly made up of Latin overweight patients above 50 years of age, with a high prevalence of metabolic comorbidities and altered transaminases, very likely reflecting the real need for further fibrosis assessment in those patients. In addition, all the patients included in the present analysis were referred to hepatologists, reinforcing the fact that said population could have a higher risk of fibrosis than patients receiving primary care.
It is difficult to compare our results with those of previously published studies because the prevalence of NASH in a given population varies considerably, as do the scores and cutoff points utilized in each study. For instance, in a population-based study conducted in the United Kingdom, FIB-4 score results were available in 40% of 176,114 patients with NAFLD, up to 36% of whom had an indeterminate or high risk of fibrosis22,23. In another population-based study conducted on a Hispanic community in Texas, where the prevalence of NAFLD reached 52%, there were findings consistent with indeterminate or high risk of fibrosis, using different noninvasive scores, in 17–63% of the entire cohort24. Similarly, when obese patients evaluated for bariatric surgery or patients with type 2 diabetes were assessed with those tools, indeterminate or high-risk results were observed in 60% and 61% of the study population, respectively25,26.
When obtaining an indeterminate or high risk of fibrosis with noninvasive scores, a careful interpretation of the findings is needed because the primary limitation of those tools is their inferior accuracy to rule in advanced fibrosis22. Other limitations are that concomitant hepatic and extrahepatic conditions can influence the results, their accuracy is lower in younger and older patients, and that a high proportion of patients are classified in an indeterminate zone. Thus, fibrosis assessment with imaging studies is mandatory in patients with an indeterminate or high risk of fibrosis6,15,17.
During the last few years, several algorithms and practice guidelines have been proposed by different experts to stratify patients with NAFLD: there is consensus in acknowledging that a combination of noninvasive scores and imaging techniques, such as elastography (in its different modalities), is useful for identifying patients at a higher risk of fibrosis that could benefit from a liver biopsy6,13,17,27. In a recent large prospective cohort study that used a two-step algorithm (the FIB-4 score combined with the ELF test, if deemed necessary), 5-times more cases of advanced fibrosis were detected, resulting in reduced secondary care referrals, when compared with standard care, providing strong new evidence of the benefits a stepwise approach offers patients and physicians alike28.
However, the manner in which those staging algorithms are incorporated into real-world practice is not well known. The scarce information available only refers to the utilization of fibrosis staging approaches in the primary care setting, but whether hepatologists adhere to those strategies has seldom been reported28,29. In our study, only 56% of the patients that were indicated for further fibrosis assessment and followed by a hepatologist underwent additional testing. A possible explanation for the low adherence to recommended algorithms is a lack of knowledge of them, but the access to information on the part of Argentinian hepatologists appears to be adequate, given that 50% of the physicians reported that they had attended an international hepatology congress or postgraduate course within the last 2 years, where NAFLD assessment was a “hot topic”. It is also thoroughly discussed in current practice guidelines13,27,30,31. Furthermore, all participating physicians declared they were familiar with the proposed fibrosis staging algorithms and were motivated to treat patients with NAFLD, ever since their involvement with ECHO-NAFLD.
The perceived barriers to staging fibrosis were related to imaging study costs, lack of medical insurance coverage and/or the distance from the patient’s place of residence to the closest available site performing elastography. Those obstacles would explain the high percentage (26%) of patients that underwent liver biopsy with no prior noninvasive imaging assessment of fibrosis, given that biopsy is a more extensively available procedure that can be carried out at any hepatology center throughout the country.
In our study, we attempted to identify patient characteristics that could be associated with having access to imaging studies for staging fibrosis. Even though we explored several clinical variables and social determinants, the only variable that was different, between patients that did or did not undergo imaging studies to stage fibrosis, was the time interval from diagnosis of NAFLD to inclusion in the study. Patients that had more follow-up visits after NAFLD diagnosis were more likely to have undergone imaging studies than patients with fewer follow-up visits. It appears that greater interaction between patients and physicians is needed for having access to further testing, such as transient elastography, which is considered a “point-of-care” technique in other regions6. That finding could correctly challenge the use of proposed staging algorithms in Argentina, given that at least two months of follow-up with a specialist are needed to complete the initial fibrosis work-up.
Our study has several strengths. First, even though it was a cross-sectional study, the researchers had data collection training and all information was recorded in a single visit, with the participation of the patient, generating consistent data and minimizing missing information. Second, to the best of our knowledge, it is the first study to address adherence to diagnostic algorithms in patients with NAFLD that are under the care of a hepatologist.
The main limitation of our study is the fact that at the time it was designed, no widely accepted two-step algorithm was available, resulting in varying degrees of adherence, depending on the strategy of choice. However, we believe said situation only underlines the current need to standardize diagnostic algorithms in patients with NAFLD.
In conclusion, we found that a high percentage of patients with NAFLD followed by hepatologists had indeterminate or high risk of fibrosis, assessed by noninvasive scores. However, only half of those patients underwent further diagnostic testing to stage fibrosis. The main barrier to adhering to current algorithms that was identified appears to be related to not having access to other noninvasive tools, such as elastography, whether due to lack of insurance coverage or increased distance to the closest center performing those studies. Efforts should be made by scientific societies and the national government to resolve those barriers and properly assess patients at risk for fibrosis, using those cost-effective algorithms. As was the case with other prevalent liver diseases, it is likely that the best way to overcome the current problem is through education and the implementation of multidisciplinary guidelines that strongly endorse the necessity and advantages of proper fibrosis risk stratification, in patients with NAFLD.
Ethical considerationsThe Institutional Review Board approved the study (protocol number 3559). All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the 1975 Declaration of Helsinki, as revised in 2008. All patients were asked for oral statements of informed consent and all patients included in the analysis were above 17 years of age. The study patients cannot be identified through the data in the article.
FundingNo specific grants were received from public sector agencies, the business sector, or non-profit organizations in relation to this study.
Conflict of interestThe authors declare that there is no conflict of interest.
The authors wish to thank the Fundación Icalma for the methodological support received for this research, as well as Project ECHO and the Health Informatics Department of the Hospital Italiano de Buenos Aires, for making it possible to keep ECHO-NAFLD active.
Please cite this article as: Marciano S, Dirchwolf M, Torres MC, Allevato J, García Dans C, García B, et al. Evaluación de fibrosis en pacientes con enfermedad por hígado graso no alcohólico: adherencia a los algoritmos propuestos y barreras para cumplir con ellos. Revista de Gastroenterología de México. 2022;87:4–12.