Eosinophilic esophagitis is a chronic antigen-mediated disease characterized by esophageal symptoms, esophageal eosinophilia, and the absence of response to proton pump inhibitors. It is the most frequent cause of dysphagia and food impaction in adults. Its incidence and prevalence is very high in the developed countries (USA, Europe, Australia), where its connotation is that of an emerging epidemic. While studies have been published with large case series in the developed countries, those published in Latin America are small or consist of isolated case reports. The differences in the prevalence of the disease between the developed and developing regions are unknown. Genetic or racial causes have been cited. Nevertheless, the epidemic nature of the disease suggests that environmental causes are the most powerful.
Based on the published hypotheses, as well as on epidemiologic studies, the present review discusses some of the possible causes of the disparity in the prevalence of eosinophilic esophagitis between the two types of countries. The ‘hygiene hypothesis’ is reviewed, together with the possible relation of Helicobacter pylori, intestinal parasites, and modifications of the esophageal microbiota in patients with eosinophilic esophagitis. In reference to studies conducted in the United States, the clinical behavior and progression of eosinophilic esophagitis in Hispanics is reviewed and a possible predominant phenotype in Mexican and other Latin American patients is discussed. Finally, based on the above, an algorithm for studying the disease in the Latin American countries is proposed.
La esofagitis eosinofílica (EEo) es una enfermedad crónica mediada por alérgenos, caracterizada por síntomas esofágicos, eosinofilia esofágica y ausencia de respuesta favorable a inhibidores de la bomba de protones (IBP). En los adultos es la causa más frecuente de disfagia e impactación alimentaria. Su incidencia y prevalencia son muy altas en los países desarrollados (EUA, Europa y Australia), en donde ha alcanzado una connotación de epidemia. Mientras que en los países desarrollados se publican estudios con grandes series de pacientes, en nuestro subcontinente se reporta en series con pocos casos o en casos aislados. Las causas de las diferencias de la prevalencia de la enfermedad entre ambas regiones se desconocen. Se han invocado causas genéticas o raciales. No obstante, el carácter epidémico de la enfermedad sugiere que las causas ambientales son más poderosas.
Con base en hipótesis publicadas, así como en los estudios epidemiológicos, en la presente revisión se discutirán algunas de las posibles causas de la disparidad de prevalencia de la EEo entre ambos tipos de países. Se revisará la «hipótesis de la higiene», así como la posible relación del Helicobacter pylori, los parásitos intestinales y las modificaciones de la microbiota esofágica con la EEo. Con base en estudios realizados en EUA, se revisará el comportamiento clínico y evolutivo de la EEo en individuos hispanos y se discutirá acerca de un posible fenotipo predominante en los pacientes de América Latina y México. Finalmente, sobre la base anterior, se propondrá un algoritmo de estudio de la enfermedad en nuestros países.
Eosinophilic esophagitis (EoE) is a chronic disease of the esophagus caused by environmental and food allergens. It is characterized by symptoms of esophageal dysfunction, eosinophilic infiltrate (≥ 15 eosinophils/high power field) in the esophageal mucosa, and absence of clinical and histologic response to treatment with proton pump inhibitors (PPIs) for 8 weeks.1,2 According to the diagnostic guidelines established by the 2007, 2011, and 2013 international consensuses on digestive eosinophilia,1–3 EoE should be differentiated from the esophageal eosinophilia caused by gastroesophageal reflux disease and PPI-responsive esophageal eosinophilia (PPI-REE).3 The latter is clinically, endoscopically, and histologically indistinguishable from EoE,2,4 and whether it is a phenotype of EoE that responds to PPIs, or an independent entity, is not known.5
Initially described in children, it was not until 1978 that EoE was reported in adults.6 In children it manifests as an inflammatory phenotype clinically characterized by vomiting, heartburn, chest pain, and difficulty to gain body weight. At endoscopy, there is predominant inflammatory exudate in the esophageal mucosa.7,8 In adults it is currently the most frequent cause of dysphagia and food impaction. It behaves as a progressively chronic disease, highly recurrent, and with a tendency toward esophageal remodeling (submucosal fibrosis) and stricture.9,10 It affects males more frequently than females, with a 4:1 ratio, and individuals in the fourth and fifth decades of life.11
The present article describes the general clinical and epidemiologic characteristics of EoE in developed and developing countries (North and South), particularly the Latin American countries, emphasizing the differences in prevalence between both regions of the world. At the same time, based on the scant epidemiologic evidence that has been published up to now, we discuss the possible causes involved in such differences. We then attempt to outline the clinical and progressive characteristics of the disease in Latin American populations, gleaned from the available clinical information, and finally, we propose measures to diagnose and treat the disease in Mexico and other Latin American countries.
Eosinophilic esophagitis. Prevalence and incidence in developed and developing countriesBased on studies conducted on general populations, since its initial description in the 1980s, EoE incidence and prevalence has significantly increased, to the degree that it is considered epidemic, in the United States (US), Canada, Western Europe, and Australia (this last country located in the South but with economic and social development comparable to that of Europe and the US).12–15 Prevalence in children and adults in the general population in those geographic regions has been determined at 10 to 90 /100,000 inhabitants with an estimated incidence of 2 to 13 cases/100,000 inhabitants. These figures are the highest, compared with those reported in the few studies published in Latin America,16–18 in China,19,20 in Japan,21,22 and in Saudi Arabia.23 No cases have been reported in India or in North Africa or Sub-Saharan Africa.
In Mexico and in other Latin American countries, incidence of the disease in the general population is not known because of the lack of studies. There have only been isolated case reports or small case series from some of the South American countries (Chile, Uruguay, Argentina, Peru).24–26 In two prospective Latin American studies, one from Mexico and the other from Brazil, in subjects that underwent esophagogastroduodenoscopy due to esophageal symptoms, prevalence was reported at 1 and 1.7%,16,18 which was lower than that reported in similar studies in the US and Spain (5 to 8%).27,28 In another Mexican study conducted by our group on patients with clinical signs of refractory gastroesophageal reflux disease, prevalence was also low, at 4%.17
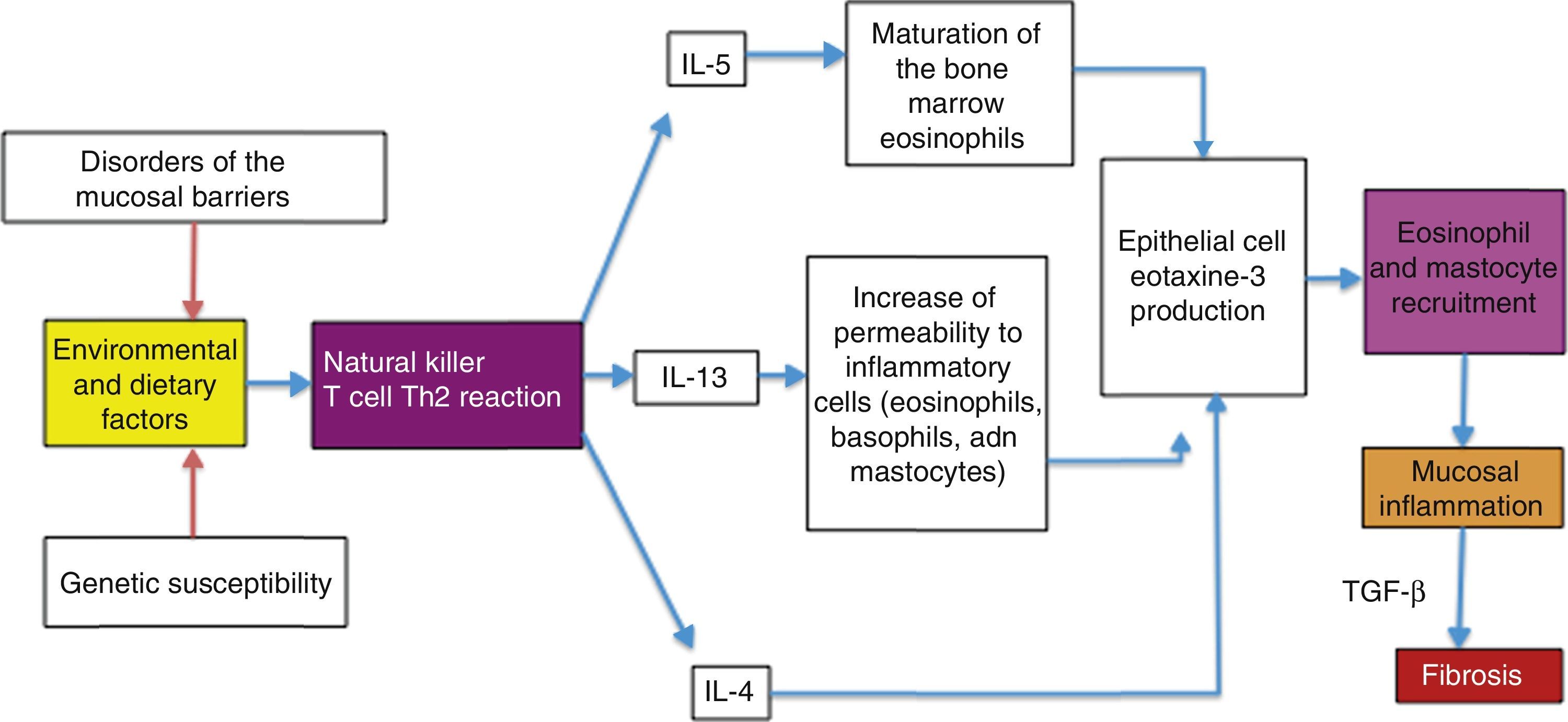
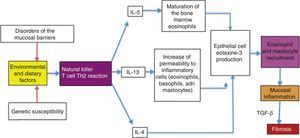
Possible causes of the eosinophilic esophagitis epidemic in developed countries and of the low prevalence in developing countriesEoE is an allergic disease with pathophysiologic mechanisms similar to those of other atopic diseases, such as bronchial asthma, allergic rhinitis, and atopic dermatitis. It is well documented that EoE is triggered by allergens contained in foods and the environment, given that the restriction of certain foods improves symptoms and the esophageal eosinophilic infiltrate. In addition, it has an elevated seasonal incidence, with greater frequency between summer and fall, when there is a greater concentration of pollen in the environment.29 These allergens cause an inflammatory reaction mediated by helper T lymphocytes, known as the Th2 reaction, which induces the production of IL-4, IL-5, and IL-13 interleukins and the secretion of eotaxine-3 by the epithelial cells of the esophageal mucosa. That agent is a powerful recruiter of eosinophils and mast cells, whose presence causes inflammation of the mucosa and disruption of the epithelial barrier (fig. 1).
Similar to what has been observed with EoE, other allergic diseases, such as asthma, allergic rhinitis, and atopic dermatitis, have had a tendency to increase in the last 30 years in Europe and the USA, with asthma becoming an epidemic phenomenon30 and atopic dermatitis doubling or tripling its prevalence.31
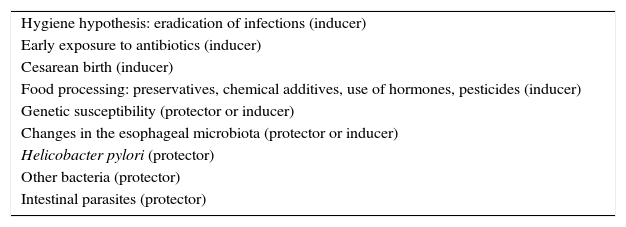
Even though the knowledge of the genetic bases of EoE has been defined in recent years,32 the epidemic behavior of the disease suggests that environmental causes are more powerful than genetic ones. It is not clear why food and environmental allergens that had been tolerated for many years in the affected countries became EoE triggers. The factors involved in the epidemic could also explain the low prevalence in developing countries, including Mexico. Those factors are most likely multiple and complex, but discussion of them presently amounts to speculation only, given that the majority have not been intentionally studied in relation to EoE, and the few existing studies are in the preliminary phase (Table 1). Nevertheless, recent epidemiologic observations have produced interesting data, which is discussed in the present article.
Factors that could be involved in eosinophilic esophagitis (EoE) as a cause of epidemic in developed countries and of low prevalence in developing countries.
| Hygiene hypothesis: eradication of infections (inducer) |
| Early exposure to antibiotics (inducer) |
| Cesarean birth (inducer) |
| Food processing: preservatives, chemical additives, use of hormones, pesticides (inducer) |
| Genetic susceptibility (protector or inducer) |
| Changes in the esophageal microbiota (protector or inducer) |
| Helicobacter pylori (protector) |
| Other bacteria (protector) |
| Intestinal parasites (protector) |
The “hygiene hypothesis” proposed by Okada et al. in 201033 could explain why individuals from developed countries have a more intense allergic reaction to foods and environmental allergens than they had in the past and why their reaction is stronger than that currently experienced by individuals from developing countries. According to this hypothesis, the reduction in the incidence of infections in individuals from the developed countries due to the pharmacologic eradication of bacteria and parasites is the origin of the increased incidence of allergic and autoimmune diseases, such as inflammatory bowel disease. In support of this hypothesis, over recent decades an increase in allergic diseases mediated by pathophysiologic mechanisms similar to EoE, such as asthma, allergic rhinitis, and atopic dermatitis,30 has been reported. The incidence of atopic dermatitis has doubled or tripled in developed countries over the past 3 decades.31 The mechanisms through which the “hygiene hypothesis” attempts to explain these phenomena are not well defined, but it proposes that the main one could be related to a redirection of the Th1 and Th2 inflammatory reactions. The helper T lymphocytes in the Th1 reaction produce inflammatory cytokines, such as interleukin IL-2, interferon-γ, and the tumor necrosis factor alpha (TNF-α), that act on cell-mediated immunity. In contrast, the helper T lymphocytes of the Th2 reaction produce the IL-4, IL-5, and IL-13 interleukins that contribute to the production of type E immunoglobulins (IgE) and eosinophil-mediated allergic responses. Some authors suggest that in developed countries, the reduced microbial burden in early childhood (that normally favors a strong Th1 inflammatory reaction) redirects the inflammatory response toward the Th2 phenotype, thus predisposing the individual to allergic disorders.
Helicobacter pyloriIn the context of the “hygiene hypothesis”, an inverse relation of Helicobacter pylori (H. pylori) and EoE has recently been reported. In an extensive study on biopsies from individuals in the US general population, Dellon et al. found that individuals with esophageal eosinophilia had a significantly reduced odds ratio of H. pylori, compared with individuals with normal biopsy. They also found a dose-response relation between the increase in the eosinophilic infiltrate and reduced prevalence of H. pylori.34 Another more recent study conducted in Germany confirmed those findings. In that case-control study with 58 patients, 5.2% of the patients with EoE had serologic evidence of H. pylori infection, compared with seroprevalence in 37.9% of the patients without EoE.35
In Mexico, H. pylori prevalence data obtained in a national survey on an open population was 66%36 and it reached 90% in some Central and South American countries.37,38 In contrast, prevalence in the developed countries was significantly lower, at 6 to 20%.39 The differences in prevalence between the two regions has been attributed to the exposure and contagion rates of H. pylori in childhood, which are high in the Latin American countries.40,41
It is interesting that since its characterization in 1980 and its consequent eradication, the prevalence of H. pylori has been significantly reduced in the US. In contrast, it has remained high or has been reduced very little in the less developed countries.42 In the US, H. pylori prevalence varies among the different racial groups that make up its population. It is lower in Caucasians (8-26%) and higher among African Americans and Latinos (52-54% and 48-64%, respectively).43–45 This intergroup difference is linked more to socioeconomic differences than to racial ones, as shown in the study by Malaty and Graham, conducted on a resident Hispanic population in the US, in which there was an inverse relation between H. pylori prevalence and socioeconomic level. The Hispanics from the high, middle, and low socioeconomic classes had prevalences of H. pylori of 6, 43, and 86%, respectively.46
In addition to the ethnic differences, it has been speculated that differences in climate and exposure to allergens could explain the unequal prevalence of EoE between developed and developing countries. The results of studies conducted in the US could be interpreted as contradicting those arguments. In those studies, in which the prevalence of EoE in different population groups was evaluated, EoE was reported to be significantly less frequent in Hispanics than in Caucasians, despite their being exposed to the same climate and the same environmental and food allergens. In a study conducted on an open population of more than 6 million individuals, of which 3,360 (0.05%) had EoE, 3,160 (94.04%) were Caucasian, 190 (5.65%) were African American, and only 10 (0.29%) were Hispanic.47 These results suggest that African American and Hispanic individuals may have “protective” factors against EoE and could also be arguments in favor of the inverse relation between H. pylori and EoE and of the hygiene hypothesis. The concomitant effect of genetic and racial susceptibility cannot be ruled out.
ParasitesBased on the “hygiene hypothesis”, intestinal parasitosis could be a protective factor against EoE, given that parasitosis prevalence is still high in the developing countries. However, this aspect has not been widely analyzed. In some studies in tropical and subtropical countries, there is evidence that Schistosoma infections have a strong protective factor against atopia,48 that Necator americanus possibly has a protective effect against asthma, and that the eradication of helminths increases atopic cutaneous sensitivity.49,50 However, in other studies, the eradication of intestinal parasites has not been observed to provide a protective factor against allergic diseases, particularly asthma.51 In addition, the proposed mechanism through which bacterial infections would reduce the risks for allergic diseases (redirection of the Th1 and Th2 inflammatory reactions) would not be the same for parasites, precisely because they induce Th2 reactions. However, other alternate mechanisms could be involved, such as antigen competence and immunoregulation. The first proposes that two immune responses caused by different antigens tend to inhibit each another.33 The second postulates that in an immunologic response, regulating T cells that suppress the immunologic responses different from the responses against the antigens in question could be activated.52 The theory about parasites is interesting, but further studies that evaluate the possible relation to EoE are required.
MicrobiotaThe human intestine is colonized with different strains of bacteria after birth. The gut microbiota is important for numerous physiologic functions, for maintaining the integrity of the mucosal barrier, and for regulating immunologic functions.53,54 In recent years it has been pointed out that alterations in the composition of the gut microbiota could be involved in the causes of some chronic diseases and neoplasias.55,56 In that context, the gut microbiota has been extensively studied. In contrast, the esophageal microbiota had not been characterized until recently. At present, there are few studies evaluating the alterations of the esophageal microbiota in EoE. In one study conducted on 33 children with EoE, 18 with active disease and 15 with inactive disease were compared with 35 controls. The modifications of the esophageal microbiome were evaluated after dietary manipulation and significant differences in microbiome composition were found in the children with active EoE, compared with the controls (Proterobacteria in EoE and Streptococcus and Atopobium genera in the controls), who experienced no modifications from dietary changes.57 In another study conducted on 70 children, there were significant differences in the composition of the esophageal microbiota in patients with EoE, compared with patients with gastroesophageal reflux and normal individuals. Hemophillus was significantly predominant in patients with EoE.58 Finally, another study found significant modifications in the esophageal microbiota with chronic PPI use in adults with gastroesophageal reflux disease, Barrett's esophagus, and esophageal and gastric adenocarcinoma.59,60 Even though chronic PPI use has not been demonstrated to be a risk factor for EoE, it has been cited as a possible cause through alternative mechanisms to those related to acid suppression.61
Although the findings of those studies are interesting, modifications in the esophageal microbiota cannot yet be ascertained to be the cause of EoE, but rather an effect of the disease.62 Further studies with respect to this are needed.
Possible predominance of an attenuated clinical phenotype of eosinophilic esophagitis in developing countriesIn developing countries, EoE is a chronic disease with an elevated clinical and pathologic recurrence rate when treatment is suspended.63,64 Recent studies have shown that it has a tendency to progress from the inflammatory phenotype (that occurs in children) to the fibrostenotic phenotype (that occurs in adults), resulting in esophageal stricture and food impaction.65,66 These complications are more frequent as the progression time of the disease increases, and are seen in 70% of the cases that have had symptomatic activity for more than two decades.67,68
In a 2011 study published by Richter et al., conducted at a single hospital center in the US on 64 patients with esophageal eosinophilia detected through pathology reports, and with a 10-year follow-up (81% Caucasians, 12% African Americans, and 6% Hispanics), the following interesting data were found: the African Americans and Hispanics had a clinically atypical form of the disease characterized by advanced age, a greater frequency of gastroesophageal reflux disease symptoms, less dysphagia for solid foods, and fewer alterations related to fibrosis (circumferential rings) at endoscopy, compared with the Caucasians. The authors concluded that those intergroup differences in the clinical behavior of esophageal eosinophilia could be due to differences in the phenotype of the disease itself or to the presence of different clinical entities.69 The results of that study were confirmed in 2016 in an extensive multicenter study conducted at 5 large US hospital centers on 793 patients with EoE (476 adults and 317 children), of which 83% were Caucasian, 10% were African American, and 7% were Hispanics and Asians. As in the study by Richter et al., the non-Caucasian groups had an atypical “benign” form of the disease, given that they presented with a significantly lower frequency of dysphagia (56 and 53% vs 74%) and esophageal food impaction (13 and 13% vs 35%) than the Caucasians. They had a lower frequency of concentric rings and linear grooves at endoscopy. The authors concluded that EoE diagnosis should be considered in African Americans and Hispanics, even if they did not present with typical symptoms.70
The findings of those two studies could have broader implications. It must be remembered that PPI-REE is a condition that responds favorably to PPIs and is clinically and histologically indistinguishable from EoE.71,72 In the last consensus of the international working group on EoE in 2016, it was suggested that this clinical entity is a part of the EoE spectrum, possibly with different clinical and progressive characteristics.73 Its frequency in the patients with esophageal eosinophilia in general is from 50 to 75%.74,75 It has been suggested that PPIs could have a restoring effect on lesions in the esophageal mucosa produced by acid, increasing their permeability and making the penetration of allergens possible.76,77 PPIs have also been observed to have anti-inflammatory properties that are independent of acid suppression, inhibiting eotaxine-3 secretion.78,79 Until recently, the long-term natural history of this entity was unknown. A retrospective study on 94 patients demonstrated that patients with PPI-REE, compared with EoE, had significantly less risk for distal stricture and narrowings of esophageal caliber after 20 years of symptoms (30.2 vs 72.3%).80 At present it is not known if there are racial differences in the distribution of this entity.
The results of the studies discussed above suggest that non-Caucasian individuals (Hispanics and African Americans) could possess “protective” or attenuating factors for EoE, particularly against the fibrostenotic phenotype of the disease. It is possible that in developing countries an attenuated inflammatory phenotype prevails that has “atypical” clinical characteristics and is sensitive to PPIs.71 In the two studies published in Mexico in 2011 and 2016, a predominance of typical clinical characteristics was described, but they were small case series.17,81 The quantity and characteristics of the underdiagnosed patients in Mexico is not known and the long-term clinical outcome has not been evaluated. In our experience with 8 patients seen at our hospital since 2007, none of them has required esophageal dilations and only one presented with food impaction at the time of diagnosis (personal data not published).
Conclusions and recommendationsIn conclusion, the incidence of EoE in developed countries has been increasing rapidly in recent years, with epidemic characteristics. In contrast, prevalence is very low in developing countries, including Mexico. The causes of this are unknown. The epidemic behavior of EoE, as in other allergic disease, suggests that environmental causes are more powerful than possible genetic or racial susceptibility. The imputable causes are new environmental reagents and/or immunologic hypersensitivity to allergens. Current theories postulate that this hypersensitivity could have been induced by exacerbation of personal and community hygiene and the eradication of non-lethal infectious diseases, as a result of improved socioeconomic levels. The eradication of H. pylori, gastrointestinal parasites, alterations in the ecology of the gut and esophageal microbiota due to indiscriminate antibiotic use, cesarean births, and the industrial processing of foods with additives, preservatives, pesticides, etc., have been cited as possible causes.82 These theories provide the mechanistic bases for future research on the relation between the etiopathogenesis and therapeutics of the disease.
On the other hand, based on evidence described in the present article, EoE in developed countries could predominantly be a more attenuated, clinically “atypical” phenotype, manifested mainly as reflux symptoms, related more to inflammation than to esophageal fibrosis (fig. 2), and with an elevated response to PPIs, similar to what is known as PPI-responsive esophageal eosinophilia. However, given its low prevalence, clinical trials, preferably multicenter ones, are needed to confirm this.
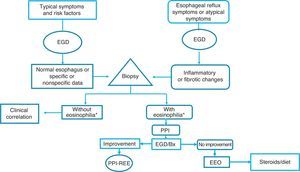
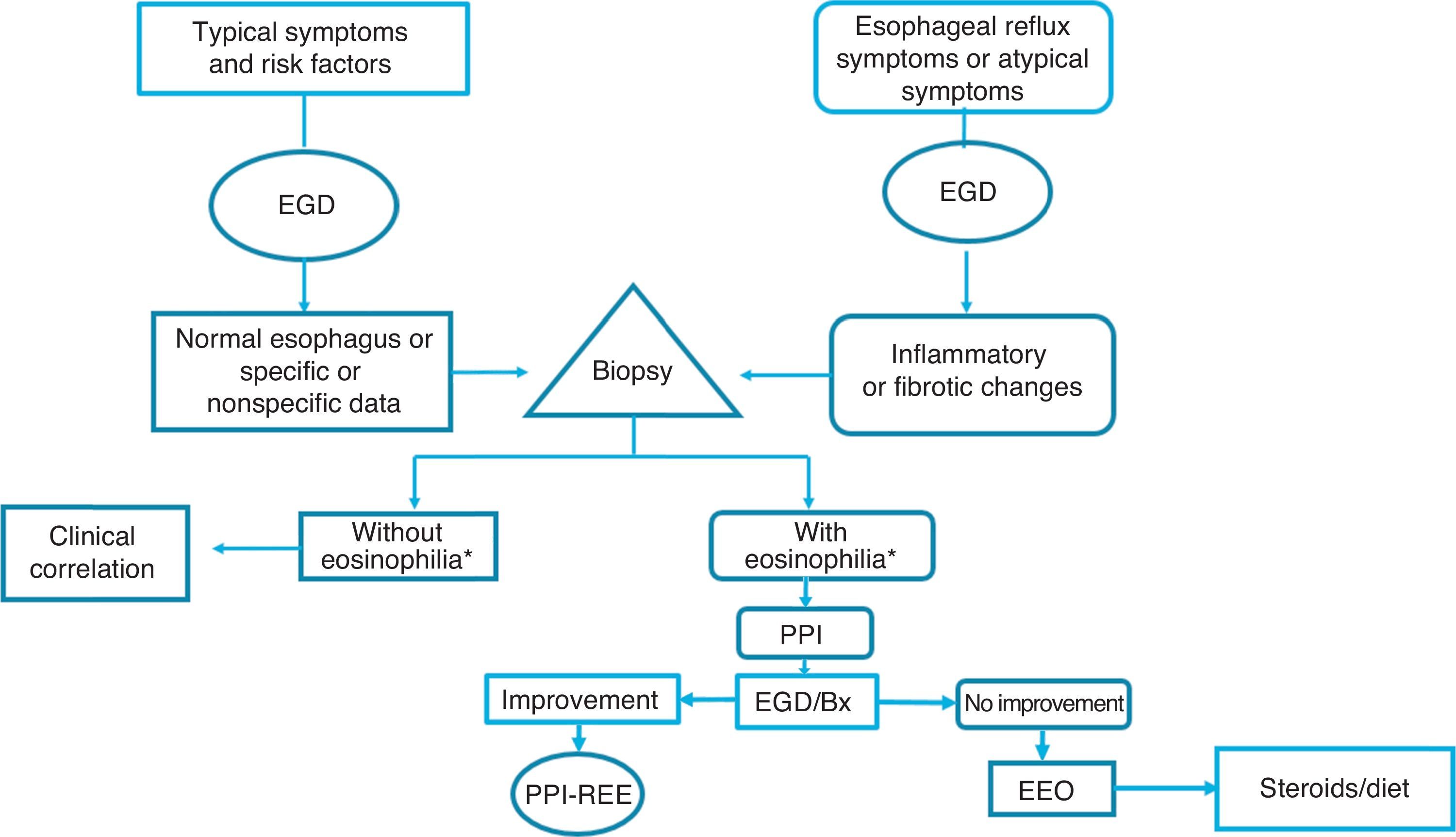
Given the above, we consider that in the Latin American countries, EoE should definitely be suspected in individuals with the classic known risk factors (young persons with dysphagia and atopy), but also in older subjects with symptoms of gastroesophageal reflux and endoscopic data of inflammation of the esophageal mucosa (whitish, spotted exudates, fragile and crepe paper mucosa), in whom biopsies should be taken from the distal and proximal esophagus. If esophageal eosinophilia is found, gastroesophageal reflux should be ruled out through 24-h outpatient esophageal pH monitoring or a PPI trial, after which it is imperative to repeat endoscopic and histologic control. If the endoscopic alterations and eosinophilia disappear, the disease should be considered PPI-REE, and EoE diagnosis should be made only if there is no histologic improvement, treating the disease with topical steroids or dietary restrictions (fig. 3).
Diagnostic and treatment algorithm for esophageal eosinophilia and eosinophilic esophagitis proposed for Latin American populations.
*Eosinophilia: ≥ 15 eosinophils/high power field; EGD: esophagogastroduodenoscopy; EoE: eosinophilic esophagitis; PPI: proton pump inhibitor; Bx: biopsy; PPI-REE: PPI-responsive esophageal eosinophilia.
The knowledge of the clinical and progressive characteristics of esophageal eosinophilia of a good number of patients in Latin America will contribute to improved clinical practice and establish the bases formulating diagnostic and treatment guidelines adapted to those populations.
Financial disclosureNo financial support was received in relation to this article.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: García-Compeán D, González-González JA, González-Moreno EI, Maldonado-Garza HJ. La esofagitis eosinofílica. ¿El Norte contra el Sur? Enfoque mecanicista bio-económico-social e implicaciones clínicas. Revista de Gastroenterología de México. 2017;82:328–336.


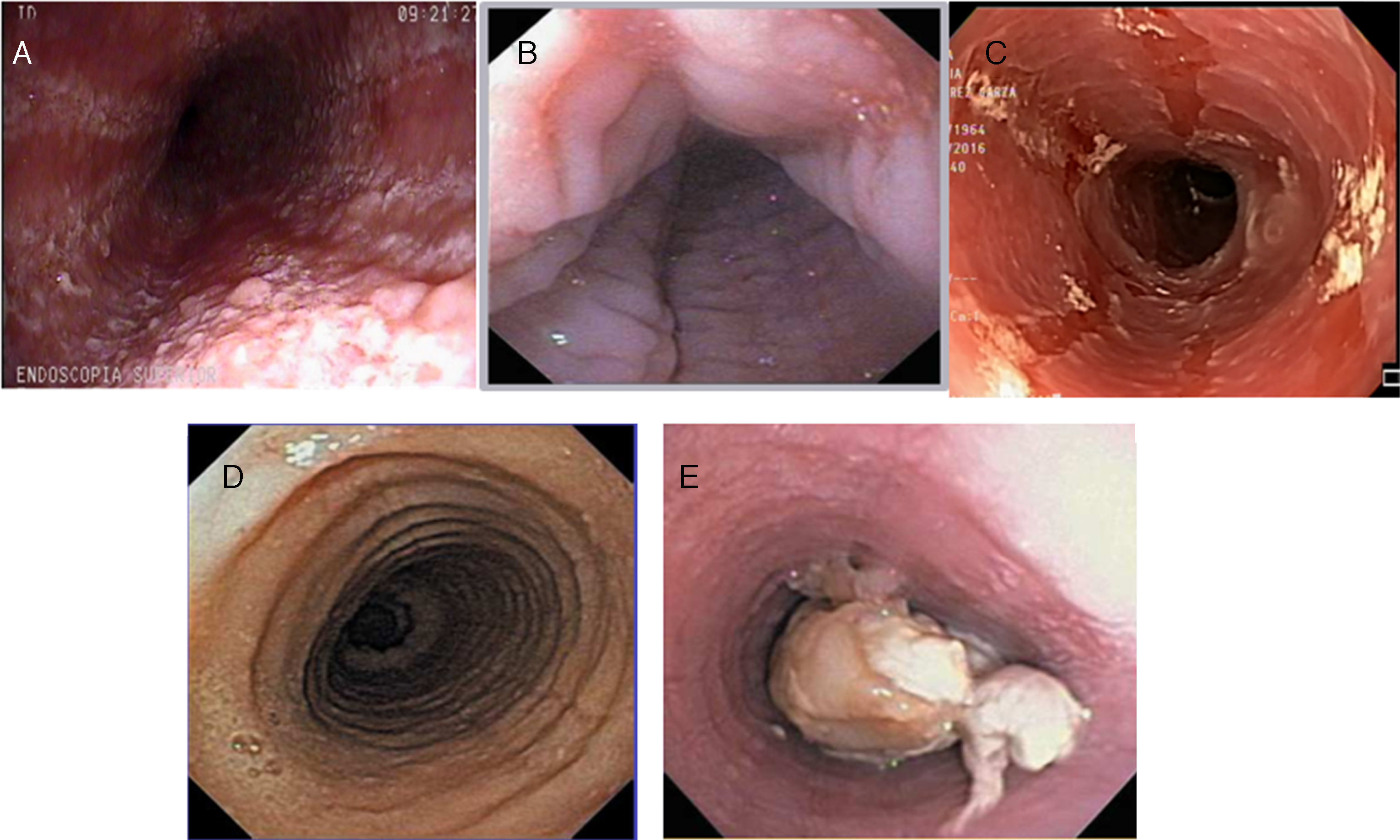
![Inflammatory phenotype (A: whitish and mottled; B: longitudinal grooves; and C: mucosal edema [crepe paper]) and fibrostenotic phenotype (D: rings; E: stricture with food impaction) of eosinophilic esophagitis at endoscopy. Inflammatory phenotype (A: whitish and mottled; B: longitudinal grooves; and C: mucosal edema [crepe paper]) and fibrostenotic phenotype (D: rings; E: stricture with food impaction) of eosinophilic esophagitis at endoscopy.](https://static.elsevier.es/multimedia/2255534X/0000008200000004/v1_201712030700/S2255534X1730066X/v1_201712030700/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w97o/wdEXW47bqlyT1CqG6R0=)